Microalgae Enhance the Resistance of Pond-Dwelling Ammonia-Oxidizing Bacteria to Light Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reactor Description and Operating Conditions
2.2. Microbial Analysis
2.3. Photoinhibition Batch Tests under Different Chlorophyll a Concentrations
2.4. Fluorescence In Situ Hybridization Analysis of Algae and Bacteria
2.5. Water Quality Analysis Methodology
2.6. Statistical Analyses
3. Results
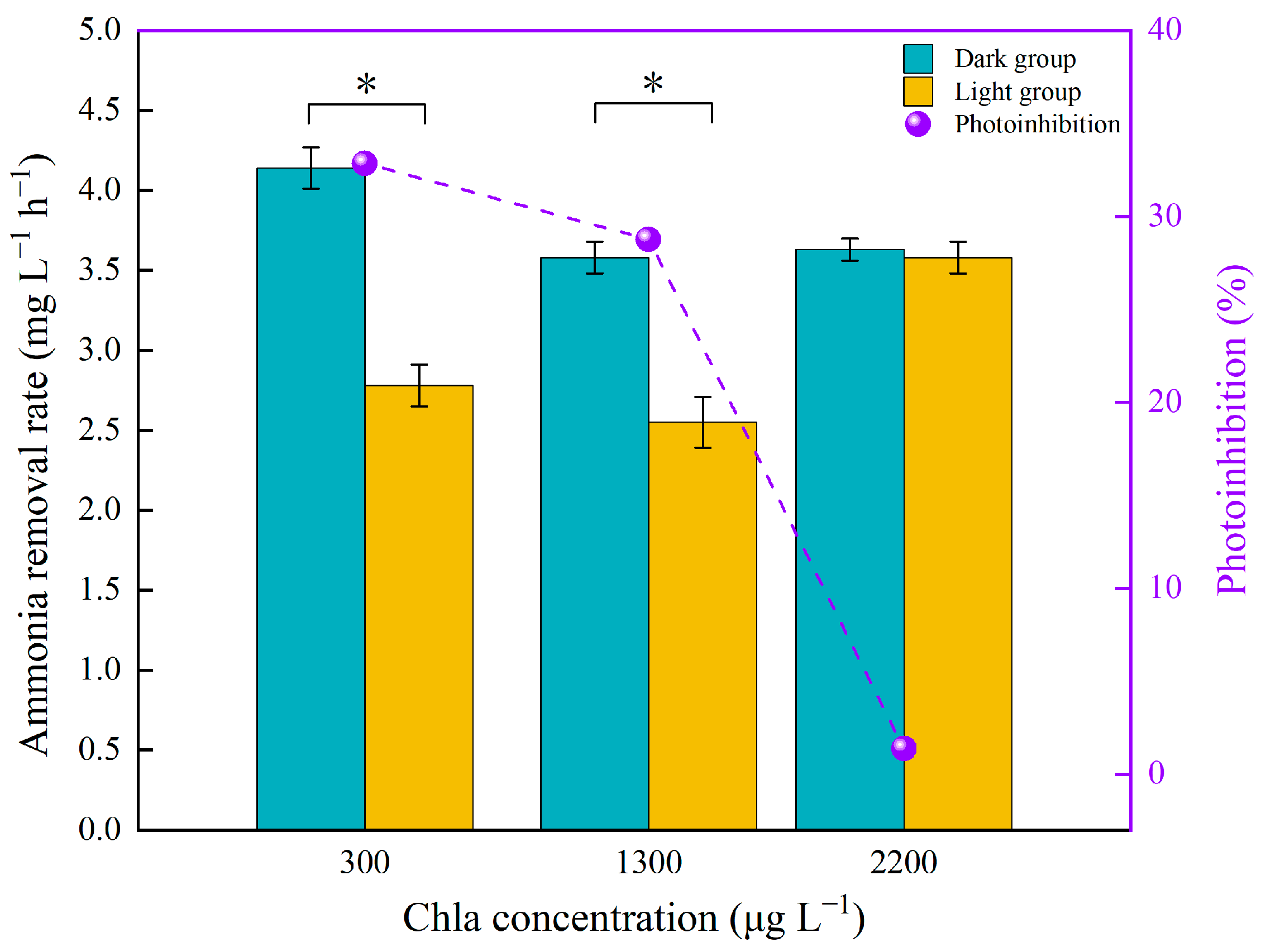
3.1. Photoinhibition of Nitrification
3.2. Microbial Analysis
3.3. Algae Enhance the Resistance of AOB to Light Irradiation
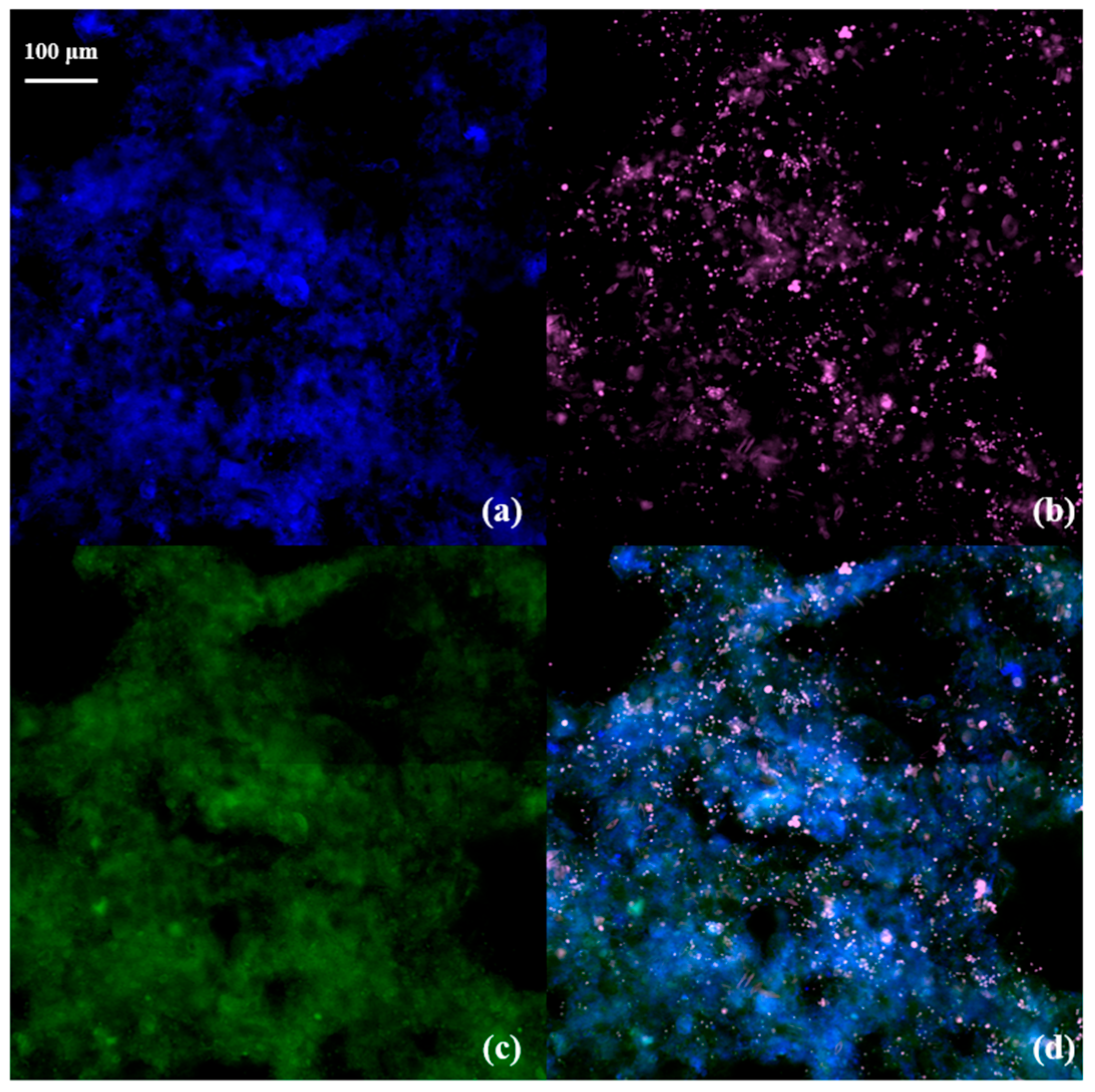
3.4. Fluorescence In Situ Hybridization
4. Discussion
5. Conclusions
- Long-term exposure to light has a serious impact on the AOR of aquaculture ponds, as well as on the potential to decrease the abundance of Nitrosomonas AOB species and their ammonia monooxygenase genes.
- In light conditions, AOB, NOB, and algae can coexist and grow together without free-growing AOB.
- Microalgae could enhance the resistance of AOB in ponds to light irradiation when Chla concentrations reach a certain value and can even completely protect AOB from the suppressive effects of light.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fisheries Bureau, Ministry of Agriculture and Rural Affairs of the People’s Republic of China. China Fisheries Statistics Yearbook; China Agriculture Press: Beijing, China, 2022. [Google Scholar]
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2024. [Google Scholar]
- Liu, X.; Shao, Z.; Cheng, G.; Lu, S.; Gu, Z.; Zhu, H.; Shen, H.; Wang, J.; Chen, X. Ecological engineering in pond aquaculture: A review from the whole-process perspective in China. Rev. Aquac. 2021, 13, 1060–1076. [Google Scholar] [CrossRef]
- Lu, S.; Liao, M.; Xie, C.; He, X.; Li, D.; He, L.; Chen, J. Seasonal dynamics of ammonia-oxidizing microorganisms in freshwater aquaculture ponds. Ann. Microbiol. 2015, 65, 651–657. [Google Scholar] [CrossRef]
- Handy, R.D.; Poxton, M.G. Nitrogen pollution in mariculture: Toxicity and excretion of nitrogenous compounds by marine fish. Rev. Fish Biol. Fish. 1993, 3, 205–241. [Google Scholar] [CrossRef]
- Lemarié, G.; Dosdat, A.; Covés, D.; Dutto, G.; Gasset, E.; Person-Le Ruyet, J. Effect of chronic ammonia exposure on growth of European seabass (Dicentrarchus labrax) juveniles. Aquaculture 2004, 229, 479–491. [Google Scholar] [CrossRef]
- Lu, S.; Liu, X.; Liu, C.; Wang, X.; Cheng, G. Review of ammonia-oxidizing bacteria and archaea in freshwater ponds. Rev. Environ. Sci. Bio-Technol. 2019, 18, 1–10. [Google Scholar] [CrossRef]
- Ruiz, P.; Miguel Vidal, J.; Sepulveda, D.; Torres, C.; Villouta, G.; Carrasco, C.; Aguilera, F.; Ruiz-Tagle, N.; Urrutia, H. Overview and future perspectives of nitrifying bacteria on biofilters for recirculating aquaculture systems. Rev. Aquac. 2020, 12, 1478–1494. [Google Scholar] [CrossRef]
- Sayavedra-Soto, L.A.; Hommes, N.G.; Alzerreca, J.J.; Arp, D.J.; Norton, J.M.; Klotz, M.G. Transcription of the amoC, amoA and amoB genes in Nitrosomonas europaea and Nitrosospira sp. NpAV. FEMS Microbiol. Lett. 1998, 167, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Witzel, K.P.; Rotthauwe, J.H. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar]
- Schoen, G.H.; Engel, H. The effect of light on Nitrosomonas europaea Win. Arch. Fur Mikrobiol. 1962, 42, 415. [Google Scholar] [CrossRef]
- Lu, S.; Li, Y.; Liu, X.; Cheng, G.; Yuan, Z.; Wu, F. Influence of Light Irradiation on Nitrification in Microalgal-Bacterial Systems for Treating Wastewater. Processes 2023, 11, 3453. [Google Scholar] [CrossRef]
- Akizuki, S.; Natori, N.; Cuevas-Rodriguez, G.; Toda, T. Application of nitrifying granular sludge for stable ammonium oxidation under intensive light. Biochem. Eng. J. 2020, 160, 107631. [Google Scholar] [CrossRef]
- Nishi, K.; Akizuki, S.; Toda, T.; Matsuyama, T.; Ida, J. Advanced light-tolerant microalgae-nitrifying bacteria consortia for stable ammonia removal under strong light irradiation using light-shielding hydrogel. Chemosphere 2022, 297, 134252. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, J.A. Photosynthetic suspended-growth systems in aquaculture. Aquac. Eng. 2006, 34, 344–363. [Google Scholar] [CrossRef]
- Zhou, R.; Li, Y.; Xiao, S.; Liu, X.; Liu, C.; Cheng, G.; Zhang, W.; Lu, S. Ecophysiological characterization of a nitrite-oxidizing bacterial culture from a freshwater aquaculture pond. Biotechnol. Biotechnol. Equip. 2022, 36, 891–901. [Google Scholar] [CrossRef]
- Wu, D.; Cheng, M.; Zhao, S.; Peng, N.; Hu, R.; Hu, J.; Liang, Y. Algal Growth Enhances Light-Mediated Limitation of Bacterial Nitrification in an Aquaculture System. Water Air Soil Pollut. 2020, 231, 1–9. [Google Scholar] [CrossRef]
- Ma, G.; Yu, D.; Zhang, J.; Miao, Y.; Zhao, X.; Li, J.; Zhang, Y.; Dong, G.; Zhi, J. A novel simultaneous partial nitrification, anammox, denitrification and fermentation process: Enhancing nitrogen removal and sludge reduction in a single reactor. Bioresour. Technol. 2023, 369, 128484. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Z.; Sun, M.; Liang, D.; Hou, L.; Zhang, J.; Wang, X.; Li, J. Optimization of nitrogen and carbon removal with simultaneous partial nitrification, anammox and denitrification in membrane bioreactor. R. Soc. Open Sci. 2020, 7, 200584. [Google Scholar] [CrossRef]
- Ge, S.; Wang, S.; Yang, X.; Qiu, S.; Li, B.; Peng, Y. Detection of nitrifiers and evaluation of partial nitrification for wastewater treatment: A review. Chemosphere 2015, 140, 85–98. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Chen, Y.W.; Gao, X.Y. Comparison of Two Methods for Phytoplankton Chlorophyll-a Concentration Measurement. J. Lakeence 2000, 12, 185–188. [Google Scholar]
- García-Martín, E.E.; Aranguren-Gassis, M.; Karl, D.M.; Martínez-García, S.; Robinson, C.; Serret, P.; Teira, E. Validation of the in vivo Iodo-Nitro-Tetrazolium (INT) Salt Reduction Method as a Proxy for Plankton. Front. Mar. Sci. 2019, 6, 220. [Google Scholar]
- Guerrero, M.A. Photoinhibition of marine nitrifying bacteria. II. Dark recovery after monochromatic or polychromatic irradiation. Mar. Ecol. Prog. 1996, 141, 193–198. [Google Scholar] [CrossRef]
- Lu, S.; Liu, X.; Liu, C.; Cheng, G.; Shen, H. Influence of photoinhibition on nitrification by ammonia-oxidizing microorganisms in aquatic ecosystems. Rev. Environ. Sci. Bio-Technol. 2020, 19, 531–542. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, S.; Guo, J.; Ge, S. Light Irradiation Enables Rapid Start-Up of Nitritation through Suppressing nxrB Gene Expression and Stimulating Ammonia Oxidizing Bacteria. Environ. Sci. Technol. 2021, 55, 13297–13305. [Google Scholar] [CrossRef]
- Akizuki, S.; Kishi, M.; Cuevas-Rodriguez, G.; Toda, T. Effects of different light conditions on ammonium removal in a consortium of microalgae and partial nitrifying granules. Water Res. 2020, 171, 115445. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhen, Y.; Mi, T.; Fu, L.; Yu, Z. Ammonia-Oxidizing Archaea and Bacteria Differentially Contribute to Ammonia Oxidation in Sediments from Adjacent Waters of Rushan Bay, China. Front. Microbiol. 2018, 9, 116. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, Y.; Liu, G.; Liu, W.; Lu, B. The effects of climate, catchment land use and local factors on the abundance and community structure of sediment ammonia-oxidizing microorganisms in Yangtze lakes. AMB Express 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Lin, X.; Zheng, P.; Zou, S.; Sun, F.; Zhang, X.; Gong, J. Seagrass (Zostera marina) promotes nitrification potential and selects specific ammonia oxidizers in coastal sediments. J. Soils Sediments 2021, 21, 3259–3273. [Google Scholar] [CrossRef]
- Arthurs, S.P.; Stamps, R.H.; Giglia, F.F. Environmental Modification Inside Photoselective Shadehouses. Hortscience 2013, 48, 975–979. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Wang, J.; Zhao, D.; Wei, J.; Peng, Y.; Miao, L. Characterizing algal-bacterial symbiotic biofilms: Insights into coexistence of algae and anaerobic microorganisms. Bioresour. Technol. 2024, 406, 130966. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.; Cheng, X.; Guo, Z. Dissipation of Eutrophic Substances in Grass Carp Aquaculture Pond Water by Ozone. Water 2023, 15, 3167. [Google Scholar] [CrossRef]
- Krieg, N.R.; Holt, J.G. Bergey’s Manual of Systemaic Bacteriology; Science Press: Beijing, China, 1984; p. 660. (In Chinese) [Google Scholar]
- Harris, S.H.; Smith, R.L. In situ measurements of microbially-catalyzed nitrification and nitrate reduction rates in an ephemeral drainage channel receiving water from coalbed natural gas discharge, Powder River Basin, Wyoming, USA. Chem. Geol. 2009, 267, 77–84. [Google Scholar] [CrossRef]
- Arun, S.; Ramasamy, S.; Pakshirajan, K. Mechanistic insights into nitrification by microalgae-bacterial consortia in a photo-sequencing batch reactor under different light intensities. J. Clean. Prod. 2021, 321, 128752. [Google Scholar] [CrossRef]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.; Li, Y.; Yuan, Z.; Liu, X.; Che, X.; Cheng, G.; Gu, Z.; Wu, F. Microalgae Enhance the Resistance of Pond-Dwelling Ammonia-Oxidizing Bacteria to Light Irradiation. Processes 2024, 12, 2261. https://doi.org/10.3390/pr12102261
Lu S, Li Y, Yuan Z, Liu X, Che X, Cheng G, Gu Z, Wu F. Microalgae Enhance the Resistance of Pond-Dwelling Ammonia-Oxidizing Bacteria to Light Irradiation. Processes. 2024; 12(10):2261. https://doi.org/10.3390/pr12102261
Chicago/Turabian StyleLu, Shimin, Yayuan Li, Zehui Yuan, Xingguo Liu, Xuan Che, Guofeng Cheng, Zhaojun Gu, and Fan Wu. 2024. "Microalgae Enhance the Resistance of Pond-Dwelling Ammonia-Oxidizing Bacteria to Light Irradiation" Processes 12, no. 10: 2261. https://doi.org/10.3390/pr12102261
APA StyleLu, S., Li, Y., Yuan, Z., Liu, X., Che, X., Cheng, G., Gu, Z., & Wu, F. (2024). Microalgae Enhance the Resistance of Pond-Dwelling Ammonia-Oxidizing Bacteria to Light Irradiation. Processes, 12(10), 2261. https://doi.org/10.3390/pr12102261






