Design of Continuous Kneading System for Active Anode Material Fabrication Using Retrofitted Assembly of Co-Rotating Screw Extruder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Needle Cokes and Binding Pitch Composition
2.1.2. Comparison of Continuous Kneading Performance of KRC-S2 and CHT-20 Extruders
2.1.3. Continuous Kneading by Retrofitted Double Co-Rotating Parallel Extruding System
2.2. Characterization of Samples
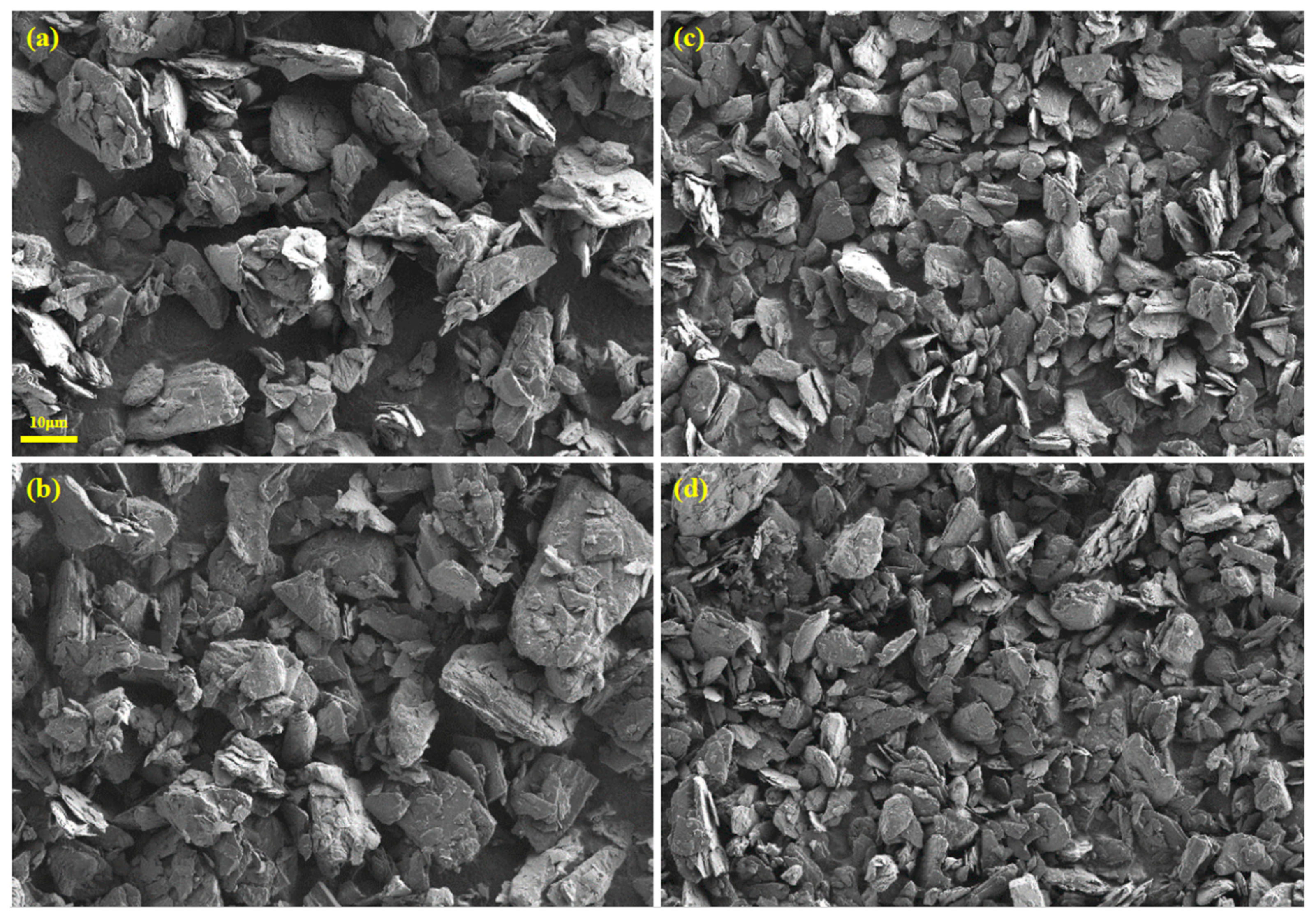
2.2.1. Surface Properties
2.2.2. Particle Size Analysis
2.2.3. Tap Density Measurement
2.2.4. Elemental Analysis
2.2.5. X-ray Diffraction Measurement
2.2.6. Topological Characterization
2.3. Electrochemical Performance of the Artificial Graphite Anode
2.3.1. Cell Preparation
2.3.2. Electrochemical Testing
3. Results and Discussion
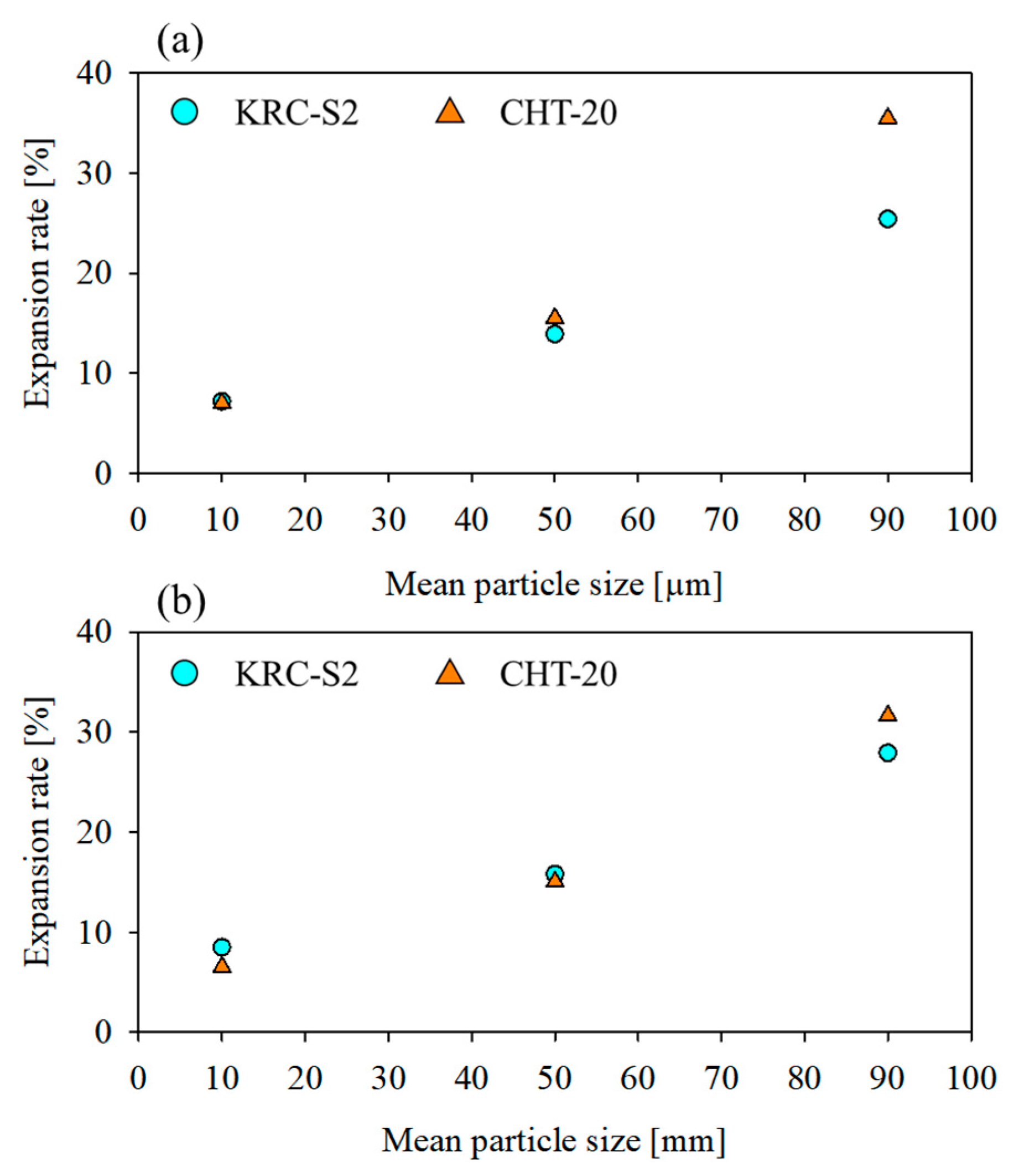
3.1. Comparison of Continuous Kneading Performance of KRC-S2 and CHT-20 Extruders
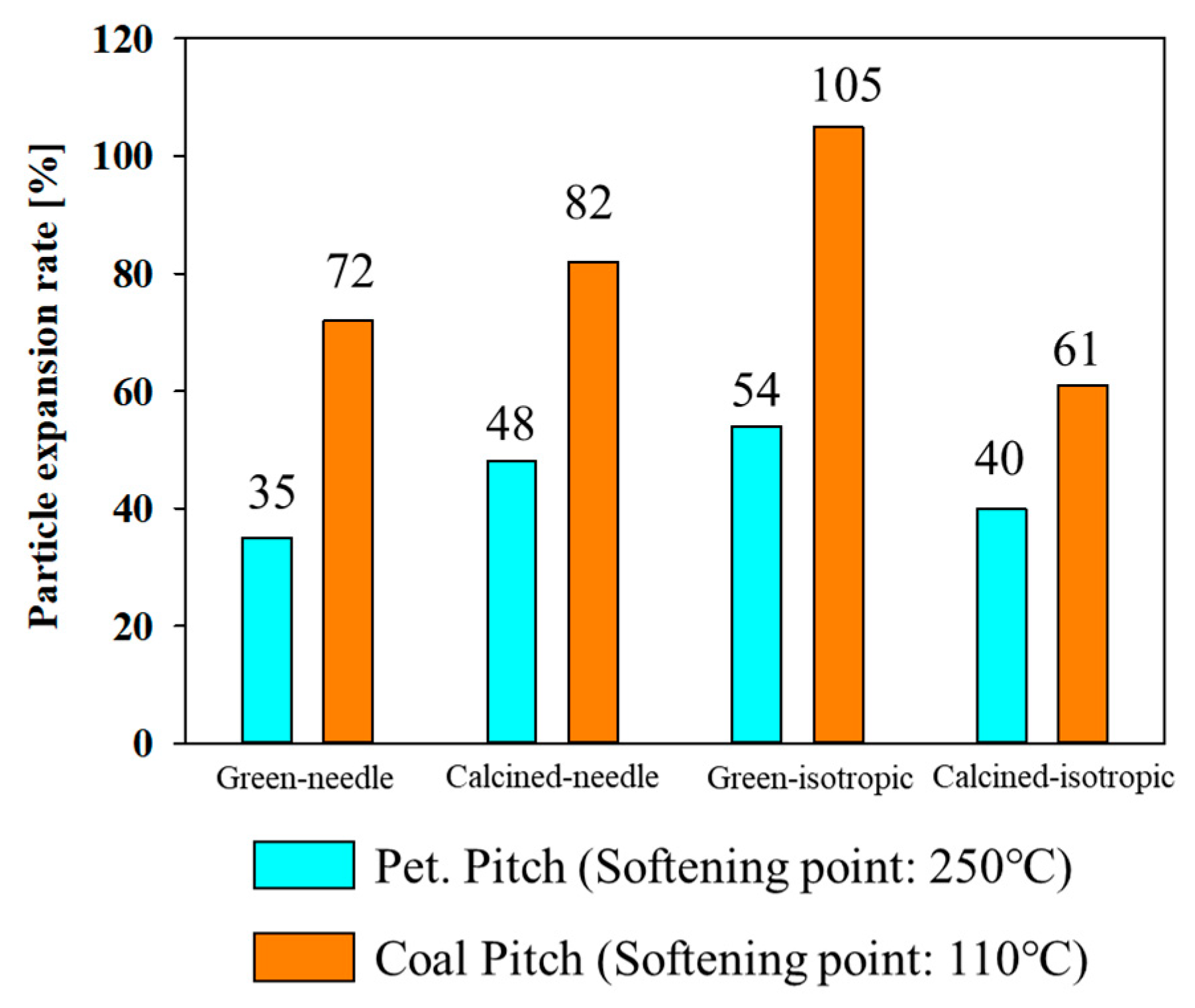
3.2. Continuous Kneading Performance of the Retrofitted Consecutive Dual Co-Rotating Parallel Extruding System
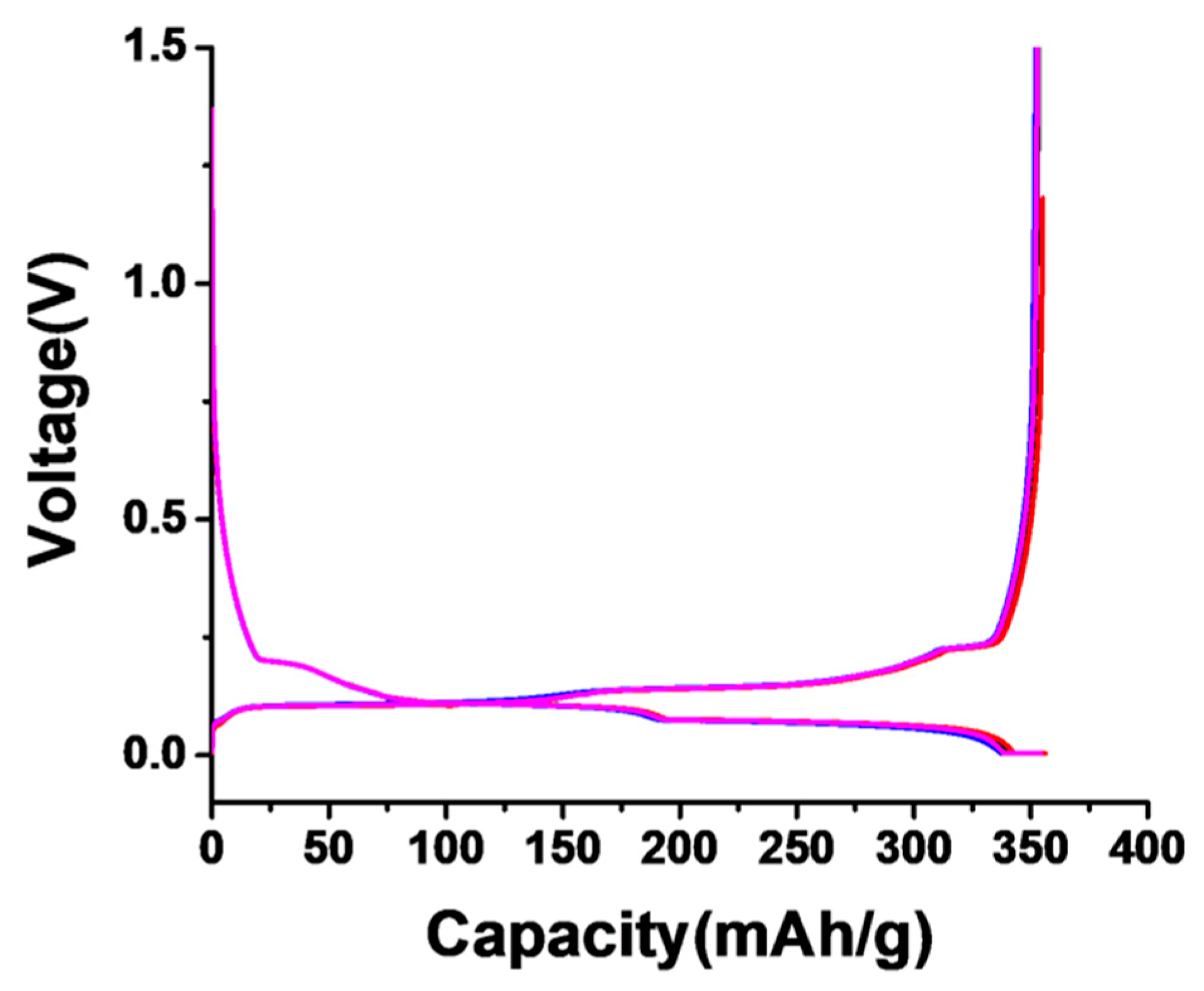
3.3. Electrochemical Testing Result
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.S.; Pfleging, W.; Kohler, R.; Seifert, H.J.; Kim, T.Y.; Byun, D.J.; Jung, H.G.; Choi, W.C.; Lee, J.K. Three-dimensional silicon/carbon core–shell electrode as an anode material for lithium-ion batteries. J. Power Source 2015, 279, 13–20. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Source 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Nishi, Y. Lithium ion secondary batteries; past 10 years and the future. J. Power Source 2001, 100, 101–106. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Andre, D.; Hain, H.; Lamp, P.; Maglia, F.; Stiaszny, B. Future high-energy density anode materials from an automotive application perspective. J. Mater. Chem. A 2017, 5, 17174–17198. [Google Scholar] [CrossRef]
- Bresser, D.; Hosoi, K.; Howell, D.; Li, H.; Zeisel, H.; Amine, K.; Passerini, S. Perspectives of automotive battery R&D in China, Germany, Japan, and the USA. J. Power Source 2018, 382, 176–178. [Google Scholar]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. JACS 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Mekonnen, Y.; Sundararajan, A.; Sarwat, A.I. A review of cathode and anode materials for lithium-ion batteries. SoutheastCon 2016, 2016, 1–6. [Google Scholar]
- Oh, Y.J.; Shin, M.C.; Kim, J.H.; Yang, S.J. Facile preparation of ZnO quantum dots@porous carbon composites through direct carbonization of metal–organic complex for high-performance lithium ion batteries. Carbon Lett. 2021, 31, 323–329. [Google Scholar] [CrossRef]
- Zanini, M.; Basu, S.; Fischer, J.E. Alternate Synthesis and Reflectivity Spectrum of Stage 1 Lithium-Graphite Interca-Lation Compound. Carbon 1978, 16, 211–212. [Google Scholar] [CrossRef]
- Guerard, D.; Herold, A. Intercalation of lithium into graphite and other carbons. Carbon 1975, 13, 337–345. [Google Scholar] [CrossRef]
- Basu, S.; Zeller, C.; Flanders, P.J.; Fuerst, C.D.; Johnson, W.D.; Fischer, J.E. Synthesis and properties of lithium-graphite intercalation compounds. Mater. Sci. Eng. 1979, 38, 275–283. [Google Scholar] [CrossRef]
- Ohta, N.; Nagaoka, K.; Hoshi, K.; Bitoh, S.; Inagaki, M. Carbon-coated graphite for anode of lithium ion rechargeable batteries: Graphite substrates for carbon coating. J. Power Source 2009, 194, 985–990. [Google Scholar] [CrossRef]
- Han, Y.J.; Kim, J.; Yeo, J.S.; An, J.C.; Hong, I.P.; Nakabayashi, K.; Miyawaki, J.; Jung, J.D.; Yoon, S.H. Coating of graphite anode with coal tar pitch as an effective precursor for enhancing the rate performance in Li-ion batteries: Effects of composition and softening points of coal tar pitch. Carbon 2015, 94, 432–438. [Google Scholar] [CrossRef]
- Zheng, H.; Kim, M.S. Performance of modified graphite as anode material for lithium-ion secondary battery. Carbon Lett. 2011, 12, 243–248. [Google Scholar] [CrossRef]
- Shaker, M.; Ghazvini, A.A.S.; Qureshi, F.R.; Riahifar, R. A criterion combined of bulk and surface lithium storage to predict the capacity of porous carbon lithium-ion battery anodes: Lithium-ion battery anode capacity prediction. Carbon Lett. 2021, 31, 985–990. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Flandrois, S.; Simon, B. Carbon materials for lithium-ion rechargeable batteries. Carbon 1999, 37, 165–180. [Google Scholar] [CrossRef]
- Lu, M.; Cheng, H.; Yang, Y. A comparison of solid electrolyte interphase (SEI) on the artificial graphite anode of the aged and cycled commercial lithium ion cells. Electrochim. Acta 2008, 53, 3539–3546. [Google Scholar] [CrossRef]
- Shi, M.; Song, C.; Tai, Z.; Zou, K.; Duan, Y.; Dai, X.; Sun, J.; Chen, Y.; Liu, Y. Coal-derived synthetic graphite with high specific capacity and excellent cyclic stability as anode material for lithium-ion batteries. Fuel 2021, 292, 120250. [Google Scholar] [CrossRef]
- Singh, B.; Mulvaney, S.J. Modeling and Process Control of Twin-Screw Cooking Food Extruders. J. Food Eng. 1994, 23, 403–428. [Google Scholar] [CrossRef]
- Kim, S.M.; Woo, J.H.; Kim, H.W.; Park, H.J. Formulation and evaluation of cold-extruded chocolate ganache for three-dimensional food printing. J. Food Eng. 2022, 314, 110785. [Google Scholar] [CrossRef]
- Snel, S.J.E.; Bellwald, Y.; Goot, A.J.V.D.; Beyrer, M. Novel rotating die coupled to a twin-screw extruder as a new route to produce meat analogues with soy, pea and gluten. Innov. Food Sci. Emerg. Technol. 2022, 81, 103152. [Google Scholar] [CrossRef]
- Ge, X.; Duan, H.; Zhou, Y.; Zhou, S.; Shen, H.; Liang, W.; Sun, Z.; Yan, W. Mechanistic insights into the supramolecular structure and physicochemical properties of twin-screw extruded high amylose corn starch with different amylose content by improved electron beam irradiation. Innov. Food Sci. Emerg. Technol. 2023, 87, 103414. [Google Scholar] [CrossRef]
- Riaz, M.N. Chapter 19 Food Extruders. In Handbook of Farm, Dairy and Food Machinery Engineering, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 483–497. [Google Scholar]
- Bertan, F.M.; Montedo, O.R.K.; Rambo, C.R.; Hotza, D.; Novaes de Oliveira, A.P. Extruded ZrSiO4 particulate-reinforced LZSA glass-ceramics matrix composite. J. Mater. Process. Technol. 2009, 209, 1134–1142. [Google Scholar] [CrossRef]
- Moon, Y.W.; Shin, K.H.; Koh, Y.H.; Choi, W.Y.; Kim, H.E. Porous alumina ceramics with highly aligned pores by heat-treating extruded alumina/camphene body at temperature near its solidification point. J. Eur. Ceram. Soc. 2012, 32, 1029–1034. [Google Scholar] [CrossRef]
- Tan, D.W.; Guo, W.M.; Lao, Z.Y.; Lin, R.L.; Lin, H.T. A novel strategy for c-axis textured silicon nitride ceramics by hot extrusion. J. Eur. Ceram. Soc. 2021, 41, 6059–6063. [Google Scholar] [CrossRef]
- Hossain, S.S.; Lu, k. Recent progress of alumina ceramics by direct ink writing: Ink design, printing and post-processing. Ceram. Int. 2023, 49, 10199–10212. [Google Scholar] [CrossRef]
- Kim, H.; Oh, K.; Seo, Y. Rheological and mechanical properties of a novel polyamide 6 synthesized by anionic polymerization of ε-caprolactam in a twin-screw extruder. Polymer 2019, 177, 196–201. [Google Scholar] [CrossRef]
- McGauran, T.; Harris, M.; Dunne, N.; Smyth, B.M.; Cunningham, E. Development and optimisation of extruded bio-based polymers from poultry feathers. Eur. Polym. J. 2021, 158, 110678. [Google Scholar] [CrossRef]
- Chen, Y.C.; Moseson, D.E.; Richard, C.A.; Swinney, M.R.; Horava, S.D.; Oucherif, K.A.; Cox, A.L.; Hawkins, E.D.; Li, Y.; DeNeve, D.F.; et al. Development of hot-melt extruded drug/polymer matrices for sustained delivery of meloxicam. J. Control. Release 2022, 342, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Roland, W.; Hammer, A.; Leimhofer, C.; Kapshammer, A.; Hild, S. Application of a novel testing device to characterize the layer adhesion of co-extruded polymer sheets. Mater. Today Proc. 2022, 62, 2652–2657. [Google Scholar] [CrossRef]
- Fujita, Y.; Fukumoto, K.; Agata, H.; Fukui, T. Development of Continuous Kneading Process for Cathode Electrode of Lithium-Ion Battery. J. Soc. Powder Technol. Jpn. 2014, 51, 206–211. [Google Scholar] [CrossRef]



| Pitch Binder | Source | QI (%) | Fixed Carbon (%) | Ash (%) | Sulfur (%) |
|---|---|---|---|---|---|
| ZL250 | Petroleum | 0.5 | 68 | 0.02 | 0.01 |
| Primary Particle Size (D50, μm) | Pitch | Secondary Particle Size (D50, μm) | After Graphitization | |||
|---|---|---|---|---|---|---|
| Span | Tap Density (g/cc) | d(002) (nm) | Surface Area (m2/g) | |||
| 10 | Pet. | 15.24 | 1.40 | 0.82 | 0.3358 | 1.48 |
| 6 | Pet. | 8.90 | 1.48 | 0.63 | 0.3358 | 2.94 |
| 10 | Coal | 14.76 | 1.25 | 0.72 | 0.3358 | 1.96 |
| 6 | Coal | 10.91 | 1.34 | 0.62 | 0.3360 | 2.70 |
| Primary Particle Size (D50, μm) | Pitch | Electrochemical Performance | |
|---|---|---|---|
| Discharging Capacity (3rd, mAh/g) | ICE (1st, %) | ||
| 10 | Pet. | 353.1 | 93.7 |
| 6 | Pet. | 355.0 | 92.7 |
| 10 | Coal | 350.3 | 92.4 |
| 6 | Coal | 352.9 | 91.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.-H.; Yi, H.; Cho, H.-R.; Kim, Y.-J.; Park, S.-M.; Yoon, S.-J.; Seo, D.-J.; Oh, K.; Yeon, J.-M.; Choi, S.-Y.; et al. Design of Continuous Kneading System for Active Anode Material Fabrication Using Retrofitted Assembly of Co-Rotating Screw Extruder. Processes 2023, 11, 2660. https://doi.org/10.3390/pr11092660
Lee G-H, Yi H, Cho H-R, Kim Y-J, Park S-M, Yoon S-J, Seo D-J, Oh K, Yeon J-M, Choi S-Y, et al. Design of Continuous Kneading System for Active Anode Material Fabrication Using Retrofitted Assembly of Co-Rotating Screw Extruder. Processes. 2023; 11(9):2660. https://doi.org/10.3390/pr11092660
Chicago/Turabian StyleLee, Gang-Ho, Hyenoseok Yi, Hye-Ryeong Cho, Yu-Jin Kim, Sei-Min Park, Seong-Jin Yoon, Dong-Jin Seo, Kyeongseok Oh, Jeong-Mi Yeon, Sun-Yong Choi, and et al. 2023. "Design of Continuous Kneading System for Active Anode Material Fabrication Using Retrofitted Assembly of Co-Rotating Screw Extruder" Processes 11, no. 9: 2660. https://doi.org/10.3390/pr11092660
APA StyleLee, G.-H., Yi, H., Cho, H.-R., Kim, Y.-J., Park, S.-M., Yoon, S.-J., Seo, D.-J., Oh, K., Yeon, J.-M., Choi, S.-Y., Yoon, S.-H., & Park, J.-I. (2023). Design of Continuous Kneading System for Active Anode Material Fabrication Using Retrofitted Assembly of Co-Rotating Screw Extruder. Processes, 11(9), 2660. https://doi.org/10.3390/pr11092660







