1. Introduction
Hydrogen is a promising clean energy source with a wide range of applications, and the development of hydrogen energy systems is also full of opportunities and challenges [
1]. In the refinery industry, many hydrotreatment and hydrogenation [
2,
3] units consume hydrogen, making the cost of purchasing fresh hydrogen a significant proportion of the total annual cost (TAC). It is crucial to consider the rational utilization of hydrogen, which is called the hydrogen network (HN), among the various units within a refinery. This should include the optimization of hydrogen allocation, reaction, and treatment. However, it is a challenge to determine the optimal HN due to the vast number of potential solutions. Consequently, HN synthesis is introduced to solve these issues with the objective of achieving the optimal utilization of hydrogen and maximizing economic benefits. There are two principal approaches for HN synthesis: graphical and mathematical methods.
The graphical method primarily utilizes pinch analysis [
4,
5,
6], which offers more interpretable results in comparison with the mathematical method. For instance, Gai et al. [
7] utilized the pinch analysis method to handle hydrogen allocation problems concerning different fresh hydrogen quality and purification systems. However, the graphical method is limited in addressing complex HN optimization issues involving multiple impurities, compressors, and process units. These challenges can be addressed using the mathematical method of building and solving the mathematical model. It was initially proposed by Hallale et al. [
8] and has undergone extensive development. The recent studies of mathematical methods can be classified into two different categories.
The first category focuses on integrating the HN mathematical model with other techniques, with the goal of extending the applicability of an HN. For instance, Da Silva et al. [
3] integrated product design with the HN model which allowed for the adjustment of hydrogen allocation based on product requirements. In addition, there are studies focusing on risk management [
9] and flexibility analysis [
10,
11] for increasing the stability of an HN with uncertain reaction processes. Other studies focus on integrating the HN with more accurate models of reactor units and purification units in order to enhance the efficiency of these process units further. Machine learning methods [
12,
13] are widely utilized to construct surrogate models of units alongside the direct adoption of rigorous mathematical models [
14,
15,
16]. However, the hydrogen allocation is typically regulated within a fixed structure of the network in these works.
In the second category, the focus is on developing more effective and solvable superstructure-based HN mathematical models. For instance, Zhao et al. [
17] conducted a study on the problem of hydrogen network synthesis containing multiple impurities in closer alignment with the actual refinery environment. In addition, Chang et al. [
18] introduced a novel HN model with high computational efficiency in 2021. In recent research, innovative superstructures have been proposed with regard to the compressor layout in HNs which can reduce the expense of the compression process [
19,
20]. In 2022, Huang et al. [
21] introduced an efficient HN model accounting for all potential hydrogen pressurizing paths. In 2023, Zhou et al. [
22] constructed an HN involving the selection strategy for various types of compressors, which is closer to the real refinery. In addition to the compressors, the purification system within the HN is of significant importance because the cost of purchasing fresh hydrogen far exceeds the cost of recovering hydrogen through the purification system [
23].
After processing with a purification unit, a hydrogen stream can be separated into two distinct streams: a product stream with a higher hydrogen purity and a tail-gas stream with a lower hydrogen purity. The major hydrogen purification units in refineries are membrane separation (MS) and pressure swing adsorption (PSA). The MS unit exhibits high recovery rates, relatively low product purity, and low equipment costs, while PSA is conversely characterized by relatively low recovery rates, high product purity, and high equipment costs [
24]. There has been a significant corpus of the literature dedicated to the mathematical modeling of MS units [
25] and PSA units [
26] in the field of gas separation.
To further enhance the recovery rate, yield, and capacity of these purification processes, it is common to optimize the relevant parameters and integrate multiple purification units. For example, multi-stage MS units are widely utilized not only for gas separation [
27,
28,
29,
30,
31,
32] but also for wastewater recycling [
33,
34]. Additionally, the coupling of PSA and MS units is also an effective strategy for gas purification [
35]. However, the optimal configuration of purification units as well as the hydrogen allocation are dependent on the specific scenario, making it difficult to identify the optimal solution through empirical means. The HN superstructure model integrated with the purification system can be utilized to solve this problem. In 2004, Liu et al. [
24] integrated a single PSA and a single MS unit into the HN to optimize its configuration and the operating pressure of the MS. In 2010, Liao et al. [
36] employed the state-space superstructure HN model to retrofit the original HN with additional MS units and compressors which further reduced the fresh hydrogen consumption. However, the parameters of the purification unit were held constant. Subsequently, in 2016, Liao et al. [
37] conducted a comparative study on four short-cut models related to PSA and MS units within HN, investigating the effects of pressure ratio, selectivity, feed concentration, and flow on the minimum usage of fresh hydrogen.
In conclusion, the preceding HN models are deficient in their comprehensive configuration of purification units, and the relevant parameters are generally set to constant values. With improvements in the quantity and quality of hydrogen consumption, there is an absence of an HN superstructure model that can adequately optimize the multi-stage and multi-technology purification system. Expanding on present research, a parameter analysis of purification models is conducted initially, which considers the impact of the parameters on its capacity and the possibility of coupling it with additional purification units. Subsequently, this work aims to introduce a comprehensive HN superstructure model by including a richer combination of purification units. The HN model is applied in a series of refinery scenarios to demonstrate further the benefits of integrating multiple purification units in terms of economic and system efficiency. The following are the key points of this article:
The impact of purification parameters on the separation effect is illuminated through a model analysis, which demonstrates the feasibility of integrating multiple purification units;
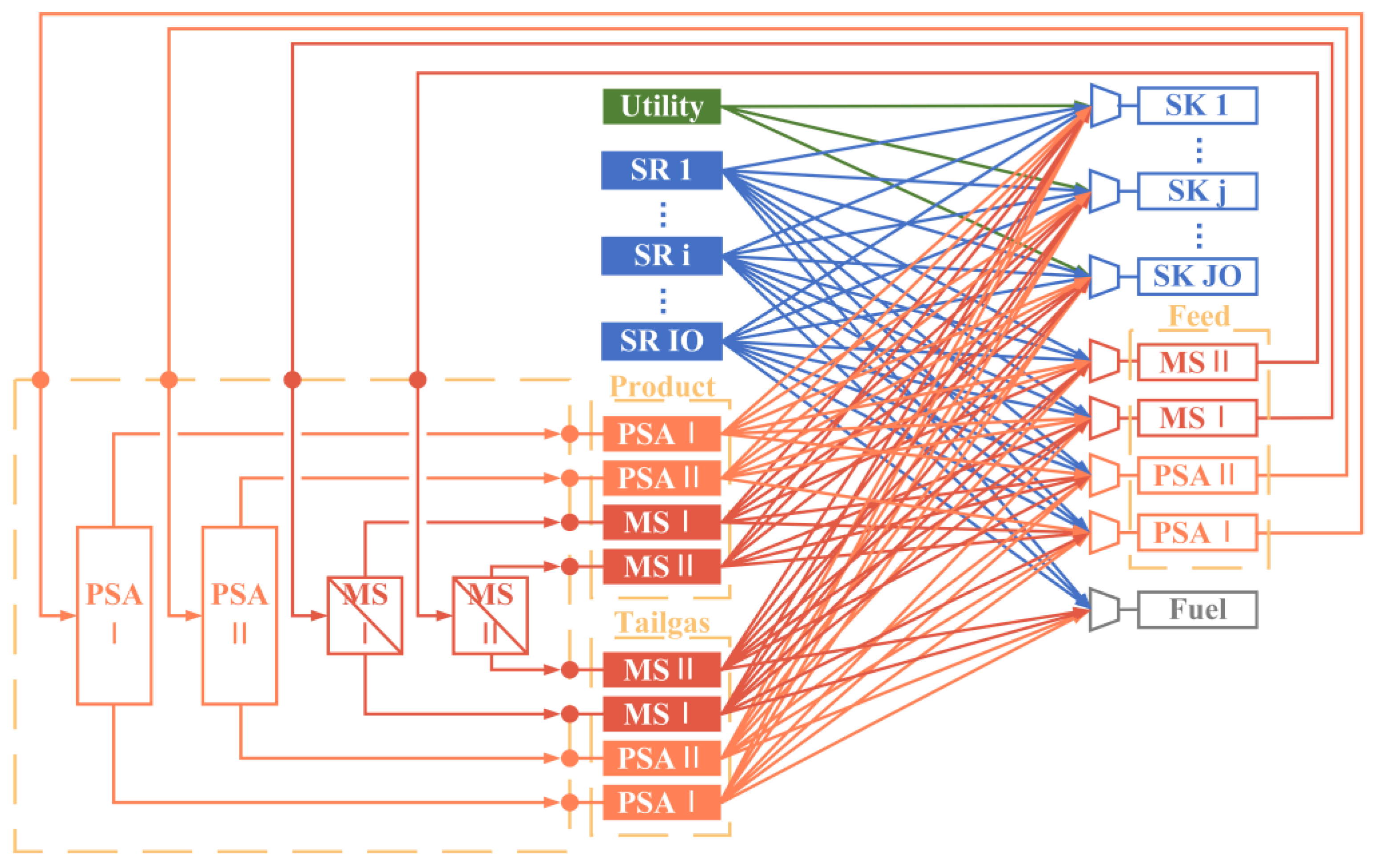
An HN superstructure model integrated with the multi-stage MS and PSA units is constructed, offering a wider range of potential solutions;
The model considers simultaneously optimizing the critical parameters of the purification system, including the operating pressures, feed flow rate, recycle ratio, and so on.
4. Conclusions
The HN synthesis considering purification systems makes an outstanding contribution to both hydrogen energy savings and improved economics. This study has two main parts to make this method better for the aforementioned sides. Firstly, it introduces a multi-unit purification system to address the broader range of problems associated with HN synthesis. Secondly, it is conducted to simultaneously optimize the structure and parameters of an intricate purification system.
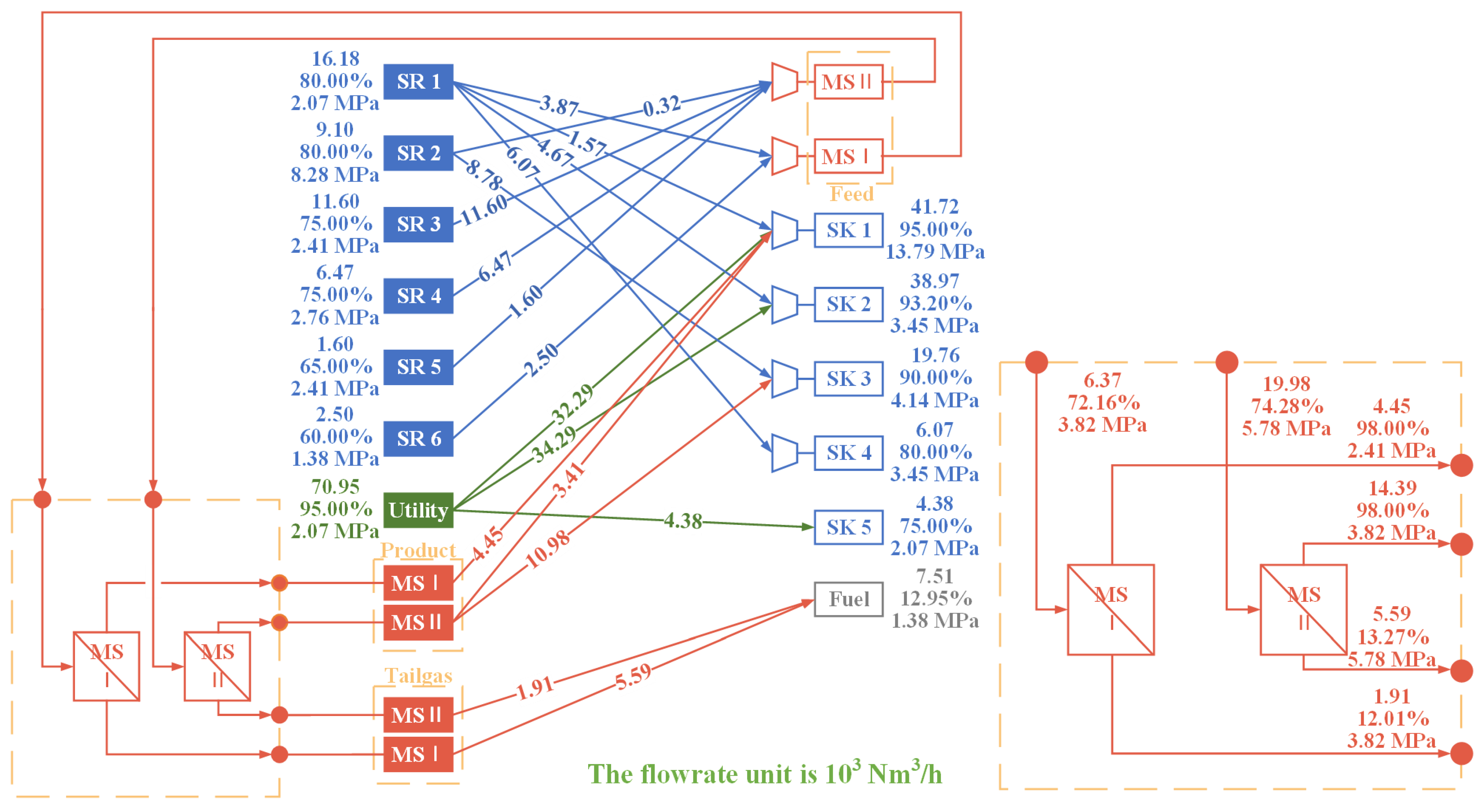
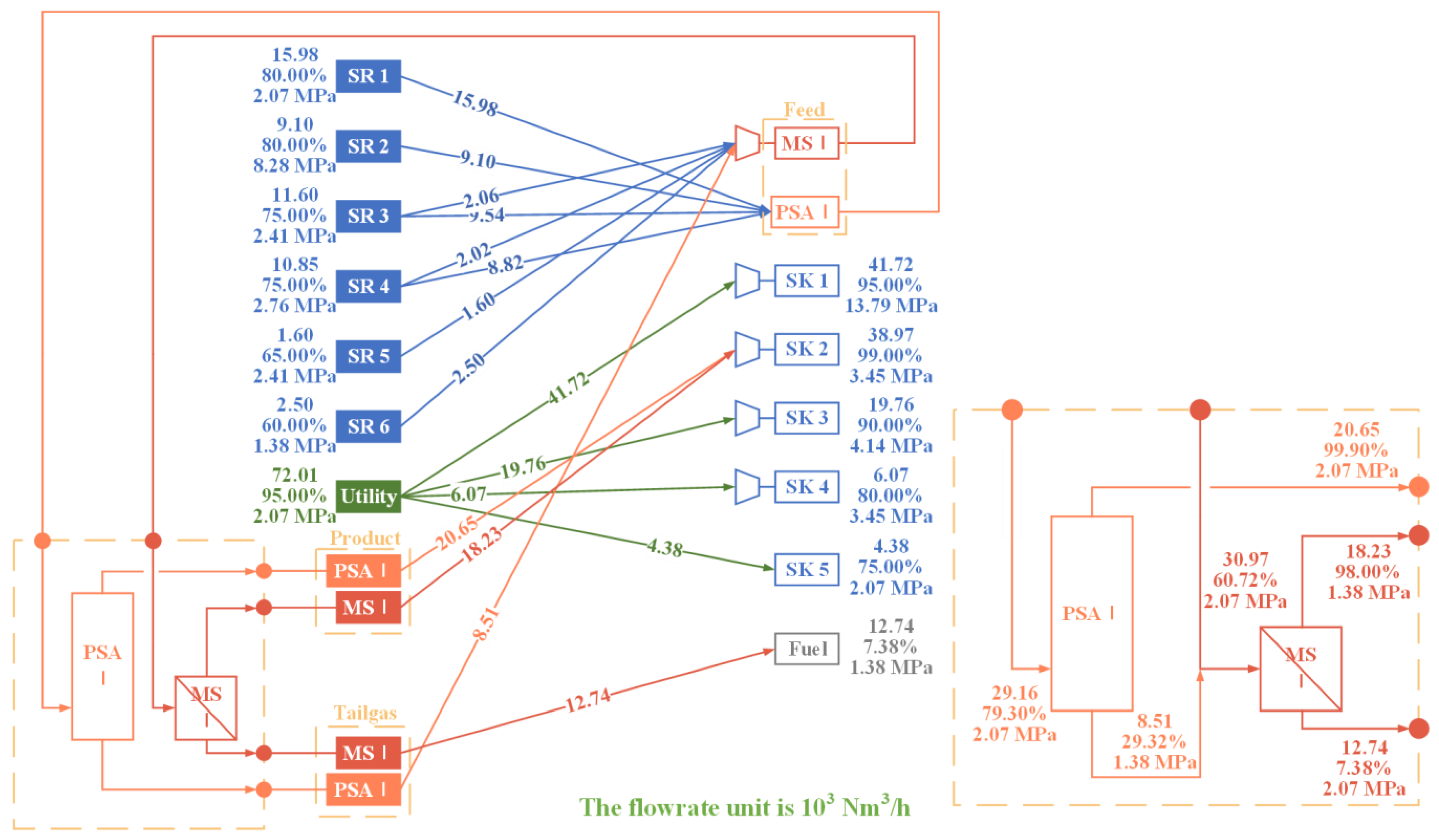
A parameter analysis is conducted first to gain further insight into the influence of operating parameters on the purification units and to illustrate the necessity of a multi-unit purification system. The findings indicate that the capacity of a single purification unit is limited, which can be enhanced by the implementation of a multi-unit purification system. Accordingly, an HN superstructure model incorporating a multi-stage and multi-technology purification system has been proposed with the objective of a minimum TAC. The model optimizes the structure and the relevant parameters, notably the operational pressures, of the purification system within the HN. In addition, the optimal results also affirm the advantages of a multi-unit purification system. It is demonstrated that PSA units are particularly effective in producing high-purity product streams. In contrast, membrane separation units offer superior economic viability in other circumstances. A low-pressure MS unit, which is characterized by low equipment cost, is typically included in the system to treat the tail gas from the other purification units. Compared to a single purification unit, it is possible to further reduce the TAC of HN with a multi-stage purification system by 2–22% and improve both the recycle ratio and yield of the purification system. Furthermore, the optimized purification system has enhanced recovery and yield, which additionally conserves hydrogen energy.
Further optimization of the HN model represents a valuable avenue for future research. Firstly, the incorporation of more accurate economic calculation and process unit models can acquire more realistic optimization results. These include the multicomponent hydrogen stream, the non-ideal compressor model, and the rigorous purification unit model. Moreover, the model functionality can be enhanced regarding fluctuations in cost and feed quality from a robustness and flexibility standpoint.