Batch and Continuous Column Adsorption of p-Nitrophenol onto Activated Carbons with Different Particle Sizes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of the Adsorbents
2.3. PNP Adsorption Studies
2.3.1. PNP Adsorption in Equilibrium Conditions
2.3.2. Dynamic PNP Adsorption in Batch Conditions
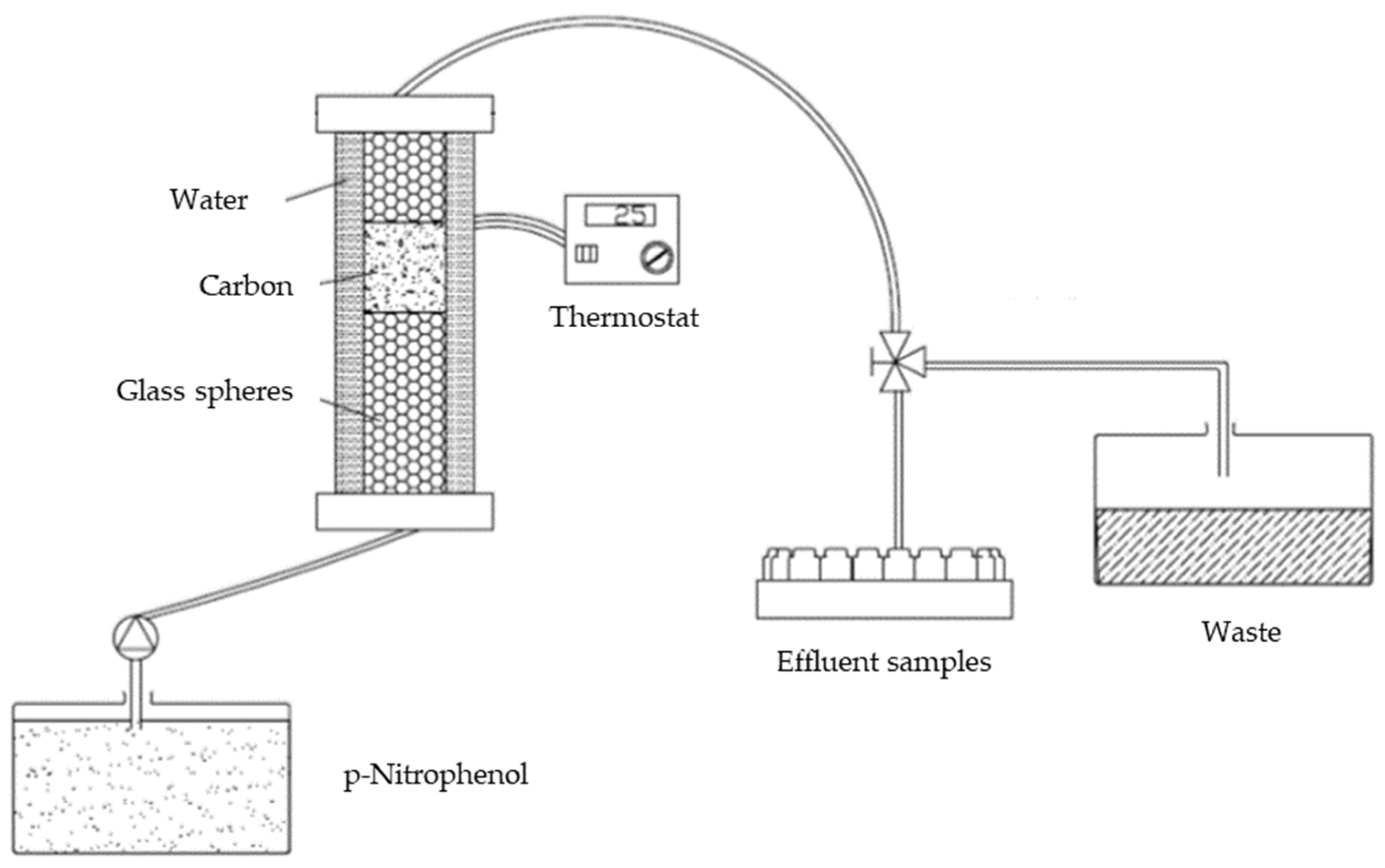
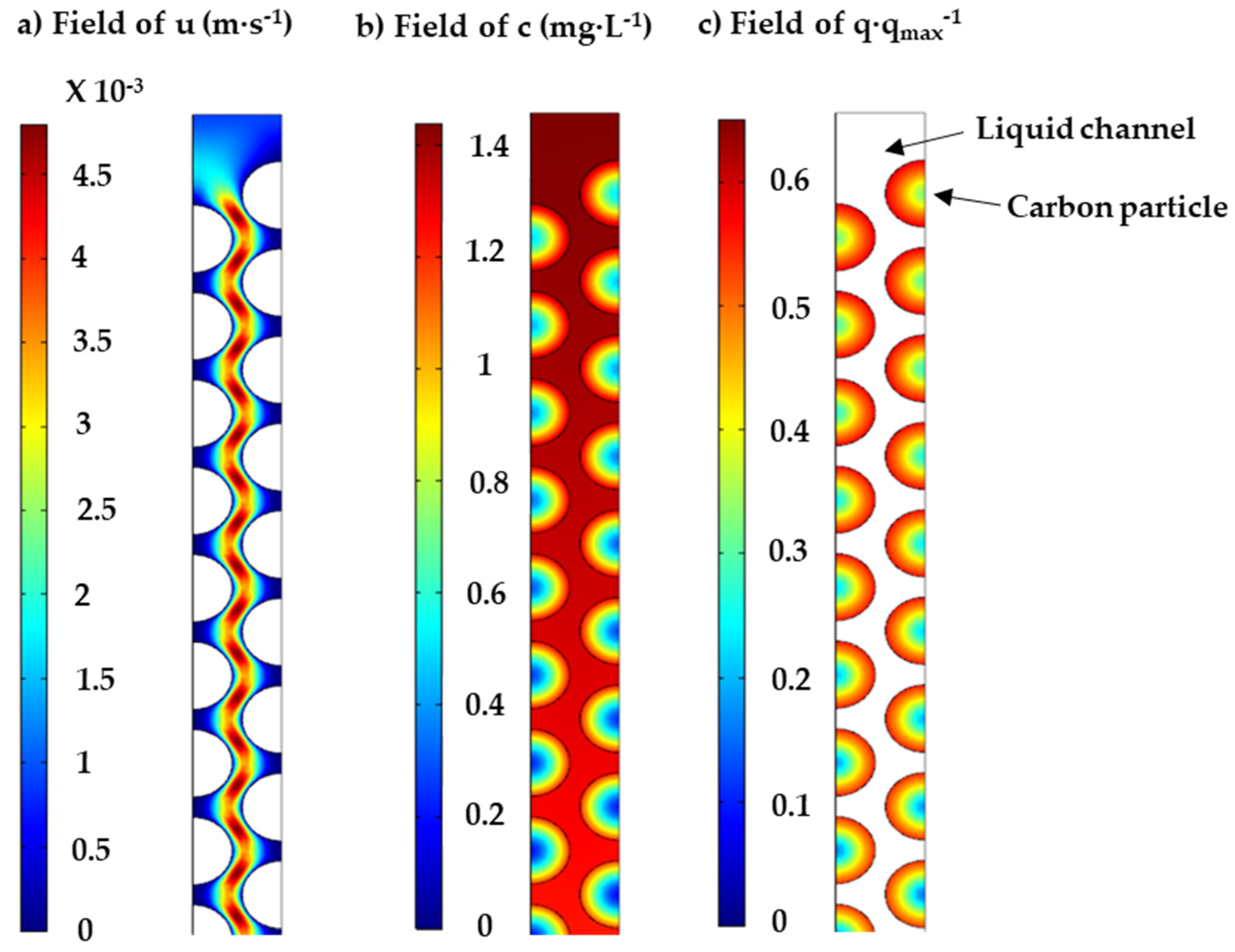
2.3.3. Dynamic PNP Adsorption in Fixed-Bed Columns
3. Results
3.1. Characterization of the Adsorbents
3.2. PNP Adsorption Studies
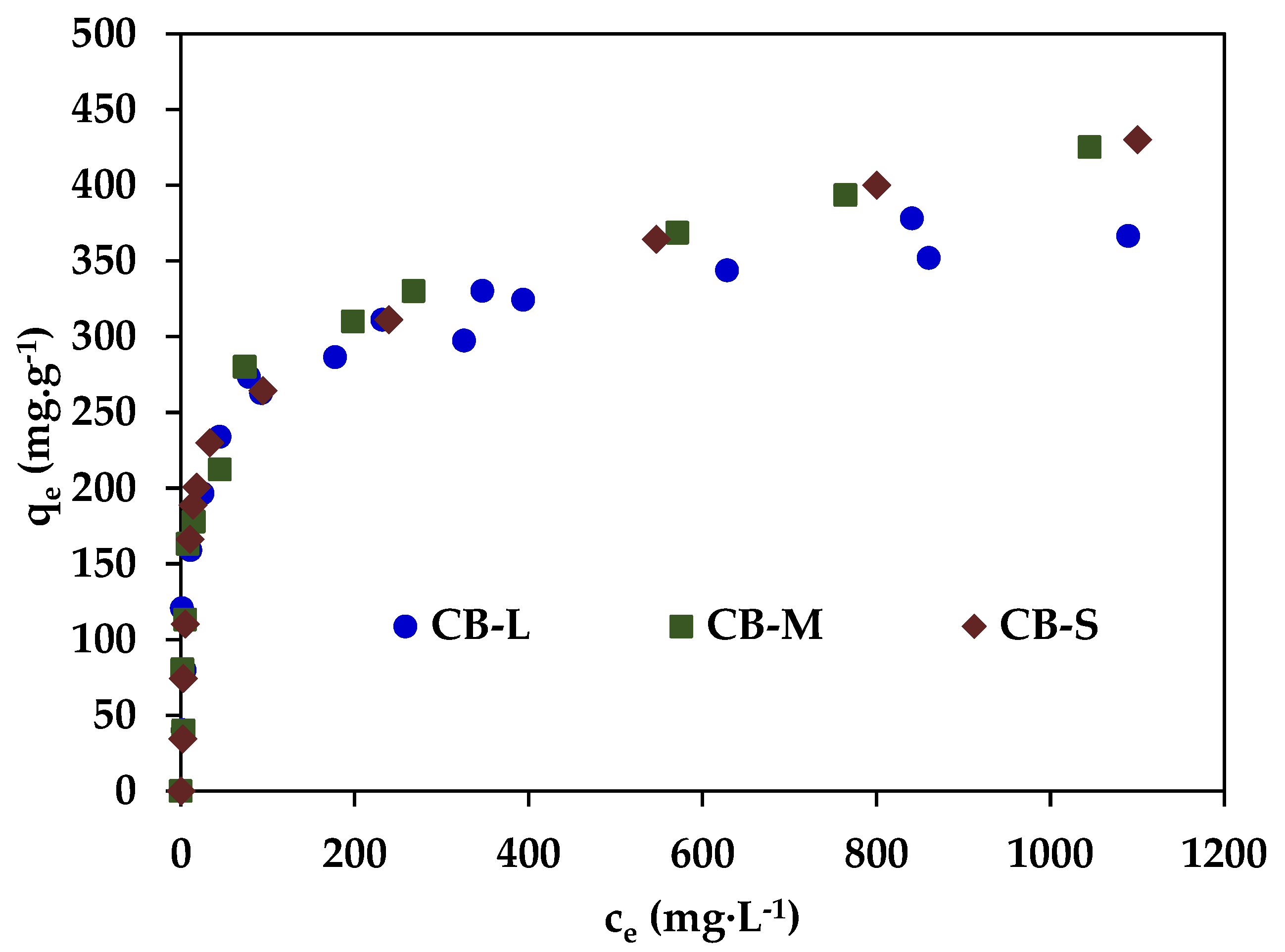
3.2.1. PNP Adsorption in Equilibrium Conditions
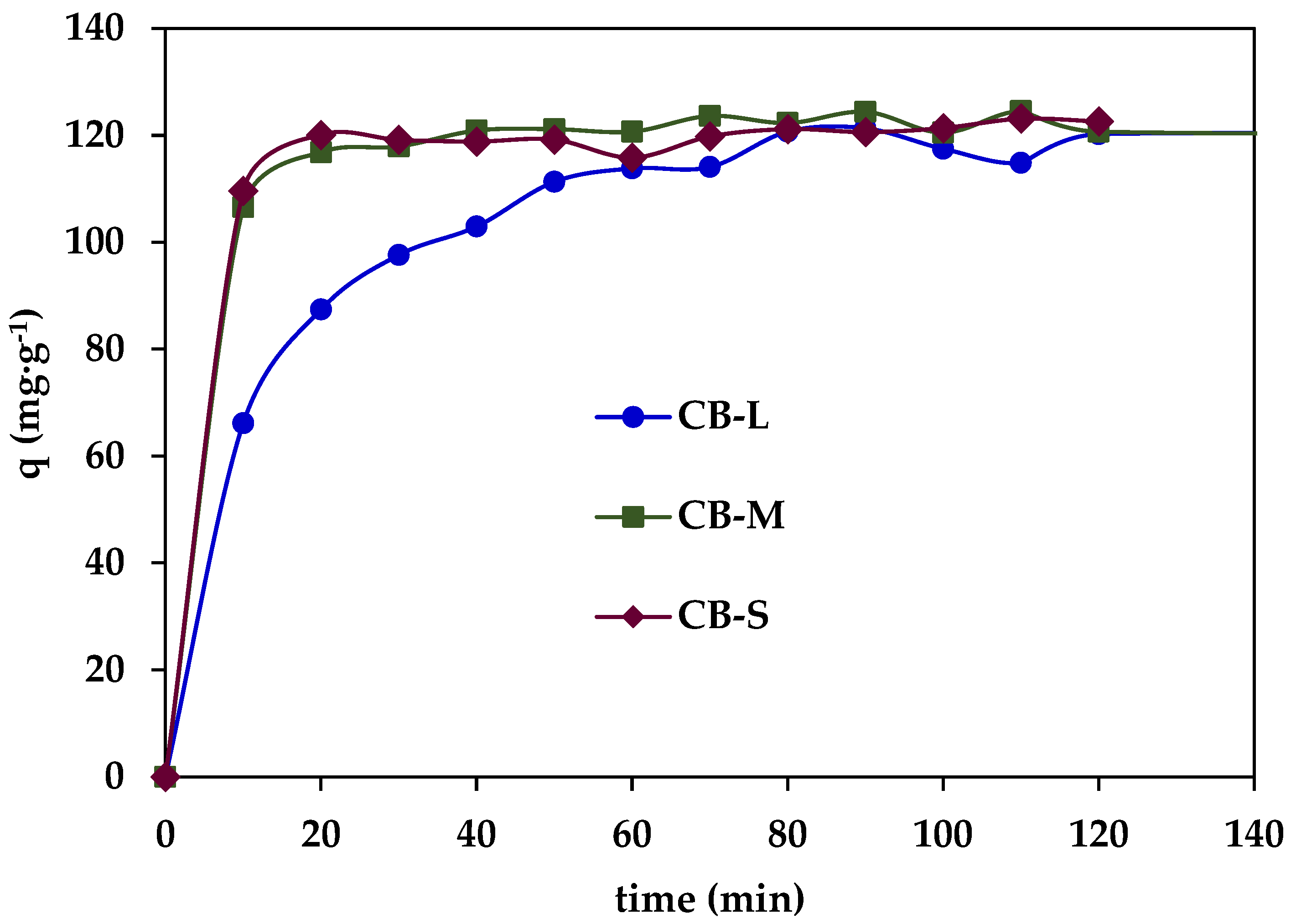
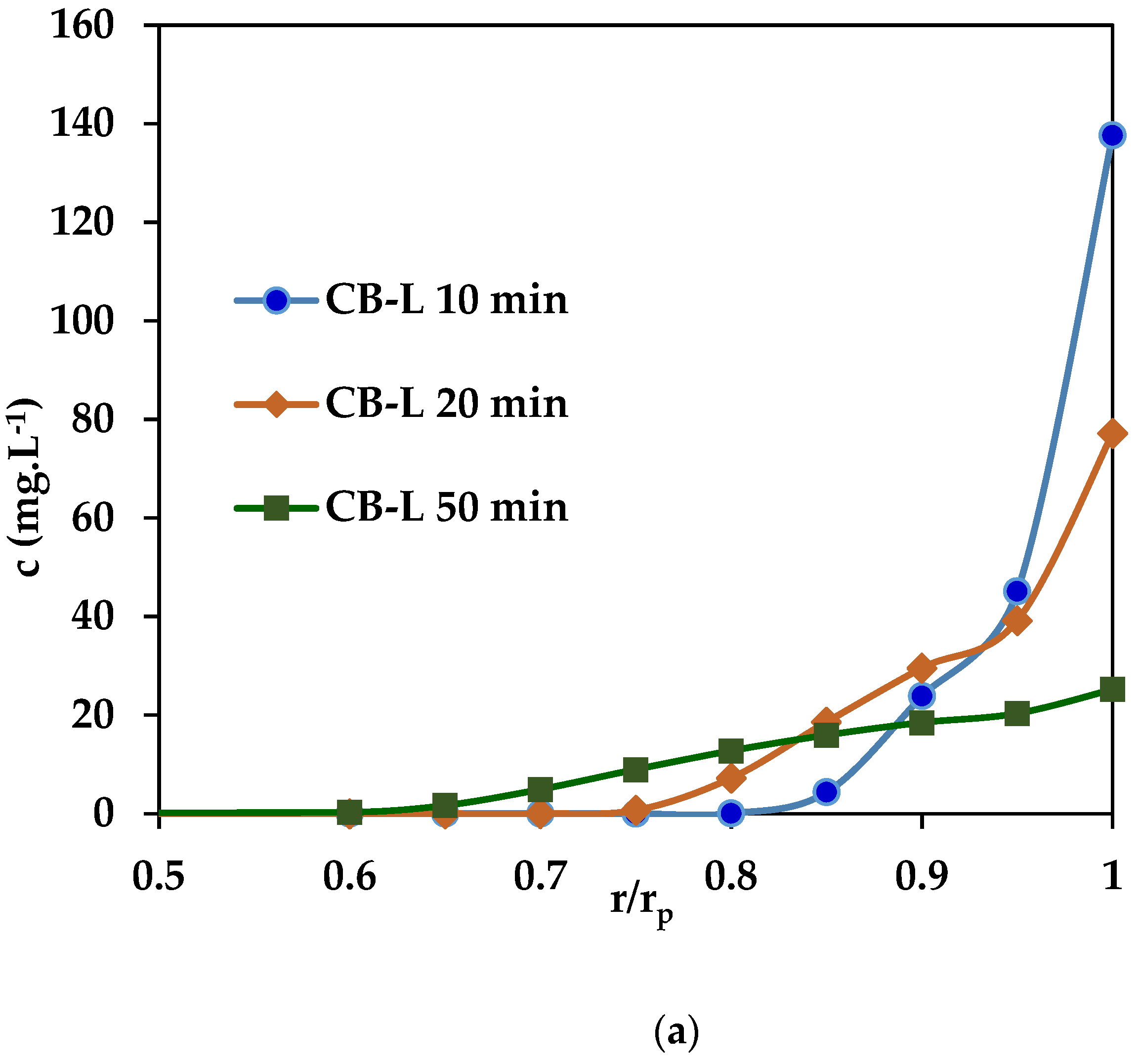
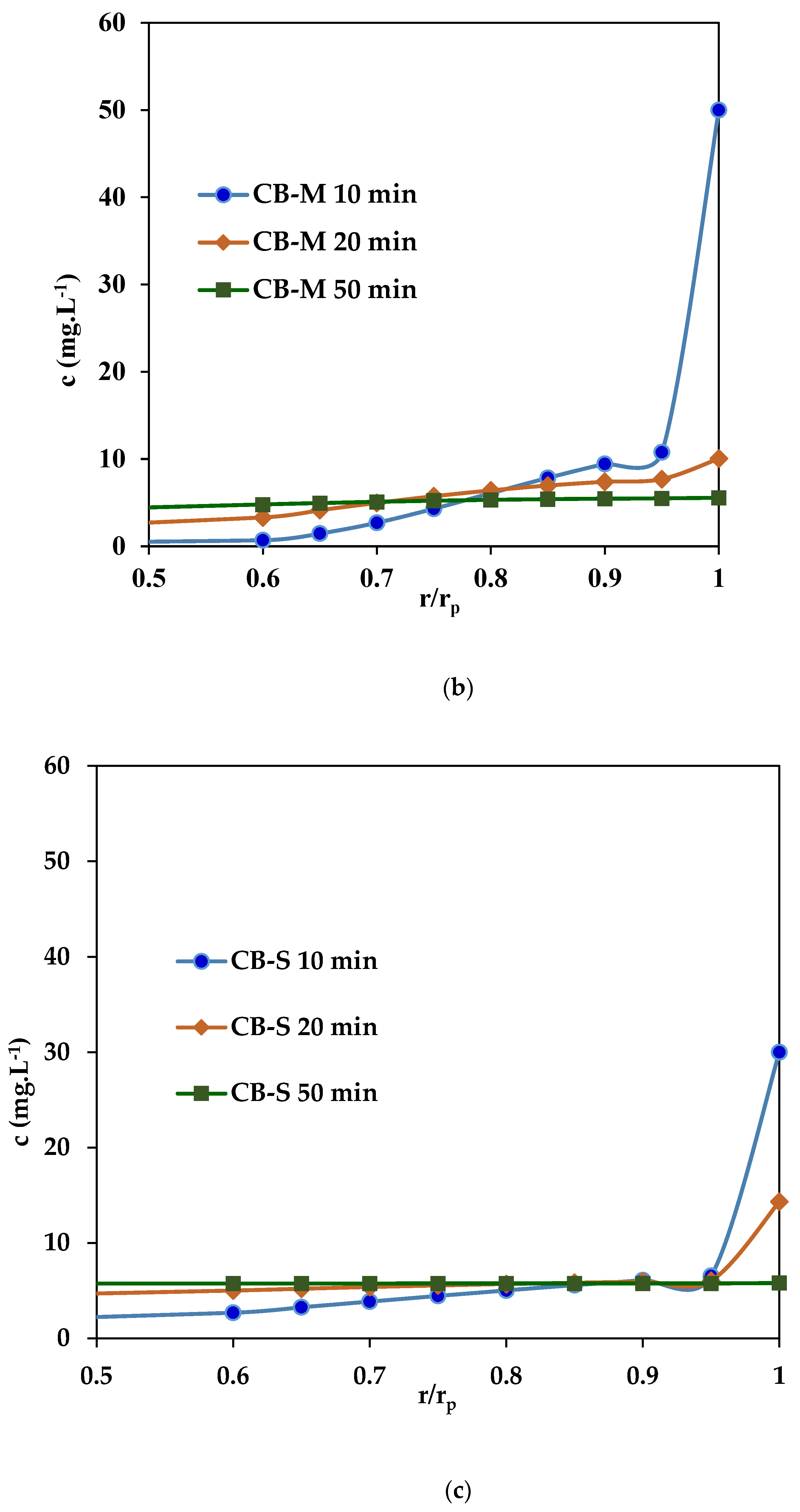
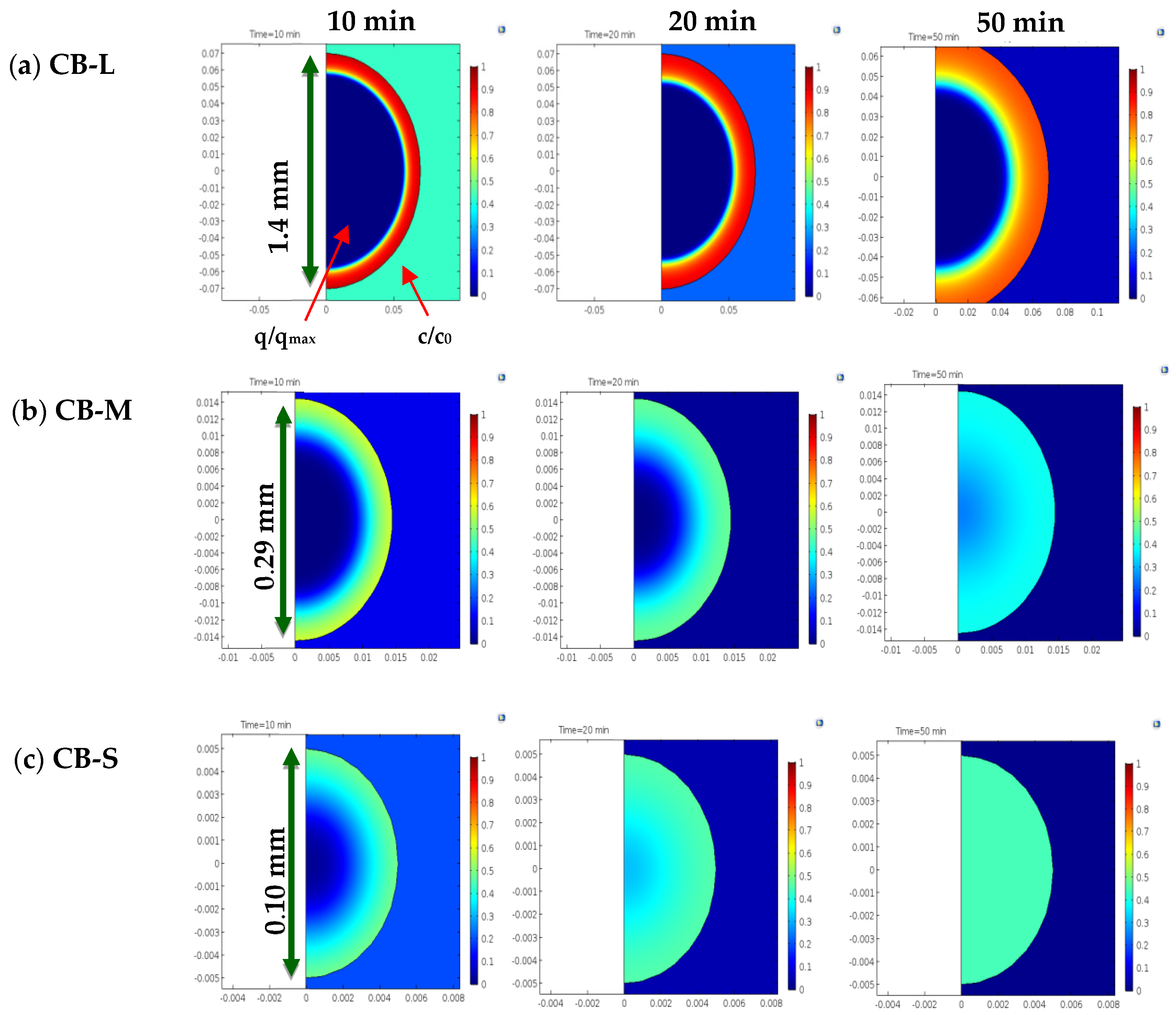
3.2.2. Dynamic PNP Adsorption in Batch Conditions
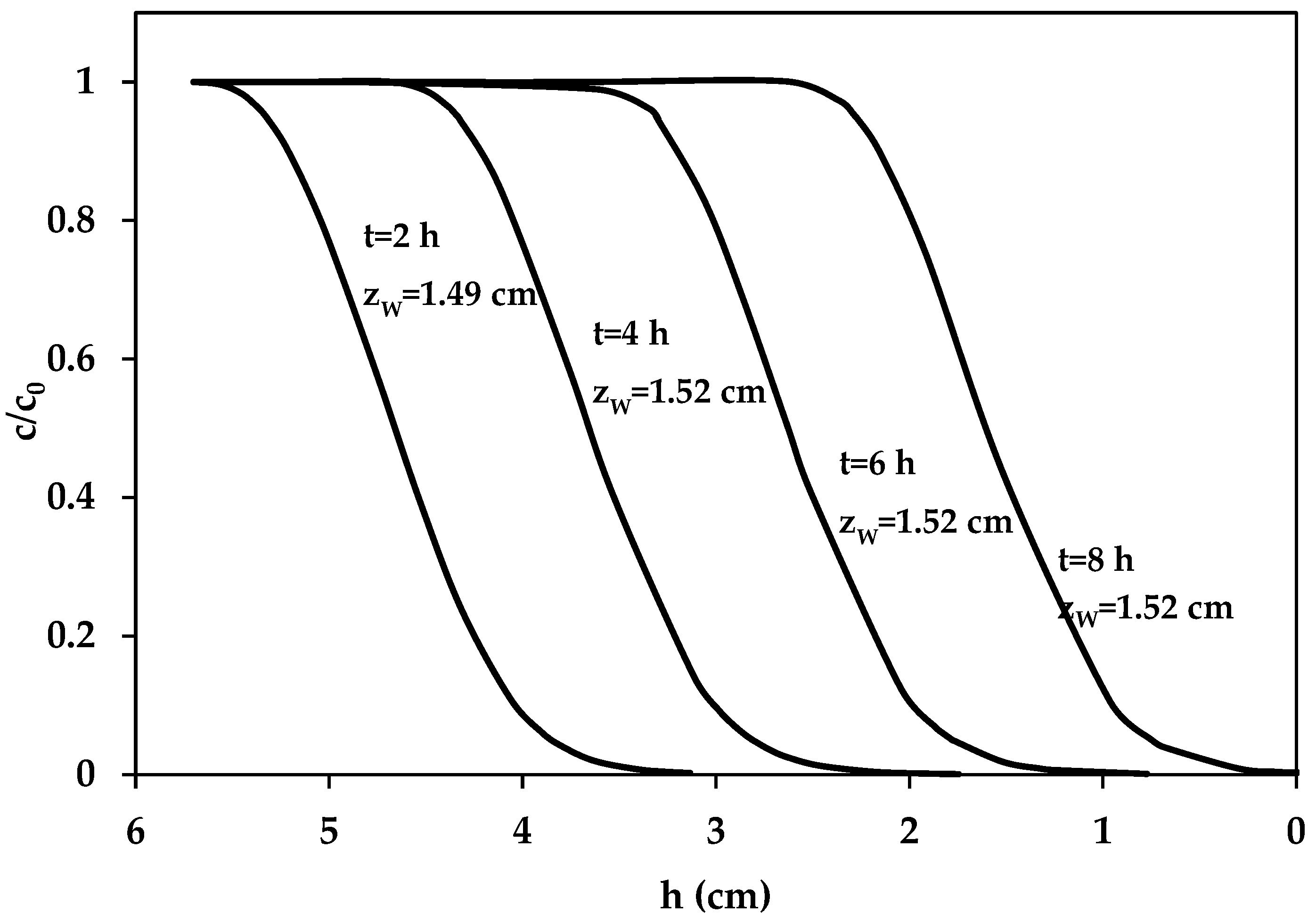
3.2.3. Dynamic PNP Adsorption in Fixed-Bed Columns
- (1)
- Particle parameters: the size, porosity, and weight of the particle (Table 1).
- (2)
- Mass transfer and isotherm parameters: the two Langmuir parameters (b and qmax), the constant of proportionality in the interphase, and the diffusion coefficient into the particle. The values of these four parameters were those provided by the batch study (Table 6).
- (3)
- Flow and bed parameters: flow rate, carbon mass, diameter, and length of the bed (Table 7).
- (1)
- Beyond the wave, loaded with the pristine carbon (q = 0, c = 0),
- (2)
- Wave, where the mass transfer is taking place, and both q and c are changing,
- (3)
- Post-wave zone, in which c is the concentration entering the column (c0) and the carbon has a constant contaminant load in equilibrium with c0(qe).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drinking Water Directive. Proposal for a Directive of the European Parliament and of the Council on the Quality of Water Intended for Human Consumption (Recast). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1519210589057&uri=CELEX:52017PC0753 (accessed on 18 January 2023).
- Kundan, S.; Saswat, M.; Md Hizbur, A. Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. [Google Scholar]
- Nan, Y.; Ji-Yu, L.; Hong-Tao, F.; Hua, S. In-situ sampling of nitrophenols in industrial wastewaters using diffusive gradients in thin films based on lignocellulose-derived activated carbons. J. Adv. Res. 2019, 15, 77–86. [Google Scholar]
- Giraldo, L.; Moreno-Piraján, J.C. Study of adsorption of phenol on activated carbons obtained from eggshells. J. Anal. Appl. Pyrolysis 2014, 106, 41–47. [Google Scholar] [CrossRef]
- Z-Flores, E.; Abatal, M.; Bassam, A.; Trujillo, L. Modeling the adsorption of phenols and nitrophenols by activated carbon using genetic programming. J. Clean. Prod. 2017, 161, 860–870. [Google Scholar] [CrossRef]
- Biao, L.; Zhi-Ying, Y.; Xiao-Na, L.; Chen, T.; Jun, Z.; Xia-Yuan, W.; Ping, W.; Hong-Hua, J.; Xiao-Yu, Y. Enhanced Bio-Electro-Fenton degradation of phenolic compounds based on a novel Fe–Mn/Graphite felt composite cathode. Chemosphere 2019, 234, 260–268. [Google Scholar]
- Alvárez-Torrellas, S.; Martín-Martinez, M.; Gomes, H.T.; Ovejero, G.; García, J. Enhancement of p-nitrophenol adsorption capacity through N2-thermal-based treatment of activated carbons. Appl. Surf. Sci. 2017, 414, 424–434. [Google Scholar] [CrossRef]
- Shaogang, L.; Jue, W.; Wanting, H.; Xuecai, T.; Huiyu, D.; Bernard, A.G.; Hanchun, D.; Fuhou, L.; Kaisheng, D. Adsorption of phenolic compounds from water by a novel ethylenediamine rosin-based resin: Interaction models and adsorption mechanisms. Chemosphere 2019, 214, 821–829. [Google Scholar]
- Sellaoui, L.; Kehili, M.; Lima, E.C.; Thue, P.S.; Bonilla-Petriciolet, A.; Lamine, A.B.; Dotto, G.L.; Erto, A. Adsorption of phenol on microwave-assisted activated carbons: Modelling and interpretation. J. Mol. Liq. 2019, 274, 309–314. [Google Scholar] [CrossRef]
- Kavitha, D. Equilibrium, kinetics, thermodynamics and desorption studies of pentachlorophenol onto agricultural waste activated carbon. Mater. Today 2020, 33, 4746–4750. [Google Scholar] [CrossRef]
- Farzaneh, F.; Ajit, K.S.; Ropru, R. Adsorption of pharmaceuticals in a fixed-bed column using tyre-based activated carbon: Experimental investigations and numerical modelling. J. Hazard. Mater. 2021, 417, 126010. [Google Scholar]
- Ositadinma Chamberlain, I.; Joseph Tagbo, N.; Christopher Chiedozie, O.; Chijioke Elijah, O. Packed bed column adsorption of phenol onto corn cob activated carbon: Linear and nonlinear kinetics modeling. S. Afr. J. Chem. Eng. 2021, 36, 80–93. [Google Scholar]
- Contreras, S.; Henríquez-Vargas, L.; Álvarez, P.I. Arsenic transport and adsorption modeling in columns using a copper nanoparticles composite. J. Water Process Eng. 2017, 19, 212–219. [Google Scholar] [CrossRef]
- Ashanendu, M.; Akanksha, M.; Ihita, B.; Koushik, G.; Nirjhar, B.; Sudip, K.D. Fixed-bed column study for removal of phenol by neem leaves-Experiment, MLR and ANN analysis. Sustain. Chem. Pharm. 2021, 23, 100514. [Google Scholar]
- Sabio, E.; Zamora, F.; Gañan, J.; González-García, C.M.; González, J.F. Adsorption of p-nitrophenol on activated carbon fixed-bed. Water Res. 2006, 40, 3053–3060. [Google Scholar] [CrossRef]
- Darweesh, T.M.; Ahmed, M.J. Adsorption of ciprofloxacin and norfloxacin from aqueous solution onto granular activated carbon in fixed bed column. Ecotoxicol. Environ. Saf. 2017, 138, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Zamora, F.; Sabio, E.; Román, S.; González-García, C.M. Modelling the Adsorption of p -Nitrophenol by the Boyd Method in Conjunction with the Finite Element Method. Adsorpt. Sci. Technol. 2010, 28, 671–687. [Google Scholar] [CrossRef]
- Sabio, E.; Zamora, F.; González-García, C.M.; Ledesma, B.; Álvarez-Murillo, A.; Román, S. Homogeneous diffusion solid model as a realistic approach to describe adsorption onto materials with different geometries. Nanoscale Res. Lett. 2016, 11, 671–687. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Dubinin, M.M. Fundamentals of the theory of adsorption in micropores of carbon adsorbents: Characteristics of their adsorption properties and microporous structures. Pure Appl. Chem. 1989, 61, 1841–1843. [Google Scholar] [CrossRef]
- Atchabarova, A.A.; Abdimomyn, S.K.; Abduakhytova, D.A.; Zhigalenok, Y.R.; Tokpayev, R.R.; Kishibayev, K.K.; Khavaza, T.N.; Kurbatov, A.P.; Zlobina, Y.V.; Djenizian, T.J.; et al. Role of carbon material surface functional groups on their interactions with aqueous solutions. J. Electroanal. Chem. 2022, 922, 116707. [Google Scholar] [CrossRef]
- Oickle, A.M.; Goertzen, S.L.; Hopper, K.R.; Abdalla, Y.O.; Andreas, H.A. Modified potentiometric titration method to distinguish and quantify oxygenated functional groups on carbon materials by pKa and chemical reactivity. Carbon 2020, 166, 436–445. [Google Scholar]
- Carrott, P.J.M.; Nabais, J.M.V.; Ribeiro Carrott, M.M.L.; Menendez, J.A. Thermal treatments of activated carbon fibers using a microwave furnace. Microporous Mesoporous Mater. 2001, 47, 243–252. [Google Scholar] [CrossRef]
- Ledesma, B.; González-García, C.M.; Román, S.; González, J.F. Arrangements of PNP molecule onto activated carbons of different acidity. Influence of pH. In Proceedings of the XXXVII Reunión Ibérica de Adsorción, Sevilla, Spain, 9 September 2012. [Google Scholar]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Saied, A.; Setareh, E. Chapter 6—Adsorption isotherms and kinetics. In Adsorption: Fundamental Processes and Applications, Interface Science and Technology; Mehrorang, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 33, pp. 445–509. [Google Scholar]
- Puccia, V.; Avena, M.J. On the use of the Dubinin-Radushkevich equation to distinguish between physical and chemical adsorption at the solid-water interface. Colloid Interface Sci. Commun. 2021, 41, 100376. [Google Scholar] [CrossRef]
- Zeynep, M.S.; Savas, K.; Selçuk, S.; Katin, K.P.; Ozer, A.; Marzouki, R. Synthesis and characterization of chitosan-vermiculite-lignin ternary composite as an adsorbent for effective removal of uranyl ions from aqueous solution: Experimental and theoretical analyses. Int. J. Biol. Macromol. 2022, 209, 1234–1247. [Google Scholar]
- Byungryul, A. Cu(II) and As(V) Adsorption Kinetic Characteristic of the Multifunctional Amino Groups in Chitosan. Processes 2020, 8, 1194. [Google Scholar]
- Sumalinog, D.A.; Capareda, S.C.; de Luna, M.D.G. Evaluation of the effectiveness and mechanisms of acetaminophen and methylene blue dye adsorption on activated biochar derived from municipal solid wastes. J. Environ. Manag. 2018, 249, 255–262. [Google Scholar] [CrossRef]
- Ahmed, A.; Binoy, S.; Nadeesh, A.; Janitha, W.; Anushka, U.R.; Yong, S.O.; Meththika, V. Sorption process of municipal solid waste biochar-montmorillonite composite for ciprofloxacin removal in aqueous media. Chemosphere 2019, 236, 124384. [Google Scholar]
- Derylo-Marczewska, A.; Blachnio, M.; Wojciech Marczewski, A.; Seczkowska, M.; Tarasiuk, B. Phenoxyacid pesticide adsorption on activated carbon—Equilibrium and kinetics. Chemosphere 2019, 214, 349–360. [Google Scholar] [CrossRef]
- Najeh, M.; Paula, O.; Manuel, R.; Achraf, G.; Mario, D. Enhanced Cu(II) adsorption using sodium trimetaphosphate–modified cellulose beads: Equilibrium, kinetics, adsorption mechanisms, and reusability. Environ. Sci. Pollut. Res. 2020, 28, 46523–46539. [Google Scholar]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Alvarez-Gutierrez, N.; Gil, M.V.; Rubiera, F.; Pevida, C. Kinetics of CO2 adsorption on cherry stone-based carbons in CO2/CH4 separations. Chem. Eng. J. 2017, 307, 249–257. [Google Scholar] [CrossRef]
- Loganathan, S.; Tikmani, M.; Edubilli, S.; Mishra, A.; Ghoshal, A.K. CO2 adsorption kinetics on mesoporous silica under wide range of pressure and temperature. Chem. Eng. J. 2014, 256, 1–8. [Google Scholar] [CrossRef]
- Viotti, P.V.; Moreira, W.M.; Andreo dos Santos, O.A.; Bergamasco, R.; Salcedo Vieira, A.M.; Vieira, M.F. Diclofenac removal from water by adsorption on Moringa oleifera pods and activated carbon: Mechanism, kinetic and equilibrium study. J. Clean. Prod. 2019, 219, 809–817. [Google Scholar] [CrossRef]
- Álvarez-Torrellas, S.; Rodríguez, A.; Ovejero, G.; García, J. Comparative adsorption performance of ibuprofen and tetracycline from aqueous solution by carbonaceous materials. Chem. Eng. J. 2016, 283, 936–947. [Google Scholar] [CrossRef]
- Lemus, J.; Moya, C.; Gilarranz, M.A.; Rodriguez, J.J.; Palomar, J. Fixed-bed adsorption of ionic liquids onto activated carbon from aqueous phase. J. Environ. Chem. Eng. 2017, 5, 5347–5351. [Google Scholar] [CrossRef]
- Bayat, M.; Alighardashi, A.; Sadeghasadi, A. Fixed-bed column and batch reactors performance in removal of diazinon pesticide from aqueous solutions by using walnut shell-modified activated carbon. Environ. Technol. Innov. 2018, 12, 148–159. [Google Scholar] [CrossRef]
- Espina de Franco, M.A.; Bonfante de Carvalho, C.; Bonetto, M.M.; de Pelegrini Soares, R.; Féris, L.A. Diclofenac removal from water by adsorption using activated carbon in batch mode and fixed-bed column: Isotherms, thermodynamic study and breakthrough curves modeling. J. Clean. Prod. 2018, 181, 145–154. [Google Scholar] [CrossRef]
- Sausen, M.G.; Scheufele, F.B.; Alves, H.J.; Adeodato Vieira, M.G.; Carlos da Silva, M.G.; Borba, F.H.; Borba, C.E. Efficiency maximization of fixed-bed adsorption by applying hybrid statistical-phenomenological modeling. Sep. Purif. Technol. 2018, 207, 477–488. [Google Scholar] [CrossRef]
- Thomas, H.C. Heterogeneous ion exchange in a flowing system. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Nelson, J.H. Application of gas adsorption kinetics I. A theorical model for respirator cartridge service life. Am. Ind. Hyg. Assoc. J. 1984, 45, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Na, L.; Xiufen, L. Phosphorus removal from wastewater using Ca-modified attapulgite: Fixed-bed column performance and breakthrough curves analysis. J. Environ. Manag. 2023, 328, 116905. [Google Scholar]
- Ronbanchob, A.; Khim, H.C. Improved fixed bed models for correlating asymmetric adsorption breakthrough curves. J. Water Process Eng. 2021, 40, 101810. [Google Scholar]
- Ehsan, S.; Mahdi, A.; Yaser, D. Novel combinatorial extensions to breakthrough curve modeling of an adsorption column—Depth filtration hybrid process. J. Ind. Eng. Chem. 2020, 86, 232–243. [Google Scholar]
- Jennifer, T.; Ricardo, A.B.; Victor, H.G.; Cristina Alejandra, V.A. Removal of caffeine using agro-industrial residues in fixed-bed columns: Improving the adsorption capacity and efficiency by selecting adequate physical and operational parameters. J. Water Process Eng. 2023, 53, 103778. [Google Scholar]
- Byron, B.R.; Warren, E.S.; Edwin, N. Lightfoot—Transport Phenomena, 2nd ed.; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]


| Parameter | CB-L | CB-M | CB-S |
|---|---|---|---|
| SBET (m2·g−1) | 930 | 861 | 893 |
| Vmi (cm3·g−1) | 0.490 | 0.438 | 0.454 |
| Vme (cm3·g−1) | 0.063 | 0.058 | 0.061 |
| ρp (g·cm−3) | 1.0241 | 1.0209 | 1.1283 |
| ρt (g·cm−3) | 2.138 | 2.138 | 2.127 |
| ε | 0.521 | 0.522 | 0.470 |
| Φp (mm) | 1.40 | 0.29 | 0.10 |
| Sp (mm2) | 6.16 | 0.26 | 3.14 × 10−2 |
| Vp (mm3) | 1.44 | 1.28 × 10−2 | 5.24 × 10−4 |
| Sp/Vp (mm−1) | 4.29 | 20.69 | 60.00 |
| mp (mg) | 1.47 | 1.30 × 10−2 | 5.91 × 10−4 |
| Carboxyl groups (mEq·g−1) | 0.02 | 0.02 | 0.02 |
| Lactone groups (mEq g−1) | 0.01 | 0.01 | 0.01 |
| Phenol groups (mEq g−1) | 0.13 | 0.13 | 0.13 |
| Carbonil groups (mEq g−1) | 0.07 | 0.07 | 0.07 |
| Total surface acidic groups (mEq g−1) | 0.23 | 0.23 | 0.23 |
| Total surface basic groups (mEq g−1) | 0.39 | 0.39 | 0.39 |
| Point Zero Charge | 10.2 | 10.2 | 10.2 |
| Langmuir | Double-Langmuir | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | qL, (mg·g−1) | b, (L·mg−1) | R2 | qL1, (mg·g−1) | b1, (L·mg−1) | qL2, (mg·g−1) | b2, (L·mg−1) | R2 |
| CB-L | 343.7 | 0.0582 | 0.9281 | 250.1 | 0.178 | 435.8 | 4.3·× 10−4 | 0.9540 |
| CB-M | 374.1 | 0.0580 | 0.9382 | 280.0 | 0.130 | 447.2 | 4.0·× 10−4 | 0.9816 |
| CB-S | 377.3 | 0.0628 | 0.9449 | 285.1 | 0.134 | 439.0 | 4.9·× 10−4 | 0.9937 |
| Langmuir | |||
|---|---|---|---|
| Sample | qRD, (mg·g−1) | EDR, (kJ·mol −1) | R2 |
| CB-L | 888.1 | 3.8 | 0.9504 |
| CB-M | 1089.7 | 3.5 | 0.9449 |
| CB-S | 1232.9 | 3.4 | 0.9500 |
| PFO Model | PSO Model | ||||||
|---|---|---|---|---|---|---|---|
| Sample | qe (exp) (mg·g−1) | qe (mg·g−1) | K1 (min−1) | R2 | qe (mg·g−1) | K2 (g mg−1 min−1) | R2 |
| CB-L | 122.4 | 119.7 ± 1 | 0.064 ± 0.04 | 0.9819 | 125.6 ± 1 | 9.91 × 10−4 ± 8 × 10−5 | 0.9890 |
| CB-M | 121.4 | 121.4 ± 0.5 | 0.205 ± 0.01 | 0.9950 | 123.1 ± 0.7 | 6.27·× 10−3 ± 1 × 10−3 | 0.9940 |
| CB-S | 121.8 | 120.2 ± 0.6 | 0.243 ± 0.02 | 0.9970 | 122.4 ± 0.9 | 7.97·× 10−3 ± 1 × 10−3 | 0.9968 |
| Sample | Line 1: t = 0–20 min | Line 2: t = 20–60 min | ||||
|---|---|---|---|---|---|---|
| kID,1 (mg g−1 min−0.5) | CID,1 (mg g−1) | R2 | kID,2 (mg g−1 min−0.5) | CID,2 (mg g−1) | R2 | |
| CB-L | 21.3 | 1.51 | 0.9786 | 7.6 | 56.1 | 0.9719 |
| CB-M | 7.8 | 81.8 | 1 | -- | -- | -- |
| CB-S | 8.0 | 84.3 | 1 | -- | -- | -- |
| Parameter | CB-L | CB-M | CB-S |
|---|---|---|---|
| qmax (mg·g−1) | 340 | 350 | 365 |
| b (L·mg−1) | 0.0142 | 0.0510 | 0.0600 |
| DF,p (mm2·s−1) | 12.0 × 10−2 | 8.0 × 10−2 | 4.5 × 10−2 |
| kBL (mm·s−1) | 10.5 × 10−2 | 5.4 × 10−2 | 2.7 × 10−2 |
| Rc2 | 0.9498 | 0.9963 | 0.9987 |
| Rq2 | 0.9916 | 0.9925 | 0.9956 |
| (1) 11-6-S | (2) 5.5-6-S | (3) 11-3-S | (4) 5.5-3-S | (5) 11-6-M | (6) 5.5-6-M | |
|---|---|---|---|---|---|---|
| (cm3·min−1) | 11.0 | 5.5 | 11.0 | 5.5 | 11.0 | 5.5 |
| mc (g) | 6.0 | 6.0 | 3.0 | 3.0 | 6.0 | 6.0 |
| hc (cm) | 5.7 | 5.7 | 2.9 | 2.9 | 5.8 | 5.8 |
| tcontact (min) | 1.04 | 2.08 | 0.53 | 1.06 | 1.06 | 2.12 |
| tb (h) | 8.50 | 19.15 | 3.8 | 10.6 | 8.2 | 18.6 |
| uw (mm·min−1) | 11 × 10−2 | 5.1 × 10−2 | 13 × 10−2 | 5.0 × 10−2 | 1.1 × 10−1 | 5.1 × 10−2 |
| Vb/mc | 0.935 | 1.017 | 0.836 | 1.056 | 0.935 | 1.034 |
| Run 1 11-6-S | Run 2 5.5-6-S | Run 3 11-3-S | Run 4 5.5-3-S | Run 5 11-6-M | Run 6 5.5-6-M | |
|---|---|---|---|---|---|---|
| kTH mL (h−1mg−1) | 0.145 ± 0.010 | 0.171 ± 0.003 | 0.172 ± 0.005 | 0.172 ± 0.005 | 0.0905 ± 0.002 | 0.143 ± 0.005 |
| qTH exp. (mg·g−1) | 222.8 | 234.5 | 254.1 | 254.1 | 240.7 | 233.4 |
| qTH cal. (mg·g−1) | 230.2 ± 1.1 | 241.0 ± 0.3 | 261.7 ± 0.3 | 261.7 ± 0.3 | 244.8 ± 1.0 | 281.4 ± 1.1 |
| R2 | 0.9971 | 0.9993 | 0.9995 | 0.9995 | 0.9990 | 0.9980 |
| kYN (h−1) | 1.67 ± 0.12 | 1.67 ± 0.12 | 2.07 ± 0.18 | 2.07 ± 0.18 | 1.09 ± 0.28 | 1.66 ± 0.19 |
| τ exp. (h) | 10.28 | 21.82 | 5.60 | 11.92 | 11.13 | 22.23 |
| τ cal. (h) | 10.46 ± 0.05 | 21.91 ± 0.24 | 5.61 ± 0.30 | 11.90 ± 0.34 | 11.13 ± 0.65 | 27.95 ± 0.65 |
| R2 | 0.9970 | 0.9992 | 0.9996 | 0.9996 | 0.9990 | 0.9980 |
| tb (h) | 8.84 | 18.7 | 3.50 | 10.33 | 8.00 | 18.08 |
| zw,aveg | 1.51 | 1.23 | 1.77 | 0.95 | 1.43 | 1.10 |
| R2 | 0.9824 | 0.989 | 0.9897 | 0.9864 | 0.9949 | 0.9986 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledesma, B.; Sabio, E.; González-García, C.M.; Román, S.; Fernandez, M.E.; Bonelli, P.; Cukierman, A.L. Batch and Continuous Column Adsorption of p-Nitrophenol onto Activated Carbons with Different Particle Sizes. Processes 2023, 11, 2045. https://doi.org/10.3390/pr11072045
Ledesma B, Sabio E, González-García CM, Román S, Fernandez ME, Bonelli P, Cukierman AL. Batch and Continuous Column Adsorption of p-Nitrophenol onto Activated Carbons with Different Particle Sizes. Processes. 2023; 11(7):2045. https://doi.org/10.3390/pr11072045
Chicago/Turabian StyleLedesma, Beatriz, Eduardo Sabio, Carmen María González-García, Silvia Román, Maria Emilia Fernandez, Pablo Bonelli, and Ana L. Cukierman. 2023. "Batch and Continuous Column Adsorption of p-Nitrophenol onto Activated Carbons with Different Particle Sizes" Processes 11, no. 7: 2045. https://doi.org/10.3390/pr11072045
APA StyleLedesma, B., Sabio, E., González-García, C. M., Román, S., Fernandez, M. E., Bonelli, P., & Cukierman, A. L. (2023). Batch and Continuous Column Adsorption of p-Nitrophenol onto Activated Carbons with Different Particle Sizes. Processes, 11(7), 2045. https://doi.org/10.3390/pr11072045







