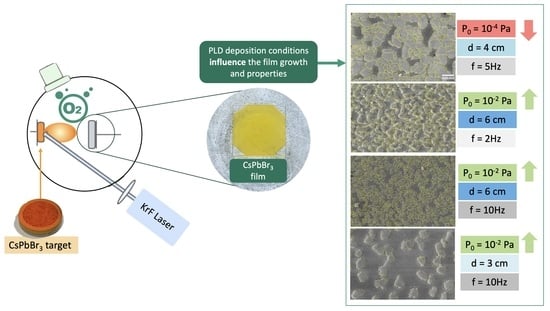
CsPbBr3 Films Grown by Pulsed Laser Deposition: Impact of Oxygen on Morphological Evolution and Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the CsPbBr Ablation Targets
2.2. Pulsed Laser Deposition Experiments
3. Characterization Analyses
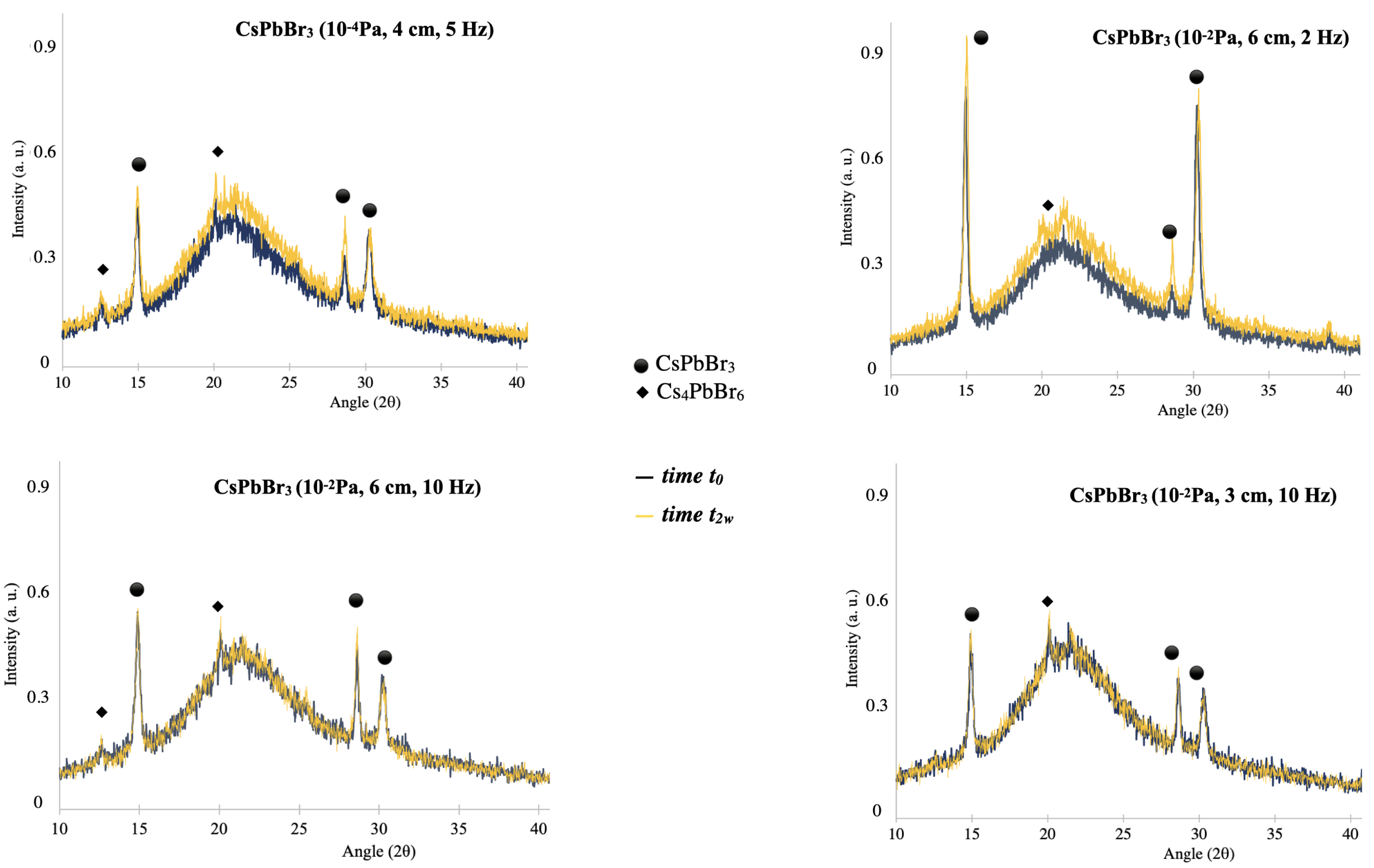
3.1. X-ray Diffraction (XRD) Analysis
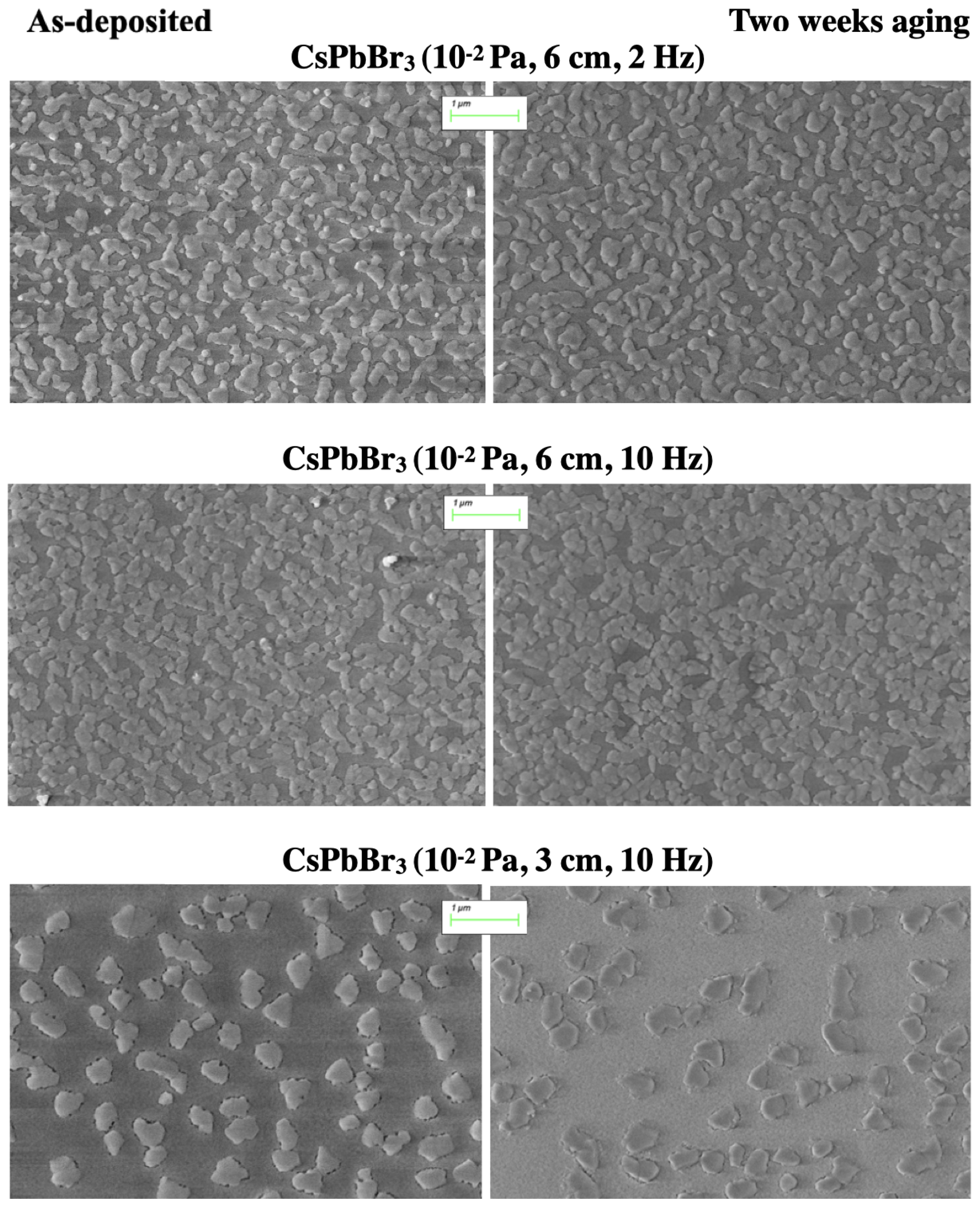
3.2. Scanning Electron Microscopy (SEM) Analysis
3.3. Absorption Measurements
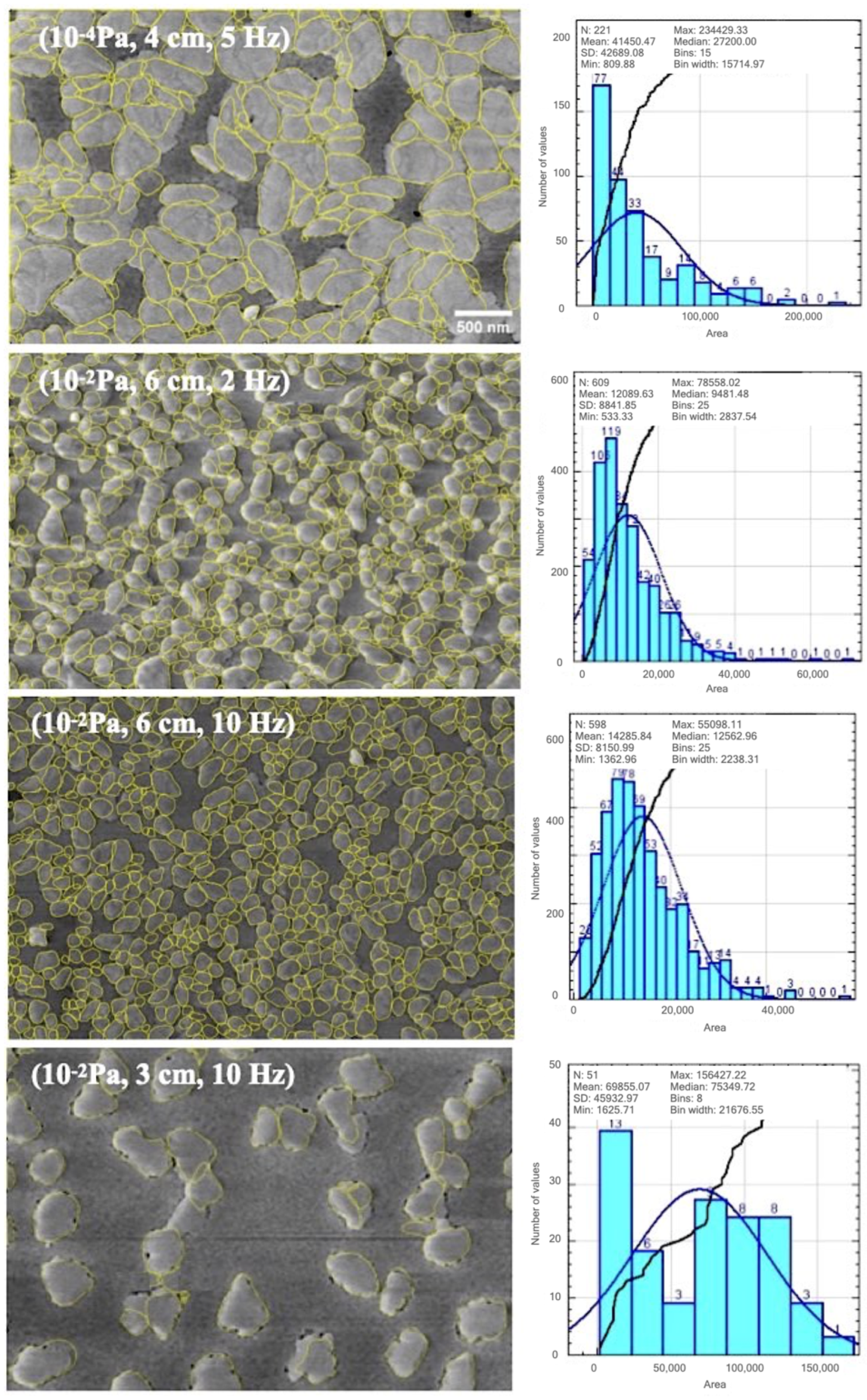
3.4. Digital Image Processing and Statistics
4. Results and Discussion
4.1. Compositional, Structural, and Morphological Properties
4.2. Digital Image Processing and Statistics
4.3. Absorbance Properties
4.4. Remarks
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitzi, D.B. Introduction: Perovskites. Chem. Rev. 2019, 119, 3033–3035. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, B.; Zhang, T.; Shi, Y.; Xu, Q.H. Photoluminescence Mechanisms of All-Inorganic Cesium Lead Bromide Perovskites Revealed by Single Particle Spectroscopy. Chem. Nanomat. 2020, 6, 327–335. [Google Scholar] [CrossRef]
- Cesaria, M.; Quarta, G.; Guascito, M.R.; Mazzeo, M.; Marra, M.; Provenzano, C.; Aziz, M.R.; Martino, M.; Calcagnile, L.; Caricato, A.P. CsPbBr3 deposited by laser ablation: Effects of post-growth aging, oxygen adsorption and annealing on film properties. Appl. Phys. A 2022, 128, 950. [Google Scholar] [CrossRef]
- Cesaria, M.; Mazzeo, M.; Quarta, G.; Aziz, M.R.; Nobile, C.; Carallo, S.; Martino, M.; Calcagnile, L.; Caricato, A.P. Pulsed Laser Deposition of CsPbBr3 Films: Impact of the Composition of the Target and Mass Distribution in the Plasma Plume. Nanomaterials 2021, 11, 3210. [Google Scholar] [CrossRef]
- Zhang, Q.; Yin, Y. All-Inorganic Metal Halide Perovskite Nanocrystals: Opportunities and Challenges. ACS Cent. Sci. 2018, 4, 668–679. [Google Scholar] [CrossRef]
- Soto-Montero, T.; Soltanpoor, W.; Morales-Masis, M. Pressing challenges of halide perovskite thin film growth. APL Mater. 2020, 8, 110903. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, Y.; Ge, Z. Understanding of perovskite crystal growth and film formation in scalable deposition processes. Chem. Soc. Rev. 2020, 49, 1653–1687. [Google Scholar] [CrossRef]
- Teng, P.; Han, X.; Li, J.; Xu, Y.; Kang, L.; Wang, Y.; Yang, Y.; Yu, T. Elegant Face-Down Liquid-Space-Restricted Deposition of CsPbBr3 Films for Efficient Carbon-Based All-Inorganic Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 9541–9546. [Google Scholar] [CrossRef]
- Parobek, D.; Dong, Y.; Qiao, T.; Son, D.H. Direct Hot-Injection Synthesis of Mn-Doped CsPbBr3 Nanocrystals. Chem. Mater. 2018, 30, 2939–2944. [Google Scholar] [CrossRef]
- Zhai, W.; Lin, J.; Li, C.; Hu, S.; Huang, Y.; Yu, C.; Wen, Z.; Liu, Z.; Fangab, Y.; Tang, C. Solvothermal synthesis of cesium lead halide perovskite nanowires with ultra-high aspect ratios for high-performance photodetectors. Nanoscale 2018, 45, 21451–21458. [Google Scholar] [CrossRef]
- Zhai, W.; Lin, J.; Li, Q.; Zheng, K.; Huang, Y.; Yao, Y.; He, X.; Li, L.; Yu, C.; Liu, C.; et al. Solvothermal Synthesis of Ultrathin Cesium Lead Halide Perovskite Nanoplatelets with Tunable Lateral Sizes and Their Reversible Transformation into Cs4PbBr6 Nanocrystals. Chem. Mater. 2018, 30, 3714–3721. [Google Scholar] [CrossRef]
- Rakita, Y.; Kedem, N.; Gupta, S.; Sadhanala, A.; Kalchenko, V.; Böhm, M.L.; Kulbak, M.; Friend, R.H.; Cahen, D.; Hodes, G. Low-temperature solution-grown CsPbBr3 single crystals and their characterization. Cryst. Growth Des. 2016, 16, 5717–5725. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Peters, J.A.; Liu, Z.; Sebastian, M.; Im, J.; Chasapis, T.C.; Wibowo, A.C.; Chung, D.Y.; Freeman, A.J.; et al. Crystal Growth of the Perovskite Semiconductor CsPbBr3: A New Material for High-Energy Radiation Detection. Cryst. Growth Des. 2013, 13, 2722–2727. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, X.; Li, D.; Cheng, H.C.; Duan, X.; Lin, Z.; Duan, X. Chemical vapor deposition growth of single-crystalline cesium lead halide microplatelets and heterostructures for optoelectronic applications. Nano Res. 2017, 10, 1223–1233. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, F.; Dong, H.; Jiao, B.; Zhang, W.; Hou, X.; Wang, S.; Gong, Q.; Wu, Z. One-Step Co-Evaporation of All-Inorganic Perovskite Thin Films with Room-Temperature Ultralow Amplified Spontaneous Emission Threshold and Air Stability. ACS Appl. Mater. Interfaces 2018, 10, 40661–40671. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Gao, F.; Wang, H.; Li, J.; Jiang, J.; Wu, X.; Gao, R.; Yang, Z.; Liu, S. Efficient planar CsPbBr3 perovskite solar cells by dual-source vacuum evaporation. Sol. Energy Mater. Sol. Cells 2018, 187, 1–8. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Wang, J.; Zhang, B.; Xin, L.; Niu, S.; Zhao, Y.; Xu, M.; Chu, X.; Zhang, D.; et al. Growth and optoelectronic application of CsPbBr3 thin films deposited by pulsed-laser deposition. Opt. Lett. 2019, 44, 1908–1911. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Ma, M.; Dong, S.; Li, Q.; Du, J.; Zhang, H.; Xu, Q. ulsed Laser Deposition of CsPbBr3 Films for Application in Perovskite Solar Cells. ACS Appl. Energy Mater. 2019, 2, 2305–2312. [Google Scholar] [CrossRef]
- Schou, J. Physical aspects of the pulsed laser deposition technique: The stoichiometric transfer of material from target to film. Appl. Surf. Sci. 2009, 255, 5191–5198. [Google Scholar] [CrossRef]
- Cesaria, M. Surface energy and nucleation modes. In Pulsed Laser Ablation: Advances and Applications in Nanoparticles and Nanostructuring Thin Films; Pan Stanford Publishing Pte Ltd.: Singapore, 2018; pp. 1–84. [Google Scholar] [CrossRef]
- Ameer, Z.; Monteduro, A.; Rizzato, S.; Caricato, A. Dielectrical performance of high-k yttrium copper titanate thin films for electronic applications. J. Mater. Sci. Mater. Electron. 2018, 29, 7090–7098. [Google Scholar] [CrossRef]
- Caricato, A.P.; Moretto, S.; Guascito, M.R.; Quarta, G.; Mazzeo, M.; Favaro, M.; Aziz, M.R.; Provenzano, C.; Marra, M.; Cesaria, M.; et al. High scintillation yield and fast response to alpha particles from thin perovskite films deposited by pulsed laser deposition. Front. Phys. 2022, 10, 895. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, Y.; Zhang, S.; Wu, J.; Song, J.; Li, X.; Xu, J.; Sow, C.H.; Zeng, H.; Sun, H. Switching excitonic recombination and carrier trapping in cesium lead halide perovskites by air. Commun. Phys. 2018, 1, 96. [Google Scholar] [CrossRef]
- Brenes, R.; Guo, D.; Osherov, A.; Noel, N.; Eames, C.; Hutter, E.; Pathak, S.; Niroui, F.; Friend, R.; Islam, M.; et al. Metal Halide Perovskite Polycrystalline Films Exhibiting Properties of Single Crystals. Joule 2017, 1, 155–167. [Google Scholar] [CrossRef]
- Howard, J.; Tennyson, E.; Barik, S.; Szostak, R.; Waks, E.; Toney, M.; Nogueira, A.; Neves, B.; Leite, M. Humidity-Induced Photoluminescence Hysteresis in Variable Cs/Br Ratio Hybrid Perovskites. J. Phys. Chem. Lett. 2018, 9, 3463–3469. [Google Scholar] [CrossRef]
- Shi, H.; Du, M.H. Shallow halogen vacancies in halide optoelectronic materials. Phys. Rev. B 2014, 90, 174103–174108. [Google Scholar] [CrossRef]
- Kang, J.; Wang, L.W. High defect tolerance in lead halide perovskite CsPbBr3. J. Phys. Chem. Lett. 2017, 8, 489–493. [Google Scholar] [CrossRef]
- Girolamo, D.D.; Ibrahim-Dar, M.; Dini, D.; Gontrani, L.; Caminiti, R.; Mattoni, A.; Graetzel, M.; Meloni, S. Dual effect of humidity on cesium lead bromide: Enhancement and degradation of perovskite films. J. Mater. Chem. A 2019, 19, 12292–12302. [Google Scholar] [CrossRef]
- Popov, A.I.; Kotomin, E.A.; Majer, J. Basic properties of the F-type centers in halides, oxides and perovskites. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 3085–3089. [Google Scholar] [CrossRef]
- Singh, R.K.; Narayan, J. Pulsed-laser evaporation technique for deposition of thin films: Physics and theoretical model. Phys. Rev. B 1990, 41, 8843–8859. [Google Scholar] [CrossRef]
- Ianno, N.J.; Erington, K.B. Thin films of uniform thickness by pulsed laser deposition. Rev. Sci. Instrum. 1992, 63, 3525–3526. [Google Scholar] [CrossRef]
- Cesaria, M.; Alfinito, E.; Arima, V.; Bianco, M.; Cataldo, R. MEED: A novel robust contrast enhancement procedure yielding highly-convergent thresholding of biofilm images. Comput. Biol. Med. 2022, 151, 106217. [Google Scholar] [CrossRef] [PubMed]
- Kampstra, P. A boxplot alternative for visual comparison of distributions. J. Stat. Softw. Code Snippets 2008, 28, 1–9. [Google Scholar] [CrossRef]
- Williamson, D.F.; Parker, R.A.; Kendrick, J.S. The box plot: A simple visual method to interpret data. Ann. Intern. Med. 1989, 110, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Hu, K. Become competent within one day in generating boxplots and violin plots for a novice without prior R experience. Methods Protoc. 2020, 3, 64. [Google Scholar] [CrossRef]
- Krzywinski, M.; Altman, N. Visualizing samples with box plots. Nat. Methods 2014, 11, 119–120. [Google Scholar] [CrossRef]
- Thrun, M.C.; Gehlert, T.; Ultsch, A. Analyzing the fine structure of distributions. PLoS ONE 2020, 15, e0238835. [Google Scholar] [CrossRef]
- Hintze, J.L.; Nelson, R.D. Violin plots: A box plot-density trace synergism. Am. Stat. 1998, 52, 181–184. [Google Scholar] [CrossRef]
- de Weerd, C.; Lin, J.; Gomez, L.; Fujiwara, Y.; Suenaga, K.; Gregorkiewicz, T. Hybridization of Single Nanocrystals of Cs4PbBr6 and CsPbBr3. J. Phys. Chem. C 2017, 121, 19490–19496. [Google Scholar] [CrossRef]
- Govindassamy, G.A.; Prentice, J.J.; Lunney, J.G.; Eason, R.W.; Mackenzie, J.I. Effect of laser repetition rate on the growth of Sc2O3 via pulsed laser deposition. Appl. Phys. A 2022, 128, 577. [Google Scholar] [CrossRef]
- Zhou, Y.; Poli, I.; Meggiolaro, D.; Angelis, F.D.; Petrozza, A. Defect activity in metal halide perovskites with wide and narrow bandgap. Nat. Rev. Mater. 2021, 6, 986–1002. [Google Scholar] [CrossRef]
- Pathak, S.; Sepe, A.; Sadhanala, A.; Deschler, F.; Haghighirad, A.; Sakai, N.; Goedel, K.C.; Stranks, S.; Noel, N.; Price, M.; et al. Atmospheric Influence upon Crystallization and Electronic Disorder and Its Impact on the Photophysical Properties of Organic-Inorganic Perovskite Solar Cells. ACS Nano 2015, 9, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Ng, A.; Shen, Q.; Gokkaya, H.C.; Wang, J.; Yang, L.; Yiu, W.K.; Bai, G.; Djurisič, A.B.; Leung, W.W.; et al. Thermal Assisted Oxygen Annealing for High Efficiency Planar CH3NH3PbI3 Perovskite Solar Cells. Sci. Rep. 2014, 4, 6752. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, M.; Plochocka, P. Excitons in Metal-Halide Perovskites. Adv. Energy Mater. 2020, 10, 1903659. [Google Scholar] [CrossRef]
- Liu, S.C.; Li, Z.; Yang, Y.; Wang, X.; Chen, Y.X.; Xue, D.J.; Hu, J.S. Investigation of Oxygen Passivation for High-Performance All-Inorganic Perovskite Solar Cells. J. Am. Chem. Soc. 2019, 141, 18075–18082. [Google Scholar] [CrossRef]
- Schneider, C.W.; Lippert, T. PLD plasma plume analysis: A summary of the PSI contribution. Appl. Phys. A 2023, 129, 138. [Google Scholar] [CrossRef]
- Sambri, A.; Christensen, D.; Trier, F.; Chen, Y.; Amoruso, S.; Pryds, N.; Bruzzese, R.; Wang, X. Plasma plume effects on the conductivity of amorphous-LaAlO3/SrTiO3 interfaces grown by pulsed laser deposition in O2 and Ar. Appl. Phys. Lett. 2012, 100, 231605. [Google Scholar] [CrossRef]





| Sample | P (Pa) | d (cm) | RR (Hz) | N |
| CsPbBr (2 × 10, 4 cm, 5 Hz) | 2 × 10 | 4 | 5 | 2000 |
| CsPbBr (2 × 10, 6 cm, 2 Hz) | 2 × 10 | 6 | 2 | 4500 |
| CsPbBr (2 × 10, 6 cm, 10 Hz) | 2 × 10 | 6 | 10 | 4500 |
| CsPbBr (2 × 10, 3 cm, 10 Hz) | 2 × 10 | 3 | 10 | 1200 |
| 2 × 10 Pa | 2 × 10 Pa | 2 × 10 Pa | 2 × 10 Pa | |
| 4 cm, 5 Hz | 6 cm, 2 Hz | 6 cm, 10 Hz | 3 cm, 10 Hz | |
| # of cluster | 221 | 609 | 598 | 51 |
| Minimum area (nm) | 0.8 × 10 | 0.5 × 10 | 1.4 × 10 | 1.6 × 10 |
| Maximum area (nm) | 2.3 × 10 | 0.7 × 10 | 0.6 × 10 | 1.6 × 10 |
| Mean area (nm) | 4.1 × 10 | 1.2 × 10 | 1.4 × 10 | 7 × 10 |
| ±0.4 × 10 | ±0.1 × 10 | ±0.8 × 10 | ±4.6 × 10 | |
| Coverage (%) | 71.99 | 57.86 | 61.45 | 25.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marra, M.; Provenzano, C.; Cesaria, M.; Cataldo, R.; Monteduro, A.G.; Caricato, A.P. CsPbBr3 Films Grown by Pulsed Laser Deposition: Impact of Oxygen on Morphological Evolution and Properties. Processes 2023, 11, 2514. https://doi.org/10.3390/pr11092514
Marra M, Provenzano C, Cesaria M, Cataldo R, Monteduro AG, Caricato AP. CsPbBr3 Films Grown by Pulsed Laser Deposition: Impact of Oxygen on Morphological Evolution and Properties. Processes. 2023; 11(9):2514. https://doi.org/10.3390/pr11092514
Chicago/Turabian StyleMarra, Marcella, Chiara Provenzano, Maura Cesaria, Rosella Cataldo, Anna Grazia Monteduro, and Anna Paola Caricato. 2023. "CsPbBr3 Films Grown by Pulsed Laser Deposition: Impact of Oxygen on Morphological Evolution and Properties" Processes 11, no. 9: 2514. https://doi.org/10.3390/pr11092514
APA StyleMarra, M., Provenzano, C., Cesaria, M., Cataldo, R., Monteduro, A. G., & Caricato, A. P. (2023). CsPbBr3 Films Grown by Pulsed Laser Deposition: Impact of Oxygen on Morphological Evolution and Properties. Processes, 11(9), 2514. https://doi.org/10.3390/pr11092514