Evaluation of the Corrosion Resistance of 904L Composite Plate in a High-Temperature and High-Pressure Gas Field Environment
Abstract
1. Introduction
2. Experiment
2.1. Experimental Materials
2.2. Experimental Methods
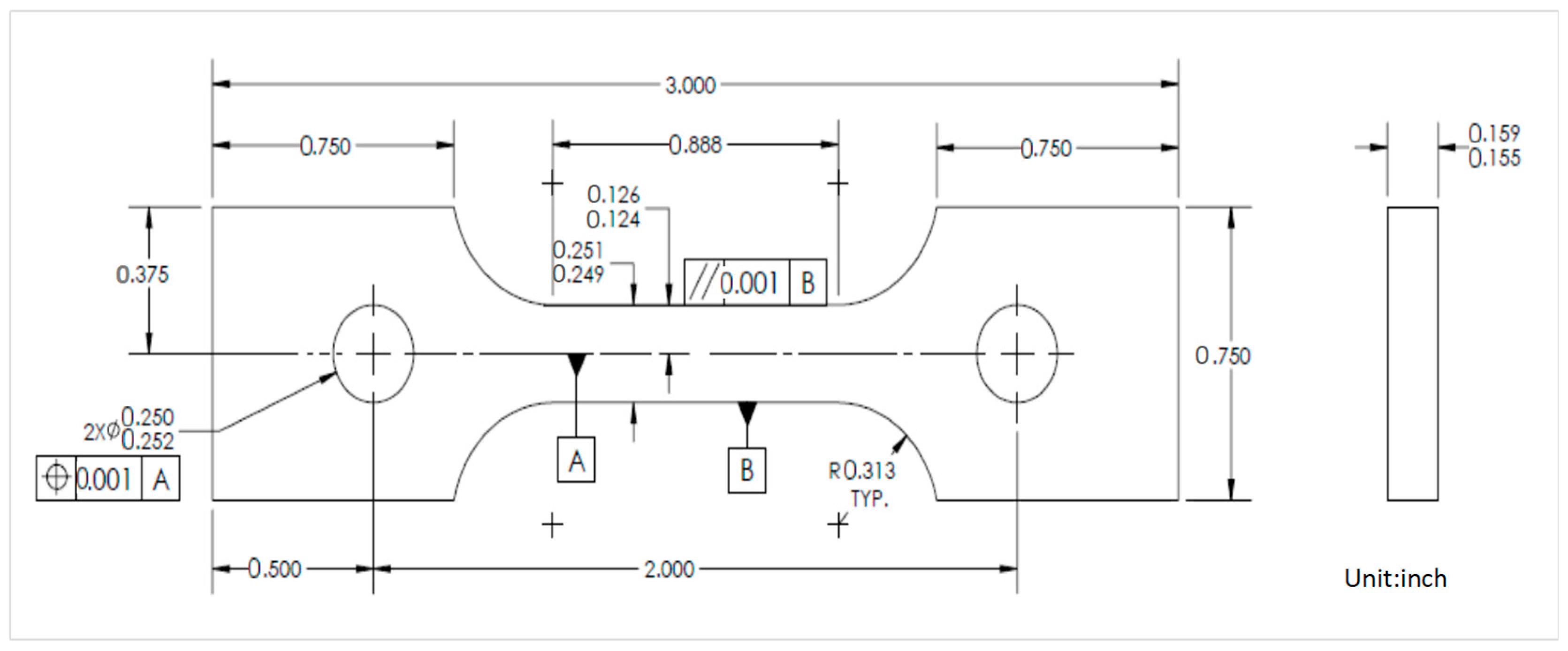
2.2.1. Electrochemical Test
2.2.2. Iron Trichloride Pitting Test
2.2.3. Weight Loss Test of High-Temperature Autoclave
2.2.4. Chloride Stress Corrosion Cracking Test
3. Results
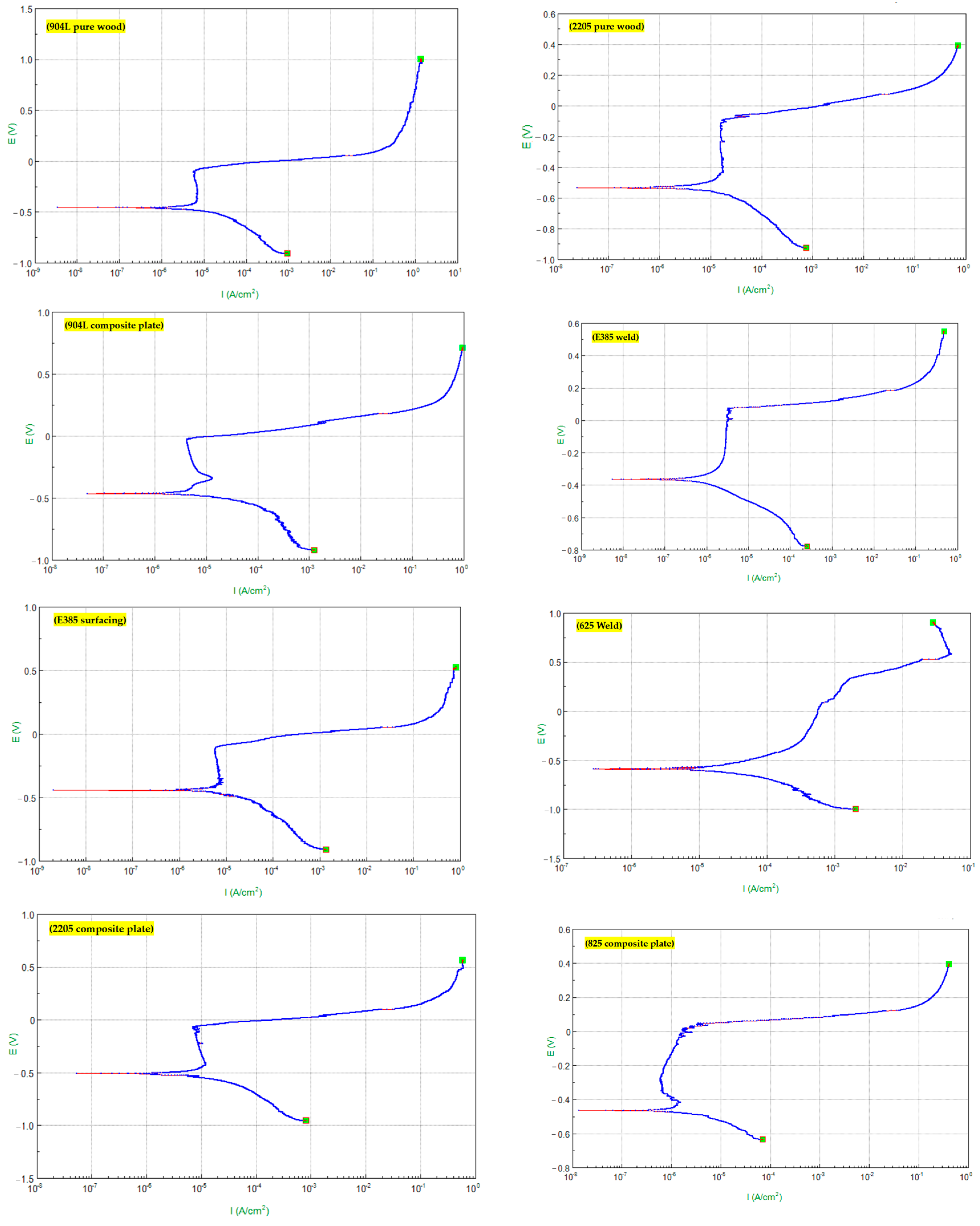
3.1. Pitting Resistance
3.2. Corrosion Resistance Under Simulated Working Conditions
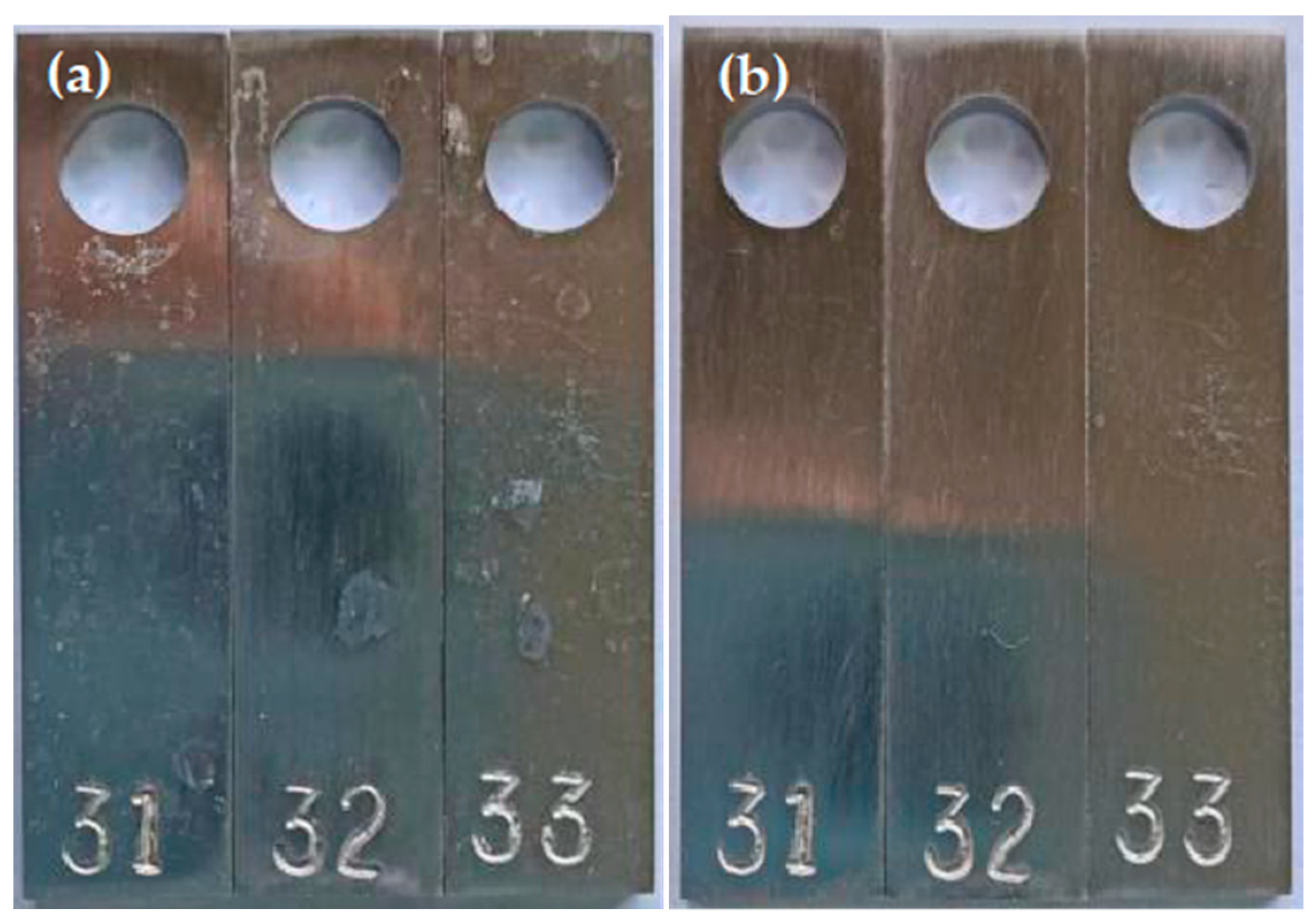
3.3. Resistance to Chloride Stress Corrosion Cracking
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, J.; Li, J.; Wang, W.; Dou, Y.; Yang, X.; Zhang, Y. Establishment and application of temperature–pressure coupling model for opening and closing wells in HTHP gas wells. Energy Explor. Exploit. 2024, 42, 1359–1385. [Google Scholar] [CrossRef]
- Ji, N.; Zhao, M.; Wu, Z.; Wang, P.; Feng, C.; Xie, J.; Long, Y.; Song, W.; Xiong, M. Collapse failure analysis of S13Cr-110 tubing in a high-pressure and high-temperature gas well. Eng. Fail. Anal. 2023, 148, 107187. [Google Scholar] [CrossRef]
- Li, L.; Chen, Q.; Fang, Y.; Li, X.; Luo, J.; Song, C.; Wang, S. Failure analysis of 2205 duplex stainless steel connecting pipe for composite plate pressure vessel in a high pressure gas field. Int. J. Press. Vessel. Pip. 2023, 202, 104921. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, S.; Xu, M.; Cheng, Y.; Dong, M. Effect of Heat Treatment on Microstructure and Properties of Clad Plates 316L/Q370qE. Materials 2019, 12, 1556. [Google Scholar] [CrossRef]
- Ceyhun, K. Effect of post-weld heat treatment on microstructure, crystallography, and mechanical properties of laser beam welded AISI 904L super austenitic stainless steel. Eng. Fail. Anal. 2024, 158, 108025. [Google Scholar]
- Xu, D.; Zhang, X.; He, X. Mechanism and evaluation method of stress corrosion susceptibility of 904L stainless steel with optimized structure in seawater. Corros. Sci. 2024, 229, 111865. [Google Scholar] [CrossRef]
- Aftimi, S.; Kerroum, Y.; Guenbour, A.; Bellaouchou, A.; Idrissi, H.; Boulif, R.; Semlal, N.; Warad, I.; Zarrouk, A. New Insights on the Corrosion–Erosion Behavior of 904L Stainless Steel in Phosphoric Acid Containing Impurities. J. Bio- Tribo-Corros. 2024, 10, 1. [Google Scholar] [CrossRef]
- Bai, L.; Peng, W.; Li, W.; Shi, X. Effect of titanium element on high temperature chlorine corrosion properties of 904 L alloy. Corros. Sci. 2023, 211, 110915. [Google Scholar] [CrossRef]
- Mändl, S.; Manova, D. Comparison of Nitriding Behavior for Austenitic Stainless Steel 316Ti and Super Austenitic Stainless Steel 904L. Metals 2024, 14, 659. [Google Scholar] [CrossRef]
- Prabu, S.S.; Muthu, S.M.; Beemkumar, N.; Sujai, S.; Arvind, M.; Kailash, K.; Midhun Chandran, P.M.; Ramkumar, S. Microstructure and mechanical assessment of Inconel 625 and AISI 904L dissimilar joints using high-density welding technique for pressure vessels. Int. J. Press. Vessel. Pip. 2024, 209, 105191. [Google Scholar] [CrossRef]
- Wang, J.; Shi, W.; Xiang, S.; Ballinger, R.G. Study of the corrosion behaviour of sensitized 904L austenitic stainless steel in Cl-solution. Corros. Sci. 2021, 181, 109234. [Google Scholar] [CrossRef]
- Kiey, S.A.; Meguid, E.A.E.; Rehim, S.S.E. Electrochemical Investigations on the Corrosion Behavior of 904L Stainless Steel in LiBr Solutions. J. Mater. Eng. Perform. 2023, 32, 9163–9173. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Q.; Yue, Z.; Hou, F. Corrosion Performance Study of 904L (N08904) Stainless Steel Composite Plate. Mater. Dev. Appl. 2017, 32, 53–58. [Google Scholar]
- ASTM G150; Standard Test Method for Electrochemical Critical Pitting Temperature Testing of Stainless Steels and Related Alloys. ASTM International: West Conshohocken, PA, USA, 2018.
- Zhang, B.; Zhou, J.; Zheng, M.; Jiang, X.; Guo, J.; Zhang, T. Study on Heat Treatment Process of 904L+14Cr1MoR (H) Composite Plate. Corros. Prot. Petrochem. Ind. 2015, 32, 12–17. [Google Scholar]
- ASTM G48-11; Standard Test Methods for Pitting and Crevice Corrosion Resistance of Stainless Steels and Related Alloys by Use of Ferric Chloride Solution. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM G111-21a; Standard Guide for Corrosion Tests in High Temperature or High Pressure Environment, or Both. ASTM International: West Conshohocken, PA, USA, 2021.
- GB/T 15970.1-2018; Corrosion of Metals and Alloys—Stress Corrosion Testing—Part 1: General Guidance on Testing Procedures. Standardization Administration of China: Beijing, China, 2018.
- Liu, X.; Zhu, X.; Liu, Z. Role of Cl/F Ions Concentration, pH and Temperature on Pitting Corrosion Behavior of 2507 Duplex Stainless Steel. J. Phys. Conf. Ser. 2024, 2731, 12053. [Google Scholar] [CrossRef]
- Nuthalapati, S.; Kee, K.E.; Ismail, M.C.; Jumbri, K.; Pedapati, S.R. Comparative study of corrosion behaviour and microstructural analysis of as-received and sensitized SS304 U-bend samples under perlite thermal insulation using chloride drip test. Eng. Fail. Anal. 2024, 159, 108054. [Google Scholar] [CrossRef]
- Yoo, J.S.; Chung, N.T.; Lee, Y.H.; Kim, Y.W.; Kim, J.G. Effect of Sulfide and Chloride Ions on Pitting Corrosion of Type 316 Austenitic Stainless Steel in Groundwater Conditions Using Response Surface Methodology. Materials 2023, 17, 178. [Google Scholar] [CrossRef]
- Geng, Y.; Liu, Z.; Zeng, W.; Feng, Y.; Ding, B. How to Choose the Suitable Steel of Wellhead, Wellbore, and Downhole Tools for Acid Gas Reinjection Flooding. Processes 2022, 10, 2685. [Google Scholar] [CrossRef]
- Hoa, L.Q.; Bäßler, R.; Bettge, D.; Buggisch, E.; Schiller, B.N.; Beck, M. Corrosion Study on Wellbore Materials for the CO2 Injection Process. Processes 2021, 9, 115. [Google Scholar] [CrossRef]
- de Souza, L.M.; Pereira, E.; Amaral, T.B.; Monteiro, S.N.; de Azevedo, A.R. Corrosion Study on Duplex Stainless Steel UNS S31803 Subjected to Solutions Containing Chloride Ions. Materials 2024, 17, 1974. [Google Scholar] [CrossRef]
- Li, B.; Lang, Y.; Chen, H.; Qu, H.; Feng, H.; Sun, X.; Tian, Z. Studies on the Cooperative Influence of Cr and Mo on the Pitting Corrosion Resistance of Super Austenitic Stainless Steels. Materials 2023, 16, 7397. [Google Scholar] [CrossRef] [PubMed]
- Kannan, T.; Murugan, N. Effect of Welding Parameters on Pitting Resistance Equivalent Number of Duplex Stainless Steel Clad Metals. Indian Weld. J. 2006, 39, 18. [Google Scholar] [CrossRef]


| Composite Plate Material | Heat Treatment Parameters After Explosive Composite | Heat Treatment Parameters After Welding |
|---|---|---|
| 904L + Q345R | 1010 °C/10~20 min, air cooling to ≤400 °C after air cooling | 580 °C/2 h, furnace cooling to ≤400 °C after air cooling |
| 825 + Q345R | 940 °C/20~30 min, air cooling to ≤400 °C after air cooling | 580 °C/2 h, furnace cooling to ≤400 °C after air cooling |
| 2205 + Q345R | 1050 °C/10~20 min air cooling to ≤400 °C after air cooling | 580 °C/2 h, furnace cooling to ≤400 °C after air cooling |
| Degree of Corrosion | Uniform Corrosion Rate (mm/a) | Maximum Pitting Rate (mm/a) |
|---|---|---|
| Light | <0.025 | <0.13 |
| moderate | 0.025–0.12 | 0.13–0.20 |
| Severe | 0.12–0.25 | 0.21–0.38 |
| Extremely severe | >0.25 | >0.38 |
| Stress Corrosion Sensitivity Coefficient | Stress Corrosion Sensitivity |
|---|---|
| >35% | Have a significant tendency to stress corrosion |
| 25~35% | Some tendency to stress corrosion |
| Less than 25% | No significant tendency to stress corrosion |
| No. | Material | Pitting Potential Eb (V) | Critical Current Density ip (μA/cm2) |
|---|---|---|---|
| (a) | Pure 904L | 0.111 | 7.08 |
| (b) | Pure 2205 | 0.098 | 16.00 |
| (c) | 904L composite plate | 0.029 | 5.29 |
| (d) | E385 weld | 0.102 | 5.65 |
| (e) | E385 surfacing | 0.112 | 6.72 |
| (f) | 625 weld | 0.259 | 3.70 |
| (g) | 2205 composite plate | 0.072 | 9.09 |
| (h) | 825 composite plate | 0.044 | 0.86 |
| No. | Material | Weight Before Test (g) | Weight After Test (g) | Weight Loss (g) | Area (cm2) | Mass Loss (g/cm2) |
|---|---|---|---|---|---|---|
| (a) | Pure 904L | 15.9671 | 15.8196 | 0.1475 | 14.9688 | 9.85 × 10−3 |
| (b) | Pure 2205 | 14.3944 | 14.1123 | 0.2821 | 15.0859 | 1.87 × 10−2 |
| (c) | 904L composite plate | 12.9069 | 12.6761 | 0.2308 | 14.8641 | 1.55 × 10−2 |
| (d) | E385 weld | 11.9554 | 11.3134 | 0.642 | 14.5075 | 4.43 × 10−2 |
| (e) | E385 surfacing | 11.1224 | 10.2061 | 0.9163 | 14.2994 | 6.41 × 10−2 |
| (f) | 625 weld | 14.6628 | 14.6323 | 0.0305 | 14.8054 | 2.06 × 10−3 |
| (g) | 2205 composite plate | 14.0658 | 13.5457 | 0.5201 | 14.7791 | 3.52 × 10−2 |
| (h) | 825 composite plate | 13.4271 | 13.4089 | 0.0182 | 14.8457 | 1.23 × 10−3 |
| Material | Sample Number | Weight Before Test (g) | Length (mm) | Width (mm) | Thickness (mm) | Weight After Test (g) | Weight Loss (g) | Uniform Corrosion Rate (mm/a) | Average (mm/a) |
|---|---|---|---|---|---|---|---|---|---|
| Pure 904L | 11 | 8.5951 | 39.9 | 9.83 | 3.02 | 8.5949 | 0.0002 | 0.0006 | 0.0006 |
| 12 | 8.3942 | 39.94 | 9.89 | 2.94 | 8.3939 | 0.0003 | 0.0009 | ||
| 13 | 8.415 | 39.94 | 9.9 | 2.92 | 8.4149 | 0.0001 | 0.0003 | ||
| Pure 2205 | 21 | 8.3305 | 39.84 | 9.91 | 2.97 | 8.3303 | 0.0002 | 0.0006 | 0.0008 |
| 22 | 8.3211 | 39.84 | 9.82 | 2.99 | 8.3208 | 0.0003 | 0.0009 | ||
| 23 | 8.1886 | 39.9 | 9.91 | 2.92 | 8.1883 | 0.0003 | 0.0009 | ||
| 904L composite plate | 31 | 8.6541 | 39.69 | 9.97 | 3.00 | 8.654 | 0.0001 | 0.0003 | 0.0003 |
| 32 | 8.6359 | 39.7 | 9.95 | 2.98 | 8.6358 | 0.0001 | 0.0003 | ||
| 33 | 8.6296 | 39.64 | 9.98 | 3.02 | 8.6295 | 0.0001 | 0.0003 | ||
| E385 weld | 3A | 7.9332 | 39.76 | 9.88 | 2.79 | 7.933 | 0.0002 | 0.0006 | 0.0009 |
| 3B | 8.0547 | 39.75 | 9.88 | 2.82 | 8.0541 | 0.0006 | 0.0018 | ||
| 3C | 8.2399 | 39.74 | 9.92 | 2.88 | 8.2398 | 0.0001 | 0.0003 | ||
| E385 surfacing | 3D | 8.531 | 40.15 | 9.99 | 2.94 | 8.5309 | 0.0001 | 0.0003 | 0.0002 |
| 3E | 8.1471 | 40.21 | 10.05 | 2.77 | 8.147 | 0.0001 | 0.0003 | ||
| 3F | 7.9005 | 40.12 | 10.08 | 2.72 | 7.9005 | 0 | 0 | ||
| 625 weld | 4A | 8.804 | 39.64 | 10 | 2.96 | 8.8036 | 0.0004 | 0.0011 | 0.0015 |
| 4B | 8.7926 | 39.73 | 9.99 | 2.97 | 8.7923 | 0.0003 | 0.0009 | ||
| 4C | 8.9265 | 39.72 | 9.98 | 2.98 | 8.9256 | 0.0009 | 0.0026 | ||
| 2205 composite plate | 51 | 8.2491 | 39.88 | 9.92 | 2.92 | 8.2483 | 0.0008 | 0.0025 | 0.0012 |
| 52 | 7.9776 | 39.87 | 9.83 | 2.90 | 7.9775 | 0.0001 | 0.0003 | ||
| 53 | 8.1173 | 39.85 | 9.89 | 2.89 | 8.117 | 0.0003 | 0.0009 | ||
| 825 composite plate | 61 | 8.6737 | 39.71 | 9.96 | 2.98 | 8.6731 | 0.0006 | 0.0018 | 0.0008 |
| 62 | 8.5877 | 39.68 | 9.95 | 2.93 | 8.5876 | 0.0001 | 0.0003 | ||
| 63 | 8.3706 | 39.72 | 9.95 | 2.87 | 8.3705 | 0.0001 | 0.0003 |
| No. | Material | Tensile Strength | Elongation | ||||
|---|---|---|---|---|---|---|---|
| Corrosion Sample (MPa) | Blank Sample (MPa) | Change Rate (%) | Corrosion Sample (%) | Blank Sample (%) | Change Rate (%) | ||
| (a) | Pure 904L | 524 | 622 | 15.76 | 42.33 | 52.04 | 18.66 |
| (b) | Pure 2205 | 671 | 782 | 14.19 | 28.49 | 35.35 | 19.41 |
| (c) | 904L composite plate | 645 | 756 | 14.68 | 38.00 | 46.93 | 19.03 |
| (d) | E385 weld | 487 | 559 | 12.88 | / | / | / |
| (e) | E385 surfacing | 489 | 561 | 12.83 | / | / | / |
| (f) | 625 weld | 699 | 775 | 9.81 | / | / | / |
| (g) | 2205 composite plate | 695 | 827 | 15.96 | 34.65 | 42.16 | 17.81 |
| (h) | 825 composite plate | 628 | 718 | 12.53 | 31.68 | 38.02 | 16.68 |
| Types of Stainless Steels | ASTM | PREN |
|---|---|---|
| 316L | S31603 | 26 |
| 2205 | S31803 | 33 |
| 904L | N08904 | 34 |
| 825 | N08825 | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Mei, P.; Chang, L.; Wu, C.; Chen, S.; Chen, Q.; Li, G. Evaluation of the Corrosion Resistance of 904L Composite Plate in a High-Temperature and High-Pressure Gas Field Environment. Processes 2024, 12, 2372. https://doi.org/10.3390/pr12112372
Wang S, Mei P, Chang L, Wu C, Chen S, Chen Q, Li G. Evaluation of the Corrosion Resistance of 904L Composite Plate in a High-Temperature and High-Pressure Gas Field Environment. Processes. 2024; 12(11):2372. https://doi.org/10.3390/pr12112372
Chicago/Turabian StyleWang, Shuai, Ping Mei, Lijing Chang, Chao Wu, Shaoyun Chen, Qingguo Chen, and Guangshan Li. 2024. "Evaluation of the Corrosion Resistance of 904L Composite Plate in a High-Temperature and High-Pressure Gas Field Environment" Processes 12, no. 11: 2372. https://doi.org/10.3390/pr12112372
APA StyleWang, S., Mei, P., Chang, L., Wu, C., Chen, S., Chen, Q., & Li, G. (2024). Evaluation of the Corrosion Resistance of 904L Composite Plate in a High-Temperature and High-Pressure Gas Field Environment. Processes, 12(11), 2372. https://doi.org/10.3390/pr12112372





