Biodiesel Production from Waste Cooking Oil Using Different Types of Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Analyses
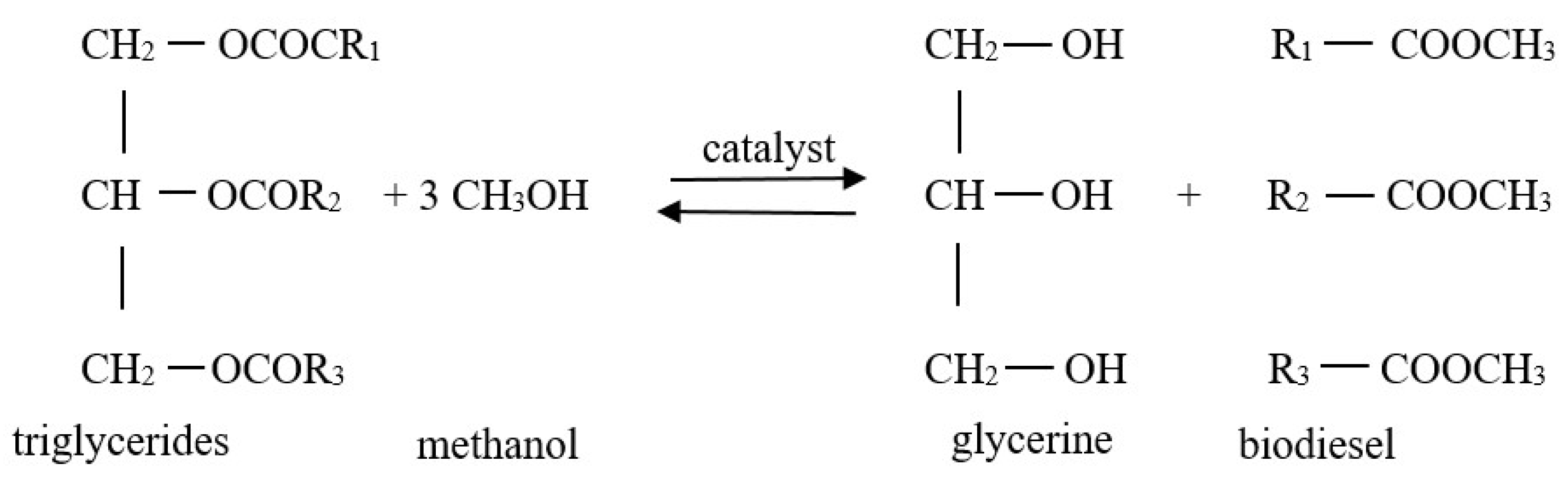
2.3. Transesterification Experiment Runs
3. Results and Discussion
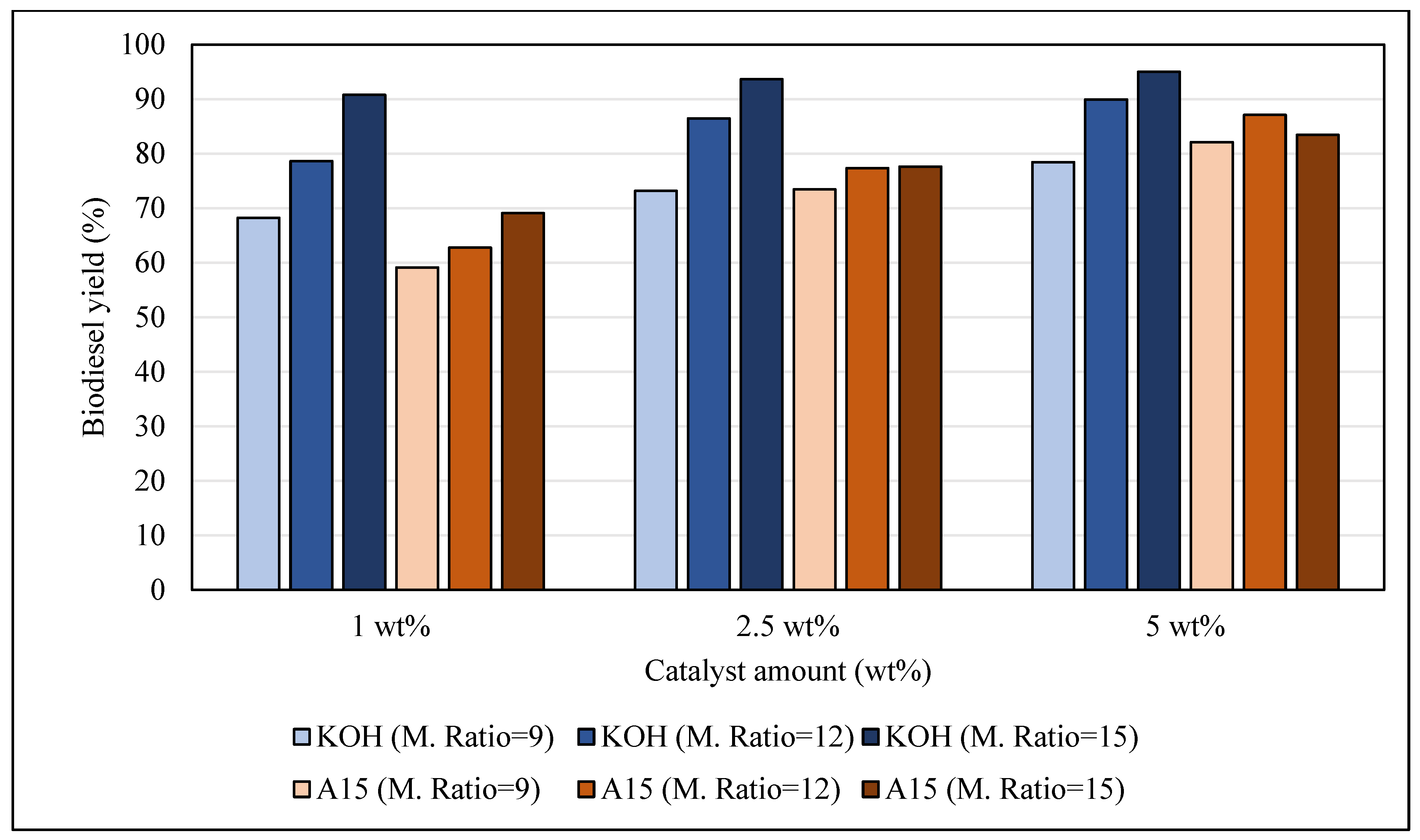
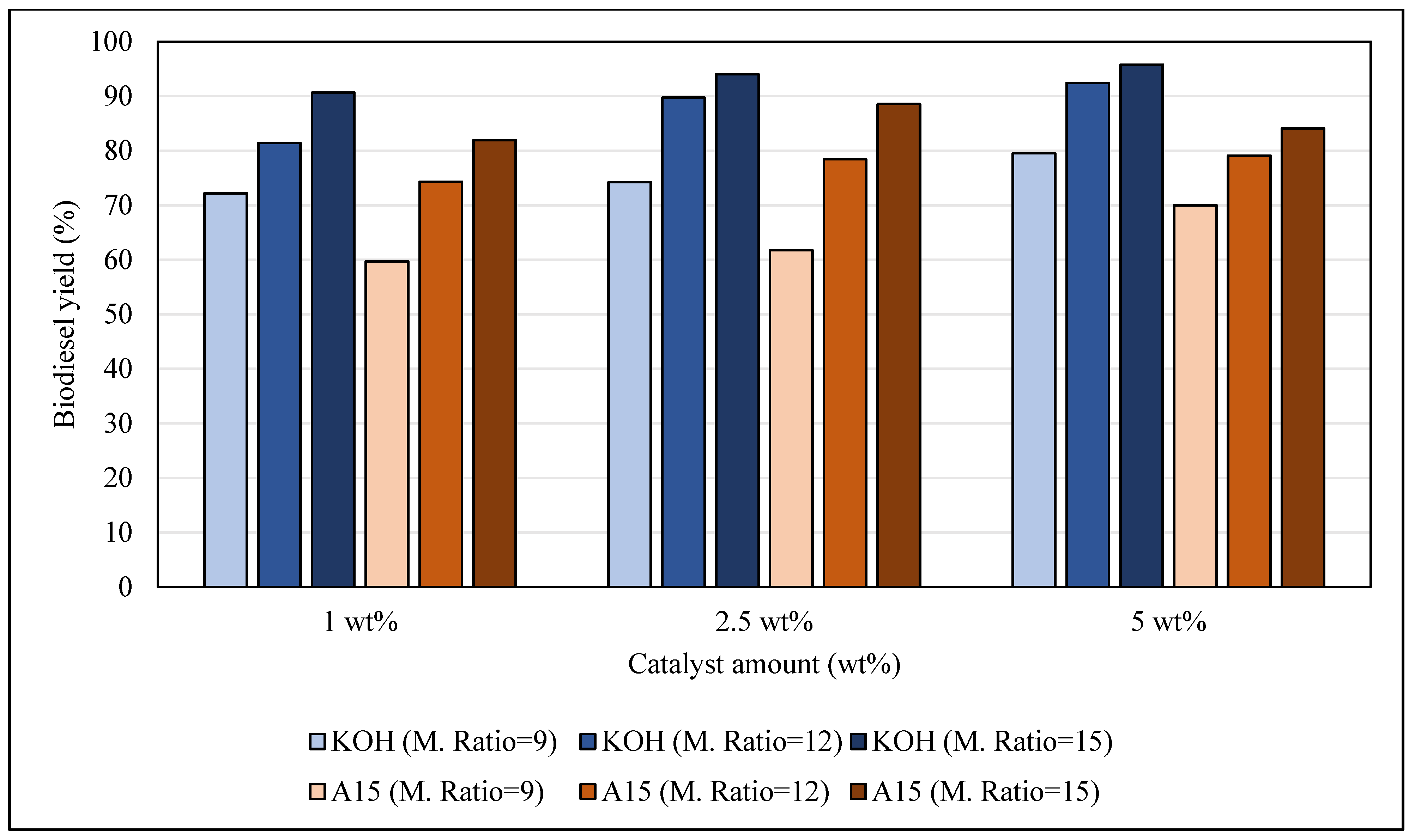
3.1. Biodiesel Yield
3.1.1. Effect of Methanol/Oil Ratio
3.1.2. Effect of Catalyst Amount
3.1.3. Reaction Time
3.2. Biodiesel Characteristics
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Tian, F.; Xu, L.; Peng, R.; Li, Y.; Deng, J. Batch and Continuous Esterification for the Direct Synthesis of High Qualified Biodiesel From Waste Cooking Oils (WCO) With Amberlyst-15/Poly (Vinyl Alcohol) Membrane as a Bifunctional Catalyst. Chem. Eng. J. 2020, 388, 124214. [Google Scholar] [CrossRef]
- Alajmi, F.; Alajmi, A.; Alrashidi, A.; Alrashidi, N.; Adam, N.M.; Hairuddin, A.A. Development of Solar Powered Biodiesel Reactor for Kuwait Sheep Tallow. Processes 2021, 9, 1623. [Google Scholar] [CrossRef]
- Soares Dias, A.P.; Ramos, M.; Rijo, B. Rendering of Beef Tallow for Biodiesel Production: Microwave versus Boiling Water and Acetone Fat Extraction. Processes 2022, 10, 666. [Google Scholar] [CrossRef]
- Kiran, K.; Hebbar, G.S. Optimization of Biodiesel Production from Waste Cooking Oil by Box Behnken Design Using Response Surface Methodology. Int. J. Renew. Energy Res. 2021, 11, 344–354. [Google Scholar]
- Zhao, Y.; Wang, C.; Zhang, L.; Chang, Y.; Hao, Y. Converting Waste Cooking Oil to Biodiesel in China: Environmental Impacts and Economic Feasibility. Renew. Sustain. Energy Rev. 2021, 140, 110661. [Google Scholar] [CrossRef]
- Dursun, N. Identification of Public Awareness on Environmental Effects and Recycling of Waste Vegetable Oils: The Case of Malatya Province. Artvin Çoruh Univ. Nat. Hazards Appl. Res. Cent. J. Nat. Hazards Environ. 2020, 6, 228–247. [Google Scholar]
- Tan, X.; Sudarsanam, P.; Tan, J.; Wang, A.; Zhang, H.; Li, H.; Yang, S. Sulfonic Acid-Functionalized Heterogeneous Catalytic Materials for Efficient Biodiesel Production: A Review. J. Environ. Chem. Eng. 2021, 9, 104719. [Google Scholar] [CrossRef]
- Sanli, H.; Yasar, F. An Investigation on Fatty Acid Compositions of Three-Generation Biodiesel Fuels. J. Turk. Chem. Soc. Sect. B 2022, 5, 195–202. [Google Scholar]
- Akgul, G.; Sozer, S.; Culfa, M. A Novel Biochar Catalyst for Biodiesel Production from Waste Cooking Oil. TÜBAV Bilim 2017, 10, 29–38. [Google Scholar]
- Ameen, M.; Zafar, M.; Nizami, A.S.; Ahmad, M.; Munir, M.; Sultana, S.; Usma, A.; Rehan, M. Biodiesel Synthesis from Cucumis Melo Varagrestis Seed Oil: Toward Non-food Biomass Biorefineries. Front. Energy Res. 2022, 10, 830845. [Google Scholar] [CrossRef]
- Mohiddin, M.N.B.; Tan, Y.H.; Seow, Y.X.; Kansedo, J.; Mubarak, N.M.; Abdullah, M.O.; Chan, Y.S.; Khalid, M. Evaluation on Feedstock, Technologies, Catalyst and Reactor for Sustainable Biodiesel Production: A Review. J. Ind. Eng. Chem. 2021, 98, 60–81. [Google Scholar] [CrossRef]
- Roushdy, M.H. Sanitary Ware Waste as a Source for a Valuable Biodiesel Catalyst. Hindawi J. Chem. 2022, 2022, 1232110. [Google Scholar] [CrossRef]
- Jayakumar, M.; Karmegam, N.; Gundupalli, M.P.; Gebeyehu, K.B.; Asfaw, B.T.; Chang, S.W.; Ravindran, B.; Awasthi, M.K. Heterogeneous Base Catalysts: Synthesis and Application for Biodiesel Production- A Review. Bioresour. Technol. 2021, 331, 125054. [Google Scholar] [CrossRef] [PubMed]
- Yasvanthrajan, N.; Sivakumar, P.; Muthukumar, K.; Murugesan, T.; Arunagiri, A. Production of Biodiesel from Waste Bio-Oil Through Ultrasound Assisted Transesterification Using Immobilized Lipase. Environ. Technol. Innov. 2021, 21, 101199. [Google Scholar] [CrossRef]
- Marwaha, A.; Rosha, P.; Mohapatra, S.K.; Mahla, S.K.; Dhir, A. Waste Materials as Potential Catalysts for Biodiesel Production: Current State and Future Scope. Fuel Process. Technol. 2018, 181, 175–186. [Google Scholar] [CrossRef]
- Okolie, J.A.; Escobar, J.I.; Umenweke, G.; Khanday, W.; Okoye, P.U. Continuous Biodiesel Production: A Review of Advances in Catalysis, Microfluidic and Cavitation Reactors. Fuel 2022, 307, 121821. [Google Scholar] [CrossRef]
- Banerjee, N.; Ramakrishnan, R.; Jash, T. Biodiesel Production from Used Vegetable Oil Collected from Shops Selling Fritters in Kolkata. Energy Procedia 2014, 54, 161–165. [Google Scholar] [CrossRef]
- Koçer, N.N.; Durmuş, B. Determination of the Biodiesel Potential and Glycerol Quantity Obtained from Waste Oil. Batman Univ. J. Life Sci. 2019, 9, 69–80. [Google Scholar]
- Al-Hamamre, Z.; Foerster, S.; Hartmann, F.; Kröger, M.; Kaltschmitt, M. Oil Extracted from Spent Coffee Grounds as a Renewable Source for Fatty Acid Methyl Ester Manufacturing. Fuel 2012, 96, 70–76. [Google Scholar] [CrossRef]
- Suzihaque, M.U.H.; Syazwina, N.; Alwi, H.; Ibrahim, U.K.; Abdullah, S.; Haron, N. A Sustainability Study of the Processing of Kitchen Waste as a Potential Source of Biofuel: Biodiesel Production from Waste Cooking Oil (WCO). Mater. Today Proc. 2022, 63, S484–S489. [Google Scholar] [CrossRef]
- Shibasaki-Kitakawa, N.; Honda, H.; Kuribayashi, H.; Toda, T.; Fukumura, T.; Yonemoto, T. Biodiesel Production Using Anionic Ion-Exchange Resin as Heterogeneous Catalyst. Bioresour. Technol. 2007, 98, 416–421. [Google Scholar] [CrossRef]
- Boz, N.; Degirmenbasi, N.; Kalyon, D.M. Esterification and Transesterification of Waste Cooking Oil Over Amberlyst 15 and Modified Amberlyst 15 Catalysts. Appl. Catal. B Environ. 2015, 165, 723–730. [Google Scholar] [CrossRef]
- Hazrat, M.A.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Silitonga, A.S.; Fattah, I.M.R.; Mahlia, T.M.I. Kinetic Modelling of Esterification and Transesterification Processes for Biodiesel Production Utilising Waste-Based Resource. Catalysts 2022, 12, 1472. [Google Scholar] [CrossRef]
- Avhad, M.R.; Marchetti, J.M. A Review on Recent Advancement in Catalytic Materials for Biodiesel Production. Renew. Sustain. Energy Rev. 2015, 50, 696–718. [Google Scholar] [CrossRef]
- Patiño, Y.; Mantecón, L.G.; Polo, S.; Faba, L.; Díaz, E.; Ordóñez, S. Effect of Sludge Features and Extraction-Esterification Technology on the Synthesis of Biodiesel from Secondary Wastewater Treatment Sludges. Bioresour. Technol. 2018, 247, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Trombettoni, V.; Lanari, D.; Prinsen, P.; Luque, R.; Marrocchi, A.; Vaccaro, L. Recent Advances in Sulfonated Resin Catalysts for Efficient Biodiesel and Bio-Derived Additives Production. Prog. Energy Combust. Sci. 2018, 65, 136–162. [Google Scholar] [CrossRef]
- Thirumarimurugan, M.; Sivakumar, V.M.; Merly Xavier, A.; Prabhakaran, D.; Kannadasan, T. Preparation of Biodiesel from Sunflower Oil by Transesterification. Int. J. Biosci. Biochem. Bioinform. 2012, 2, 441–445. [Google Scholar] [CrossRef]
- Komintarachat, C.; Chuepeng, S. Solid Acid Catalyst for Biodiesel Production from Waste Used Cooking Oils. Ind. Eng. Chem. Res. 2009, 48, 9350–9353. [Google Scholar] [CrossRef]
- Mohadesi, M.; Aghel, B.; Maleki, M.; Ansari, A. Production of Biodiesel from Waste Cooking Oil Using a Homogeneous Catalyst: Study of Semi-Industrial Pilot of Microreactor. Renew. Energy 2019, 136, 677–682. [Google Scholar] [CrossRef]
- Pradhan, P.; Chakraborty, S.; Chakraborty, R. Optimization of Infrared Radiated Fast and Energy-Efficient Biodiesel Production from Waste Mustard Oil Catalyzed by Amberlyst 15: Engine Performance and Emission Quality Assessments. Fuel 2016, 173, 60–68. [Google Scholar] [CrossRef]
- Peter, A.S.; Alias, M.P.; Iype, M.P.; Jolly, J.; Sankar, V.; Babu, K.J.; Baby, D.K. Optimization of Biodiesel Production by Transesterification of Palm Oil and Evaluation of Biodiesel Quality. Mater. Today Proc. 2021, 42, 1002–1007. [Google Scholar] [CrossRef]
- Chiang, C.L.; Lin, K.S.; Shu, C.W.; Wu, J.C.S.; Wu, K.C.W.; Huang, Y.T. Enhancement of Biodiesel Production via Sequential Esterification/Transesterification Over Solid Superacidic and Superbasic Catalysts. Catal. Today 2020, 348, 257–269. [Google Scholar] [CrossRef]
- Naeem, A.; Khan, I.W.; Farooq, M.; Mahmood, T.; Din, I.U.; Ghazi, Z.A.; Saeed, T. Kinetic and Optimization Study of Sustainable Biodiesel Production from Waste Cooking Oil Using Novel Heterogeneous Solid Base Catalyst. Bioresour. Technol. 2021, 328, 124831. [Google Scholar] [CrossRef]
- Mathew, G.M.; Raina, D.; Narisetty, V.; Kumar, V.; Saran, S.; Pugazhendi, A.; Sindhu, R.; Pandey, A.; Binod, P. Recent Advances in Biodiesel Production: Challenges and Solutions. Sci. Total Environ. 2021, 794, 148751. [Google Scholar] [CrossRef] [PubMed]
- Özbay, N.; Oktar, N.; Tapan, N.A. Esterification of Free Fatty Acids in Waste Cooking Oils (WCO): Role of Ion-Exchange Resins. Fuel 2008, 87, 1789–1798. [Google Scholar] [CrossRef]
- Gan, S.; Ng, H.K.; Chan, P.H.; Leong, F.L. Heterogeneous Free Fatty Acids Esterification in Waste Cooking Oil Using Ion-Exchange Resins. Fuel Process. Technol. 2012, 102, 67–72. [Google Scholar] [CrossRef]
- Farooq, M.; Ramli, A. Biodiesel Production from Low FFA Waste Cooking Oil Using Heterogeneous Catalyst Derived from Chicken Bones. Renew. Energy 2015, 76, 362–368. [Google Scholar] [CrossRef]
- Yaakob, Z.; Mohammad, M.; Alherbawi, M.; Alam, Z.; Sopian, K. Overview of the Production of Biodiesel from Waste Cooking Oil. Renew. Sustain. Energy Rev. 2013, 18, 184–193. [Google Scholar] [CrossRef]
- Degfie, T.A.; Mamo, T.T.; Mekonnen, Y.S. Optimized Biodiesel Production from Waste Cooking Oil (WCO) Using Calcium Oxide (CaO) Nanocatalyst. Sci. Rep. 2019, 9, 18982. [Google Scholar] [CrossRef]
- Sharma, A.; Kodgire, P.; Kachhwaha, S.S. An Experimental Investigation of the Performance of Biodiesel Production Techniques: Optimization, Kinetics, and Energy Analysis. Therm. Sci. Eng. Prog. 2021, 22, 100842. [Google Scholar] [CrossRef]
- Takase, M. Biodiesel Yield and Conversion Percentage from Waste Frying Oil Using Fish Shell at Elmina as a Heterogeneous Catalyst and the Kinetics of the Reaction. Hindawi Int. J. Chem. Eng. 2022, 2022, 8718638. [Google Scholar] [CrossRef]
- Sungyup Jung, S.; Kim, M.; Lin, K.A.; Park, Y.K.; Kwon, E.E. Biodiesel Synthesis from Bio-Heavy Oil Through Thermally Induced Transesterification. J. Clean. Prod. 2021, 294, 126347. [Google Scholar] [CrossRef]
- Singh, V.; Belova, L.; Singh, B.; Sharma, Y.C. Biodiesel Production Using a Novel Heterogeneous Catalyst, Magnesium Zirconate (Mg2Zr5O12): Process Optimization Through Response Surface Methodology (RSM). Energy Convers. Manag. 2018, 174, 198–207. [Google Scholar] [CrossRef]
- Almasi, S.; Najafi, G.; Ghobadian, B.; Jalili, S. Biodiesel Production from Sour Cherry Kernel Oil as Novel Feedstock Using Potassium Hydroxide Catalyst: Optimization Using Response Surface Methodology. Biocatal. Agric. Biotechnol. 2021, 35, 102089. [Google Scholar] [CrossRef]
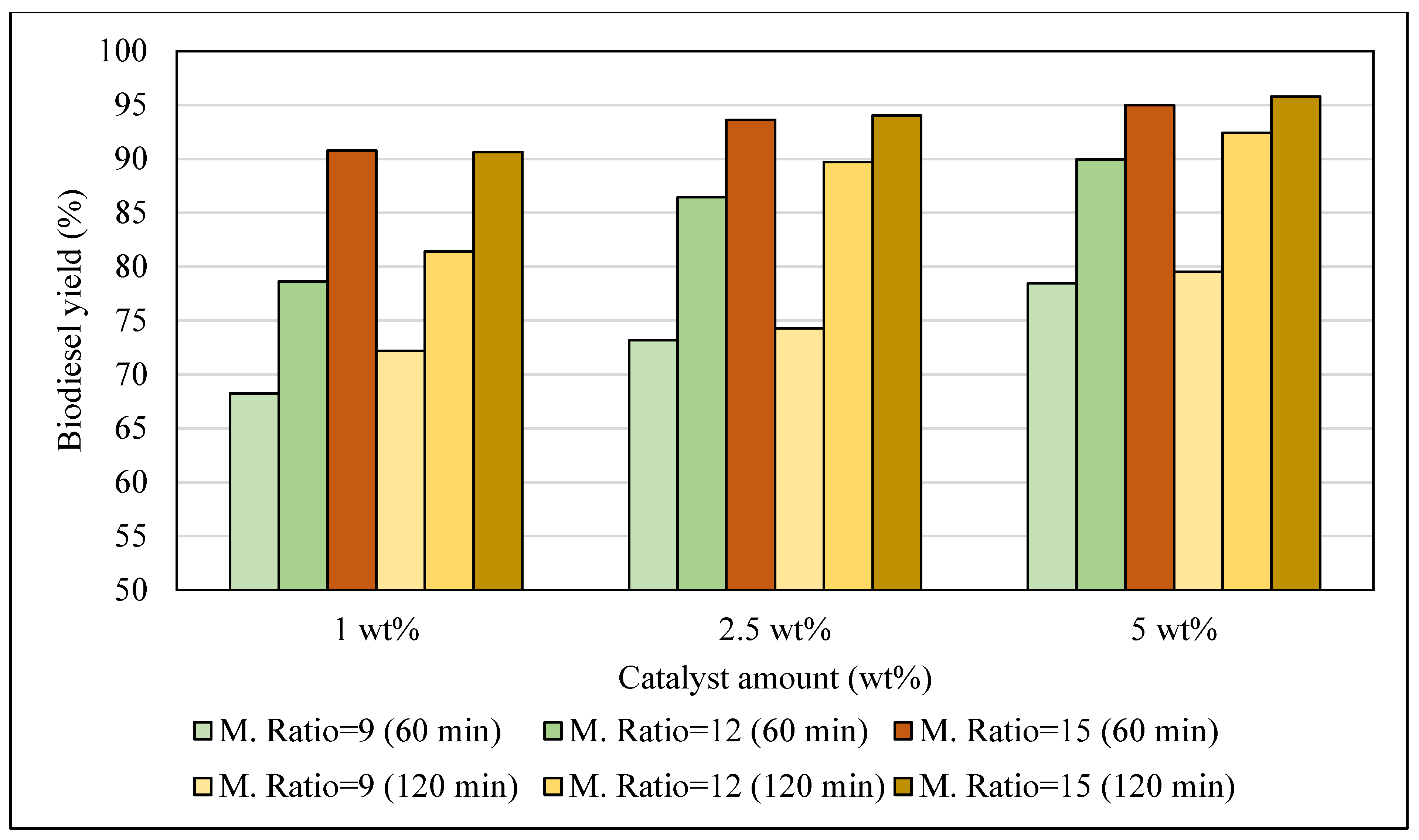

| Fatty Acid Composition | (wt.%) | |
|---|---|---|
| Name | Formula | |
| Oleic acid | C18H34O2 | 40.78 |
| Linoleic acid | C18H32O2 | 34.63 |
| Myristic acid | C14H28O2 | 12.5 |
| Palmitic acid | C16H32O2 | 3.98 |
| Stearic acid | C18H38O2 | 3.33 |
| Eicosenoic acid | C24H48O2 | 1.98 |
| 11-Eicosenoic acid | C20H38O2 | 1.32 |
| Behenic acid | C22H44O2 | 0.73 |
| Tetracosanoic acid | C24H48O2 | 0.45 |
| Others | 0.3 | |
| Catalyst Type | Methanol/Oil Ratio (Molar) | Catalyst Amount (wt.%) | Time (min) |
|---|---|---|---|
| KOH and A15 | 9 | 1 | 60 |
| 120 | |||
| 2.5 | 60 | ||
| 120 | |||
| 5 | 60 | ||
| 120 | |||
| 12 | 1 | 60 | |
| 120 | |||
| 2.5 | 60 | ||
| 120 | |||
| 5 | 60 | ||
| 120 | |||
| 15 | 1 | 60 | |
| 120 | |||
| 2.5 | 60 | ||
| 120 | |||
| 5 | 60 | ||
| 120 |
| Fatty Acid Composition | (wt.%) | ||
|---|---|---|---|
| Name | Formula | KOH | A15 |
| Oleic acid | C18H34O2 | 40.78 | 39.72 |
| Linoleic acid | C18H32O2 | 34.63 | 36.69 |
| Myristic acid | C14H28O2 | 12.5 | 10.45 |
| Palmitic acid | C16H32O2 | 3.98 | 4.02 |
| Stearic acid | C18H38O2 | 3.33 | 4.25 |
| Eicosenoic acid | C24H48O2 | 1.98 | 1.97 |
| 11- Eicosenoic acid | C20H38O2 | 1.32 | 1.45 |
| Behenic acid | C22H44O2 | 0.73 | 0.61 |
| Tetracosanoic acid | C24H48O2 | 0.45 | 0.65 |
| Others | 0.3 | 0.19 | |
| Property | Limits [4,12,14,29,43,44] | WCO Biodiesel (KOH) | WCO Biodiesel (A15) |
|---|---|---|---|
| Density (kg/m3) | 860–900 | 879 | 901 |
| Kinematic viscosity (40 °C) (mm2/s) | 3.5–5 | 4.79 | 4.98 |
| Saponification value (mg KOH/g oil) | --- | 203 | 190 |
| Acid value (mg KOH/g oil) | <0.5 | 0.2 | 0.23 |
| Iodine value (Wijs g/100 g oil) | <120 | 91.73 | 132 |
| Peroxide value (meq g/kg) | 2.1–4.1 | 2.8 | 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulukardesler, A.H. Biodiesel Production from Waste Cooking Oil Using Different Types of Catalysts. Processes 2023, 11, 2035. https://doi.org/10.3390/pr11072035
Ulukardesler AH. Biodiesel Production from Waste Cooking Oil Using Different Types of Catalysts. Processes. 2023; 11(7):2035. https://doi.org/10.3390/pr11072035
Chicago/Turabian StyleUlukardesler, Ayse Hilal. 2023. "Biodiesel Production from Waste Cooking Oil Using Different Types of Catalysts" Processes 11, no. 7: 2035. https://doi.org/10.3390/pr11072035
APA StyleUlukardesler, A. H. (2023). Biodiesel Production from Waste Cooking Oil Using Different Types of Catalysts. Processes, 11(7), 2035. https://doi.org/10.3390/pr11072035






