Abstract
It is known that highly efficient catalysts for the catalytic oxidation of aromatic hydrocarbons can be obtained based on magnetic nanoparticles. The development of nanosized magnetically controlled catalysts for the oxidation of aromatic hydrocarbons with oxygen deserves especially close attention in the territory of the Republic of Kazakhstan, which does not have its own industrial production of oxygen-containing compounds. The aim of this work is to create catalysts based on Fe and Co nanoparticles stabilized with polymers: polyvinylpyrrolidone, chitosan, and polyethylenimine, study them by methods of physico-chemical research, and conduct preliminary tests of catalysts to predict their effectiveness. Magnetic nanoparticles were prepared by the co-precipitation method. Based on the results of the SEM analysis, it was concluded that polymers form composites together with metal nanocrystals. According to preliminary data, the most efficient oxidation of phenol in a non-flowing glass gradient-free thermostated duck-type reactor occurs on Fe3O4/chitosan, with the phenol conversion being 55–60%. Tests on the oxidation of phenol with oxygen showed a favorable prognosis for the use of such catalysts for the oxidative conversion of aromatic hydrocarbons in order to obtain valuable intermediates.
1. Introduction
The creation of nanoscale magnetically controlled catalysts for the oxidation of aromatic hydrocarbons with oxygen is a very topical issue in Kazakhstan since the republic does not have its own industrial production of oxygen-containing compounds [1,2]. Catalysts based on cobalt and iron nanoparticles are environmentally friendly and allow for subsequent oxidation of hydrocarbons in the liquid phase under fairly mild conditions. Oxygen is the cheapest and most widely used oxidizing agent that can be used for the syntheses of many oxygen-containing compounds [3,4,5,6,7,8,9,10].
Research devoted to methods of obtaining ferromagnetic nanomaterials, as well as the study of their structure and properties, attract more attention. The most studied and applied nanoparticles in electronics, chemistry, catalysis, and medicine are the iron oxides magnetite (Fe3O4) and magnetite (Fe2O3). Iron oxide Fe3O4 has adsorption properties due to the presence of coordinatively unsaturated Fe3+ and Fe2+ ions and O2− and OH− ions. Iron atoms can be both donors and acceptors of electrons. OH groups are able to interact with stabilizing polymers, which contribute to the modification of the surface state by reactive amino groups. Such modified Fe3O4 nanoparticles can easily join the original molecules. It is known that Fe3O4 magnetic nanoparticles with sizes up to 200 nm have superparamagnetic properties. In general, nanoparticles are very prone to oxidation and agglomeration due to their large surface area and high reactivity, which violates their special properties. Under environmental conditions, the surfaces of nanoparticles undergo oxidation and a thin layer of an oxide film is formed. To reduce or completely prevent such consequences, a method is proposed for their “sealing” (“stabilization”) with the help of carbon, silicon, noble metals, metal oxides, organic polymers, and surfactants. Stabilization procedures, i.e., preventing the formation of aggregates of magnetic nanoparticles, can be achieved by using electrostatic forces or steric factors.
A change in the particle size of starting nanoparticles, for example, Fe3O4, leads to a change saturation magnetization, coercive force, and other characteristics. This feature enables researchers to improve the characteristics of the materials used based on iron nanoparticles and create fundamentally new functional materials. An important task for the subsequent use of colloidal nanosystems is their stability, which is of particular importance when nanoparticles are used in catalytic reactions, for example, in the oxidation of aromatic hydrocarbons with oxygen. In order to effectively stabilize nanoparticles, ensuring the presence of individual nanoparticles rather than agglomerates in solutions while maintaining the magnetic properties of the material and the surface of the particles involved in the catalytic reaction and further functionalization, various stabilizers have been used in recent years. To stabilize magnetic nanoparticles, their surface is coated with materials of both organic and inorganic nature. Such coatings solve a dual problem: firstly, they prevent the aggregation of nanoparticles and the oxidation of magnetite (both problems are, for example, fundamental in the transport of nanomaterials); secondly, they allow for modification of the surface of nanoparticles with various specific ligands for the intended use [11,12,13,14,15,16,17,18,19,20,21,22,23,24]. Currently, a promising direction is the synthesis and application of magnetic nanoparticles stabilized by modifiers: polymers of natural and artificial origin. It is known that nanoscale magnetic composites of transition metals immobilized on a polymer matrix have a large surface area, ease of separation from the reaction mixture, and that their activity and selectivity can be controlled by a magnetic field. When the metal complex is bound to the polymer, some of the ligands in the complex are replaced by functional groups of the polymer chain. In this case, the catalytic properties of the metal complex change, and it becomes possible to purposefully influence the course of the catalytic process by changing both the ligand environment of the central atom and the nature of the functional groups of the polymer [25,26,27,28,29].
The high sensitivity of polymeric materials to various factors determines the prospects for their use in various fields of science: medicine, physics, chemistry, biotechnology (purification of proteins and enzymes), membrane technologies for separating liquids and gases, microencapsulation, electronics, and robotics. Polymers, due to their structure and the presence of functional groups of various compositions and structures, contribute to the stabilization of metal nanoparticles with the formation of metal-polymer systems, which can act as catalysts for many processes of organic synthesis, including oxidation reactions. The presence of polymer macromolecules imparts new properties for catalysts, among them: flexibility and mobility of polymer chain segments and the possibility of good swelling, i.e., the process of absorption by a polymer of a low molecular weight liquid or its vapor, accompanied by an increase in the volume of the polymer and a change in the conformations (shape) of its macromolecules [30,31,32].
In our research, the polymers chitosan, polyvinylpyrrolidone (PVP), and polyethylene-imine (PEI) were used as a stabilizer of nanoparticles of Fe and Co. The natural biopolymer chitosan (Figure 1a) is a promising material for changing the properties, structure, and functional characteristics of magnetic nanoparticles (MNPs) used in catalytic reactions, biological experiments, medicine, and pharmacy since chitosan has unique properties, such as biocompatibility, biodegradability, low toxicity, and low allergenicity.
Figure 1.
Polymers used in our research as a stabilizer of nanoparticles: (a)—chitosan, (b)—polyvinylpyrrolidone (PVP); (c,d)—polyethyleneimine (PEI).
Chitosan is obtained from chitin from the shells of red-legged crabs or from lower fungi by removing the acyl (carboxylic compound) that stiffens chitin. Chitosan was first obtained in 1859 by the physiologist Charles Rouget. Shellfish are a source of chitosan. The only source of chitosan is chitin. In nature, it is found in the cell walls of zygomycetes, in particular, mucor. MNPs coated with chitosan retain their superparamagnetic properties and make it possible to separate the sorbent from the liquid matrix by the action of a permanent magnet. The presence of NH2 groups in the chitosan macromolecule makes it possible to adsorb many metals and, upon protonation of amino groups, also organic compounds containing negatively charged sulfo- and carboxyl groups [24,25,32,33,34,35]. The macromolecules of this polysaccharide have an asymmetric structure and contain highly polar groups in their structure that can interact with the formation of hydrogen bonds. The melting point of chitosan is higher than the decomposition temperature. Chitosan is able to adsorb a large amount of water, and with a decrease in the degree of crystallinity, its sorption properties, as for other polysaccharides, increase.
PVP (Figure 1b) acts as a surface stabilizer, nanoparticle expansion modifier, and dispersant. It has protective properties due to its unique structure. The molecule contains a highly polar amide group, which provides hydrophilic properties, as well as non-polar methyl groups in both the backbone and the ring, which provide hydrophobic properties. PVP is a vinyl polymer, it can be obtained by radical vinyl polymerization from vinylpyrrolidone monomer [36,37,38].
PEI or polyaziridine is a polymer with a repeating unit consisting of an amino group and two aliphatic carbons H[CH2-CH2-NH]nH. Linear PEIs contain all secondary amines (Figure 1c), while branched PEIs contain primary, secondary, and tertiary amino groups (Figure 1d). PEI is produced commercially and finds many uses, usually due to its poly-cationic nature.
The aim of this study is to create composites for the catalytic oxidation of aromatic hydrocarbons with oxygen based on Fe and Co nanoparticles stabilized with polymers: PVP, natural chitosan polysaccharide, and polyethylenimine (PEI), to study the properties of catalysts by physicochemical research methods and carry out preliminary tests in conditions of phenol oxidation with oxygen.
2. Materials and Methods
Magnetic Fe3O4 nanoparticles can be obtained by various methods. One of the methods for producing magnetic nanoparticles is the thermal decomposition of iron salts or their complexes (oleate, acetylacetonate, N-nitrosophenylhydroxylamine (C6H5N(NO)O-) and iron carbonyl) in the presence of reducing agents and stabilizing agents. The disadvantage of this method is that the synthesis is carried out in an organic solvent, and as a consequence, poses the need for an additional stage—transferring nanoparticles from the organic to the aqueous phase. Another method for producing magnetic nanoparticles is synthesis in reverse micelles. Its essence lies in the use of reverse micelles as nanoreactors that capture nanoparticles entering an aqueous environment. This method makes it possible to obtain nanoparticles with a very narrow size distribution and to easily vary this indicator by changing the ratio of components in the micellar system. However, this method has a narrow scope of application and low yield compared to coprecipitation and thermal decomposition, and also requires a high consumption of reagents [39,40,41,42,43]. Another common method for the synthesis of magnetic nanoparticles is the hydrothermal method. It is based on the ability of water and aqueous solutions to dissolve substances that are practically insoluble under normal conditions at high temperatures (up to 500 °C) and pressure (10–80 MPa, sometimes up to 300 MPa). Obviously, the co-precipitation method is preferable due to its methodological simplicity and convenience.
In our work the co-deposition (co-precipitation) method was used. The initial nanocrystalline magnetite was obtained by the coprecipitation reaction of bi- and ferric iron in an alkaline medium according to the following reaction:
Fe2+ + 2Fe3+ + 8OH− → Fe(OH)2 + 2FeOOH + 2H2O → Fe3O4 + 4H2O
It should be noted that the order of addition of reagents in the synthesis of magnetite by co-deposition affects the size, shape, and monodispersity of nanoparticles. Thus, when an acidic solution of iron salts is added dropwise to an alkaline solution, nanoparticles ~10 nm in size with a narrow size distribution are obtained. The formation of magnetite occurs through an intermediate stage of formation of ferrihydrite. The advantages of this method are high product yield, narrow distribution of nanoparticles in size, and resistance to aggregation and sedimentation [13,23,32,39,40,41,42,43,44,45,46,47,48]. Co-precipitation occurs in two stages: first, the nucleation of crystals (when critical supersaturation is reached) occurs; the second stage is the slow growth of embryos through the process of diffusion of dissolved substances to the crystal surface.
The list of chemical reagents used for the synthesis of magnetite and cobalt ferrite stabilized with polymers is as follows: iron (II) sulfate heptahydrate (FeSO4·7H2O); iron (III) chloride hexahydrate (FeCl3·6H2O); Co(NO3)2∙6H2O; 25% aqueous solution of ammonia (NH4OH).
Before the stabilization of nanocomposites with polymers, cobalt nitrate salt was added to iron oxide to synthesize cobalt ferrite, CoFe2O4, and increase the catalytic activity of the initial magnetite in the oxidation of aromatic hydrocarbons.
Nanocrystals of iron magnetite (Fe3O4) stabilized with PVP were obtained by co-precipitation of ferrous and trivalent iron ions in an alkaline solution in a 500 mL mini reactor with four necks equipped with a stirrer, a thermometer, and a dividing funnel. Aqueous solutions of ferrous and trivalent iron (Fe(II)/Fe(III) = 1:2) stirred and bubbled with argon gas for 20 min at 25 °C at 200 rpm. Then at 40 °C, with intensive stirring (700 rpm), a PVP solution was added and stirred for 15 min, and a 25% solution of NH4OH was poured in (pH = 9.5–10). When the NH4OH solution was introduced, a large amount of black colloidal precipitate was formed, which indicated the formation of iron oxide. The reaction mixture was stirred for another 25 min, and then allowed to cool at room temperature (~23 °C). The resulting nanocrystals were separated using a neodymium magnet and washed with water until the pH above the sedimentary liquid became neutral and dried at a temperature of 90–100 °C.
To obtain CoFe2O4 stabilized with PVP, a mixture of aqueous solutions of FeCl3·6H2O and Co(NO3)2·6H2O and PVP was mixed for 3 min in the vortex mode in the VEG reactor. The resulting suspension was mixed in a mini reactor in an inert medium (argon) at 700 rpm, 40 °C for 15 min. Then, a 25% solution of NH4OH (pH = 9.5–10) was added and mixed under the same conditions. The resulting precipitates were washed by decantation and dried at a temperature of 90–100 °C.
To identify the initial chitosan, PVP, PEI, and the resulting polymer-stabilized magnetic composites, a Vertex 70v IR Fourier spectrometer (Bruker, Germany) with a computer system for recording and processing spectra was used. The diffraction patterns were measured in the Bragg–Brentano geometry in the angle range 2θ = 15–100°. All measurements were carried out under normal conditions.
The SEM study was carried out at the following parameters: FW: 17 µm, mode: 10 kV—Image, detector: BSD Full. To determine the particle size distribution and area of the Fe3O4 catalysts stabilized with chitosan and polyvinylpyrrolidone, catalyst samples were ground in an agate mortar with intensive manual grinding for 10 min. Next, the resulting dry crushed powder, using a Nebula Phenom unit, was dispersed without forming clusters on the graphite tape of the sample holder. To improve the contrast when shooting in an SEM, 20 nm of gold was deposited onto the dispersed powder by magnetron sputtering. Using Phenom Particle Metric 1.2.3.0. Version number 1.2.3.0., 113 particles were identified on the obtained SEM micrographs.
The oxidation reactions of phenol with oxygen were carried out in a non-flowing glass gradient-free thermostated duck-type reactor equipped with a potentiometric device. The kinetic regime was ensured by intense shaking of the reactor (300–400 vibrations per minute), and the volume of the liquid phase was no more than 40 cm3, with a total reactor volume of 180 cm3. The oxidation of phenol was studied at a temperature of T = 353 K, at a constant oxygen pressure of 1.0 MPa. The reaction rate was monitored by the absorption of oxygen from a thermostated burette connected to the reactor. The components of the system were added in the following order: the catalyst was loaded into the reactor, then the phenol solution was poured. Then, a specified oxygen atmosphere was created in the reactor. The reactor was shaken until a constant, within the experimental error, volume of the gas phase was established. The temperature was maintained with an accuracy of 0.5 °C using a thermostat. When the rate of oxygen absorption fell below 0.1 mL/min, the reaction was considered complete, and the solution from the reactor was drained and analyzed.
The direct method of measuring the absorption spectrum of phenol makes it possible to determine phenol in water. The sensitivity of the method for the least sensitive absorption band during measurement allows for the determination of phenols in water. Due to this, the phenol content was determined by UV spectrophotometry. Based on the study of the UV spectrum of solutions during the oxidation process with a given concentration of phenol with oxygen in the presence of magnetic composites, it was determined by the decrease in optical density D at a phenol absorption wavelength of 270 nm over time. Phenol concentrations were determined using a calibration graph using the optical densities of the solution at a phenol absorption wavelength of 270 nm. In the UV spectra, the absorption bands of phenol are as follows: 210 (ɛ = 6200 L/mol∙cm) and 270 nm (ɛ = 1450 L/mol∙cm) [49,50,51,52,53,54,55,56,57]. The dependence of optical density on phenol concentration obeys the Bouguer–Lambert–Beer law in the plateau region at 270–280 nm. Calculations were performed based on the deviation in the optical density of the mixture at 270–280 nm. UV spectra of solutions in quartz cuvettes (l = 10 mm) were recorded on a SHIMADZU UV-1240 spectrophotometer, which is a single-beam device. The wavelength can vary in the range from 190 to 1100 nm.
Also, a detailed description of the methodology for obtaining catalysts and equipment for physical and chemical research is described in our previous articles [16,45].
3. Results
The X-ray diffraction pattern of the synthesized Fe3O4 (Figure 2a) proves the crystal structure of magnetite, and the peaks in the diffraction pattern at 35°/43°/57°/63°/74° correspond to the lattice planes (311), (400), (422), (511), and (440) characteristic of Fe3O4. The diffraction pattern of CoFe2O4 (a) shows diffraction peaks of iron oxide and cobalt (2θ = 50 ÷ 55°) and a weakly distinguishable region in the region 2θ = 40 ÷ 45°. The resulting spectrum of the nanoparticle powder was compared with the database spectra. The location of the peaks and the ratio of their intensities in the diffraction pattern confirm the formation of a pure magnetite Fe3O4 phase. Based on the shape of the peaks (narrow distribution and good intensity), it can be argued that the resulting nanoparticles have a high crystallinity.
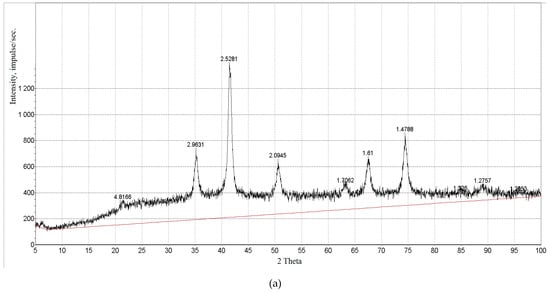
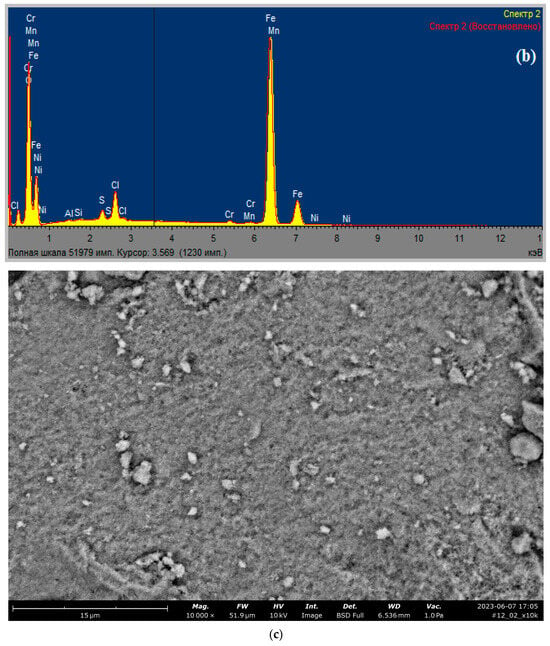
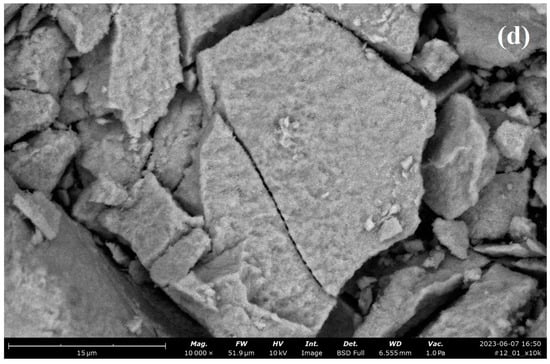
Figure 2.
Data of analyses of synthesized Fe3O4: (a) X-ray diffractogram; (b) X-ray fluorescence spectrum; (c,d) SEM-images. Designations in the figure (c): Spectrum 2. Spectrum 2 (Restored). Full scale 51979 pulses. Cursor: 3.569 (1230 pulses). The abscissa axis: keV.
According to elemental analysis (Figure 2b), the content of elements (in %) in the synthesized magnetite was as follows: O (26.51), Al (0.15), Si (0.13), S (1.13), Cl (1.27), Cr (0.47) Mn (0.40), Fe (69.94). The results of the SEM analysis of the synthesized magnetic composite Fe3O4 show that the nanoparticles prepared by the co-precipitation method have granular inhomogeneity (Figure 2c,d).
In the case of cobalt oxide, diffraction peaks of spinel Co3O4 were revealed (Figure 3b,c).
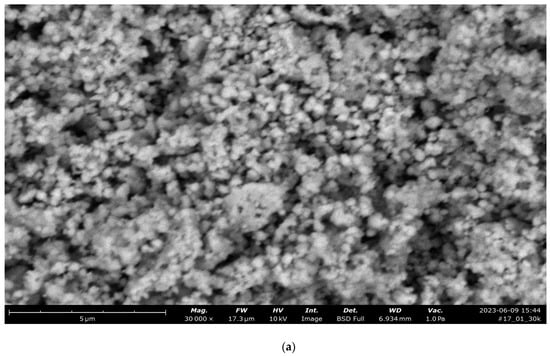
Figure 3.
Data of analyses of synthesized CoFe2O4: (a) SEM image of CoFe2O4 composite; (b) X-ray diffractogram of CoFe2O4 composite; (c) X-ray diffractogram of CoFe2O4/PEI.
After obtaining a sample stabilized with PEI, diffraction peaks also appeared for other phases of iron oxide—γ-, α, and ε-Fe2O3.
X-ray analysis results for the synthesized CoFe2O4:
Phases:
Fe2O3—hematite; a, nm—0.503607; s, nm 1.37445; concentration—30.78%. X-ray density—5.270 g/cm3. Space group-R-3c.
CoFe2O4—spinel; a, nm—0.838933; concentration—69.22%; X-ray density—5.278 g/cm3. Space group-Fd-3m.
The content of elements in CoFe2O4 (%) according to elemental analysis (Figure 4) were as follows: O (21.16), Al (0.17), Si (0.12), Cl (14.68), Ca (0.03), Cr (0, 19), Mn (0.22), Fe (43.28), Co (20.15).
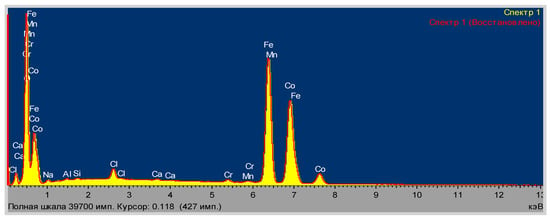
Figure 4.
Data of elemental analysis of CoFe2O4. Spectrum 1. Spectrum 1 (Restored). Full scale 39,700 pulses. Cursor: 0.118 (427 pulses). The abscissa axis: keV.
Figure 5 shows the SEM micrograph of Fe3O4 stabilized with chitosan.
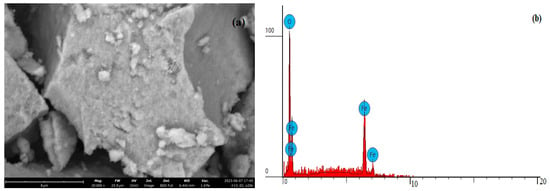
Figure 5.
Data of analyses of Fe3O4 stabilized with chitosan: (a) SEM image; (b) determination of elements concentrations.
Results of determination of elements concentrations of Fe3O4 composite stabilized with chitosan (Figure 5a):
Carbon: atomic concentration/weight concentration—14.384 and 6.194, respectively.
Oxygen: atomic concentration/weight concentration—54.334 and 31.169, respectively.
Iron: atomic concentration/weight concentration—31.281 and 62.637, respectively.
Using Phenom Particle Metric 1.2.3.0. Version number 1.2.3.0, 113 particles were identified on the obtained SEM micrographs. The results of averaging the main geometric parameters of Fe3O4/chitosan are presented in Table 1.

Table 1.
Average geometric parameters of crushed particles (particle summary based on count) for Fe3O4/chitosan.
Distribution histograms were plotted for the resulting crushed Fe3O4/chitosan particles (Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10).
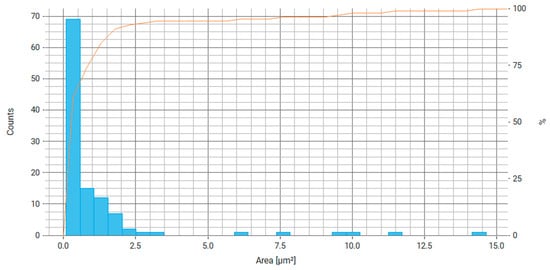
Figure 6.
Particle area distribution histogram for Fe3O4/chitosan, where the right axis is the number of particles and the orange line is the cumulative percentage curve.
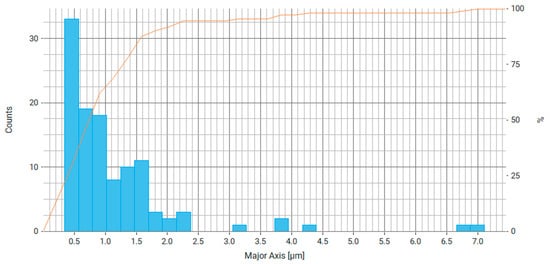
Figure 7.
Histogram of distribution of the main axis of particles (largest chord) for Fe3O4/chitosan, where the right axis is the number of particles and the orange line is the cumulative percentage curve.
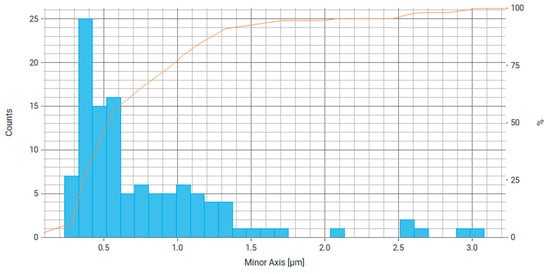
Figure 8.
Histogram of the distribution of the minor axis of particles (smallest chord) for Fe3O4/chitosan, where the right axis is the number of particles and the orange line is the cumulative percentage curve.
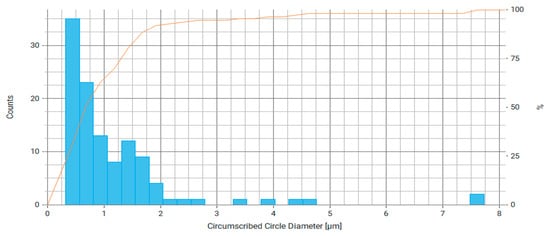
Figure 9.
Histogram of circumcircle diameter distribution for measured particles of Fe3O4/chitosan, where the right axis is the number of particles and the orange line is the cumulative percentage curve.
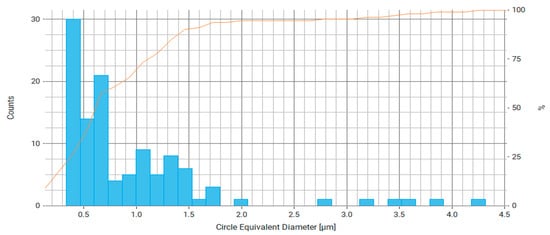
Figure 10.
Histogram of equivalent circle diameter distribution for measured particles of Fe3O4/chitosan, where the right axis is the number of particles and the orange line is the cumulative percentage curve.
From the data obtained (Figure 7 and Figure 8), it can be stated that about 60% of the particles have a major axis value (largest chord, diameter) in the range of 0.5–1 µm and about 60% of particles have a minor axis size (smallest chord, diameter) in the range of 0.3–0.6 µm. This difference may be due to the average aspect ratio of 0.737, which indicates the elongated shape of the particles. Most likely, magnetic nanoparticles form agglomerates that are connected by electrostatic and magnetic forces.
Data of analyzes of Fe3O4 stabilized with PVP are demonstrated in Figure 11 and Table 2. Disabled elements are Au, Nb, Tl.
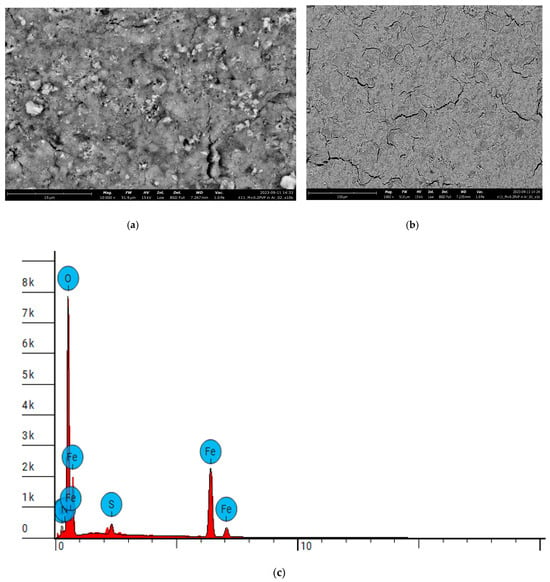
Figure 11.
Data of analyses of Fe3O4 stabilized with PVP: (a) SEM image, ×20,000 magnification; (b) SEM image, ×1000 magnification; (c) determination of elements concentrations.

Table 2.
Results of elemental analysis of catalyst based on Fe3O4 stabilized with PVP.
The results of averaging the main geometric parameters of Fe3O4/PVP are presented in Table 3.

Table 3.
Average geometric parameters of crushed particles (particle summary based on count) for Fe3O4/PVP.
Distribution histograms were constructed for the resulting crushed Fe3O4/PVP particles (Figure 12, Figure 13, Figure 14, Figure 15 and Figure 16).
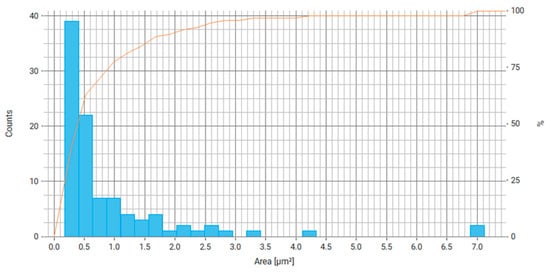
Figure 12.
Histogram of particle area distribution for Fe3O4/PVP, where the right axis is the number of particles and the orange line is the cumulative percentage curve.
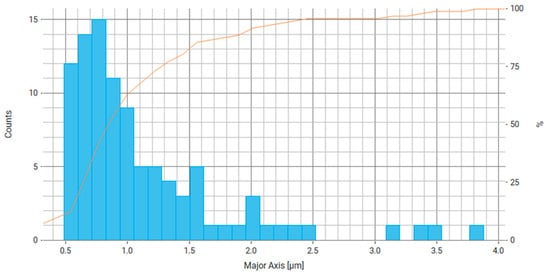
Figure 13.
Histogram of the distribution of the main axis of particles (largest chord) for Fe3O4/PVP, the right axis is the number of particles and the orange line is the cumulative percentage curve.
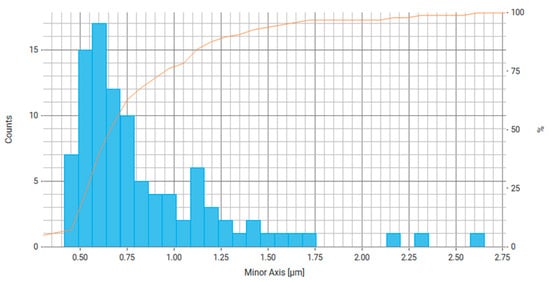
Figure 14.
Histogram of the distribution of the minor axis of particles (smallest chord) for Fe3O4/PVP, where the right axis is the number of particles and the orange line is the cumulative percentage curve.
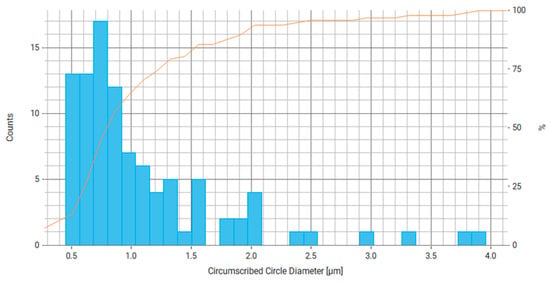
Figure 15.
Histogram of circumcircle diameter distribution for measured particles of Fe3O4/PVP, where the right axis is the number of particles and the orange line is the cumulative percentage curve.
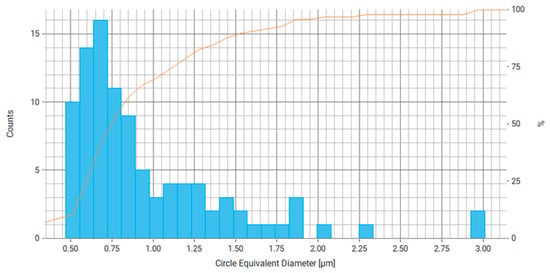
Figure 16.
Histogram of equivalent circle diameter distribution for measured particles, where the right axis is the number of particles and the orange line is the cumulative percentage curve.
Based on the data obtained for Fe3O4/PVP (Table 3, Figure 13 and Figure 14), we can conclude that at least about 70% of particles have a main axis value (largest chord, diameter) in the range of 0.5–0.9 μm and about 70% of particles have a minor axis size (smallest chord, diameter) in the range of 0.5–0.7 µm. From the literature, it is known that reducing the Fe particle size by stabilizing the particles with PVP leads to improved catalytic system activity, performance, and conversion, as seen, for example, in the reaction mechanism of a Fenton reaction [51].
Figure 17 shows data of analyses of a synthesized catalyst based on CoFe2O4 stabilized with PVP.
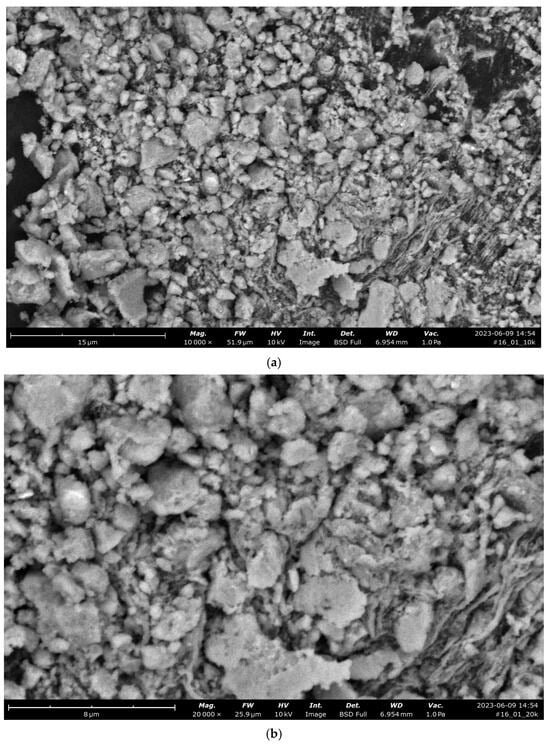
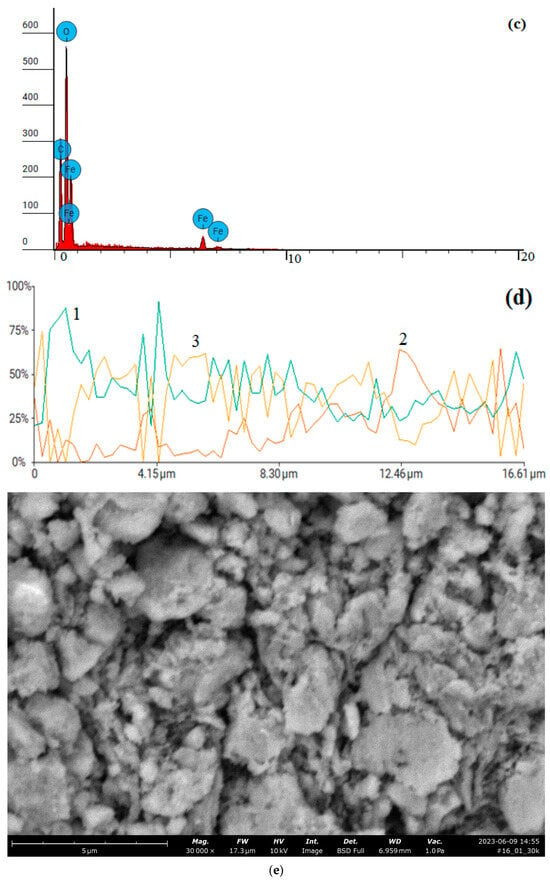
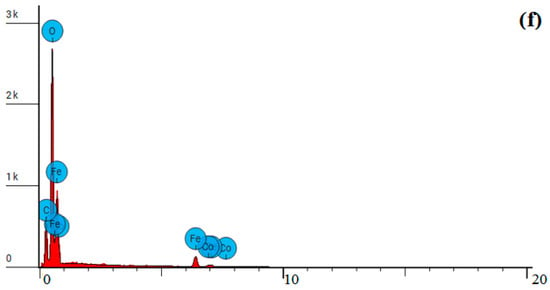
Figure 17.
Data of analyses of a synthesized composite based on CoFe2O4 stabilized with PVP: SEM images: (a) magnification: ×10,000; (b) magnification: ×20,000; (c) X-ray fluorescence spectrum; (d) combined line scan—weight of elements: 1—oxygen, 2—carbon, 3—iron; (e) SEM image of CoFe2O4 composite stabilized with PVP for a CoFe2O4 stabilized with PVP; (f) X-ray fluorescence spectrum for CoFe2O4 stabilized with PVP.
Elements concentrations of CoFe2O4 composite stabilized with PVP for a line scan (Figure 17b,c):
Carbon: atomic concentration/weight concentration—39.154/20.080.
Oxygen: atomic concentration/weight concentration—38.309/26.174.
Iron: atomic concentration/weight concentration—22.536/53.746.
The element concentrations of CoFe2O4 composite stabilized with PVP in a spot scan on Figure 17e,f are presented in Table 4.

Table 4.
Results of elemental analysis of composite based on CoFe2O4 stabilized with PVP.
Detection of hydrocarbons by elemental analysis in both cases of catalysts based on iron and cobalt nanoparticles stabilized with both PVP and chitosan show that there is an interaction between nanoparticles and polymers and suggests efficient formation of nanocomposites. Based the results of SEM-analysis, it was shown that PVP and chitosan form composites together with the metal nanocrystals. PVP can be adsorbed on iron nanoparticles through a weak coordination bond that stabilize it [46,47,48,55,56,57,58,59,60,61,62].
For nanocomposites, the bands in the region of 600–800 cm−1 are due to stretching vibrations of the Fe-O bond in oxides. The absorption bands at 735, 663, 649, and 626 cm−1 are natural vibrations for composite nanoparticles embedded in a PVP matrix. Bands characteristic of PVP at 1657 (amide Raman band), 1498, 1461, 1423, and 1372 (deformation vibrations of CH2 groups in the pyrrolidone cycle) and 1287 cm–1 (Amide III–C–H bending vibrations) were found in the polymer matrix, with slight shifts compared to pure PVP. This may indicate that PVP forms a composite together with the ferrite nanocrystal. The inclusion of CoFe2O4 nanoparticles in the polymer matrix leads to a shift of some bands in the nanocomposites.
According to the IR data, after treatment of the Fe3O4 and CoFe2O4 samples with chitosan, in addition to bands identifying the vibrations of the original composites, bands that are characteristic of deformation (in the range of 1575–1650 cm−1) and stretching (3030–3130 cm−1) vibrations of the NH3+ group of chitosan, δ C-H (1448 cm−1), were revealed.
We assume the efficient formation of nanocomposites and that the main active centers of complex formation in the nanocomposites with chitosan are the hydroxyl or amino groups of chitosan.
The synthesized nanocomposites of composition, Fe3O4, CoFe2O4 and CoFe2O4/PEI, were tested in the oxidation reaction of phenol with oxygen (Cphenol = 0.003 mol/L) [45]. The UV spectrum of the sample during the reaction and the IR spectra of phenol and reaction products after oxidation with oxygen showed a favorable prognosis for the use of such catalysts in the production of oxygen-containing compounds using the catalytic reaction of oxidation with oxygen.
Catalysts with a composition of Fe3O4/chitosan and Fe3O4/PVP were also used for the oxidation of phenol with oxygen. Figure 18 shows typical conversion curves for the oxidation of phenol with oxygen, in the coordinates WO2 = f(QO2). W is oxygen absorption rate in mol/L × min; Q is amount of absorbed O2, mol/L. The initial rate of phenol oxidation on Fe3O4/chitosan was approximately one order of magnitude higher than on Fe3O4/PVP.
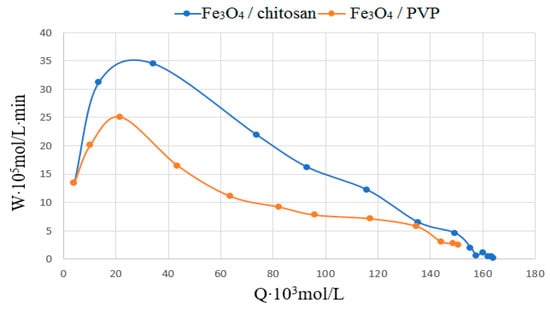
Figure 18.
Oxidation of phenol with oxygen in the presence of Fe3O4/chitosan and Fe3O4/PVP; CC6H5OH—0.001 mol/L; T-353K.
Figure 19 shows the absorption spectra of the original solution of phenol (0.01 mol/L). In the wavelength range 190–320 nm, there is an absorption band of phenol at 209 nm and 275 nm. By the decrease in optical density D at a given wavelength and the appearance of an absorption band at a wavelength of 240–245 nm, as well as a weak band at 435 nm, characteristic of benzoquinone, one can judge the decrease in the phenol content in the solution as a result of its oxidation to benzoquinone. Additional absorption bands that could correspond to possible intermediate products of phenol degradation, such as pyrocatechin, hydroquinone, and quinone, were not detected on the spectrum. This fact is confirmed by the IR spectrum data of the product. In the IR spectrum of the reaction product, absorption bands were observed in the region of 1630–1644 cm−1, indicating the presence of a C=O bond characteristic of p-benzoquinone.
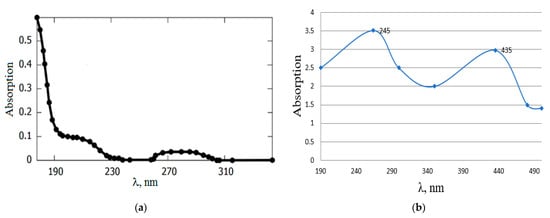
Figure 19.
Absorption spectra of the initial aqueous solution of phenol (a) and the reaction product (b).
According to the preliminary data, the most efficient oxidation of phenol occurs on Fe3O4/chitosan, with the phenol conversion being 55–60%. This research will continue.
4. Conclusions
Sorbents and catalysts based on magnetic nanoparticles are widely used in medicine, chemistry, physics, ecology, and electronics, and are described in detail by various scientific schools. A promising direction is the synthesis and application of magnetic nanoparticles stabilized by modifiers: polymers of natural and artificial origin. Such nanocomposites with catalytic properties make it possible to carry out the process of hydrocarbon oxidation under mild conditions in the liquid phase. In this research, the polymers chitosan, polyvinylpyrrolidone (PVP), and polyethyleneimine (PEI) were used as a stabilizer of nanoparticles of Fe and Co. The synthesized catalysts were investigated by a complex of physicochemical studies. The data of the SEM analysis have shown that polymers form composites together with the metal nanocrystals. The samples of the catalysts tested in the reaction of phenol oxidation with oxygen demonstrated good activity.
Author Contributions
Conceptualization, B.T.D. and L.R.S.; methodology, B.T.D. and T.V.S.; software, L.R.S., M.S.I., A.R.S., A.A.B. and B.B.B.; validation, B.T.D., L.R.S., M.S.I. and Z.M.Z.; formal analysis, B.T.D., L.R.S., A.R.S., A.A.B., U.N.D. and B.B.B.; investigation, M.S.I., U.N.D., B.T.D., L.R.S., A.R.S., A.A.B. and B.B.B.; resources, T.V.S. and B.T.D.; data curation, T.V.S. and B.T.D.; writing—original draft preparation, B.T.D. and L.R.S.; writing—review and editing, L.R.S. and B.T.D.; visualization, T.V.S. and Z.M.Z.; supervision, T.V.S., B.T.D. and L.R.S.; project administration, T.V.S.; funding acquisition, T.V.S. and B.T.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan, grant No. AP14870308 “Development of technology for catalytic petrochemical synthesis of oxygen-containing compounds from aromatic hydrocarbons in the presence of nanoscale magnetic composites”.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Portnyagina, N. What Problems Are Experienced by the Kazakhstan Oil Industry and How Can One Get Rid of Them? 19 April 2022. Available online: https://kazpravda.kz/n/kakie-problemy-ispytyvaet-kazahstanskaya-neftyanka-i-kak-mozhno-ot-nih-izbavitsya/ (accessed on 1 July 2023).
- Amaniyazova, G.D. Problems and Prospects for the Use of Hydrocarbon Resources in Kazakhstan. Bull of KazEU. 2012. Available online: https://articlekz.com/article/13950 (accessed on 1 July 2023).
- Datsko, T.; Zelentsov, V.; Dvornikov, D. Advanced Nanotechnology-Based Approaches to Waste Water Purification from Organic Pollutants. IFMBE Proc. 2024, 91, 134–146. [Google Scholar]
- Naĭden, E.P.; Zhuravlev, V.A.; Itin, V.I.; Terekhova, O.G.; Magaeva, A.A.; Ivanov, Y.F. Magnetic properties and structural parameters of nanosized oxide ferrimagnet powders produced by mechanochemical synthesis from salt solutions. Phys. Solid State 2008, 50, 894–900. [Google Scholar] [CrossRef]
- López-Ortega, A.; Lottini, E.; Fernández, C.D.J.; Sangregorio, C. Exploring the Magnetic Properties of Cobalt-Ferrite Nanoparticles for the Development of a Rare-Earth-Free Permanent Magnet. Chem. Mater. 2015, 27, 4048–4056. [Google Scholar] [CrossRef]
- Chithra, M.; Anumol, C.N.; Argish, V.; Sahu, B.N.; Sahoo, S.C. Magnetic properties of co-ferrite nanoparticles prepared by co-precipitation method. J. Mater. Sci. Mater. Electron. 2023, 34, 806. [Google Scholar] [CrossRef]
- Nguyen, T.K.C.; Nguyen, A.T. Structural, optical and magnetic properties of Y-doped CoFe2O4 nanoparticles prepared by a simple co-precipitation method. J. Mater. Sci. Mater. Electron. 2023, 34, 448. [Google Scholar] [CrossRef]
- Kakati, S.; Rendale, M.K.; Mathad, S.N. Synthesis, Characterization, and Applications of CoFe2O4 and M-CoFe2O4 (M = Ni, Zn, Mg, Cd, Cu, RE) Ferrites: A Review. Int. J. Self-Propagating High-Temp. Synth. 2021, 30, 189–2019. [Google Scholar] [CrossRef]
- Cabot, A.; Puntes, V.F.; Shevchenko, E.; Yin, Y.; Balcells, L.; Marcus, M.A.; Hughes, S.M.; Alivisatos, A.P. Vacancy Coalescence during Oxidation of Iron Nanoparticles. J. Am. Chem. Soc. 2007, 129, 10358–10360. [Google Scholar] [CrossRef]
- Maaz, K.; Mumtaz, A.; Hasanain, S.K.; Ceylan, A. Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J. Magn. Magn. Mater. 2007, 308, 289–295. [Google Scholar] [CrossRef]
- Siva, K.V.; Kumar, A.; Arockiarajan, A. Structural, magnetic and magnetoelectric investigations on CoFe2O4 prepared via various wet chemical synthesis route: A Comparative Study. J. Magn. Magn. Mater. 2021, 535, 168065. [Google Scholar] [CrossRef]
- Dhand, C.; Dwivedi, N.; Loh, X.J.; Ying, A.N.J.; Verma, N.K.; Beuerman, R.W.; Lakshminarayanan, R.; Ramakrishna, S. Methods and strategies for the synthesis of diverse nanoparticles and their applications: A comprehensive overview. RSC Adv. 2015, 5, 105003–105037. [Google Scholar] [CrossRef]
- Sashko, N.; Vaitulevich, E.; Yurmazova, T. Synthesis and Properties of Iron-Based Magnetic Nanoparticles. Key Eng. Mater. 2016, 712, 282–287. [Google Scholar] [CrossRef]
- Tartaj, P.; Morales, M.P.; Veintemillas-Verdaguer, S.; González-Carreño, T.; Serna, C.J. The preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D 2003, 36, R182–R197. [Google Scholar] [CrossRef]
- Mmelesi, O.K.; Masunga, N.; Kuvarega, A.; Nkambule, T.T.; Mamba, B.B.; Kefeni, K.K. Cobalt ferrite nanoparticles and nanocomposites: Photocatalytic, antimicrobial activity and toxicity in water treatment. Mater. Sci. Semicond. 2021, 123, 105523. [Google Scholar] [CrossRef]
- Sassykova, L.R.; Dossumova, B.T.; Ilmuratova, M.S.; Shakiyeva, T.V.; Baizhomartov, B.B.; Sassykova, A.R.; Zhaxibayeva, Z.M.; Abildin, T.S. Development of nanostructured catalysts for catalytic oxidative water purification from organic impurities, including phenolic compounds. Chim. Tech. Acta 2023, 10, 202310309. [Google Scholar] [CrossRef]
- Alaerts, L.; Wahlen, J.; Jacobs, P.A.; De Vos, D.E. Recent progress in the immobilization of catalysts for selective oxidation in the liquid phase. Chem. Commun. 2008, 15, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Shokouhimehr, M. Magnetically Separable and Sustainable Nanostructured Catalysts for Heterogeneous Reduction of Nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Usov, N.A. Expert Opinion: Magnetic Nanoparticles: Theory and Modern Technological Applications. 5 February 2016. Available online: https://habr.com/ru/companies/misis/articles/390127/ (accessed on 1 July 2023).
- Zhang, W.X. Nanoscale iron particles for environmental remediation: An overview. J. Nanopart. Res. 2003, 5, 323–332. [Google Scholar] [CrossRef]
- Yi, D.K.; Lee, S.S.; Ying, J.Y. Synthesis and applications of magnetic nanocomposite catalysts. Chem. Mater. 2006, 18, 2459–2461. [Google Scholar] [CrossRef]
- Taghipour, S.; Hosseini, S.M.; Ataie-Ashtiani, B. Engineering nanomaterials for water and wastewater treatment: Review of classifications, properties and applications. New J. Chem. 2019, 43, 7902–7927. [Google Scholar] [CrossRef]
- Khabibullin, V.R.; Stepanov, G.V. Effect of a Low-Frequency Magnetic Field on the Release of Heat by Magnetic Nanoparticles of Different Shapes. Russ. J. Phys. Chem. 2020, 94, 439–444. [Google Scholar] [CrossRef]
- Bolm, C.; Legros, J.; Le Paih, J.; Zani, L. Iron-catalyzed reactions in organic synthesis. Chem. Rev. 2004, 104, 6217–6254. [Google Scholar] [CrossRef] [PubMed]
- Chekanova, A.E.; Sorkina, T.A.; Dubov, A.L.; Nikiforov, V.N.; Davydova, G.A.; Selezneva, I.I.; Goodilin, E.A.; Trusov, L.A.; Korolev, V.V.; Aref’ev, I.M.; et al. New environmental nontoxic agents for the preparation of core-shell magnetic nanoparticles. Mendeleev Commun. 2009, 19, 72–74. [Google Scholar] [CrossRef]
- Sassykova, L.R.; Shakiyeva, T.V.; Ilmuratova, M.S.; Muktaly, D.; Zhaxibayeva, Z.M.; Sassykova, A.R.; Baizhomartov, B. Catalysts, magnetic composites for removal of phenol-containing compounds from wastewater. Rasayan J. Chem. 2023, 16, 1605–1612. [Google Scholar] [CrossRef]
- Koksharov, Y.A.; Gubin, S.P.; Taranov, I.V.; Khomutov, G.B.; Gulyaev, Y.V. Magnetic Nanoparticles in Medicine: Progress, Problems, and Advances. J. Commun. Technol. Electron. 2022, 67, 101–116. [Google Scholar] [CrossRef]
- Baranov, D.A.; Gubin, S.P. Magnetic nanoparticles: Advances and problems of chemical synthesis. Radioelektron. Nanosistemy Inf. Tehnol. 2009, 1, 129–147. [Google Scholar]
- Kritika, R.I. Therapeutic applications of magnetic nanoparticles: Recent advances. Mater. Adv. 2022, 3, 7425–7444. [Google Scholar] [CrossRef]
- Tashmukhambetova, Z.K.; Sassykova, L.R.; Aubakirov, Y.A.; Dangaliyeva, A.K.; Kanatbayeva, M.A.; Rustem, A.E. New catalysts for toluene oxidation technology in the liquid phase. Mater. Today Proc. 2020, 31, 529–553. [Google Scholar] [CrossRef]
- Habibi, D.; Faraji, A.R.; Arshadi, M.; Veisi, H.; Gil, A. Manganese nanocatalyst and N-hydroxyphthalimide as an efficient catalytic system for selective oxidation of ethylbenzene, cyclohexene and oximes under aerobic condition. J. Mol. Catal. A Chem. 2014, 382, 41–54. [Google Scholar] [CrossRef]
- Mohammadi-Samani, S.; Miri, R.; Salmanpour, M.; Khalighian, N.; Sotoudeh, S.; Erfani, N. Preparation and assessment of chitosan-coated superparamagnetic Fe3O4 nanoparticles for controlled delivery of methotrexate. Res. Pharm. Sci. 2013, 8, 25–33. [Google Scholar]
- Iorio, E.D.; Colombo, C.; Cheng, Z.; Capitani, G.; Mele, D.; Ventruti, G.; Angelico, R. Characterization of magnetite nanoparticles synthetized from Fe(II)/nitrate solutions for arsenic removal from water. J. Environ. Chem. Eng. 2019, 7, 102986. [Google Scholar] [CrossRef]
- Singh, A.N.; Singh, S.; Suthar, N.; Dubey, V.K. Glutaraldehyde-Activated Chitosan Matrix for Immobilization of a Novel Cysteine Protease, Procerain B. J. Agric. Food Chem. 2011, 59, 6256–6262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Zhu, A.; Yuan, L.; Dai, S. Preparation of Well-Dispersed Superparamagnetic Iron Oxide Nanoparticles in Aqueous Solution with Biocompatible N-Succinyl-O-carboxymethylchitosan. J. Phys. Chem. C 2008, 112, 5432–5438. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Kuznetsov, N.T.; Meshalkin, V.P.; Salerno, M.; Fabiano, B. Systematical analysis of chemical methods in metal nanoparticles synthesis. Theor. Found. Chem. Eng. 2016, 50, 59–66. [Google Scholar] [CrossRef]
- Reverberi, A.; Salerno, M.; Lauciello, S.; Fabiano, B. Synthesis of Copper Nanoparticles in Ethylene Glycol by Chemical Reduction with Vanadium (+2) Salts. Materials 2016, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Sorensen, C.M.; Klabunde, K.J.; Hadjipanayis, G.C. Magnetic Properties of nanophase cobalt particles synthesized in inversed micelles. J. Appl. Phys. 1994, 76, 6316–6318. [Google Scholar] [CrossRef]
- Chen, K.; Bakuzis, A.F.; Luo, W. Improving surfactant grafting in magnetic colloids. Appl. Surf. Sci. 2006, 252, 6379–6382. [Google Scholar] [CrossRef]
- Pileni, M.P. Reverse micelles: A microreactor. J. Phys. Chem. 1993, 97, 9661–9668. [Google Scholar] [CrossRef]
- Pileni, M.P. Magnetic Fluids: Fabrication, Magnetic Properties, and Organization of Nanocrystals. Adv. Funct. Mater. 2001, 11, 323–336. [Google Scholar] [CrossRef]
- Pileni, M.P. The Role of soft colloidal templates in controlling the size and shape of inorganic nanocrystals. Nat. Mater. 2003, 2, 145–150. [Google Scholar] [CrossRef]
- Baabu, P.R.S.; Kumar, H.K.; Gumpu, M.B.; Babu, K.J.; Kulandaisamy, A.J.; Rayappan, J.B.B. Iron Oxide Nanoparticles: A Review on the Province of Its Compounds, Properties and Biological Applications. Materials 2023, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Dossumova, B.T.; Shakiyeva, T.V.; Muktaly, D.; Sassykova, L.R.; Baizhomartov, B.B.; Subramanian, S. Synthesis, Characterization of Magnetic Composites and Testing of Their Activity in Liquid-Phase Oxidation of Phenol with Oxygen. ChemEngineering 2022, 6, 68. [Google Scholar] [CrossRef]
- Narasimharao, K.; Ali, T.T.; Abu-Zied, B.M.; Alfaifi, S.Y. Combustion synthesis of nanocrystalline porous CoFexAl2-xO4 spinels: Structural, textural, magnetic, and electrical properties. Ceram. Int. 2023, 49, 13238–13248. [Google Scholar] [CrossRef]
- Sijo, A.K. Magnetic and structural properties of CoCrxFe2−xO4 spinels prepared by solution self combustion method. Ceram. Int. 2017, 43, 2288–2290. [Google Scholar] [CrossRef]
- Bantz, C.; Koshkina, O.; Lang, T.; Galla, H.-J.; Kirkpatrick, C.J.; Stauber, R.H.; Maskos, M. The surface properties of nanoparticles determine the agglomeration state and the size of the particles under physiological conditions. Beilstein J. Nanotechnol. 2014, 5, 1774–1786. [Google Scholar] [CrossRef] [PubMed]
- Aluker, N.L.; Lavrentieva, A.L.; Suzdaltseva, Y.M. Direct Optical Research Methods in the Analytics of Phenol. Opt. Spectrosc. 2020, 128, 422–428. [Google Scholar] [CrossRef]
- Vorob’eva, T.V.; Terletskaya, A.V.; Kushchevskaya, N.F. Standardized and unified methods for determining phenols in natural and drinking waters and main trends of their development. J. Water Chem. Technol. 2007, 29, 203–213. [Google Scholar] [CrossRef]
- Li, H.; Wang, N.; Li, H.; Ren, Z.; Ma, W.; Li, J.; Du, Y.; Xu, Q. Polyvinylpyrrolidone-induced size-dependent catalytic behavior of Fe sites on N-doped carbon substrate and mechanism conversion in Fenton-like oxidation reaction. Appl. Catal. B 2024, 341, 123323. [Google Scholar] [CrossRef]
- Sapir, L.; Stanley, C.B.; Harries, D. Properties of Polyvinylpyrrolidone in a Deep Eutectic Solvent. J. Phys. Chem. A 2016, 120, 3253–3259. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part A: Theory and Application in Inorganic Chemistry; John Willey & Sons: Hoboken, NJ, USA, 2008; pp. 240–295. [Google Scholar]
- Ryberg, R. Infrared spectroscopy of adsorbed molecules: Some experimental aspects. J. Phys. Colloq. 1983, 44, C10-421–C10-427. [Google Scholar] [CrossRef]
- Semko, L.S.; Storozhuk, L.P.; Khutornoi, S.V.; Abramov, N.V.; Gorbik, P.P. Template synthesis, structure, and properties of magnetically controlled, large surface area Fe3O4/TiO2 adsorbents. Inorg. Mater. 2015, 51, 430–435. [Google Scholar] [CrossRef]
- Paukshtis, E.A.; Yurchenko, E.N. Study of the Acid–Base Properties of Heterogeneous Catalysts by Infrared Spectroscopy. Russ. Chem. Rev. 1983, 52, 242–258. [Google Scholar] [CrossRef]
- Tsyganenko, A.A.; Smirnov, K.S.; Rzhevskij, A.M.; Mardilovich, P.P. Infrared spectroscopic evidence for the structural OH groups of spinel alumina modifications. Mater. Chem. Phys. 1990, 26, 35–46. [Google Scholar] [CrossRef]
- Davydov, A. Molecular Spectroscopy of Oxide Catalyst Surfaces; John Wiley & Sons: Hoboken, NJ, USA, 2003; 690p, ISBN 978-0-471-98731-4. [Google Scholar]
- Lázár, K.; Nimz, M.; Lietz, G.; Guczi, L. Formation of PdFe alloys on silica supported catalysts. Hyperfine Interact. 1988, 41, 657–660. [Google Scholar] [CrossRef]
- Balcı, S.; Tomul, F. Catalytic wet peroxide oxidation of phenol through mesoporous silica-pillared clays supported iron and/or titanium incorporated catalysts. J. Environ. Manag. 2023, 326 Pt B, 116835. [Google Scholar] [CrossRef]
- Zambrzycki, C.; Shao, R.; Misra, A.; Streb, C.; Herr, U.; Güttel, R. Iron based core-shell structures as versatile materials: Magnetic support and solid catalyst. Catalysts 2021, 11, 72. [Google Scholar] [CrossRef]
- Tomita, K.; Oshima, Y. Stability of manganese oxide in catalytic supercritical water oxidation of phenol. Ind. Eng. Chem. Res. 2004, 43, 7740–7743. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).