Carbonization of Invasive Plant Species—Novel Route for Removal of Active Pharmaceutical Ingredients via Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Activated Biochar Preparation
2.3. Activated Biochar Characterization
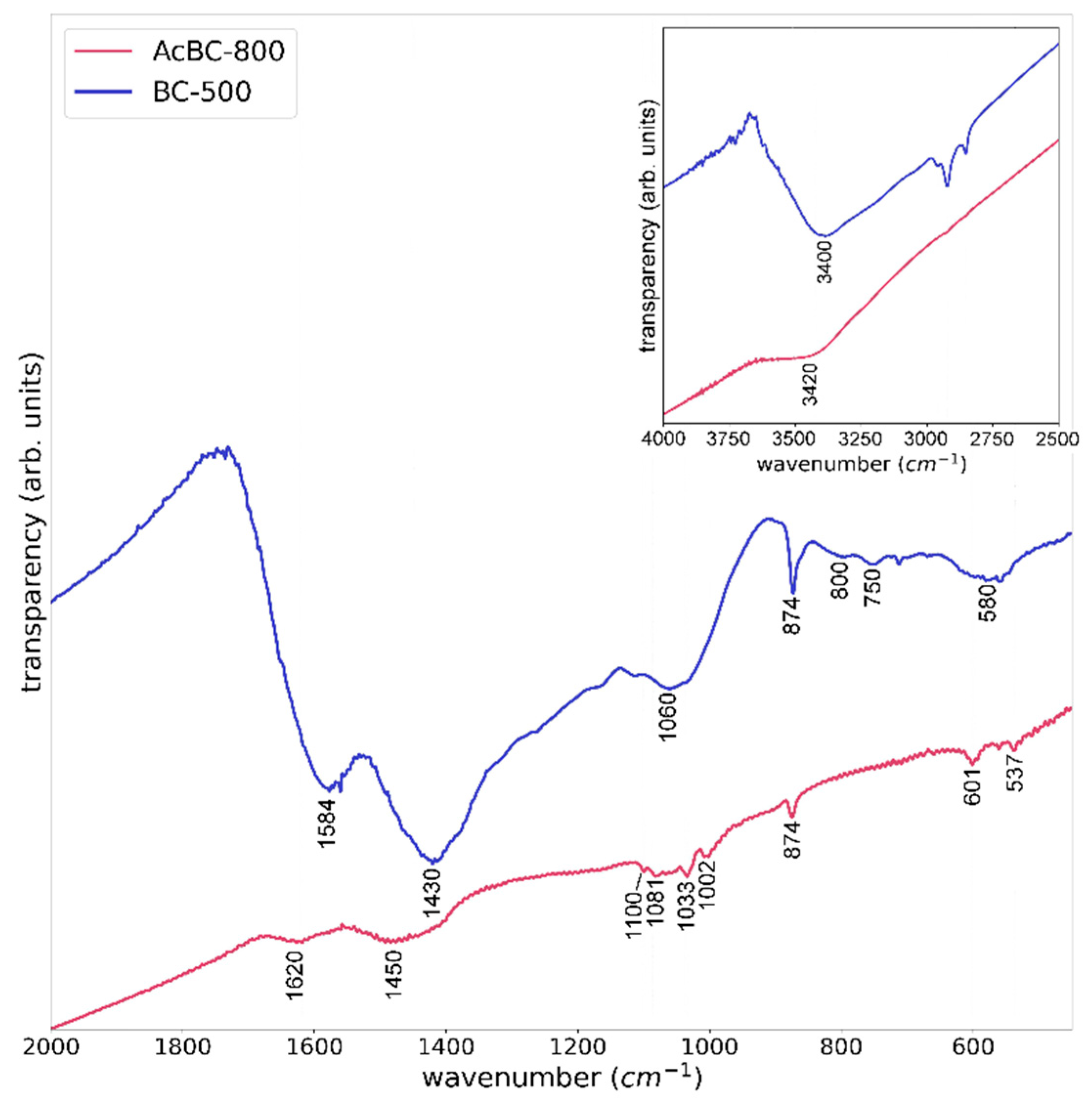
2.3.1. Fourier-Transform Infrared (FTIR) Spectroscopy
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. X-ray Photoelectron Spectroscopy (XPS)
2.3.4. Raman Spectroscopy
2.4. Adsorption Tests
2.4.1. Preparation of Standard Solutions
2.4.2. Adsorption Test Procedure
2.4.3. Adsorption Kinetics
2.4.4. Adsorption Isotherms
2.5. Software
3. Results and Discussion
3.1. Evaluation of the Reproducibility of the Production of Activated Biochar by FTIR Spectroscopy
3.2. Activated Biochar Characterization
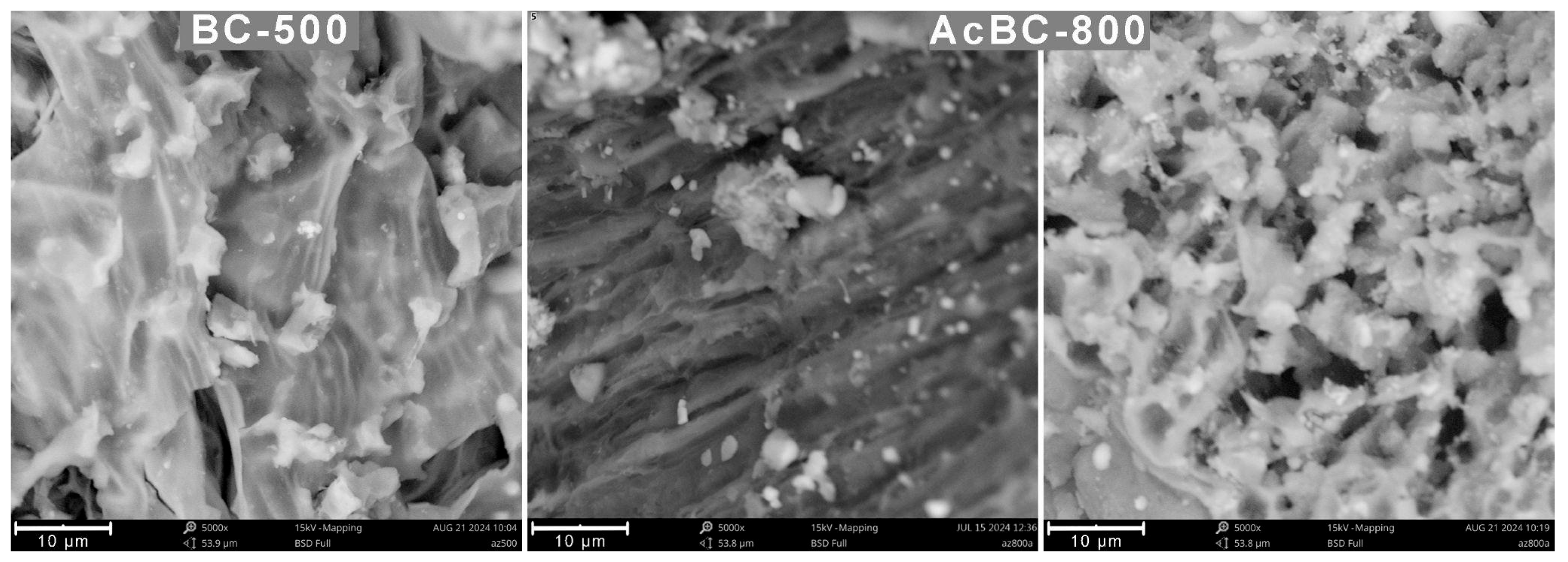
3.2.1. Morphology
3.2.2. X-ray Photoelectron Spectroscopy (XPS)
3.2.3. Raman Spectroscopy
3.3. Adsorption of the Selected APIs on AcBC-800
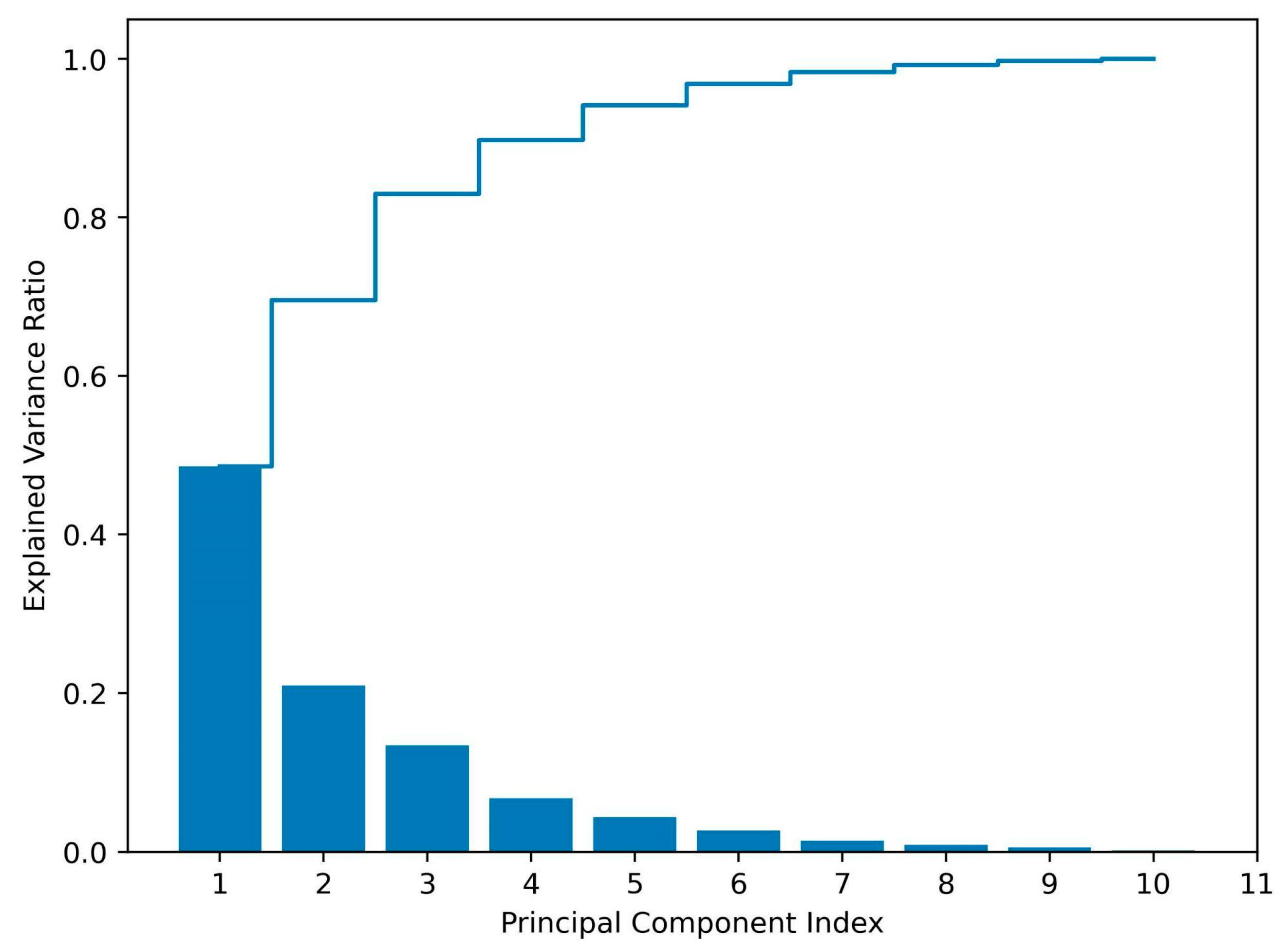
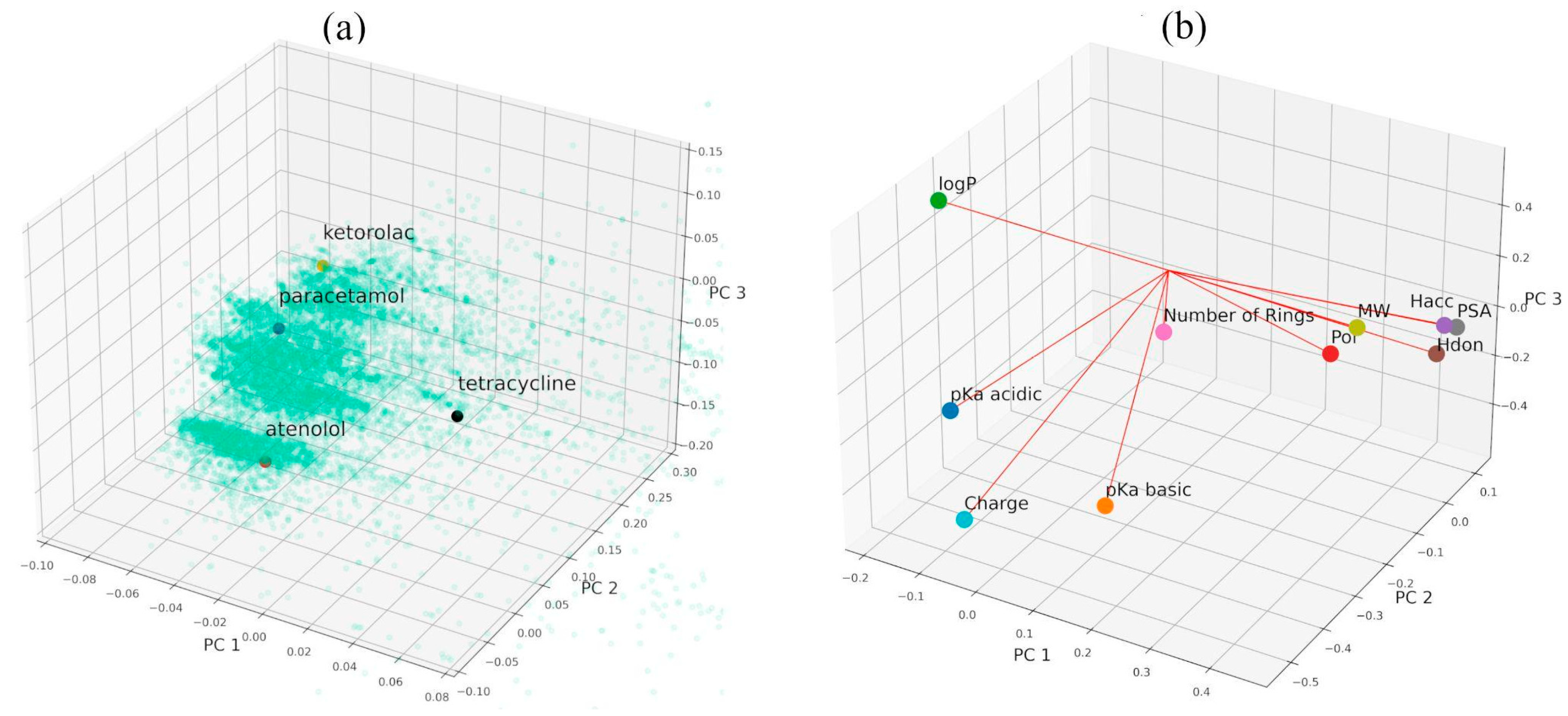
3.3.1. The Selection of Representative APIs
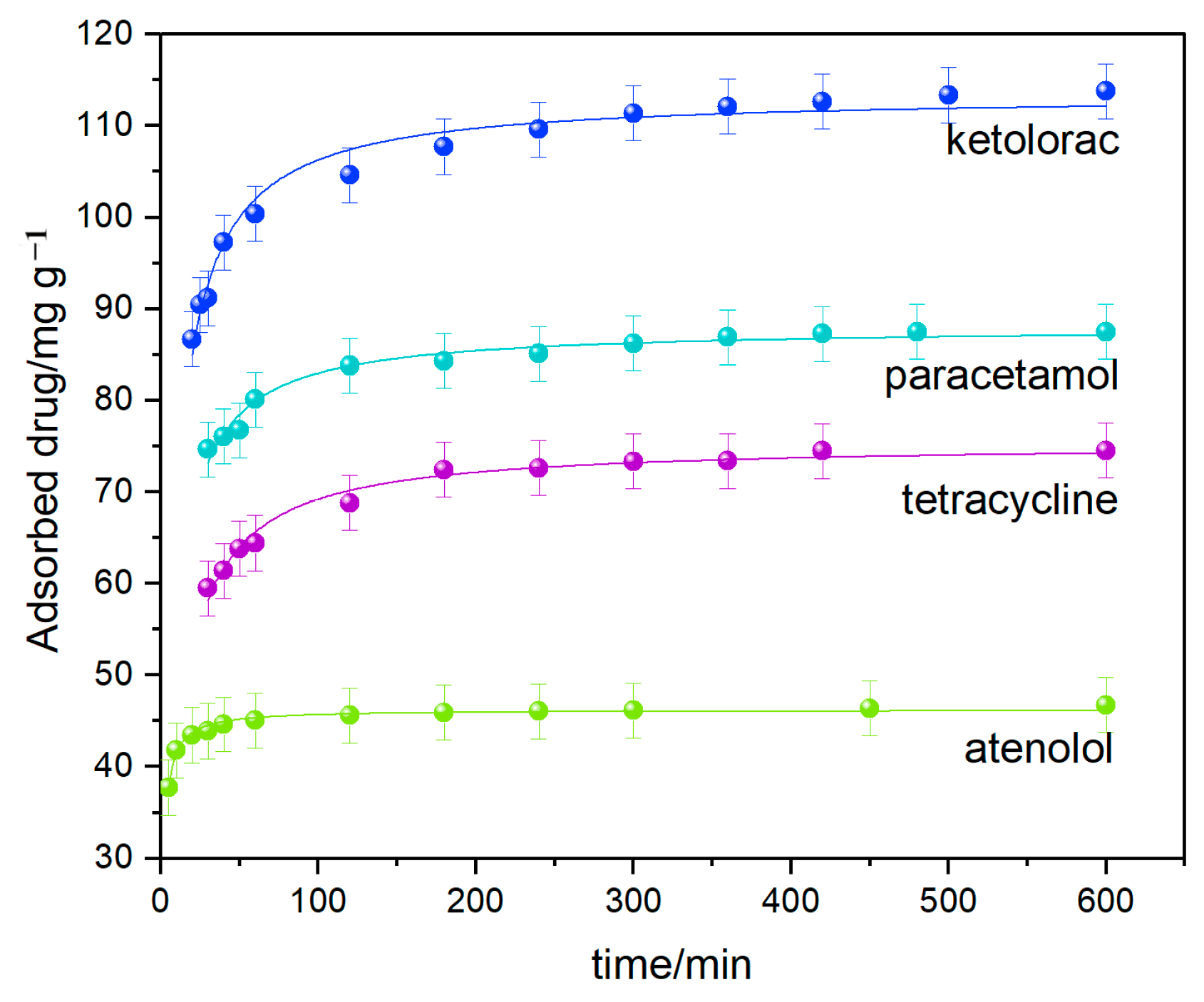
3.3.2. Adsorption Kinetics
3.3.3. Adsorption Isotherms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Analyte | Injection Volume (µL) | Detection Wavelength (nm) | Run Time (min) | Mobile Phase Composition | ||
|---|---|---|---|---|---|---|
| Organic Modifier | Aqueous Phase | Modifier vol. % | ||||
| atenolol | 10 | 225 | 3.5 | acetonitrile | 30 mM HClO4 pH 2.5 | 15 |
| paracetamol | 5 | 242 | 3 | water | 15 | |
| ketorolac tromethamine | 5 | 323 | 3.5 | 10 mM HCOOH/HCOONH4 buffer pH 2.8 | 50 | |
| tetracycline hydrochloride | 10 | 270 | 4 | 100 mM HClO4 pH 2.5 | 30 | |
Appendix B
References
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental Application of Biochar: Current Status and Perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Deng, Y.; Shu, Z.; Chen, Q.; Yang, H.; Tan, X. Application of Invasive Plants as Biochar Precursors in the Field of Environment and Energy Storage. Front. Environ. Sci. 2022, 10, 902915. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Thompson, K.A.; Shimabuku, K.K.; Kearns, J.P.; Knappe, D.R.U.; Summers, R.S.; Cook, S.M. Environmental Comparison of Biochar and Activated Carbon for Tertiary Wastewater Treatment. Environ. Sci. Technol. 2016, 50, 11253–11262. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of Emerging Contaminants from Water and Wastewater by Modified Biochar: A Review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A. Influence of Activation Conditions on the Physicochemical Properties of Activated Biochar: A Review. Biomass Convers. Biorefinery 2022, 12, 925–947. [Google Scholar] [CrossRef]
- Kalinke, C.; Oliveira, P.R.; Oliveira, G.A.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Activated Biochar: Preparation, Characterization and Electroanalytical Application in an Alternative Strategy of Nickel Determination. Anal. Chim. Acta 2017, 983, 103–111. [Google Scholar] [CrossRef]
- da Silva Medeiros, D.C.C.; Nzediegwu, C.; Benally, C.; Messele, S.A.; Kwak, J.-H.; Naeth, M.A.; Ok, Y.S.; Chang, S.X.; Gamal El-Din, M. Pristine and Engineered Biochar for the Removal of Contaminants Co-Existing in Several Types of Industrial Wastewaters: A Critical Review. Sci. Total Environ. 2022, 809, 151120. [Google Scholar] [CrossRef]
- van Kleunen, M.; Bossdorf, O.; Dawson, W. The Ecology and Evolution of Alien Plants. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 25–47. [Google Scholar] [CrossRef]
- Gioria, M.; Hulme, P.E.; Richardson, D.M.; Pyšek, P. Why Are Invasive Plants Successful? Annu. Rev. Plant Biol. 2023, 74, 635–670. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Tran, T.V.; Kumar, P.S.; Din, A.T.M.; Jalil, A.A.; Vo, D.-V.N. Invasive Plants as Biosorbents for Environmental Remediation: A Review. Environ. Chem. Lett. 2022, 20, 1421–1451. [Google Scholar] [CrossRef] [PubMed]
- Soler, J.; Izquierdo, J. The Invasive Ailanthus Altissima: A Biology, Ecology, and Control Review. Plants 2024, 13, 931. [Google Scholar] [CrossRef] [PubMed]
- Kowarik, I.; Säumel, I. Biological Flora of Central Europe: Ailanthus altissima (Mill.) Swingle. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 207–237. [Google Scholar] [CrossRef]
- Sladonja, B.; Sušek, M.; Guillermic, J. Review on Invasive Tree of Heaven (Ailanthus altissima (Mill.) Swingle) Conflicting Values: Assessment of Its Ecosystem Services and Potential Biological Threat. Environ. Manag. 2015, 56, 1009–1034. [Google Scholar] [CrossRef]
- Enescu, C.; Durrant, T.; Caudullo, G. Ailanthus altissima in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; ISBN 978-92-79-36740-3. [Google Scholar]
- Caramelo, D.; Pedro, S.I.; Marques, H.; Simão, A.Y.; Rosado, T.; Barroca, C.; Gominho, J.; Anjos, O.; Gallardo, E. Insights into the Bioactivities and Chemical Analysis of Ailanthus altissima (Mill.) Swingle. Appl. Sci. 2021, 11, 11331. [Google Scholar] [CrossRef]
- Constán-Nava, S.; Soliveres, S.; Torices, R.; Serra, L.; Bonet, A. Direct and Indirect Effects of Invasion by the Alien Tree Ailanthus altissima on Riparian Plant Communities and Ecosystem Multifunctionality. Biol. Invasions 2015, 17, 1095–1108. [Google Scholar] [CrossRef]
- Stojanović, J.; Milojević-Rakić, M.; Bajuk-Bogdanović, D.; Ranđelović, D.; Sokić, M.; Otašević, B.; Malenović, A.; Ležaić, A.J.; Protić, A. Chemometrically-Aided General Approach to Novel Adsorbents Studies: Case Study on the Adsorption of Pharmaceuticals by the Carbonized Ailanthus altissima Leaves. Heliyon 2024, 10, e34841. [Google Scholar] [CrossRef]
- López-Díaz, D.; López Holgado, M.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and Inconsistencies Regarding Adsorption of Contaminants from Aqueous Solutions: A Critical Review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Schrödinger, LLC. The PyMOL Molecular Graphics System, Version 1.8 2015; Schrödinger, LLC.: New York, NY, USA, 2015. [Google Scholar]
- Axet, M.R.; Dechy-Cabaret, O.; Durand, J.; Gouygou, M.; Serp, P. Coordination Chemistry on Carbon Surfaces. Coord. Chem. Rev. 2016, 308, 236–345. [Google Scholar] [CrossRef]
- Pinheiro, R.F.; Grimm, A.; Oliveira, M.L.S.; Vieillard, J.; Silva, L.F.O.; De Brum, I.A.S.; Lima, É.C.; Naushad, M.; Sellaoui, L.; Dotto, G.L.; et al. Adsorptive Behavior of the Rare Earth Elements Ce and La on a Soybean Pod Derived Activated Carbon: Application in Synthetic Solutions, Real Leachate and Mechanistic Insights by Statistical Physics Modeling. Chem. Eng. J. 2023, 471, 144484. [Google Scholar] [CrossRef]
- Grimm, A.; dos Reis, G.S.; Dinh, V.M.; Larsson, S.H.; Mikkola, J.-P.; Lima, E.C.; Xiong, S. Hardwood Spent Mushroom Substrate–Based Activated Biochar as a Sustainable Bioresource for Removal of Emerging Pollutants from Wastewater. Biomass Convers. Biorefinery 2024, 14, 2293–2309. [Google Scholar] [CrossRef]
- Jovanovic, Z.; Bajuk-Bogdanović, D.; Jovanović, S.; Mravik, Ž.; Kovač, J.; Holclajtner-Antunović, I.; Vujković, M. The Role of Surface Chemistry in the Charge Storage Properties of Graphene Oxide. Electrochim. Acta 2017, 258, 1228–1243. [Google Scholar] [CrossRef]
- Anto, S.; Sudhakar, M.P.; Shan Ahamed, T.; Samuel, M.S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Activation Strategies for Biochar to Use as an Efficient Catalyst in Various Applications. Fuel 2021, 285, 119205. [Google Scholar] [CrossRef]
- Breitenbach, S.; Gavrilov, N.; Pašti, I.; Unterweger, C.; Duchoslav, J.; Stifter, D.; Hassel, A.W.; Fürst, C. Biomass-Derived Carbons as Versatile Materials for Energy-Related Applications: Capacitive Properties vs. Oxygen Reduction Reaction Catalysis. C 2021, 7, 55. [Google Scholar] [CrossRef]
- High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database (Beamson, G.; Briggs, D.). J. Chem. Educ. 1993, 70, A25. [CrossRef]
- Rabchinskii, M.K.; Shnitov, V.V.; Brzhezinskaya, M.; Baidakova, M.V.; Stolyarova, D.Y.; Ryzhkov, S.A.; Saveliev, S.D.; Shvidchenko, A.V.; Nefedov, D.Y.; Antonenko, A.O.; et al. Manifesting Epoxide and Hydroxyl Groups in XPS Spectra and Valence Band of Graphene Derivatives. Nanomaterials 2023, 13, 23. [Google Scholar] [CrossRef]
- Duan, X.-L.; Yuan, C.-G.; Jing, T.-T.; Yuan, X.-D. Removal of Elemental Mercury Using Large Surface Area Micro-Porous Corn Cob Activated Carbon by Zinc Chloride Activation. Fuel 2019, 239, 830–840. [Google Scholar] [CrossRef]
- Kwan, Y.C.G.; Ng, G.M.; Huan, C.H.A. Identification of Functional Groups and Determination of Carboxyl Formation Temperature in Graphene Oxide Using the XPS O 1s Spectrum. Thin Solid Film. 2015, 590, 40–48. [Google Scholar] [CrossRef]
- Zhou, J.-H.; Sui, Z.-J.; Zhu, J.; Li, P.; Chen, D.; Dai, Y.-C.; Yuan, W.-K. Characterization of Surface Oxygen Complexes on Carbon Nanofibers by TPD, XPS and FT-IR. Carbon 2007, 45, 785–796. [Google Scholar] [CrossRef]
- Stevens, J.S.; Byard, S.J.; Zlotnikov, E.; Schroeder, S.L.M. Detection of Free Base Surface Enrichment of a Pharmaceutical Salt by X-Ray Photoelectron Spectroscopy (XPS). J. Pharm. Sci. 2011, 100, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Rupar, J.; Bajuk-Bogdanović, D.; Milojević-Rakić, M.; Krstić, J.; Upadhyay, K.; Gavrilov, N.; Janošević Ležaić, A. Tailored Porosity Development in Carbons via Zn2+ Monodispersion: Fitting Supercapacitors. Microporous Mesoporous Mater. 2022, 335, 111790. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- McDonald-Wharry, J.; Manley-Harris, M.; Pickering, K. Carbonisation of Biomass-Derived Chars and the Thermal Reduction of a Graphene Oxide Sample Studied Using Raman Spectroscopy. Carbon 2013, 59, 383–405. [Google Scholar] [CrossRef]
- Tsaneva, V.N.; Kwapinski, W.; Teng, X.; Glowacki, B.A. Assessment of the Structural Evolution of Carbons from Microwave Plasma Natural Gas Reforming and Biomass Pyrolysis Using Raman Spectroscopy. Carbon 2014, 80, 617–628. [Google Scholar] [CrossRef]
- Shagali, A.A.; Hu, S.; Wang, Y.; Li, H.; Wang, Y.; Su, S.; Xiang, J. Comparative Study on One-Step Pyrolysis Activation of Walnut Shells to Biochar at Different Heating Rates. Energy Rep. 2021, 7, 388–396. [Google Scholar] [CrossRef]
- Abbaci, F.; Nait-Merzoug, A.; Guellati, O.; Harat, A.; El Haskouri, J.; Delhalle, J.; Mekhalif, Z.; Guerioune, M. Bio/KOH Ratio Effect on Activated Biochar and Their Dye Based Wastewater Depollution. J. Anal. Appl. Pyrolysis 2022, 162, 105452. [Google Scholar] [CrossRef]
- Varela, C.F.; Moreno-Aldana, L.C.; Agámez-Pertuz, Y.Y. Adsorption of Pharmaceutical Pollutants on ZnCl2-Activated Biochar from Corn Cob: Efficiency, Selectivity and Mechanism. J. Bioresour. Bioprod. 2024, 9, 58–73. [Google Scholar] [CrossRef]
- Maged, A.; Dissanayake, P.D.; Yang, X.; Pathirannahalage, C.; Bhatnagar, A.; Ok, Y.S. New Mechanistic Insight into Rapid Adsorption of Pharmaceuticals from Water Utilizing Activated Biochar. Environ. Res. 2021, 202, 111693. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, Y.; Zhang, L.; Zhang, S.; Westerhof, R.J.M.; Liu, Q.; Jia, P.; Li, Q.; Wang, Y.; Hu, X. Impacts of Temperature on Evolution of Char Structure during Pyrolysis of Lignin. Sci. Total Environ. 2020, 699, 134381. [Google Scholar] [CrossRef]
- Sagues, W.J.; Jain, A.; Brown, D.; Aggarwal, S.; Suarez, A.; Kollman, M.; Park, S.; Argyropoulos, D.S. Are Lignin-Derived Carbon Fibers Graphitic Enough? Green Chem. 2019, 21, 4253–4265. [Google Scholar] [CrossRef]
- Shao, Y.; Guizani, C.; Grosseau, P.; Chaussy, D.; Beneventi, D. Biocarbons from Microfibrillated Cellulose/Lignosulfonate Precursors: A Study of Electrical Conductivity Development during Slow Pyrolysis. Carbon 2018, 129, 357–366. [Google Scholar] [CrossRef]
- Chauhan, S.; Shafi, T.; Dubey, B.K.; Chowdhury, S. Biochar-Mediated Removal of Pharmaceutical Compounds from Aqueous Matrices via Adsorption. Waste Dispos. Sustain. Energy 2023, 5, 37–62. [Google Scholar] [CrossRef] [PubMed]
- Milojević-Rakić, M.; Janošević, A.; Krstić, J.; Nedić Vasiljević, B.; Dondur, V.; Ćirić-Marjanović, G. Polyaniline and Its Composites with Zeolite ZSM-5 for Efficient Removal of Glyphosate from Aqueous Solution. Microporous Mesoporous Mater. 2013, 180, 141–155. [Google Scholar] [CrossRef]
- Kumar, K.V.; Gadipelli, S.; Wood, B.; Ramisetty, K.A.; Stewart, A.A.; Howard, C.A.; Brett, D.J.L.; Rodriguez-Reinoso, F. Characterization of the Adsorption Site Energies and Heterogeneous Surfaces of Porous Materials. J. Mater. Chem. A 2019, 7, 10104–10137. [Google Scholar] [CrossRef]
- Xiao, K. Analytical Chemistry and Separation Science at Molecular Level. In Analytical Scientists in Pharmaceutical Product Development; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 101–149. ISBN 978-1-119-54778-5. [Google Scholar]
- Tran, H.N.; Tomul, F.; Thi Hoang Ha, N.; Nguyen, D.T.; Lima, E.C.; Le, G.T.; Chang, C.-T.; Masindi, V.; Woo, S.H. Innovative Spherical Biochar for Pharmaceutical Removal from Water: Insight into Adsorption Mechanism. J. Hazard. Mater. 2020, 394, 122255. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Din, A.T.M.; Yahaya, N.K.E.M.; Karim, J.; Ahmad, M.A. Atenolol Sequestration Using Activated Carbon Derived from Gasified Glyricidia Sepium. Arab. J. Chem. 2020, 13, 7544–7557. [Google Scholar] [CrossRef]
- Rizkallah, B.M.; Galal, M.M.; Matta, M.E. Characteristics of Tetracycline Adsorption on Commercial Biochar from Synthetic and Real Wastewater in Batch and Continuous Operations: Study of Removal Mechanisms, Isotherms, Kinetics, Thermodynamics, and Desorption. Sustainability 2023, 15, 8249. [Google Scholar] [CrossRef]
- Haro, N.K.; Del Vecchio, P.; Marcilio, N.R.; Féris, L.A. Removal of Atenolol by Adsorption—Study of Kinetics and Equilibrium. J. Clean. Prod. 2017, 154, 214–219. [Google Scholar] [CrossRef]
- To, M.-H.; Hadi, P.; Hui, C.-W.; Lin, C.S.K.; McKay, G. Mechanistic Study of Atenolol, Acebutolol and Carbamazepine Adsorption on Waste Biomass Derived Activated Carbon. J. Mol. Liq. 2017, 241, 386–398. [Google Scholar] [CrossRef]
- Ferreira, R.C.; de Araújo, T.P.; Dias, D.; Bernardo, M.; Lapa, N.; Fonseca, I.M.; de Barros, M.A.S.D. Removal of Paracetamol and Cu2+ from Water by Using Porous Carbons Derived from Agrowastes. Processes 2023, 11, 2146. [Google Scholar] [CrossRef]
- Bursztyn Fuentes, A.L.; Canevesi, R.L.S.; Gadonneix, P.; Mathieu, S.; Celzard, A.; Fierro, V. Paracetamol Removal by Kon-Tiki Kiln-Derived Biochar and Activated Carbons. Ind. Crops Prod. 2020, 155, 112740. [Google Scholar] [CrossRef]
- Bursztyn Fuentes, A.L.; Benito, D.E.; Montes, M.L.; Scian, A.N.; Lombardi, M.B. Paracetamol and Ibuprofen Removal from Aqueous Phase Using a Ceramic-Derived Activated Carbon. Arab. J. Sci. Eng. 2023, 48, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Erdem, H.; Yildiz, B.; Şahin, M.; Erdem, M. Etodolac Adsorption onto Activated Carbon Prepared by Chemical Activation and Pyrolysis of Biomasses Mixture. Biomass Convers. Biorefinery 2020, 10, 1153–1165. [Google Scholar] [CrossRef]
- Jang, H.M.; Yoo, S.; Choi, Y.-K.; Park, S.; Kan, E. Adsorption Isotherm, Kinetic Modeling and Mechanism of Tetracycline on Pinus Taeda-Derived Activated Biochar. Bioresour. Technol. 2018, 259, 24–31. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, J.; Li, Z.; Xia, H.; Liu, H.; Yang, Y. Highly Enhanced Adsorption Performance of Tetracycline Antibiotics on KOH-Activated Biochar Derived from Reed Plants. RSC Adv. 2020, 10, 5066–5076. [Google Scholar] [CrossRef]
- Alsawy, T.; Rashad, E.; El-Qelish, M.; Mohammed, R.H. A Comprehensive Review on the Chemical Regeneration of Biochar Adsorbent for Sustainable Wastewater Treatment. NPJ Clean Water 2022, 5, 29. [Google Scholar] [CrossRef]
- Greiner, B.G.; Shimabuku, K.K.; Summers, R.S. Influence of Biochar Thermal Regeneration on Sulfamethoxazole and Dissolved Organic Matter Adsorption. Environ. Sci. Water Res. Technol. 2018, 4, 169–174. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar] [CrossRef]




| Sample | D Band | G Band | ID/IG | ||||
|---|---|---|---|---|---|---|---|
| Position (cm−1) | FWHM (cm−1) | Area | Position (cm−1) | FWHM (cm−1) | Area | Area Ratio | |
| BC-500 | 1376 | 181 | 123 | 1596 | 83 | 85 | 1.4 |
| AcBC-800 | 1357 | 180 | 175 | 1607 | 79 | 77 | 2.3 |
| Derived PSO Parameters | Atenolol | Tetracycline | Paracetamol | Ketorolac |
|---|---|---|---|---|
| qe (mg g−1) | 46.2 ± 0.2 | 75.3 ± 0.5 | 88.0 ± 0.4 | 113.4 ± 0.7 |
| k2 (min−1) | 0.88 ± 0.05 | 0.113 ± 0.007 | 0.16 ± 0.01 | 0.150 ± 0.009 |
| Adj. R-Square | 0.98 | 0.98 | 0.97 | 0.97 |
| Derived LF Parameters | Tetracycline | Paracetamol | Atenolol | Ketorolac |
|---|---|---|---|---|
| qm (mg g−1) | 210 ± 60 | 160 ± 30 | 60.1 ± 0.7 | 160 ± 20 |
| KLF (L mg−1)1/n | 0.10 ± 0.02 | 0.36 ± 0.05 | 0.03 ± 0.02 | 0.64 ± 0.09 |
| 1/n | 0.6 ± 0.2 | 0.39± 0.08 | 1.9 ± 0.3 | 0.40 ± 0.08 |
| R-Square (COD) | 0.98 | 0.99 | 0.97 | 0.97 |
| Adj. R-Square | 0.97 | 0.98 | 0.96 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanović, J.; Milojević-Rakić, M.; Bajuk-Bogdanović, D.; Ranđelović, D.; Otašević, B.; Malenović, A.; Janošević Ležaić, A.; Protić, A. Carbonization of Invasive Plant Species—Novel Route for Removal of Active Pharmaceutical Ingredients via Adsorption. Processes 2024, 12, 2149. https://doi.org/10.3390/pr12102149
Stojanović J, Milojević-Rakić M, Bajuk-Bogdanović D, Ranđelović D, Otašević B, Malenović A, Janošević Ležaić A, Protić A. Carbonization of Invasive Plant Species—Novel Route for Removal of Active Pharmaceutical Ingredients via Adsorption. Processes. 2024; 12(10):2149. https://doi.org/10.3390/pr12102149
Chicago/Turabian StyleStojanović, Jevrem, Maja Milojević-Rakić, Danica Bajuk-Bogdanović, Dragana Ranđelović, Biljana Otašević, Anđelija Malenović, Aleksandra Janošević Ležaić, and Ana Protić. 2024. "Carbonization of Invasive Plant Species—Novel Route for Removal of Active Pharmaceutical Ingredients via Adsorption" Processes 12, no. 10: 2149. https://doi.org/10.3390/pr12102149
APA StyleStojanović, J., Milojević-Rakić, M., Bajuk-Bogdanović, D., Ranđelović, D., Otašević, B., Malenović, A., Janošević Ležaić, A., & Protić, A. (2024). Carbonization of Invasive Plant Species—Novel Route for Removal of Active Pharmaceutical Ingredients via Adsorption. Processes, 12(10), 2149. https://doi.org/10.3390/pr12102149










