Optimization and Efficiency of Novel Magnetic-Resin-Based Approaches for Enhanced Nickel Removal from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Materials
2.3. Synthesis of Magnetic Resin
2.4. Resins Characterization
2.5. Adsorption Experiments
2.5.1. Adsorption Kinetics
2.5.2. Adsorption Isotherms
2.6. Definitive Screening Design
2.7. Analytical Methods
3. Results and Discussion
3.1. Resin Characterization
3.2. Adsorption Performance
3.2.1. Adsorption Kinetics
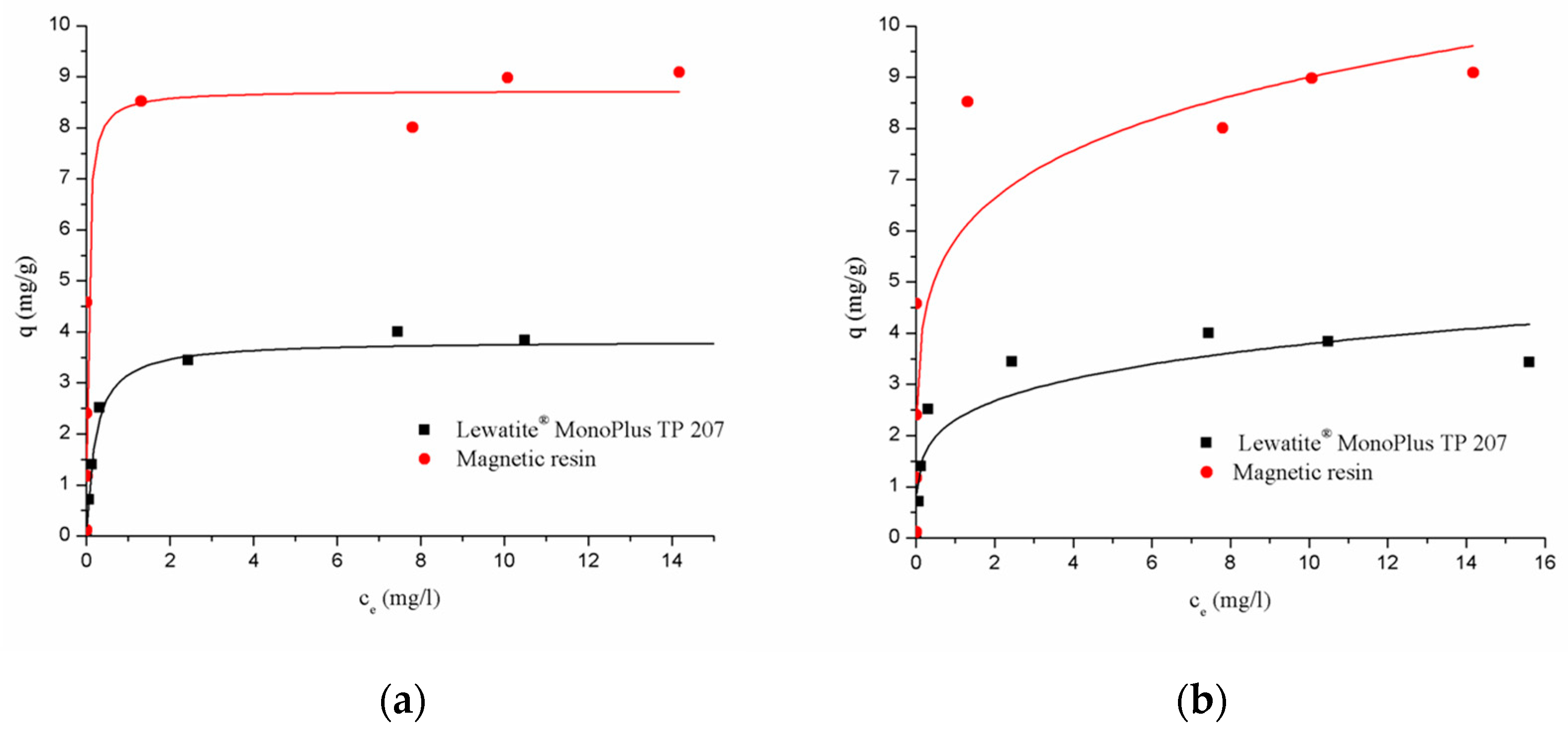
3.2.2. Adsorption Isotherms
3.3. Proposed Mechanism of Ni(II) Removal by Commercial Resin and Magnetic Resin
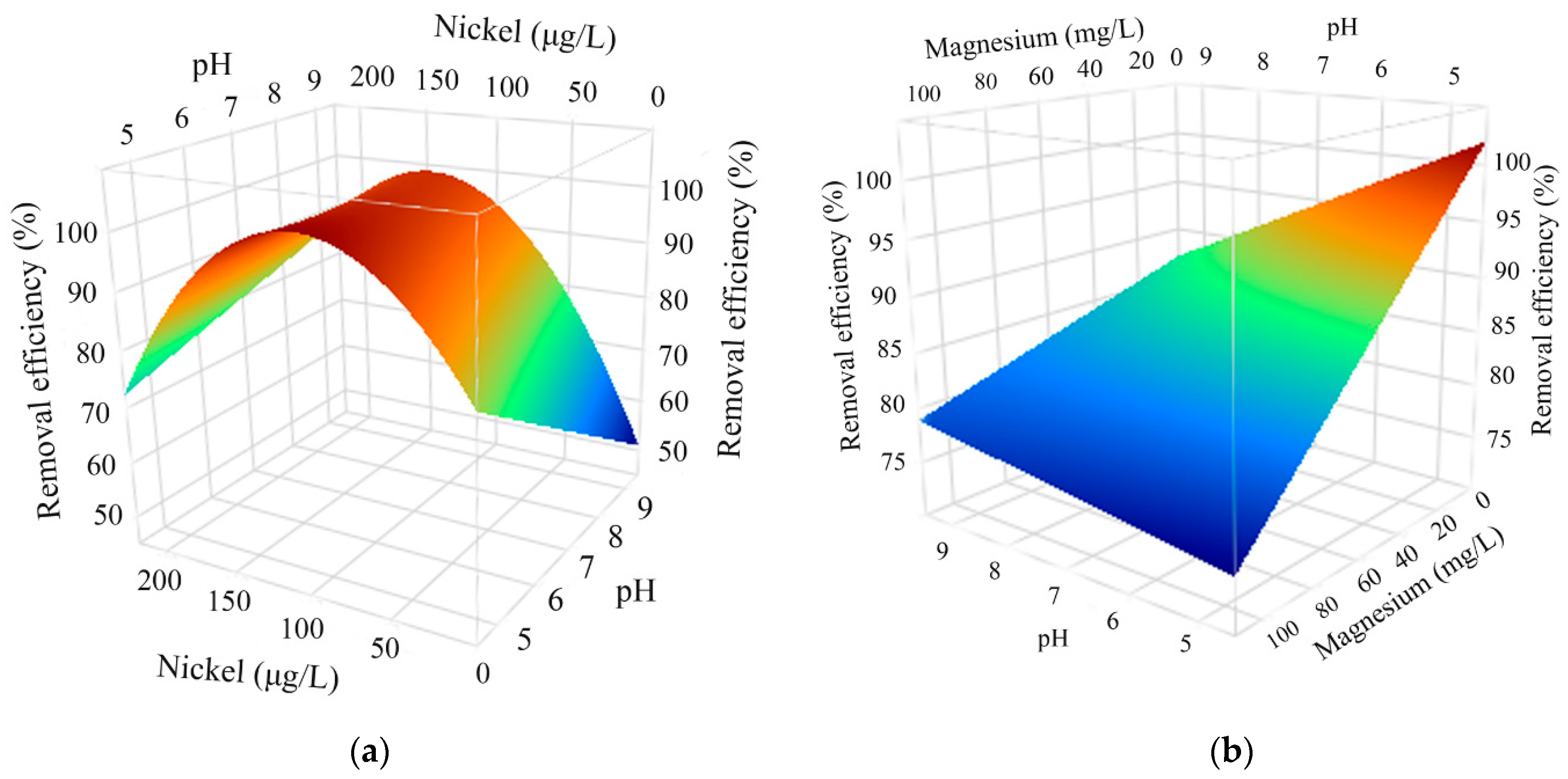
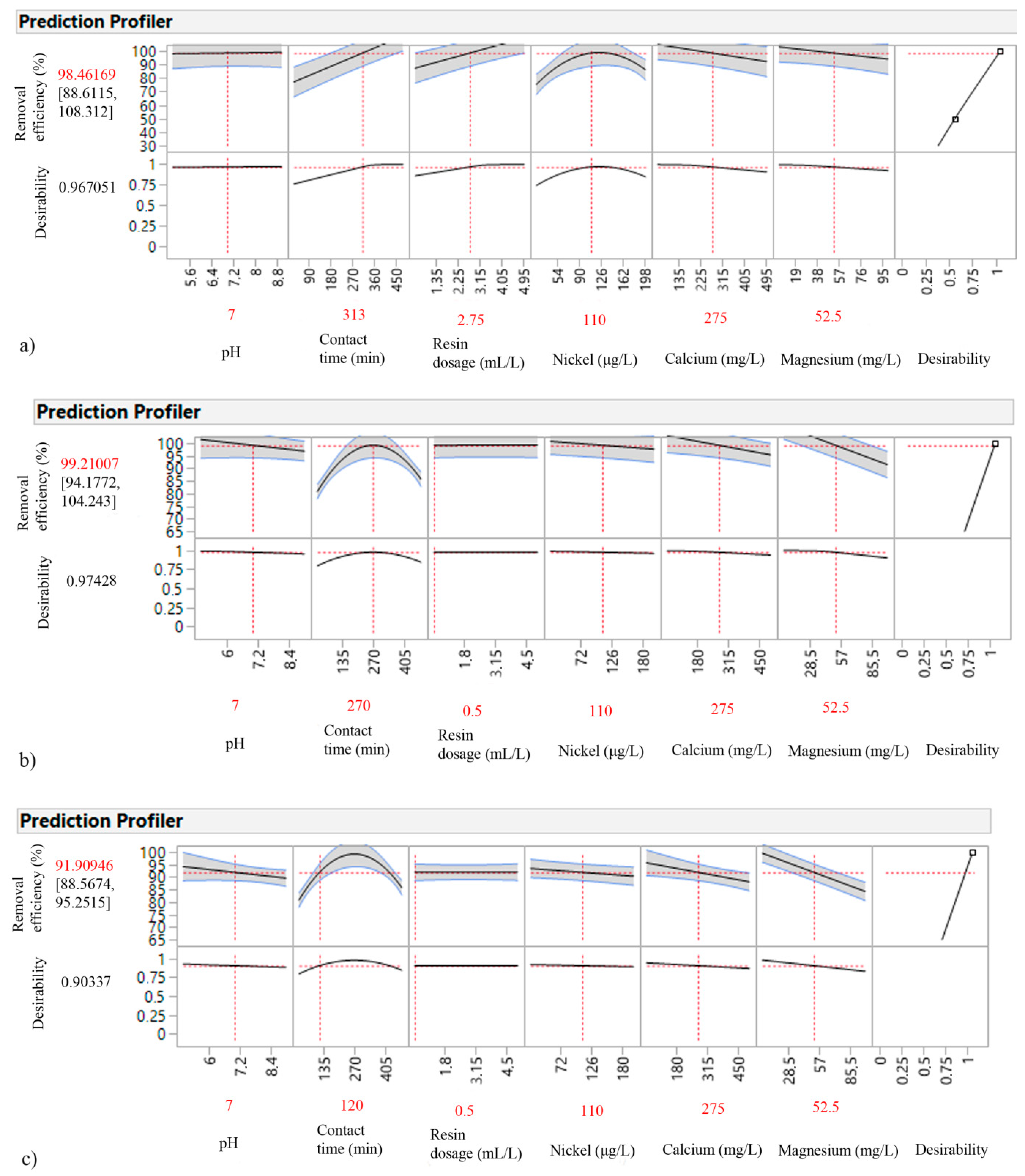
3.4. DSD Model Evaluation and Process Optimization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Basheer, A.A. New generation nano-adsorbents for the removal of emerging contaminants in water. J. Mol. Liq. 2018, 261, 583–593. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Yuan, J.; Zwain, H.M.; Mojiri, A.; Gholami, Z.; Gholami, F.; Wang, W.; Giwa, A.S.; Yu, Y.; et al. Nickel ion removal from aqueous solutions through the adsorption process: A review. Rev. Chem. Eng. 2020, 37, 755–778. [Google Scholar] [CrossRef]
- Hussain, C.M.; Keçili, R. Environmental pollution and environmental analysis. In Modern Environmental Analysis Techniques for Pollutants; Hussain, C.M., Keçili, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–36. [Google Scholar]
- Deng, Y.; Shi, H.; Yu, X.; Penghui, S.; Liming, Y.; Yu, K.; Keke, H.; Xubiao, L. Simultaneous Heavy Metals Removal via in Situ Construction of Multivariate Metal-Organic Gels in Actual Wastewater and the Reutilization for Sb(V) Capture. Chem. Eng. J. 2020, 400, 125359. [Google Scholar] [CrossRef]
- Schaumlöfel, D. Nickel species: Analysis and toxic effects. J. Trace Elem. Med. Biol. 2012, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zambelli, B.; Uversky, V.N.; Ciurli, S. Nickel impact on human health: An intrinsic disorder perspective. Biochim. Biophys. Acta Proteins Proteom. 2016, 1864, 1714–1731. [Google Scholar] [CrossRef]
- Rinklebe, J.; Antoniadis, V.; Shaheen, S.M.; Rosche, O.; Altermann, M. Health risk assessment of potentially toxic elements in soils along the Central Elbe River, Germany. Environ. Int. 2019, 126, 76–88. [Google Scholar] [CrossRef]
- Shaheen, S.M.; El-Naggar, A.; Antoniadis, V.; Moghanm, F.S.; Zhang, Z.; Tsang, D.C.W.; Ok, Y.S.; Rinklebe, J. Release of toxic elements in fishpond sediments under dynamic redox conditions: Assessing the potential environmental risk for a safe management of fisheries systems and degraded waterlogged sediments. J. Environ. Manag. 2020, 255, 109778. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer IARC. Monographs on the Identification of Carcinogenic Hazards to Human; International Agency for Research on Cancer IARC: Lyon, France, 2020. [Google Scholar]
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. Available online: https://eur-lex.europa.eu/eli/dir/2020/2184/oj (accessed on 5 September 2024).
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; Nebbia, C.S.; et al. Update of the risk assessment of nickel in food and drinking water. EFSA J. 2020, 18, 6268. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Kumar, R.; Rauwel, P.; Rauwel, E. Nanoadsorbants for the Removal of Heavy Metals from Contaminated Water: Current Scenario and Future Directions. Processes 2021, 9, 1379. [Google Scholar] [CrossRef]
- Tahoon, M.A.; Siddeeg, S.M.; Salem Alsaiari, N.; Mnif, W.; Ben Rebah, F. Effective Heavy Metals Removal from Water Using Nanomaterials: A Review. Processes 2020, 8, 645. [Google Scholar] [CrossRef]
- Dhanda, N.; Kumar, S. Smart and Innovative Nanotechnology Applications for Water Purification. Hybrid Adv. 2023, 3, 100044. [Google Scholar] [CrossRef]
- Ali, Q.; Ahmed Zia, M.; Kamran, M.; Shabaan, M.; Zulfiqar, U.; Ahmad, M.; Iqbal, R.; Maqsood, M.F. Nanoremediation for heavy metal contamination: A review. Hybrid Adv. 2023, 4, 100091. [Google Scholar] [CrossRef]
- Ethaib, S.; Al-Qutaifia, S.; Al-Ansari, N.; Zubaidi, S.L. Function of Nanomaterials in Removing Heavy Metals for Water and Wastewater Remediation: A Review. Environments 2022, 9, 123. [Google Scholar] [CrossRef]
- Baby, R.; Hussein, M.Z.; Abdullah, A.H.; Zainal, Z. Nanomaterials for the Treatment of Heavy Metal Contaminated Water. Polymers 2022, 14, 583. [Google Scholar] [CrossRef]
- Castelo-Grande, T.; Augusto, P.A.; Rico, J.; Marcos, J.; Iglesias, R.; Hernández, L.; Barbosa, D. Magnetic Water Treatment in a Wastewater Treatment Plant: Part I—Magnetic Water Treatment in a Wastewater Treatment Plant: Part I—Sorption and Magnetic Particles. J. Environ. Manag. 2021, 281, 111872. [Google Scholar] [CrossRef]
- Castelo-Grande, T.; Augusto, P.A.; Rico, J.; Marcos, J.; Iglesias, R.; Hernández, L.; Barbosa, D. Magnetic Water Treatment in a Wastewater Treatment Plant: Part II—Processing Waters and Kinetic Study. J. Environ. Manag. 2021, 285, 112177. [Google Scholar] [CrossRef]
- Liosis, C.; Papadopoulou, A.; Karvelas, E.; Karakasidis, T.E.; Sarris, I.E. Heavy Metal Adsorption Using Magnetic Nanoparticles for Water Purification: A Critical Review. Materials 2021, 14, 7500. [Google Scholar] [CrossRef]
- Shukla, S.; Khan, R.; Daverey, A. Synthesis and Characterization of Magnetic Nanoparticles, and Their Applications in Wastewater Treatment: A Review. Environmetal. Technol. Innov. 2021, 24, 101924. [Google Scholar] [CrossRef]
- Ali, I.; Peng, C.; Naz, I.; Amjed, M.A. Water Purification Using Magnetic Nanomaterials: An Overview. In Magnetic Nanostructures. Nanotechnology in the Life Sciences; Abd-Elsalam, K., Mohamed, M., Prasad, R., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Tan, Y.H.; Karri, R.R.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Mazari, A.; Nizamuddin, S. Magnetic Nanoparticles Incorporation into Different Substrates for Dyes and Heavy Metals Removal—A Review. Environ. Sci. Pollut. Res. Int. 2020, 27, 43526–43541. [Google Scholar] [CrossRef] [PubMed]
- Goswami, M.K.; Srivastava, A.; Dohare, R.K.; Tiwari, A.K.; Srivastav, A. Recent advances in conducting polymer-based magnetic nanosorbents for dyes and heavy metal removal: Fabrication, applications, and perspective. Environ. Sci. Pollut. Res. Int. 2023, 30, 73031–73060. [Google Scholar] [CrossRef]
- Nikić, J.; Watson, M.; Tubić, A.; Šolić, M.; Agbaba, J. Recent trends in the application of magnetic nanocomposites for heavy metals removal from water: A review. Sep. Sci. Technol. 2024, 59, 293–331. [Google Scholar] [CrossRef]
- Sikora, E.; Hajdu, V.; Muránszky, G.; Katona, K.K.; Kocserha, I.; Kanazawa, T.; Fiser, B.; Viskolcz, B.; Vanyorek, L. Application of ion-exchange resin beads to produce magnetic adsorbents. Chem. Pap. 2020, 75, 1187–1195. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Z.; Wu, H.; Pan, S.; Cheng, X.; Sun, Y.; Xu, Y. Effective and simultaneous removal of organic/inorganic arsenic using polymer-based hydrated iron oxide adsorbent: Capacity evaluation and mechanism. Sci. Total Environ. 2020, 742, 140508. [Google Scholar] [CrossRef]
- Perlova, O.; Dzyazko, Y.; Halutska, I.; Perlova, N.; Palchik, A. Anion exchange resin modified with nanoparticles of hydrated zirconium dioxide for sorption of soluble U(VI) compounds. In Nanooptics, Nanophotonics, Nanostructures, and Their Applications; Springer International Publishing: New York, NY, USA, 2018; pp. 3–15. [Google Scholar] [CrossRef]
- Manjare, S.B.; Chaudhari, R.A.; Thopate, S.R.; Risbud, K.P.; Badade, S.M. Resin loaded palladium nanoparticle catalyst, characterization and application in –C–C– coupling reaction. SN Appl. Sci. 2020, 2, 988. [Google Scholar] [CrossRef]
- Sodzidzi, Z.; Phiri, Z.; Nure, J.F.; Msagati, T.A.M.; de Kock, L.-A. Adsorption of Toxic Metals Using Hydrous Ferric Oxide Nanoparticles Embedded in Hybrid Ion-Exchange Resins. Materials 2024, 17, 1168. [Google Scholar] [CrossRef]
- Dizge, N.; Keskinler, B.; Barlas, H. Sorption of Ni(II) ions from aqueous solution by Lewatit cation-exchange resin. J. Hazard. Mater. 2009, 167, 915–926. [Google Scholar] [CrossRef]
- Tan, J.; Huang, Y.; Wu, Z.; Chen, X. Ion Exchange Resin on Treatment of Copper and Nickel Wastewater. IOP Conf. Ser. Earth Environ. Sci. 2017, 94, 012122. [Google Scholar] [CrossRef]
- Božecka, A.M.; and Rydlewska, S.S. The use of ion exchangers for removing cobalt and nickel ions from water solutions. Arch. Min. Sci. 2018, 63, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Wołowicz, A.; and Wawrzkiewicz, M. Screening of Ion Exchange Resins for Hazardous Ni(II) Removal from Aqueous Solutions: Kinetic and Equilibrium Batch Adsorption Method. Processes 2021, 9, 285. [Google Scholar] [CrossRef]
- Lebron, Y.A.R.; Moreira, V.R.; Amaral, M.C.S. Metallic ions recovery from membrane separation processes concentrate: A special look onto ion exchange resins. Chem. Eng. J. 2021, 425, 131812. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. The use of the chelating resin of a new generation Lewatit MonoPlus TP-220 with the bis-picolylamine functional groups in the removal of selected metal ions from acidic solutions. Chem. Eng. J. 2012, 197, 493–508. [Google Scholar] [CrossRef]
- Abbasi, P.; McKevitt, P.; Dreisinger, D.B. The kinetics of nickel recovery from ferrous containing solutions using an Iminodiacetic acid ion exchange resin. Hydrometallurgy 2018, 175, 333–339. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Alshehri, S.M. Fabrication of magnetic polymeric resin for the removal of toxic metals from aqueous medium: Kinetics and adsorption mechanisms. J. Water Process Eng. 2020, 36, 101284. [Google Scholar] [CrossRef]
- Atia, A.A.; Donia, A.M.; Yousif, A.M. Removal of some hazardous heavy metals from aqueous solution using magnetic chelating resin with iminodiacetate functionality. Sep. Purif. Technol. 2008, 61, 348–357. [Google Scholar] [CrossRef]
- Aranda-García, E.; Chávez-Camarillo, G.M.; Cristiani-Urbina, E. Effect of Ionic Strength and Coexisting Ions on the Biosorption of Divalent Nickel by the Acorn Shell of the Oak Quercus crassipes Humb. & Bonpl. Processes 2020, 8, 1229. [Google Scholar] [CrossRef]
- Charazińska, S.; Burszta-Adamiak, E.; Lochyński, P. Recent trends in Ni(II) sorption from aqueous solutions using natural materials. Rev. Environ. Sci. Bio/Technol. 2022, 21, 105–138. [Google Scholar] [CrossRef]
- Badawi, A.H.M.; Elkodous, A.; Ali, G.A.M. Recent advances in dye and metal ion removal using efficient adsorbents and novel nano-based materials: An overview. RSC Adv. 2021, 11, 36528–36553. [Google Scholar] [CrossRef]
- Lamidi, S.; Olaleye, N.; Bankole, Y.; Obalola, A.; Aribike, E.; Adigun, I. Applications of Response Surface Methodology (RSM) in Product Design, Development, and Process Optimization; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar] [CrossRef]
- Reji, M.; Kumar, R. Response surface methodology (RSM): An overview to analyze multivariate data. Indian J. Microbiol. Res. 2022, 9, 241–248. [Google Scholar] [CrossRef]
- Shamkhi, H.A.; Abdulhasan, M.J.; Raheem, S.A.; Al-Zubaidi, H.A.M.; Janabi, A.S.K. Optimization of heavy metals removal from wastewater by magnetic nano-zeolite using response surface methodology. Desalination Water Treat. 2023, 306, 63–74. [Google Scholar] [CrossRef]
- Fertu, D.I.; Bulgariu, L.; Gavrilescu, M. Modeling and Optimization of Heavy Metals Biosorption by Low-Cost Sorbents Using Response Surface Methodology. Processes 2022, 10, 523. [Google Scholar] [CrossRef]
- Sasidharan, R.; Kumar, A. Response surface methodology for optimization of heavy metal removal by magnetic biosorbent made from anaerobic sludge. J. Indian Chem. Soc. 2022, 99, 9100638. [Google Scholar] [CrossRef]
- Khoshraftar, Z.; Masoumi, H.; Ghaemi, A. Experimental, response surface methodology (RSM) and mass transfer modeling of heavy metals elimination using dolomite powder as an economical adsorbent. Case Stud. Chem. Environ. Eng. 2023, 7, 100329. [Google Scholar] [CrossRef]
- Mohrazi, A.; Ghasemi-Fasaei, R.; Mojiri, A.; Safarzadeh, S. Identification of influential parameters and conditions in heavy metals adsorption onto Cal-LDH-PC using optimization approaches of RSM and Taguchi. Sci. Rep. 2024, 14, 13225. [Google Scholar] [CrossRef]
- Boulika, H.; El Hajam, M.; Hajji Nabih, M.; Riffi Karim, I.; Idrissi Kandri, N.; Zerouale, A. Definitive screening design applied to cationic & anionic adsorption dyes on Almond shells activated carbon: Isotherm, kinetic and thermodynamic studies. Mater. Today Proc. 2023, 72, 3336–3346. [Google Scholar] [CrossRef]
- Jokić Govedarica, J.; Tomašević Pilipović, D.; Gvoić, V.; Kerkez, Đ.; Leovac Maćerak, A.; Slijepčević, N.; Bečelić-Tomin, M. Cost-effective method of simultaneous removal of copper and phosphate on environmentally friendly nanomaterial. J. Serbian Chem. Soc. 2024, 89, 581–595. [Google Scholar] [CrossRef]
- Bayuo, J.; Rwiza, M.; Mtei, K. Response surface optimization and modeling in heavy metal removal from wastewater—A critical review. Environ. Monit. Assess. 2022, 194, 351. [Google Scholar] [CrossRef]
- Hayasaka, R.; Hänisch, J.; Cayado, P. DSDApp: An Open-Access Tool for Definitive Screening Design. J. Open Res. Softw. 2024, 12, 2–8. [Google Scholar] [CrossRef]
- Jokić Govedarica, J.; Tomašević Pilipović, D.; Gvoić, V.; Kerkez, Đ.; Leovac Maćerak, A.; Slijepčević, N.; Bečelić-Tomin, M. Eco-friendly nanoparticles: Mechanisms and capacities for efficient removal of heavy metals and phosphate from water using definitive screening design approach. Environ. Geochem. Health 2024, 46, 118. [Google Scholar] [CrossRef]
- Hundie, K.B.; and Akuma, D.A. Optimization of biodiesel production parameters from Prosopisjulifera seed using definitive screening design. Heliyon 2022, 8, e08965. [Google Scholar] [CrossRef]
- Jones, B.; and Nachtsheim, C.J. Definitive screening designs with added two-level categorical factors. J. Qual. Technol. 2013, 45, 121–129. [Google Scholar] [CrossRef]
- El-Sharkawy, R.M.; Khairy, M.; Abbas, M.H.H.; Zaki, M.E.A.; El-Hadary, A.E. Innovative optimization for enhancing Pb2+ biosorption from aqueous solutions using Bacillus subtilis. Front. Microbiol. 2024, 15, 1384639. [Google Scholar] [CrossRef] [PubMed]
- Hamdzah, M.; Ujang, Z.; Nasef, M.; Abdullah, N.; Dahalan, F. Removal of Ni(II), Zn(II) and Pb(II) from aqueous solutions using cation-exchange resin in fixed-bed column. Desalination Water Treat. 2015, 57, 1095118. [Google Scholar] [CrossRef]
- Botelho Junior, A.B., Jr.; de Vicente, A.A.; Espinosa, D.C.R.; Tenório, J.A.S. Recovery of metals by ion exchange process using chelating resin and sodium dithionite. J. Mater. Res. Technol. 2019, 8, 4464–4469. [Google Scholar] [CrossRef]
- Monteserin, C.; Blanco, M.; Aranzabe, E.; Aranzabe, A.; Jose, L.; Aitor, L.; Vilas, J. Effects of graphene oxide and chemically-reduced graphene oxide on the dynamic mechanical properties of epoxy amine composites. Polymers 2017, 9, 449. [Google Scholar] [CrossRef]
- Tahereh, K.; Sharif, M.; Pourabas, B. Polythiophene-graphene oxide doped epoxy resin nanocomposites with enhanced electrical, mechanical and thermal properties. RSC Adv. 2016, 6, 93680–93693. [Google Scholar] [CrossRef]
- Bertolucci, E. Chemical and magnetic properties characterization of magnetic nanoparticles. In Proceedings of the IEEE International Instrumentation and Measurement Technology Conference (I2MTC) Proceedings, Pisa, Italy, 11–14 May 2015; pp. 1492–1496. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen Thi, Q.A.; Le, N.H. Synthesis of a novel porous Ag2O nanomaterial on ion exchange resin and its application for COD determination of high salinity water. Sci. Rep. 2021, 11, 11487. [Google Scholar] [CrossRef]
- Dhumal, J.; Bandgar, S.; Zipare, K.; Shahane, G. Fe3O4 Ferrofluid Nanoparticles: Synthesis and Rheological Behavior. Int. J. Mater. Chem. Phys. 2015, 1, 141–145. [Google Scholar]
- Munasir, M.; Setyaningsih, S.; Yanasin, S.; Supardi, Z.A.I.; Taufiq, A.; Sunaryono, S. Phase and Magnetic Properties of Fe3O4/SiO2 Natural Materials-Based Using Polyethylene Glycol Media. IOP Conf. Ser. Mater. Sci. Eng. 2019, 515, 012017. [Google Scholar] [CrossRef]
- Kristiansen, A.B.; Church, N.; Uçar, Ş. Investigation of magnetite particle characteristics in relation to crystallization pathways. Powder Technol. 2023, 415, 118145. Available online: https://hdl.handle.net/11511/103007 (accessed on 23 August 2024). [CrossRef]
- Trakal, L.; Veselská, V.; Šafařík, I.; Vítková, M.; Číhalová, S.; Komárek, M. Lead and cadmium sorption mechanisms on magnetically modified biochars. Bioresour. Technol. 2016, 203, 318–324. [Google Scholar] [CrossRef]
- Raji, Z.; Karim, A.; Karam, A.; Khalloufi, S. Adsorption of Heavy Metals: Mechanisms, Kinetics, and Applications of Various Adsorbents in Wastewater Remediation—A Review. Waste 2023, 1, 775–805. [Google Scholar] [CrossRef]
- Qiu, X.; Hu, H.; Yang, J.; Wang, C.; Cheng, Z. Selective removal of copper from the artificial nickel electrolysis anolyte by a novel chelating resin: Batch, column and mechanisms. J. Dispers. Sci. Technol. 2019, 41, 137–147. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Y.; Zhou, X.; Ren, J.; Zhong, C. Removal of mercury ions from aqueous solution by thiourea-functionalized magnetic biosorbent: Preparation and mechanism study. J. Colloid Interface Sci. 2017, 507, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hossain, F.; Duan, C.; Lu, J.; Tsang, Y.F.; Islam, S.; Zhou, Y. Isotherm models for adsorption of heavy metals from water—A review. Chemosphere 2022, 307, 135545. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M.; Citak, D.; Soylak, M. Equilibrium, kinetic and thermodynamic studies of adsorption of Pb(II) from aqueous solution onto Turkish kaolinite clay. J. Hazard. Mater. 2007, 149, 283–291. [Google Scholar] [CrossRef]
- Wang, X.S.; Huang, J.; Hu, H.Q.; Wang, J.; Qin, Y. Determination of kinetic and equilibrium parameters of the batch adsorption of Ni(II) from aqueous solutions by Na-mordenite. J. Hazard. Mater. 2007, 142, 468–476. [Google Scholar] [CrossRef]
- Fadel, D.; El-Bahy, S.; Abdelaziz, Y. Heavy metals removal using iminodiacetate chelating resin by batch and column techniques. Desalination Water Treat. 2016, 57, 25718–25728. [Google Scholar] [CrossRef]
- Nastasović, A.; Marković, B.; Suručić, L.; Onjia, A. Methacrylate-Based Polymeric Sorbents for Recovery of Metals from Aqueous Solutions. Metals 2022, 12, 814. [Google Scholar] [CrossRef]
- Stefan, D.S.; and Meghea, I. Mechanism of simultaneous removal of Ca2+, Ni2+, Pb2+ and Al3+ ions from aqueous solutions using Purolite® S930 ion exchange resin. Comptes Rendus Chim. 2014, 17, 496–502. [Google Scholar] [CrossRef]
- Yu, Z.; Qi, T.; Qu, J.; Wang, L.; Chu, J. Removal of Ca(II) and Mg(II) from potassium chromate solution on Amberlite IRC 748 synthetic resin by ion exchange. J. Hazard. Mater. 2009, 167, 406–412. [Google Scholar] [CrossRef]
- Zainol, Z.; and Nicol, M.J. Ion-exchange equilibria of Ni2+, Co2+, Mn2+ and Mg2+ with iminodiacetic acid chelating resin Amberlite IRC 748. Hydrometallurgy 2009, 99, 175–180. [Google Scholar] [CrossRef]

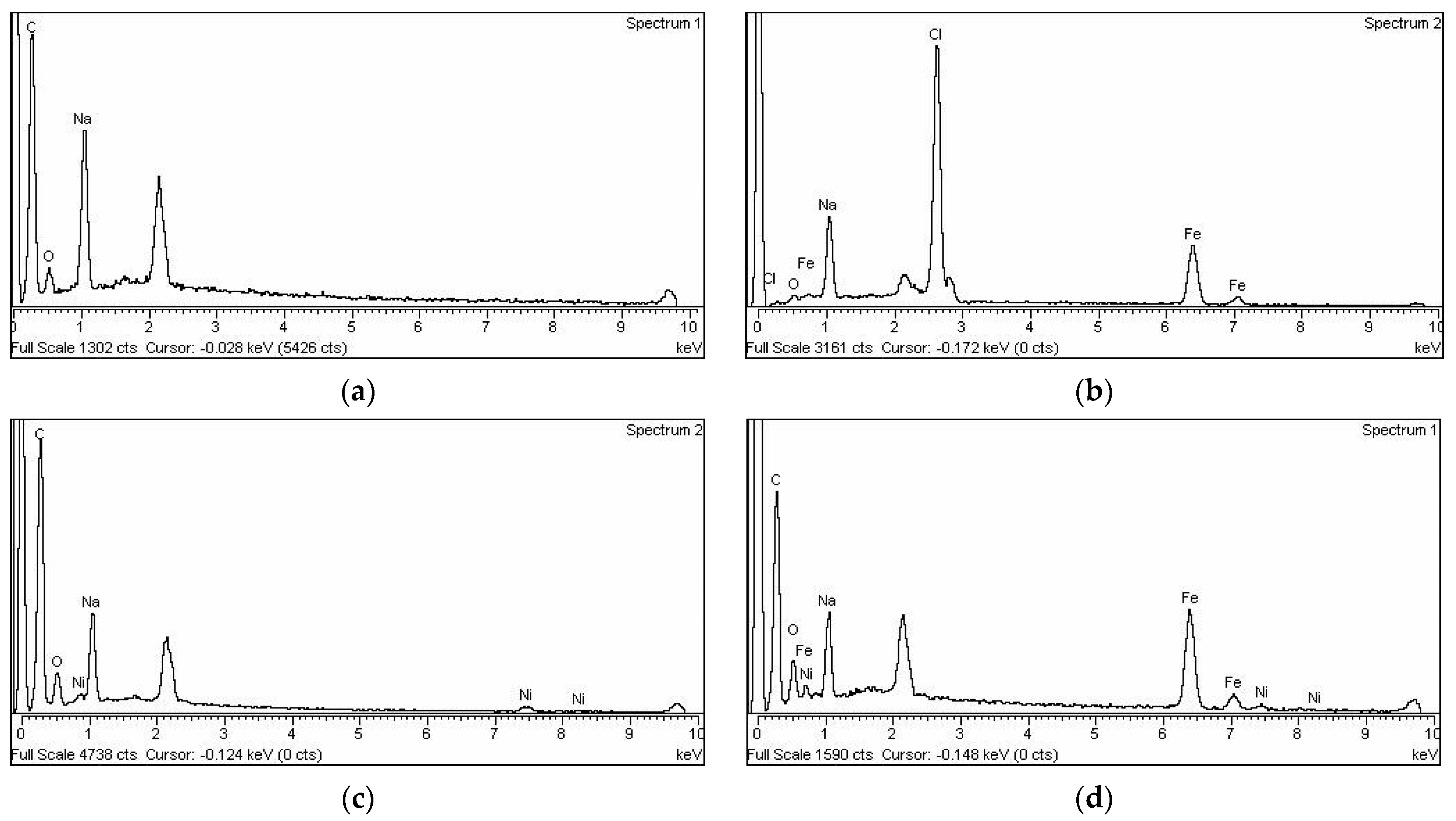

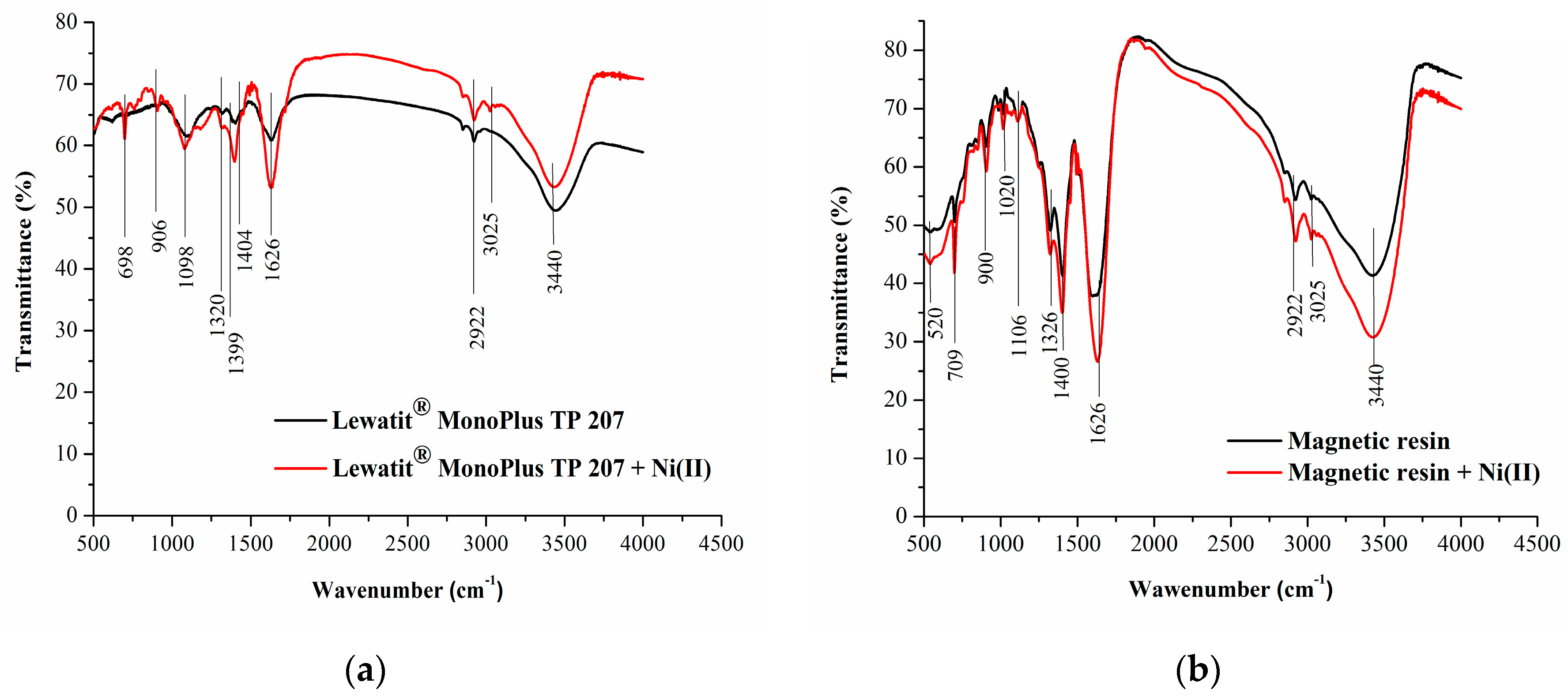
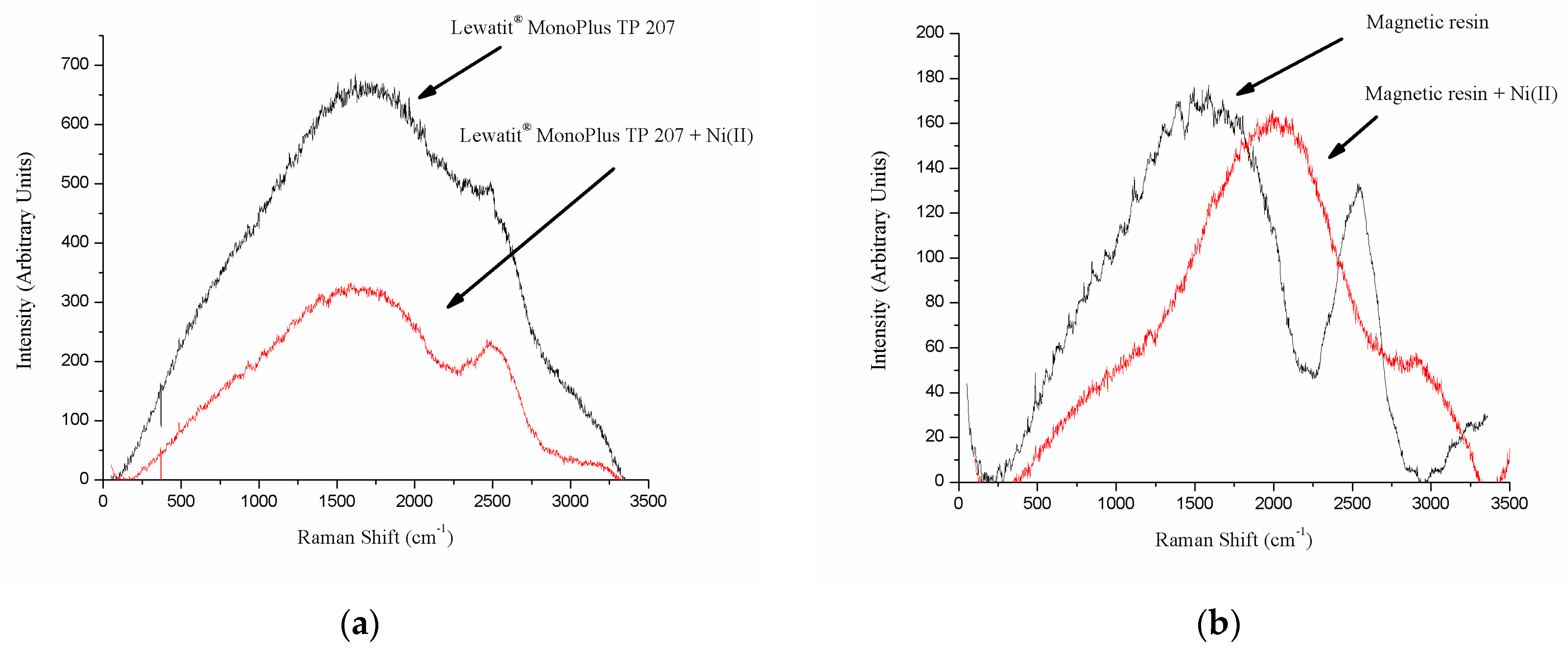
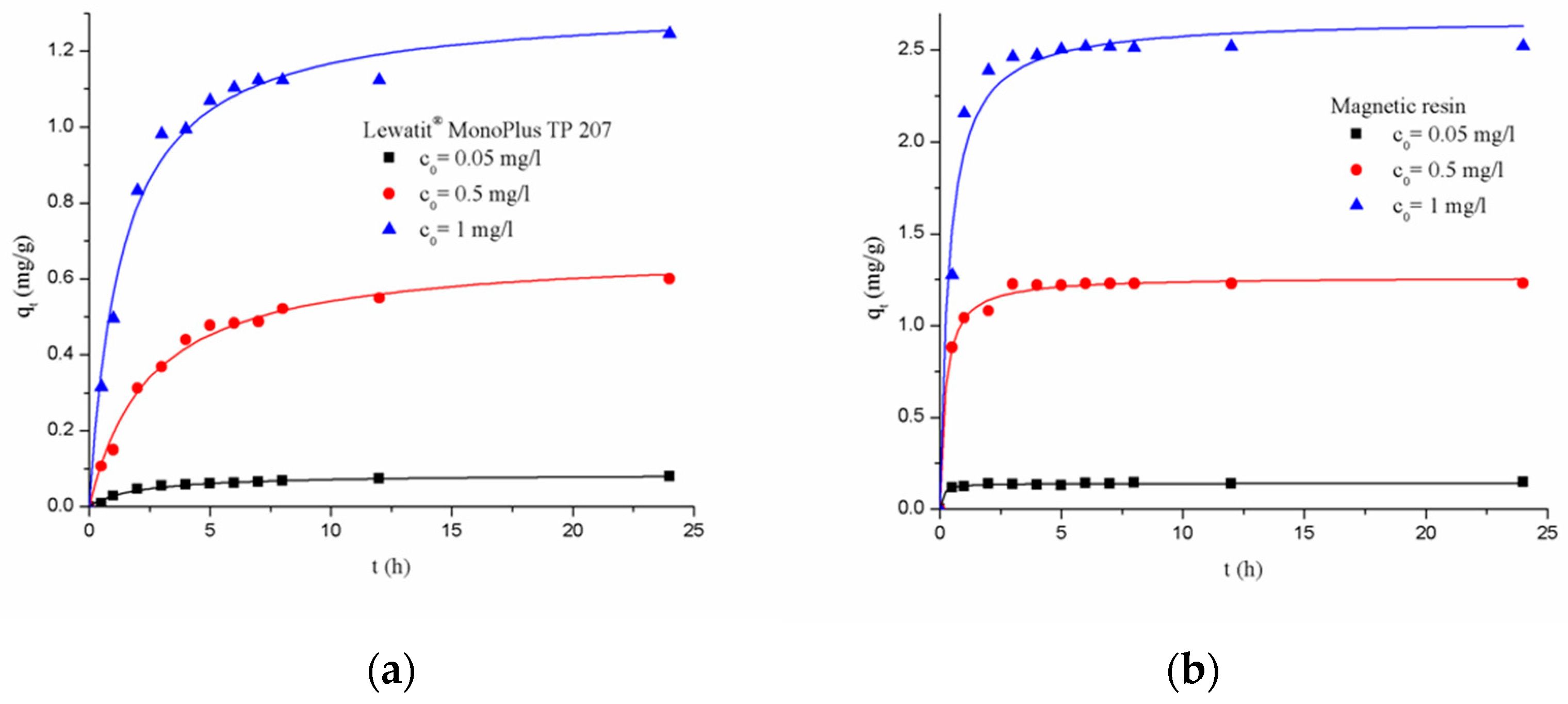

| Resin Type | Chelating Resin |
|---|---|
| Matrix | Polystyrene |
| Structure | Macroporous |
| Functional group | Iminodiactetic |
| Uniformity coefficient | 1.1 |
| Mean bead size (mm) | 0.61 ± 0.05 |
| Water retention (%) | 55–60 |
| Capacity (eq/L) | 2.0 |
| Factor | Unit | Coded Value | Level | ||
|---|---|---|---|---|---|
| Low (−1) | Central (0) | High (+1) | |||
| pH | - | X1 | 5 | 7 | 9 |
| Contact time | min | X2 | 30 | 255 | 480 |
| Resin dosage | mL/L | X3 | 0.5 | 2.75 | 5 |
| Ni concentration | µg/L | X4 | 20 | 110 | 200 |
| Ca concentration | mg/L | X5 | 50 | 275 | 500 |
| Mg concentration | mg/L | X6 | 5 | 52.5 | 100 |
| Sample Description | Mass [g] | ρ = m/V [kg/m3] | k [10−4 SI] | χlf ×10−3 [m3/kg] | Magnetic Status |
|---|---|---|---|---|---|
| Magnetite | 6.2873 | 982.4 | 9038 | 0.9200 | Ferromagnetic |
| Magnetic resin | 5.4391 | 849.9 | 37 | 0.0044 | Paramagnetic |
| Langmuir Isotherm | Freundlich Isotherm | ||||||
|---|---|---|---|---|---|---|---|
| qmax (mg/g) | KL (L/mg) | R2 | RL | n | KF (mg/g)/(mg/L)n | R2 | |
| Lewatit® MonoPlus TP 207 | 18.13 | 4.74 | 0.9801 | 0.79–0.012 | 0.216 | 2.305 | 0.8059 |
| Magnetic resin | 229.4 | 26.26 | 0.8720 | 0.42–0.002 | 5.825 | 0.188 | 0.7892 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maletin, M.; Nikić, J.; Gvoić, V.; Pešić, J.; Cvejić, Ž.; Tubić, A.; Agbaba, J. Optimization and Efficiency of Novel Magnetic-Resin-Based Approaches for Enhanced Nickel Removal from Water. Processes 2024, 12, 2287. https://doi.org/10.3390/pr12102287
Maletin M, Nikić J, Gvoić V, Pešić J, Cvejić Ž, Tubić A, Agbaba J. Optimization and Efficiency of Novel Magnetic-Resin-Based Approaches for Enhanced Nickel Removal from Water. Processes. 2024; 12(10):2287. https://doi.org/10.3390/pr12102287
Chicago/Turabian StyleMaletin, Marija, Jasmina Nikić, Vesna Gvoić, Jovana Pešić, Željka Cvejić, Aleksandra Tubić, and Jasmina Agbaba. 2024. "Optimization and Efficiency of Novel Magnetic-Resin-Based Approaches for Enhanced Nickel Removal from Water" Processes 12, no. 10: 2287. https://doi.org/10.3390/pr12102287
APA StyleMaletin, M., Nikić, J., Gvoić, V., Pešić, J., Cvejić, Ž., Tubić, A., & Agbaba, J. (2024). Optimization and Efficiency of Novel Magnetic-Resin-Based Approaches for Enhanced Nickel Removal from Water. Processes, 12(10), 2287. https://doi.org/10.3390/pr12102287









