Abstract
Different extraction techniques were used to exploit fruit processing residues for their use as a source of phenolic compounds. Three different extraction methods, namely microwave-assisted extraction (MAE), subcritical water extraction (SWE), and maceration (M), were assessed to gauge their respective efficacies. Total phenolic content (TPC) and total flavonoid content (TFC), ferric reducing antioxidant power (FRAP), and radical scavenging activity ABTS assay were evaluated. High-performance liquid chromatography was used to assess the polyphenolic profile. MAE was the extraction technique that allowed the highest recovery of polyphenolic compounds. Concerning the fruit by-products analyzed, the extract of pomegranate peels obtained using M60C and MAE had the highest TPC (313 ± 24 mg GAE/g dry weight (dw)) and TFC (36.0 ± 2.8 mg EE/g dw), respectively, and the highest antioxidant activity (FRAP = 740 ± 67 mg AAE/g dw and ABTS (628 ± 27 mg TE/g dw) corresponded to M60C. The phenolic composition obtained for this sample using high-performance liquid chromatographic–diode array detection (HPLC–DAD) showed that gallic acid, protocatechuic acid, β-resorcylic acid, (+)-cathechin, and rutin were the main phenolics found. The findings underscore the capacity of agricultural by-products to act as a source of phenolic compounds. This offers a feasible solution to enhance the nutritional content in food while simultaneously minimizing environmental waste.
1. Introduction
A third of all food produced globally, which is enough to feed 2 billion people (about 25% of the world’s population), either goes to waste or is lost before it can be consumed. The 12th goal of the United Nations Sustainable Development till 2030 is to “halve per capita global food waste at the retail and consumer levels and reduce food losses along production and supply chains, including post-harvest losses.” [1,2]. With the aim of creating a more food-sustainable world, waste valorization and its sustainable management have become significant in recent years. The production and processing of fruit results in significant volumes of by-products, potentially providing a rich source of antioxidant polyphenols [1,3,4]. Currently, fruit by-products are typically discarded, often incurring costs for manufacturers. Thus, leveraging these by-products as a source of polyphenols could offer substantial economic advantages for food processors [1,4]. Moreover, the antioxidant activity of polyphenols found in fruit by-products is paramount to highlighting their potential health benefits in human nutrition. Recent epidemiological research has suggested that a regular consumption of fruits is associated with the prevention of multiple chronic degenerative illnesses, including cancer, atherosclerosis, cardiovascular diseases, central nervous system disorders, and aging [5,6]. Consequently, the identification of the most effective method for extracting and separating the complete bioactive compound characterization obtained from natural by-product matrices is a major challenge for food, pharmaceutical, and cosmetic researchers [4,7].
Food waste, such as peels, roots, and seeds, which are sometimes removed before reaching consumers, represents a significantly underutilized waste material capable of yielding diverse valuable chemicals. Phytochemicals, minerals, vitamins, and other bioactive substances can influence disease prevention and bolster the immune system, regulate gastrointestinal transit, and other substances can be obtained from these residues. Recent studies have focused on discovering eco-friendly solutions to combat food waste, primarily aiming to reintegrate these materials into the food cycle, fostering a circular economy and enhancing sustainability [3]. However, the physical and chemical properties of target biocompounds are highly variable. Thus, using the most efficient extraction methods is essential, considering that yields and the extract composition depend on the extraction conditions, such as extracting solvent, the solid:liquid ratio, pressure, extraction time, and temperature [7].
Most published papers concerning fruit by-products compare different extraction techniques to determine which is best for recovering phenolic compounds [1,7,8,9].
In recent decades, energy efficient and eco-friendly extraction methods such as subcritical water extraction (SWE), ultrasound-assisted extraction (UAE), and microwave-assisted extraction (MAE) have been introduced. These techniques offer cost-effective solutions to produce high-quality extracts [1,3,4,7]. Despite their high solvent, time, and energy demands, traditional extraction techniques such as Soxhlet and classical extraction (maceration) are widely utilized [3,7,10]. Moreover, using toxic solvents such as methanol and chloroform to extract bioactive compounds limits their incorporation into food and pharmaceutical products, a constraint not encountered with water and ethanol extracts [7]. Ideally, the optimal extraction method should be straightforward, safe, consistently replicable, cost-effective, and readily applicable for industrial purposes [11]. This work aspires to lay the groundwork for advancing and endorsing sustainable food waste valorization practices for various by-products by applying green chemical technologies while comparing them with conventional methods.
Regarding conventional extraction, Contini et al. [10] found that using aqueous acetone as a solvent yielded the highest extraction (around 30%) of antioxidant phenolic compounds from hazelnut by-products. Whole roasted hazelnut skin showed the most significant amount of phenolics, up to 502 mg/g in gallic acid equivalents (GAE). Fernández-Agulló et al. [12] found that water yielded the highest extraction from the walnut green husk (44.11%) and samples extracted with water/ethanol (1:1) showed high bioactive potential (84.46 mg GAE/g extract; median effective concentration (EC50) = 0.95 mg/mL for reducing power and EC50 = 0.33 mg/mL for 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) assay). Yuan et al. [13] reported that optimal conditions for the phenolics extraction of hazelnut shells involved a shell particle size less than 0.5 mm, 50 °C, a 10 g/L liquid to solid ratio, 58% acetone, and a 12 h extraction time.
Using green extraction technologies, He et al. [14] employed SWE to extract phenolic compounds from pomegranate seed residues, resulting in a TPC range of 651.7 to 4854.7 (mg GAE/100 g dry weight (dw)). Kim et al. [15] combined SWE with intense pulsed light to optimize the extraction of quercetin from onion skin waste. Using SWE, Jokić et al. [16] separated valuable compounds from cocoa shell waste and identified active compounds, sugars, and sugar degradation products. Finally, Gonçalves Rodrigues et al. [17] reported the highest total yield, phenolic content (TPC), Maillard reaction products (MRPs), and antioxidant activity when using SWE at 150 °C for 5 min to extract phenolic compounds from papaya seeds.
In the study by Moreira et al. [18], the antioxidant activity and phenolic profile of apple tree (Malus domestica) bark, core, and roots were characterized. Phenolic compounds were extracted using conventional extraction (CE) and MAE methods. MAE was the most efficient extraction method, with samples presenting the highest phenolic content and antioxidant activity. After optimizing MAE conditions, Mellinas et al. [19] used MAE on cocoa bean shell (CBS) waste. The study assessed the influence of various factors such as pH, duration, temperature, and solid-to-liquid ratio on both the yield and composition of the resulting extracts. These authors concluded that MAE efficiently extracts bioactive compounds from CBS and has potential applications in the food industry.
The efficiency of these extraction techniques can be evaluated by utilizing different methods to determine the antioxidant capacity of biological substances [20,21]. Commonly employed techniques include those that involve radical chromogen compounds which trigger reductive oxygen species. These techniques are popular due to their simplicity, speed, and sensitivity. The presence of antioxidants results in the elimination of radical chromogens. Specifically, the ABTS and DPPH radical scavenging methods are the most extensively utilized [20].
Fruit by-products rich in polyphenols could be transformed into ingredients that serve as beneficial components in nutritional food formulations [9,22]. Besides their possible health advantages, natural extracts with high antioxidant activity can enhance food products by preserving their color and taste and extending their shelf life [23].
The main goal of this study was to investigate and compare the potential of green extraction techniques, namely MAE and SWE, against the conventional extraction’s maceration with agitation at different temperatures (at 20 °C (M20C) and 60 °C (M60C)). The extraction efficiency was evaluated using different optical assays, namely the total phenolic content (TPC) and the total flavonoid content (TFC) in extracts prepared from under-utilized fruits: cherry (Prunus avium L.) seeds and stems, mulberry (Morus nigra L.) leaves, and pomegranate (Punica granatum L.) peels. Furthermore, the antioxidant activities of all samples were measured using the ferric reducing antioxidant power (FRAP) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid assay (ABTS•+) assays. In addition, the phenolic profile for all extracts was evaluated using high-performance liquid chromatography with diode array detection (DAD). According to our knowledge, this is the first study to investigate the influence of these extraction techniques in the recovery of polyphenols from these fruit by-products, except for pomegranate peels [24].
2. Materials and Methods
2.1. Chemicals and Reagents
The phenolic compound standards (gallic acid (≥99%), protocatechuic acid (99.63%), (+)-catechin (≥98%), (−)-epicatechin (≥97%), vanillic acid (≥97%), 4-hydroxybenzoic acid (≥99%), 4-hydroxybenzaldehyde (98%), chlorogenic acid (>95%), 4-hydroxyphenylacetic acid (98%), caffeic acid (≥98%), syringic acid (≥98%), p-coumaric acid (≥98%), ferulic acid (≥99%), sinapic acid (≥99%), rutin hydrate (≥94%), quercetin (95%), kaempferol (≥98%), naringin (≥95%), naringenin (98%), cinnamic acid (≥99%), myricetin (≥96%), pinocembrin (95%), quercetin-3-O-glucopyranoside (≥99%), kaempferol-3-O-rutinoside (≥98%), kaempferol-3-O-glucoside (≥95%), phloridzin (99%) and phloretin (≥98.5%)), ABTS (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), ascorbic acid, and Folin–Ciocalteu reagents were obtained from Sigma–Aldrich (Madrid, Spain). All chromatographic solvents were high-performance liquid chromatographic (HPLC) grade from Merck (Darmstadt, Germany). Aluminum chloride (p.a.) was also acquired from Merck. All aqueous solutions were prepared using ultrapure water with 18.2 MΩ/cm resistivities from a Milli-Q Plus water system (Millipore, Burlington, MA, USA, Simplicity 185).
Individual phenolic compounds were dissolved in methanol to create stock solutions with a concentration of 2 g/L. These solutions were then stored in a dark environment at a temperature of −20 °C. The working standard solutions were prepared in a methanol–water (50:50, v/v) mixture via the dilution of the respective stock solutions and stored under similar conditions.
2.2. Samples
Cherry (Prunus avium L.) seeds from white and black sweet cherries (white cherry seeds (WCSs) and black cherry seeds (BCSs)) and cherry stems (CS), mulberry (Morus nigra) leaves (MLs), and pomegranate (Punica granatum L.) peels (PP) were provided from a local market (Porto, Portugal). The fruits underwent washing and selection to ensure a uniform group, and the different parts were manually separated and dried at 50 °C for 48 h. After drying, the samples were powdered in a mill (ZM 200, Retsch, Haan, Germany).
The samples’ moisture was measured using a precision infrared balance (Kern DAB 100-3, Balingen, Germany). Each measurement was made in triplicate. Samples were frozen at −20 °C until further analysis.
2.3. Polyphenols Extraction
2.3.1. Maceration at Room Temperature (M20C)
Maceration was carried out by weighing 0.4 g of the sample and placing them into an Erlenmeyer flask containing 20.0 mL of 50% aqueous ethanol for 24 h in the dark at room temperature, as described previously [25]. To prevent solvent evaporation, the extraction solution was covered with a plastic paraffin film. After filtration of the supernatant through a cellulose filter (0.45 μm; Whatman, Clinton, NJ, USA), the samples were preserved at −20 °C until further analysis. The extraction was performed in triplicate.
2.3.2. Maceration at Higher Temperature (M60C)
Fruit by-products (0.4 g) were mixed with 50% aqueous ethanol (20.0 mL) in an Erlenmeyer flask and were placed in a heating plate (VELP Scientifica, Usmate, Italy) with stirring (200 rpm) for 30 min at 60 °C [25]. The separation and subsequent processing of the filtrates followed the same procedures outlined in the previous section. The extractions were performed in triplicate.
2.3.3. Microwave-Assisted Extraction (MAE)
MAE was conducted using a sealed system in a Microwave Accelerated Reaction System, MARS-X, 1500 W (CEM, Mathews, NC, USA). The extraction was made with 0.40 g of the sample mixed with 20.0 mL of 50% aqueous ethanol in a Teflon vessel for 28 min at 120 °C. The fixed operational parameters for MAE were as follows: magnetron power set to 100%, medium speed stirring, and a maximum vessel pressure cut off at 150 psi [25]. After cooling to room temperature, extracts were centrifuged at 4000 rpm for 10 min, filtered (cellulose filter 0.45 μm), and stored at −20 °C, unless otherwise stated. The extractions were performed in triplicate.
2.3.4. Subcritical Water Extraction (SWE)
In accordance with previously described procedures and apparatus, SWE was performed in a homemade extractor with an internal volume of 1.7 L [26]. To prevent potential oxidation, the extraction vessel was pressurized with nitrogen. The selected samples’ extraction conditions have been described in previous studies [26,27,28,29,30] and the optimal conditions are presented in Table 1. Agitation frequency and extraction time were set at 3 Hz and 30 min, respectively, except for MLs, which was 40 min. Subsequently, the extraction vessels were cooled and depressurized, and the resulting extracts were filtered (cellulose filter 0.45 μm) and stored at −20 °C until analysis. The extraction procedure was performed in duplicate for each specimen.

Table 1.
Subcritical conditions for the extraction of polyphenolic compounds from the selected samples. The solvent used was water, and the extraction time was 30 min, except for pomegranate peels, which was 40 min.
2.4. Total Phenolic (TPC) and Total Flavonoid Content (TFC)
For the rapid quantification of the total phenolic content (TPC) and total flavonoid content (TFC), widely accepted spectrophotometric methods utilizing the Folin–Ciocalteu reagent and aluminum chloride were employed, respectively [31]. Measurements were conducted using a Synergy HT W/TRF multimode microplate reader (BioTek Instruments, Winooski, VT, USA) and analyzed using Gen5 2.0 software (BioTek Instruments). The assays were performed in triplicate, and the results for the total phenolic content (TPC) and total flavonoid content (TFC) were expressed as mg of gallic acid equivalents (GAE) per g of the dw of the extract (mg GAE/g dw) and mg of epicatechin equivalents (EE) per g of the dw of the extract (mg EE/g dw), respectively.
2.5. Radical Scavenging Activity
The antiradical activity of the extracts was evaluated using the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) assay (ABTS•+) according to established procedures [32]. The absorbance at 734 nm was recorded using a microplate reader (BioTek Instruments), with Trolox serving as the standard. The results were expressed as mg Trolox equivalents (TE) per g of the dw of the extract (mg TE/g dw). Triplicate measurements were performed for all extracts.
2.6. Ferric Reducing Antioxidant Power (FRAP)
The in vitro antioxidant activity of the fruit by-product extracts was determined using ferric reduction activity power (FRAP). For the FRAP assay, the absorbance was measured at 593 nm and 37 °C in a microplate reader (BioTek Instruments), and the calibration curve was prepared with ascorbic acid (AA) [31]. The results were expressed as mg AA equivalents per g of the dw of the extract (mg AAE/g dw). Triplicate measurements were made for all extracts.
2.7. HPLC–DAD Analysis
The HPLC analyses of the fruit by-product extracts were performed on a Shimadzu LC-20A HPLC system consisting of an LC-20AD prominence pump, a DGU-20AS prominence degasser, a CTO-10AS VP column oven, an SIL-20A HT prominence autosampler, and an SPD-M20A diode array detector (DAD), controlled by Lab Solutions software, under previously described conditions and procedures (Rubilar et al., 2007). A Phenomenex Gemini C18 column (250 mm × 4.6 mm, 5 μm) and a guard column with the same characteristics was used. The flow rate was 1.0 mL/min, and the eluates were monitored at 280, 320, and 360 nm at 25 °C. The mobile phase comprised solvent A (methanol) and solvent B (water), with 0.1% formic acid. The elution gradient began with 85% solvent B and 15% solvent A; then, a gradient program as follows was used: from 85% to 70% B in 20 min, from 70% to 55% B in 20 min, from 55% to 50% B in 5 min, from 50% to 45% B in 5 min, from 45% to 30% B in 15 min, from 30% to 0% B in 10 min, followed by 100% A for 5 min and back to 85% B in 10 min and 10 min of reconditioning before the next injection. The injection amount was 20 µL, and, before injection into the chromatography system, an aliquot of 1.0 mL of extract was filtered through a 0.20 μm polytetrafluoroethylene (PTFE) syringe filter (Teknokroma, Barcelona, Spain). The identification of phenolic compounds in by-product extracts was performed by comparing their retention times and UV-Vis spectra with those of standard compounds. Peak purity was assessed to exclude any contribution from interfering peaks. The concentrations of the phenolic compounds in the extracts were determined using external standard calibration curves in the concentration range of 1 to 50 mg/L using a mixture of 18 standards. The analytical parameters of the calibration curves were calculated with the Excel program. The calibration equation, coefficient of correlation (R2), the limit of detection (LOD, mg/L), and limit of quantification (LOQ, mg/L) are shown in Table 2. Results were expressed as mg/100 g of dw.

Table 2.
Calibration equation (m (±Δm) + b (±Δb), n = 3), coefficient of correlation (R2), limit of detection (LOD, mg/L), and limit of quantification (LOQ, mg/L) for the antioxidants analyzed using HPLC–DAD.
2.8. Statistical Analysis
The data collected were analyzed using inferential analysis, including comparisons among groups and correlation analysis. A two-way analysis of variance (ANOVA) was carried out on TPC, TFC, FRAP, and ABTS using the agricultural by-product and method (SWE, MAE, M20C, and M60C). Group comparisons were performed using Tukey’s honestly significant difference (HSD) post hoc tests. A Pearson’s correlation analysis was performed to check for a linear correlation and its direction between the values. A two-tailed p-value < 0.05 was considered to indicate statistical significance. Principal component analysis (PCA) was set using the HPLC analysis variables. Components with eigenvalues higher than 1 were retained for further analysis. Statistical analyses were conducted using R/RStudio software version 4.2.0. The greenness of the extraction techniques was assessed using AGREEprep, an analytical metric tool for sample preparation [33,34].
3. Results and Discussion
The extraction techniques utilized for extracting bioactive compounds from agricultural food wastes exhibit variations in their operational parameters and can target distinct compounds and bioactivities [7]. Therefore, the antioxidant content and power of the obtained extracts were measured using several spectrophotometric methods, and HPLC was used to assess the antioxidant profile. For each method (TPC, TFC, FRAP, ABTS), a two-way ANOVA was carried out considering the agricultural food waste (CSs, WCSs, BCSs, MLs, PPs) and extraction method (SWE, MAE, M20C, M60C). A statistically significant interaction between the effects of the waste type and quantification method on the quantity extracted for each quantification method was observed: TPC: F(12, 401) = 344.1, p < 0.001; TFC: F(12, 290) = 371.7; p < 0.001; FRAP F(12, 275) = 545.96; p < 0.001; ABTS: F(12, 244) = 568.3; p < 0.001]. In addition, Tukey’s HSD post hoc tests were carried out to assess differences between groups, and the results are presented in Table 3. Statistical analysis results suggested significant differences in the recovery of most compounds in extracts obtained using different extraction techniques and between agricultural by-products.

Table 3.
Total phenolic content (TPC) and total flavonoid content (TFC), ferric reduction antioxidant power (FRAP), and radical scavenging activity (ABTS) in agricultural by-products extracts obtained through various extraction techniques.
The extraction techniques present different characteristics, but all allow for the extraction of polyphenols, as seen in Table 3. In general, MAE was the technique that showed a higher polyphenolic content and room-temperature maceration (M20C); however, it was the lowest for CSs, WCSs, BCSs, and MLs. However, the PP sample differed from the other samples, with M60C presenting the highest TPC value (313 ± 24 mg GAE/g dw), followed by MAE (310 ± 28 mg GAE/g dw) and finally SWE (92.5 ± 8.1 mg GAE/g dw). A similar trend was observed for TFC values, with PPs differing from the other samples. Again, the PP sample presented the highest TPC and TFC values, and the WCS and BCS samples were the lowest.
Afonso et al. [35] determined the bioactive potential and the phenolic profile of the stems and seeds of different sweet cherries varieties after extraction with 70% methanol at 70 °C for 30 min. These authors reported TPC values for cherry stems between 23.59 ± 0.14 and 32.49 ± 5.23 mg GAE/g and TFC values from 13.06 ± 1.97 to 24.75 ± 1.14 mg catechin equivalents (CE)/g. Babotă et al. [36] extracted polyphenols from cherry stems and reported TPC values of 37.63 ± 2.75 mg GAE/g and TFC values of 12.03 ± 0.72 mg quercetin equivalents (QE)/g for extracts obtained through maceration and 19.11 ± 1.52 mg GAE/g for TPC and 5.34 ± 0.23 mg QE/g for TFC values when using decoction to prepare the extracts. Regarding cherry seeds, the values found varied between 1.17 ± 0.13 and 2.76 ± 0.14 mg GAE/g for TPC and 0.23 ± 0.03 to 2.59 ± 0.44 mg CE/g for TFC. In another study, antioxidants from cherry seeds were extracted using maceration, and the best conditions at 35 °C were water:ethanol 50:50 (1.54 ± 0.14 mg GAE/g) and 60:40 (1.41 ± 0.01 mg GAE/g) [37]. These results agree with those reported in our study.
PPs were extracted using sonication and maceration with varying solvents and concentrations in another study [9]. TPC and TFC varied depending on the method and conditions used. TPC values varied between 32.05 mg GAE/g (maceration, acetone 50%) and 72.21 mg GAE/g (sonication, methanol 50%), while TFC varied between 17.43 mg QE/g (maceration, acetone 50%) and 39.21 mg QE/g (sonication, methanol 50%) [9]. Vladić et al. [24] used both SWE and MAE, with MAE resulting in a higher extraction of phenolic compounds from PPs (TPC: 4.69 ± 0.32–14.16 ± 0.51 g GAE/100 g and TFC: 1.23 ± 0.05–2.13 ± 0.08 g CE/100 g for SWE; TPC: 13.34 ± 0.2–20.6 ± 0.65 g GAE/100 g and TFC: 2.89 ± 0.05–3.34 ± 0.04 g CE/100 g for MAE). MAE was able to extract more phenolic compounds compared to SWE. Our PP results are higher when compared with those reported in the literature; however, a similar trend was observed when comparing SWE and MAE extraction. The extraction efficiency can be affected by temperature, solvent, and irradiation power [24]. Considering that the temperatures used in SWE are higher than MAE or maceration (20 and 60 °C), the possible thermal degradation of polyphenols can result in a lower extraction and justify the differences between the extraction techniques [24]. Despite the potential for thermal degradation with higher temperatures in SWE and MAE, these techniques proved faster, more eco-friendly, and yielded better extraction efficiency than traditional methods such as maceration [7]. The results of the study support the current research focusing on improving biomass extraction processes and yields.
Regarding MLs, TPC values were reported as 61.89 ± 5.67 mg GAE/g (SWE at 160 °C) [30], 11 mg GAE/g (maceration with methanol at room temperature) [38], 8.76±0.01 to 20.26 ± 0.07 mg GAE/g (ultrasonic bath, methanol 80%, room temperature during 45 min) [39], and 19.7 ± 2.0 mg GAE/g (MAE, ethanol 50%, 120 °C, 28 min) [25]. The TFC reported for MLs were 6.27 mg rutin equivalents (RE)/g (maceration, liquid–solid ratio of 20 mL/g, temperature of 80 °C and 80% ethanol) [40] and between 10.15 and 39.09 mg QE/g of extract (ultra-sonication with methanol for 20 min) [41]. Our results are similar to the ones reported.
An understanding of the antioxidant capacity of agricultural by-products is of great interest when assessing their potential application in several industries. Regarding the antioxidant activity measured using FRAP and ABTS, the results were more variable than TPC and TFC measurements. Nevertheless, in general, MAE and SWE had the highest activity, except for PP; moreover, the maceration presented higher FRAP and ABTS values than MAE and SWE. For CSs, the FRAP values were in the order of MAE > M60C > M20C > SWE; for WCSs and BCSs, MAE ≈ SWE > M20C > M60C; for MLs, SWE > MAE > M60C > M20C; for PPs, M60C > M20C > MAE > SWE. The highest FRAP value was 740 ± 67 mg AAE/g for PPs (M60C), and the lowest was 5.60 ± 0.49 mg AAE/g dw for WCSs (M60C). Considering the ABTS values, CSs can be ordered as MAE > M60C > M20C > SWE; for WCSs, MAE > M60C > SWE > M20C; for BCSs, MAE > M60C > M20C > SWE; for MLs, SWE > M60C > M20C > MAE; for PPs, M60C > MAE > M20C > SWE. The highest ABTS value was 628 ± 27 mg TE/g for PPs (M60C), and the lowest was 7.89 ± 0.45 mg TE/g for WCSs (M20C). Considering FRAP and ABTS, there are no statistical differences (p > 0.05) between extraction techniques and sample types for WCSs and BCSs.
Afonso et al. [35] determined the FRAP values of cherry stems and seeds after extraction using maceration (70% methanol, 70 °C, 30 min) and reported values between 12.66 ± 2.48 and 26.66 ± 2.48 µg TE/g and from 0.19 ± 0.01 to 0.59 ± 0.04 µg TE/g, respectively. In another study, SWE was used to extract polyphenols from CSs, and the authors reported ABTS values of 70.38 ± 3.89 mmol TE/ 100 g (176.2 mg TE/g) [42]. Dulyanska et al. [43] studied the effect of different extraction conditions, such as solvent and temperature. They reported maximum values at 70 °C with ABTS values of 2.16 mg TE/g for the water:ethanol 60:40 (v/v) and a FRAP value of 3.43 mg TE/g for the water:ethanol 60:40 (v/v). In another study, CS extracts were prepared using maceration and decoction, and the authors reported 107.14 ± 1.43 mg TE/g (ABTS) and 111.87 ± 4.14 mg TE/g (FRAP), as well as 55.65 ± 3.62 mg TE/g (ABTS) and 61.07 ± 2.83 mg TE/g (FRAP), respectively [36].
Radojković et al. [25] reported FRAP values of 15.3 ± 1.0 mg AAE/g (MAE, ethanol 50%, 28 min, 120 °C) and 12.4 ± 1.4 mg AAE/g (maceration, ethanol 50%, 24 h, room temperature) for MLs. For MLs, Yu et al. [39] reported FRAP values from 91.62 ± 6.17 to 149.15 ± 4.05 µmol AAE/g (16.14 to 26.27 mg AAE/g) and ABTS values between 51.28 ± 1.2 and 70.84 ± 0.94 µmol TE/g (12.84 to 17.73 mg TE/g) after extraction using an ultrasonic bath (80% methanol, 300 W, 45 min). Nastić et al. [30] used SWE to extract bioactive compounds from medicinal plants traditionally used in Serbia and documented a FRAP value of 55.0 ± 1.2 mg AAE/g for MLs.
Alexandre et al. [44] extracted bioactive compounds from PPs using high-pressure-assisted extraction, the optimal conditions (40% of ethanol at 0.1 MPa during 30 min) of which were a FRAP value of 436 ± 8.3 mg ammonium iron (II) sulfate (AIS)/g dw and an ABTS value of 269 ± 25.5 mg TE/g dw. In another study, conventional extraction was optimized to extract bioactive compounds from PP; moreover, at the optimal condition (50 °C for 10 min using methanol as the solvent), the maximum FRAP value was 785.67 ± 4.92 mg AAE/100 g dw [45].
The differences observed between our results and the ones reported can be due to the differences in the extraction conditions and, in some cases, in the use of other standards while performing antioxidant activity studies [44]. High temperature mainly affects the extraction of compounds, causing them to decrease the solvent’s viscosity and increase its penetration into the matrix, as well as improve the extraction process of both polar and less-polar compounds, resulting in higher TEAC values [46]. Besides the temperature, the solvent type can affect the total antioxidant activity of polyphenols due to possible polymerization, depolymerization, and H-bonding of antioxidants between them and with solvent molecules such as water and methanol [47]. Finally, the antioxidant activity of phenolic compounds is also dependent on the reaction mechanism, solubility parameters, and electron transfer capability [47,48,49,50,51]. Figure 1 provides a brief overview of the general mechanisms of these antioxidants’ actions.
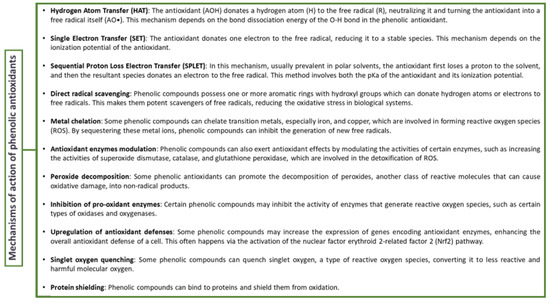
Figure 1.
Mechanism of action of antioxidant activity of polyphenolic compounds.
The solubility and electron transfer capability of phenolic compounds are key determinants of their antioxidant efficiency. Solubility influences their distribution in varied environments, while electron transfer capability indicates their readiness to donate electrons to neutralize free radicals. Their molecular structure, notably the number and position of hydroxyl groups and other functional groups, also impacts their antioxidant activity. Thus, these traits enable phenolic compounds to effectively neutralize free radicals, explaining their widespread use as antioxidants in food preservation, cosmetics, and pharmaceuticals [48,49,50].
This study demonstrates that, although the agricultural by-products have evidence of antioxidant activity in vitro, the antioxidant potencies are different. Some by-products with lower potency would need to be used in much larger quantities to match the antioxidant potency of PPs. An overall antioxidant potency composite index (APCI) was determined according to Seeram et al. [52] by assigning equal weight to all assays. An index value of 100 was attributed to the best score for each test (FRAP and ABTS), and the corresponding index score was then calculated for each sample. Antioxidant (FRAP or ABTS) index score = (sample score/best score) × 100 [52]. The average index scores for all tests for a specific extract were defined as its APCI [52]. Table 4 presents the APCI determined for the agricultural by-products based on the ranking of the antioxidant assays ABTS and FRAP.

Table 4.
The antioxidant index score for FRAP and ABTS and the antioxidant potency composite index (APCI) of the agricultural by-products.
There are various antioxidant capacity assessments, each with its own advantages, disadvantages, and resulting outcomes [53,54,55]. In our study (Table 4), PPs showed the highest APCI of 81.6%—significantly higher than other by-products studied, all of which presented APCI values of less than 10%. Among cherry by-products, cherry seeds exhibited the highest APCI (6.18%). Seeram et al. [52] used multiple antioxidant capacity tests to evaluate a range of beverages, determining that pomegranate juice demonstrated the greatest APCI—over 20% higher than any other beverage tested. However, they emphasized that in vitro antioxidant potency does not guarantee in vivo biological activity [52]. Compared to Seeram et al.’s findings [52], our results align in showcasing pomegranate (in our case, its peel) as having the highest APCI, underscoring its potent antioxidant potential.
Research indicates that agricultural by-products with strong antioxidant activity may have beneficial health impacts [6,22]. For instance, cherry fruits and by-products, such as stems and seeds, are known for their health benefits [56]. Cherry seed (CS) preparations are used in traditional medicine for urinary tract disorders [36,57]. Studies show that CS extracts have diuretic effects linked to their phenolic secondary metabolites. However, they should be used cautiously in patients with calcium, sodium, or chloride deficiency [36,57]. Morus nigra leaves (MLs) have been found to possess anti-diabetic, antinociceptive, anticancer, and hepatoprotective properties [58]. Their phenolic compounds, including anthocyanins, flavonols, and phenolic acids, contribute to their liver-protecting activity [59]. Pomegranate components, rich in bioactive compounds, have traditionally been used to treat gastrointestinal, cardiovascular, and endocrine diseases [60]. Clinical trials confirm the efficacy of pomegranate in managing diabetes, cardiovascular disease, oral disorders, endocrine issues, and cancer [60].
A polyphenolic profile of the extracts was obtained using HPLC–DAD analysis. The presence of phenolic acids and flavonoids in foods and plants is highly important due to their antioxidative, anti-inflammatory, anti-mutagenic, and anti-carcinogenic activities, as well as their positive impact on human health [61,62]. The phenolic compound content for each agricultural by-product extract obtained using the different extraction techniques applied is presented in Table 5.

Table 5.
Content of the detected phenolic compounds in agricultural by-products extracts obtained using different extraction techniques. Results are expressed as mg/100 g dry sample.
The agricultural by-product that presented the highest total amount of polyphenols according to the HPLC analysis was PPs, with the following order: M20C (7195 mg/100 g) > SWE (6290 mg/100 g) > MAE (5463 mg/100 g) > M60C (4675 mg/100 g). These results are different and much lower than the ones obtained through TPC analysis (M20C > M60C ≈ MAE >> SWE). MLs presented the lowest amount of total phenolic compounds: M60C (445 mg/100 g) > M20C (370 mg/100 g) > MAE (350 mg/100 g) > SWE (262 mg/100 g), while TPC values were in the following order: MAE > SWE > M60C > M20C. Spectrophotometric methods, such as TPC and TFC, can be affected by interferences from non-phenolic compounds, such as sugars and proteins, overestimating the overall content. The results obtained from the chromatographic method could be considered interference-free, explaining the lower values and different values obtained through HPLC [63].
The phenolic profile is significantly different for all samples considering the extraction technique (p < 0.05). Gallic acid was the most abundant antioxidant quantified in almost all samples except for M20C and M60C in the PP extracts, where protocatechuic acid was present in higher amounts. In the case of MLs, chlorogenic acid was the major phenolic compound when using the same extraction methods. Rutin was only present in MLs and PPs, while kaempferol was only present in CSs and in the MAE extract of PPs.
Regarding CSs, the highest total amount of polyphenolic compounds was in the following order: SWE (1390 mg/100 g) > MAE (1090 mg/100 g) > M60C = M20C (184 mg/100 g). Gallic acid was the main phenolic, followed by protocatechuic acid and catechin in all extracts. Chlorogenic acid was not present in the extract obtained from M20C. Cinnamic acid was only present in the SWE extract, while syringic acid was not detected in this extract. Ferulic and sinapic acids were only present in the MAE extract, while naringin was not found in this extract. Aires et al. [64] evaluated the phytochemical composition of cherry stem extracts using HPLC–DAD–UV/VIS obtained through conventional extraction (CE) and ultrasonic extraction. Their analysis identified neochlorogenic and chlorogenic acids (14.5% of total polyphenol (TP) content identified), p-coumaric and p-coumaroylquinic acids (13.6% TP), ferulic acid (11.6% TP), and (+) catechin (+) epicatechin (12.5% TP), with UAE extracts presenting a higher polyphenol amount than CE extracts [64].
WCSs and BCSs presented similar polyphenolic profiles, with the highest total amount for SWE, followed by MAE. Gallic acid was the antioxidant with the highest amount (809 ± 39 (SWE) to 151 ± 10 mg/100 g dw (M60C) for WCSs and 945 ± 39 (SWE) to 131 ± 10 mg/100 g dw (M20C) for BCSs). Other phenolics identified in these extracts were catechin, vanillic acid, caffeic acid, ferulic acid, and sinapic acid. Epicatechin and naringin were only detected in SWE, while p-coumaric acid was detected in MAE, M20C, and M60C. Afonso et al. [35] quantified phenolic compounds in cherry kernel extracts (maceration, 70% methanol, 70 °C during 30 min) ranging from 16.58 mg/100 g to 75.58 mg/100 g in different varieties. These authors identified the major phenolic compound as catechin in most samples, while protocatechuic acid had the highest amount in another sample. Gallic acid and p-coumaric acid + isomers were also detected in lower amounts [35].
Our study found that all extracts contained gallic acid, catechin, chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, and rutin. Other compounds such as protocatechuic acid, epicatechin, syringic acid, β-resorcylic acid, quercetin, and cinnamic acid were found in specific extracts (SWE and MAE). Radojković et al. [25] reported that rutin and chlorogenic acid are the main contributors to the phenolic content of MLs. Besides the differences between extract profiles, our work found significant differences in the quantity of individual compounds. In another study, ML extracts prepared with SWE, gallic, protocatechuic, caffeic and β-resorcylic acids, and the flavonoids naringin and rutin were identified and quantified in considerable amounts [30]. In our work, chlorogenic acid and rutin were the main contributors to the total phenolic content in the extracts obtained through maceration: 141 ± 9 (M20C) and 181 ± 13 (M60C) and 128 ± 3 (M20C) and 157 ± 3 (M60C) mg/100 g, respectively. Besides these two phenolic compounds, gallic acid and protocatechuic acid were the major contributors to the total phenolic content for the SWE and MAE extracts. In another study where MLs were extracted using supercritical CO2 coupled with maceration, it was reported that caffeic acid, quercetin derivatives, and rutin were the major compounds, and gallic acid, protocatechuic acid, catechin and epicatechin were also identified and quantified [65].
Regarding PPs, gallic acid, protocatechuic acid, catechin, and rutin were the major phenolic contributors, aligning with previously reported literature. However, our study noted the presence of quercetin and kaempferol in certain extracts. Quercetin was present in all extracts except for SWE, while kaempferol was only found in the MAE extract. PP total phenolic content was reported to range between 18 and 510 mg/g dry matter [66], values similar to the ones reported in the present study. The phenolic profile includes gallic acid, catechin, and ellagic acid with a content of 12.58–25.90, 8.68–12.65, and 0.44–3.04 mg/g dw, respectively [66]. These results, in combination with the reported phenolic content of PPs, suggest their superiority over other agro-industrial by-products such as rice hulls, olive leaves, and grape pomace in terms of total phenolic content [66].
The efficacy of the extraction technique significantly influenced the total phenolic content. For example, SWE demonstrated superior recoveries for gallic acid across all samples (Table 5). Each agricultural by-product responded differently to the extraction techniques, influencing the phenolic acid and flavonoid content. The CS extracts derived from SWE showed the highest content of phenolic acids, whereas the MAE extracts exhibited the highest concentration of flavonoids. The SWE extracts of WCSs and BCSs were the richest in phenolic acids and flavonoids. In contrast, MLs and PPs showed higher levels of phenolic acids and flavonoids in the maceration extracts, M60C and M20C, respectively.
Several extraction techniques were employed to extract bioactive compounds from different plant materials. High pressure and temperature extraction (HPTE) yielded the highest total phenolic content (TPC) and total flavonoid content (TFC) in grape seeds and skins [67]. In contrast, microwave-assisted extraction (MAE) demonstrated the highest antiradical power. Yet, the MAE method was less suitable for extracting certain polyphenolic compounds than pressurized liquid extraction (PLE). However, MAE efficiently obtained high amounts of kaempferol, quercetin, and their derivatives [67].
In extracting polyphenols from Teucrium montanum, subcritical water extraction (SWE) showed the highest TPC, ABTS, and DPPH values [68]. Nonetheless, it was less effective in extracting targeted polyphenols due to potential thermodegradation than conventional extraction and MAE. Both SWE and MAE were effective in extracting phenolic compounds from Chaya leaves [69]. Still, MAE outperformed in terms of antioxidant activity and was also faster and used less solvent than the SWE extraction. Comparisons to other studies suggest that extraction methods and conditions, such as the sample pretreatment, cultivar, and cultivation conditions, potentially explain the quantitative differences observed between our results and the previously reported literature [30,66]. Overall, our results largely align with previously reported studies but offer additional insight into the diversity of phenolic compounds in these extracts.
Principal component analysis (PCA) was applied to the logarithm of the amount extracted (very skewed distributions) from each of the 18 polyphenols (analyzed using HPLC) (18 variables) and 20 cases (five agricultural by-products × four techniques). The data were centered and “scaled” (the average was subtracted and divided by the standard deviation) to determine if the quantity of antioxidants varied with the analyzed agricultural by-products and if the number of antioxidants varied with the extraction technique. The scree plot of the components obtained from the PCA analysis of the HPLC values is presented in Figure 2.
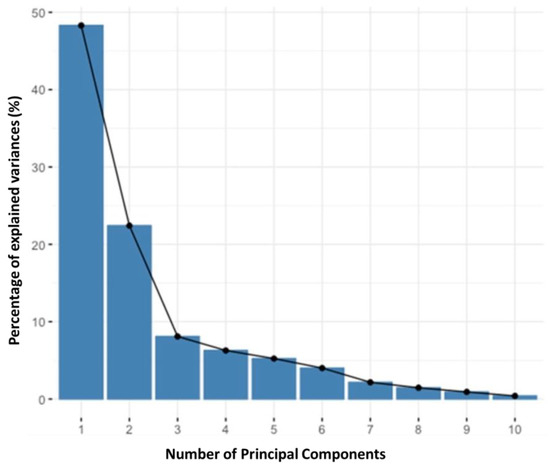
Figure 2.
Scree plot of the components obtained from the PCA analysis of the HPLC values.
In Figure 2, the x-axis shows the principal components (dimensions), of which there are 10 in this case. The y-axis shows the percentage of the explained variance per principal component. The elbow appears to occur at the third principal component. The scree plot analysis illustrates that the first two principal components already represent a very reasonable percentage of the total variability (71%); therefore, only these two components were considered in the following analyses.
The biplot in Figure 3 shows the scatter plot of the first two principal components (they explain ±71% of the total variability) regarding the agricultural by-products. Arrows indicate the contribution of the polyphenols (variables) to each component.
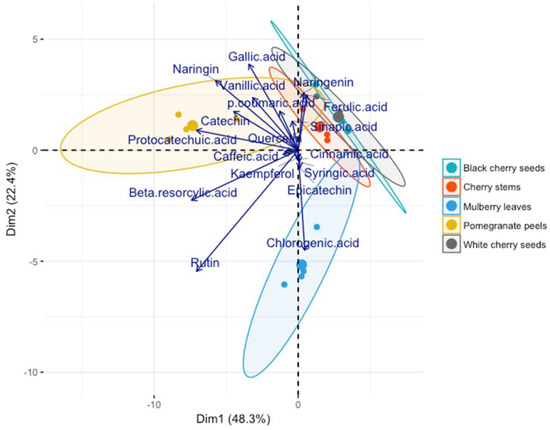
Figure 3.
Scatter plot of the first two principal components differentiated by agricultural by-product samples.
The points (20 points, the largest point representing the average) were differentiated by agricultural by-product. The graph suggests that the sample influences the number of antioxidants.
Five clusters can be distinguished regarding the five agricultural by-products, with the cherry by-products overlapping each other. The cluster of MLs is indifferent to Dim 1 and negatively correlated with Dim 2, favoring the accumulation of chlorogenic acid in the final extract. On the other hand, the CS, WCS, and BCS clusters are closely correlated and positively related to Dim 1 and Dim 2. Their main composition includes gallic, sinapic, and ferulic acids and catechin. The PCA also indicated that the PP cluster is negatively related to Dim 1 and positively to Dim 2. Its cluster has high quantities of gallic, protocatechuic acids, and catechin.
The biplot represented in Figure 4 shows the scatter plot of the first two principal components (explaining ±71% of the total variability). The points were differentiated using an extraction technique.
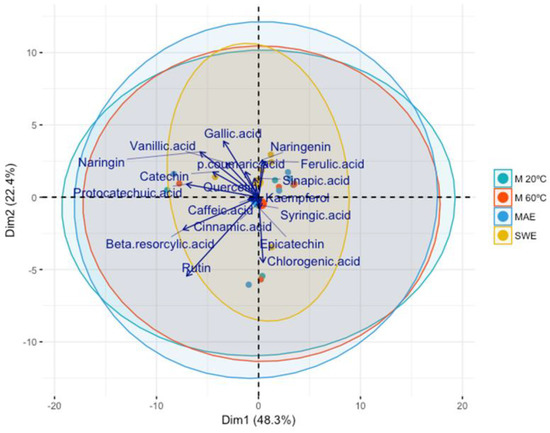
Figure 4.
Scatter plot of the first two principal components differentiated using an extraction technique.
Figure 4 shows that the extraction technique does not affect the number of antioxidants because no cluster can be distinguished. Vladić et al. [24] compared SWE and MAE in the extraction of bioactive compounds from PPs. They used PCA to identify potential clusters regarding the various extraction techniques and categorized them based on their yield and phenolic content. These authors reported a negative correlation between MAE loadings and PC1. In contrast, a positive correlation was observed with PC2, and the cluster of MAE was related to TPC, TFC, ellagic acid, and punicalagin. In contrast, the loadings of the SWE extraction showed no significant correlation with PC1 and a slight negative correlation with PC2. The cluster of the SWE extraction was closely associated with gallic acid, punicalin, and the extraction yield. These authors also concluded that a temperature above 160 °C was not favorable for extracting phenolic compounds. Conversely to our results, these authors reported differences between the two extraction techniques.
The correlation between TPC, TFC, FRAP, and ABTS is shown in Figure 5. As observed, for all samples, the correlations between methods are positive.
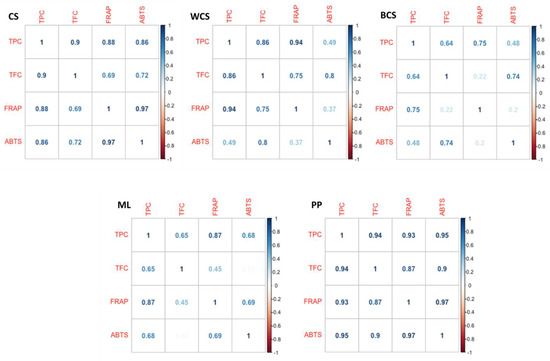
Figure 5.
Correlation plots for the results obtained from TPC, TFC, FRAP, and ABTS applied to agricultural by-products.
For CSs, there is a strong correlation (R2 > 0.85) between TPC and TFC and TPC and FRAP and ABTS, while it is lower between TFC, FRAP, and ABTS (R2 > 0.69). There was also a strong correlation between ABTS radical scavenging activity and the FRAP assay. Considering WCSs, there are strong correlations between TPC and TFC and between TPC and FRAP (R2 > 0.85), while the correlation between TFC and FRAP is weaker (R2 = 0.75). The correlation between TPC and ABTS is weak (R2 = 0.49). Likewise, the correlation between FRAP and ABTS is also low (R2 = 0.37). Regarding BCSs, the strongest correlation was observed between TPC and FRAP (R2 = 0.75) and TFC and ABTS (R2 = 0.74). Between TPC and TFC, ABTS is weak or very weak (R2 > 0.2 and <0.48). Conversely, Afonso et al. [35] reported a strong correlation between all the identified compounds and the antioxidant activity for cherry kernels, namely for FRAP and DPPH, with R2 > 0.81.
The moderate and low correlations observed in the cherry seed samples may be influenced by various factors. The antioxidant activity observed cannot be solely attributed to the phenolic content, as other antioxidants such as carotenoids, ascorbic acid, terpenes, tocopherols, carbohydrates, proteins, and other phytochemicals, which were not quantified in this study but extracted under the employed extraction conditions, may contribute to the overall antioxidant activity of the extracts [45].
The highest correlation was observed for MLs between TPC and FRAP (R2 = 0.87). No correlation was observed between TFC and ABTS (R2 = 0.04).
In our study, for PP samples, a strong correlation was observed between the total phenolic content (TPC) and the antioxidant activity, measured using FRAP (R2 = 0.93) and ABTS (R2 = 0.95). This indicates that the polyphenolic content is highly associated with the overall antioxidant capacity. These findings suggest that the phenolic compounds present in pomegranate peels may contribute to the ABTS radical scavenging activity and the FRAP-reducing ability. Pearson’s correlation coefficient (R) was also used to assess the relationship between TPC and antioxidant activities in freeze-dried pomegranate seeds and peels [45]. Contrary to our results, the authors reported a moderately positive correlation between the TPC and DPPH and TPC and FRAP assays.
The variable correlations between methods can be explained by the compounds that react with the ABTS radical and those reacting with the Fe3+-TPTZ complex [35]. The ABTS assay measures the ability of antioxidants to scavenge the ABTS radical generated by reacting with a strong oxidizing agent with the ABTS salt in aqueous systems. The neutralization of the ABTS radical blue-green coloration is due to hydrogen-donating antioxidants. The ABTS method is rapid, works over a broad interval of pH in both aqueous and organic solvent systems, and measures both hydrophilic and lipophilic compounds [46]. On the contrary, in the FRAP method, the Fe3+-TPTZ complex is reduced to TPTZ-Fe2+ through an electron transfer mechanism by compounds with redox potential below + 0.7 V and appears unable to measure compounds that act through hydrogen transfer (radical quenching) [53,54]. Moreover, the reducing power of compounds seems to depend on the degree of hydroxylation and the extent of conjugation in polyphenols [37]. It has been reported that compounds that show high antioxidant activity have a catechol group (such as flavonoids), numerous hydroxyl substitutions and conjugation, and are oxidized below +0.4 V, while compounds with an oxidation potential higher than +0.5 V are almost inactive [30]. The flavonoids catechin, rutin, and quercetin present the lowest oxidation potentials; moreover, although gallic acid is a phenolic acid, it has an oxidation potential of ∼+0.274 V, all of which being described as the most potent antioxidants regarding the FRAP assay [30]. On the contrary, hesperetin, naringenin, daidzein, apigenin, and genistein present weak and reducing activities [55]. In another study, regarding the relation between polyphenols and the ABTS assay, it was assessed that the flavonoids quercetin and isorhamnetin were the best radical scavengers, followed by myricetin, taxifolin, and kaempferol, all of which had a higher level of radical scavenging activity than Trolox [55].
The current work shows that different extraction techniques can be successfully applied to obtain bioactive compounds from agricultural by-products for maximizing TPC, TFC, and antioxidant activity. In general, MAE and SWE are good choices for extracting bioactive compounds, considering the phenolic profile of the extracts.
The AGREEPrep, an analytical greenness metric for sample preparation, was utilized in this study to evaluate the environmental sustainability of the chosen extraction technique. This assessment can aid in determining the most suitable extraction method for a given situation and sample type [33,34]. The results obtained are presented in Figure 6.
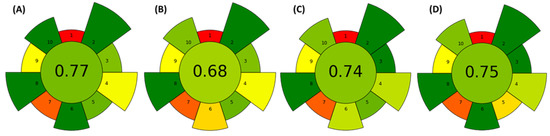
Figure 6.
Results of the AGREEPrep analysis considering the extraction technique employed: (A) M20C, (B) M60C, (C) MAE, and (D) SWE. Default weights for each input value were used in the assessment.
Figure 6 presents an overall evaluation of the procedures, while the color distinctions allow a comparison of threat and hazard types. A direct comparison indicates that the M20C (A), MAE (C), and SWE (D) procedures were the most environmentally friendly methods evaluated, with final scores of 0.77, 0.74, and 0.75, respectively. On the other hand, the M60C (B) procedure was less environmentally friendly; however, its score (0.68) was still competitive. This procedure, however, could benefit from improvements in energy consumption and sample processing efficiency.
The extraction method could be selected based on the isolated target compounds and the valorized agricultural by-product. Although MAE extracts generally presented higher TPC, TFC, and antioxidant activities, this technique does not seem as suitable as SWE for the extraction of phenolic compounds from cherry seeds and stems. Even though MAE and SWE have proven to be fast and user-friendly techniques, maceration allowed for the extraction of higher amounts of phenolic acids and flavonoids in samples such as MLs and PPs, suggesting that the high temperatures used in SWE and MAE are inadequate for these samples.
In general, each method—M20C, MAE, and SWE—could potentially be used for the sustainable extraction of polyphenolic compounds. They each present different strengths and considerations, such as efficiency, energy consumption, and environmental impact. Maceration at 60C can also be used for the sustainable extraction of polyphenolic compounds. Despite its limitations, it still produced a close result to the other techniques and may be chosen depending on the specific circumstances and requirements of the extraction process. In practice, the choice of method may depend on the specific context, including the type of sample, available resources, and environmental or sustainability goals. Optimizing the extraction method is a critical step to obtain high-value extracts considering the complexity and chemical characteristics of the agricultural by-products.
4. Conclusions
Due to the toxicity linked to conventional food additives, there is a growing necessity for additives from natural sources (such as antioxidants and preservatives). Additionally, consuming functional foods has been related to the boost of the immune system and the increased resistance of the human body against viruses, which was magnified during the COVID-19 pandemic.
Currently, designing and commercializing novel functional products while simultaneously valorizing ingredients recovered from by-products and wastes is very appealing. The choice of the extraction technique is critical and crucial to extract bioactive compounds from agricultural by-products that can have applications in several industries, contributing to their sustainable valorization and the principles of a circular economy. It is essential to employ environmentally friendly extraction methods that adopt a sustainable approach, aiming for the maximum recovery of bioactive compounds while using safer solvents and minimizing processing costs. SWE and MAE are recognized as environmentally friendly extraction techniques that address the limitations of classical extractions. These methods offer advantages such as speed, convenience, low solvent consumption, and the absence of the need for additional extract purification besides filtration. However, the selection and suitability of these technologies depend on the specific agricultural by-products and the targeting of the desired compounds.
The research presented in this work significantly contributes to the study of phenolic compound extraction from various plant materials using different extraction techniques. Some of the key advantages of this study include: (1) A comparison of extraction techniques: The research compared several extraction methods, including maceration, microwave-assisted extraction (MAE), and subcritical water extraction (SWE). This comprehensive comparison provides valuable insights into the efficiencies and limitations of each method when extracting bioactive compounds. (2) Detailed analysis of phenolic compounds: The study not only quantified the total phenolic content (TPC) but also analyzed individual phenolic compounds in the extracts. This detailed characterization helps to understand the extraction efficiency and the resultant chemical profile of the extracts from different methods. (3) Highlighting the influence of extraction conditions: The research illustrated how different extraction conditions, including temperature, can affect the yield and composition of the extracted phenolic compounds. This knowledge can guide the optimization of extraction procedures in the future. (4) Evaluation of antioxidant activity: The study also investigated the antioxidant activity of the extracts, which is an essential aspect considering the potential health benefits and industrial applications of these compounds.
Overall, this research offers valuable information for optimizing the extraction of phenolic compounds from plant materials, contributing to the efficient production of these valuable bioactive compounds for various industrial applications.
The results of this work show that high-pressure and temperature-optimized conditions, such as SWE, for bioactive compound extraction from sweet cherry seeds and stems, can incorporate a usually rejected residue from the food industry, adding value as a result. However, the cost of using high-pressure techniques must be considered against the advantages observed to see if such an extraction method is economically viable.
PPs and MLs are agricultural by-products that are abundant in bioactive compounds. The optimizations obtained in this work indicate that conventional extraction is a potential and straightforward process for scale-up and for obtaining polyphenolic-rich extracts.
To assess the potential application of any extraction technique, pilot plants with higher scale tests, using greater biomass amounts and industrial production conditions, are essential to determine the economic viability and sustainability of the process. The compounds recovered from the agricultural by-products can be used in the food, pharmaceutical, or cosmetic industries. However, bioavailability studies and the metabolism of the extracted phenolic compounds should be considered in evaluating the impact of “phenolic/antioxidant-rich” products on human health. Furthermore, toxicity and safety data are required to establish maximum limits for the obtained extract use.
Author Contributions
Conceptualization, M.M.M., M.J.R., M.C., C.D.-M. and M.F.B.; Formal analysis, C.S., M.M.M., S.R. and M.F.B.; Funding acquisition, J.S.-G. and C.D.-M.; Investigation, C.S., M.M.M. and M.F.B.; Methodology, C.S., M.M.M., S.R., J.S.-G. and M.F.B.; Resources, J.S.-G. and C.D.-M.; Software, S.R.; Supervision, M.J.R., M.C. and C.D.-M.; Validation, C.S., M.M.M., S.R., M.J.R., M.C., J.S.-G., C.D.-M. and M.F.B.; Writing—original draft, C.S. and M.M.M.; Writing—review and editing, C.S., M.M.M., S.R., M.J.R., M.C., J.S.-G., C.D.-M. and M.F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by projects REQUIMTE/LAQV—UIDB/50006/2020, UIDP/50006/2020, and LA/P/0008/2020 and financed by FCT/Ministério da Ciência, Tecnologia e Ensino Superior (MCTES) through national funds and the SYSTEMIC project, “An integrated approach to the challenge of sustainable food systems: adaptive and mitigatory strategies to address climate change and malnutrition”. The Knowledge hub on Nutrition and Food Security received funding from national research funding parties in Belgium (FWO), France (INRA), Germany (BLE), Italy (MIPAAF), Latvia (IZM), Norway (RCN), Portugal (FCT), and Spain (AEI) in a joint action of JPI HDHL, JPI-OCEANS, and FACCE-JPI, launched in 2019 under the ERA-NET ERA-HDHL (n° 696295). This work was also funded by the Science Fund of the Republic of Serbia (Grant No. 7747845) titled: In situ pollutants removal from waters by sustainable green nanotechnologies-CleanNanoCatalyze.
Data Availability Statement
Not applicable.
Acknowledgments
Manuela M. Moreira (CEECIND/02702/2017) is thankful for her contract financed by FCT/MCTES—CEEC Individual Program Contract. The Ministry of Science, Technological Development and Innovation of the Republic of Serbia project 451-03-47/2023-01/200134 is also acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Corrado, S.; Caldeira, C.; Eriksson, M.; Hanssen, O.J.; Hauser, H.-E.; van Holsteijn, F.; Liu, G.; Östergren, K.; Parry, A.; Secondi, L.; et al. Food Waste Accounting Methodologies: Challenges, Opportunities, and Further Advancements. Glob. Food Secur. 2019, 20, 93–100. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO) Overview|Sustainable Development Goals|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/sustainable-development-goals/overview/en/ (accessed on 24 November 2022).
- Ueda, J.M.; Pedrosa, M.C.; Heleno, S.A.; Carocho, M.; Ferreira, I.C.F.R.; Barros, L. Food Additives from Fruit and Vegetable By-Products and Bio-Residues: A Comprehensive Review Focused on Sustainability. Sustainability 2022, 14, 5212. [Google Scholar] [CrossRef]
- Luque, R.; Clark, J.H. Valorisation of Food Residues: Waste to Wealth Using Green Chemical Technologies. Sustain. Chem. Process. 2013, 1, 10. [Google Scholar] [CrossRef]
- Saiwal, N.; Dahiya, M.; Dureja, H. Nutraceutical Insight into Vegetables and Their Potential for Nutrition Mediated Healthcare. Curr. Nutr. Food Sci. 2019, 15, 441–453. [Google Scholar] [CrossRef]
- Islam, A.; Alam, F.; Solayman, M.; Khalil, M.I.; Kamal, M.A.; Gan, S.H. Dietary Phytochemicals: Natural Swords Combating Inflammation and Oxidation-Mediated Degenerative Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 9060649. [Google Scholar] [CrossRef] [PubMed]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, B.; Christen, P. Recent Extraction Techniques for Natural Products: Microwave-Assisted Extraction and Pressurised Solvent Extraction. Phytochem. Anal. 2002, 13, 105–113. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Amjad, S.; Ashraf, S.; Khawar, L.; Safdar, M.N.; Jabbar, S.; Nadeem, M.; Mahmood, S.; Murtaza, M.A. Extraction of Polyphenols from Apple and Pomegranate Peels Employing Different Extraction Techniques for the Development of Functional Date Bars. Int. J. Fruit Sci. 2020, 20, S1201–S1221. [Google Scholar] [CrossRef]
- Contini, M.; Baccelloni, S.; Massantini, R.; Anelli, G. Extraction of Natural Antioxidants from Hazelnut (Corylus Avellana L.) Shell and Skin Wastes by Long Maceration at Room Temperature. Food Chem. 2008, 110, 659–669. [Google Scholar] [CrossRef]
- Vongsak, B.; Sithisarn, P.; Mangmool, S.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W. Maximizing Total Phenolics, Total Flavonoids Contents and Antioxidant Activity of Moringa Oleifera Leaf Extract by the Appropriate Extraction Method. Ind. Crop. Prod. 2013, 44, 566–571. [Google Scholar] [CrossRef]
- Fernández-Agulló, A.; Pereira, E.; Freire, M.S.; Valentão, P.; Andrade, P.B.; González-álvarez, J.; Pereira, J.A. Influence of Solvent on the Antioxidant and Antimicrobial Properties of Walnut (Juglans Regia L.) Green Husk Extracts. Ind. Crop. Prod. 2013, 42, 126–132. [Google Scholar] [CrossRef]
- Yuan, B.; Lu, M.; Eskridge, K.M.; Isom, L.D.; Hanna, M.A. Extraction, Identification, and Quantification of Antioxidant Phenolics from Hazelnut (Corylus Avellana L.) Shells. Food Chem. 2018, 244, 7–15. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, X.; Xu, H.; Xu, C.; Yuan, F.; Knez, Ž.; Novak, Z.; Gao, Y. Subcritical Water Extraction of Phenolic Compounds from Pomegranate (Punica Granatum L.) Seed Residues and Investigation into Their Antioxidant Activities with HPLC–ABTS+ Assay. Food Bioprod. Process. 2012, 90, 215–223. [Google Scholar] [CrossRef]
- Kim, S.-W.; Ko, M.-J.; Chung, M.-S. Extraction of the Flavonol Quercetin from Onion Waste by Combined Treatment with Intense Pulsed Light and Subcritical Water Extraction. J. Clean. Prod. 2019, 231, 1192–1199. [Google Scholar] [CrossRef]
- Jokić, S.; Gagić, T.; Knez, Ž.; Šubarić, D.; Škerget, M. Separation of Active Compounds from Food By-Product (Cocoa Shell) Using Subcritical Water Extraction. Molecules 2018, 23, 1408. [Google Scholar] [CrossRef]
- Gonçalves Rodrigues, L.G.; Mazzutti, S.; Vitali, L.; Micke, G.A.; Ferreira, S.R.S. Recovery of Bioactive Phenolic Compounds from Papaya Seeds Agroindustrial Residue Using Subcritical Water Extraction. Biocatal. Agric. Biotechnol. 2019, 22, 101367. [Google Scholar] [CrossRef]
- Moreira, M.M.; Barroso, M.F.; Boeykens, A.; Withouck, H.; Morais, S.; Delerue-Matos, C. Valorization of Apple Tree Wood Residues by Polyphenols Extraction: Comparison between Conventional and Microwave-Assisted Extraction. Ind. Crop. Prod. 2017, 104, 210–220. [Google Scholar] [CrossRef]
- Mellinas, A.C.; Jiménez, A.; Garrigós, M.C. Optimization of Microwave-Assisted Extraction of Cocoa Bean Shell Waste and Evaluation of Its Antioxidant, Physicochemical and Functional Properties. LWT 2020, 127, 109361. [Google Scholar] [CrossRef]
- Florin Danet, A. Recent Advances in Antioxidant Capacity Assays. In Antioxidants—Benefits, Sources, Mechanisms of Action; IntechOpen: London, UK, 2021. [Google Scholar]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Román-Aguirre, M.; Cruz-Alcantar, P.; Pérez-Urizar, J.T.; Saavedra-Leos, M.Z. Application of Antioxidants as an Alternative Improving of Shelf Life in Foods. Polysaccharides 2021, 2, 594–607. [Google Scholar] [CrossRef]
- Vladić, J.; Janković, T.; Živković, J.; Tomić, M.; Zdunić, G.; Šavikin, K.; Vidović, S. Comparative Study of Subcritical Water and Microwave-Assisted Extraction Techniques Impact on the Phenolic Compounds and 5-Hydroxymethylfurfural Content in Pomegranate Peel. Plant Foods Hum. Nutr. 2020, 75, 553–560. [Google Scholar] [CrossRef]
- Radojković, M.; Moreira, M.M.; Soares, C.; Fátima Barroso, M.; Cvetanović, A.; Švarc-Gajić, J.; Morais, S.; Delerue-Matos, C. Microwave-Assisted Extraction of Phenolic Compounds from Morus Nigra Leaves: Optimization and Characterization of the Antioxidant Activity and Phenolic Composition. J. Chem. Technol. Biotechnol. 2018, 93, 1684–1693. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Cerdà, V.; Clavijo, S.; Suárez, R.; Mašković, P.; Cvetanović, A.; Delerue-Matos, C.; Carvalho, A.P.; Novakov, V. Bioactive Compounds of Sweet and Sour Cherry Stems Obtained by Subcritical Water Extraction. J. Chem. Technol. Biotechnol. 2018, 93, 1627–1635. [Google Scholar] [CrossRef]
- Nastić, N.; Lozano-Sánchez, J.; Borrás-Linares, I.; Švarc-Gajić, J.; Segura-Carretero, A. New Technological Approaches for Recovering Bioactive Food Constituents from Sweet Cherry (Prunus Avium L.) Stems. Phytochem. Anal. 2020, 31, 119–130. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Cvetanović, A.; Segura-Carretero, A.; Linares, I.B.; Mašković, P. Characterisation of Ginger Extracts Obtained by Subcritical Water. J. Supercrit. Fluids 2017, 123, 92–100. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Zeković, Z.; Jerković, J.; Zengin, G.; Gašić, U.; Tešić, Ž.; Mašković, P.; Soares, C.; Fatima Barroso, M.; et al. The Influence of the Extraction Temperature on Polyphenolic Profiles and Bioactivity of Chamomile (Matricaria Chamomilla L.) Subcritical Water Extracts. Food Chem. 2019, 271, 328–337. [Google Scholar] [CrossRef]
- Nastić, N.; Švarc-Gajić, J.; Delerue-Matos, C.; Barroso, M.F.; Soares, C.; Moreira, M.M.; Morais, S.; Mašković, P.; Gaurina Srček, V.; Slivac, I.; et al. Subcritical Water Extraction as an Environmentally-Friendly Technique to Recover Bioactive Compounds from Traditional Serbian Medicinal Plants. Ind. Crop. Prod. 2018, 111, 579–589. [Google Scholar] [CrossRef]
- Barroso, M.F.; Ramalhosa, M.J.; Alves, R.C.; Dias, A.; Soares, C.M.D.; Oliva-Teles, M.T.; Delerue-Matos, C. Total Antioxidant Capacity of Plant Infusions: Assessment Using Electrochemical DNA-Based Biosensor and Spectrophotometric Methods. Food Control. 2016, 68, 153–161. [Google Scholar] [CrossRef]
- Mendes, M.; Carvalho, A.P.; Magalhães, J.M.C.S.; Moreira, M.; Guido, L.; Gomes, A.M.; Delerue-Matos, C. Response Surface Evaluation of Microwave-Assisted Extraction Conditions for Lycium Barbarum Bioactive Compounds. Innov. Food Sci. Emerg. Technol. 2016, 33, 319–326. [Google Scholar] [CrossRef]
- Wojnowski, W.; Tobiszewski, M.; Pena-Pereira, F.; Psillakis, E. AGREEprep – Analytical Greenness Metric for Sample Preparation. Trac Trends Anal. Chem. 2022, 149, 116553. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Tobiszewski, M.; Wojnowski, W.; Psillakis, E. A Tutorial on AGREEprep an Analytical Greenness Metric for Sample Preparation. Adv. Sample Prep. 2022, 3, 100025. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.V.; Meyer, A.S.; Aires, A.; Saavedra, M.J.; Gonçalves, B. Phenolic Profile and Bioactive Potential of Stems and Seed Kernels of Sweet Cherry Fruit. Antioxidants 2020, 9, 1295. [Google Scholar] [CrossRef]
- Babotă, M.; Voştinaru, O.; Păltinean, R.; Mihali, C.; Dias, M.I.; Barros, L.; Ferreira, I.C.F.R.; Mocan, A.; Crişan, O.; Nicula, C.; et al. Chemical Composition, Diuretic, and Antityrosinase Activity of Traditionally Used Romanian Cerasorum Stipites. Front. Pharmacol. 2021, 12, 647947. [Google Scholar] [CrossRef]
- Dulyanska, Y.; Cruz-Lopes, L.P.; Esteves, B.; Ferreira, J.V.; Domingos, I.; Lima, M.J.; Correia, P.M.R.; Ferreira, M.; Fragata, A.; Barroca, M.J.; et al. Extraction of Phenolic Compounds from Cherry Seeds: A Preliminary Study. Agronomy 2022, 12, 1227. [Google Scholar] [CrossRef]
- Žugić, A.; Dordević, S.; Arsić, I.; Marković, G.; Živković, J.; Jovanović, S.; Tadić, V. Antioxidant Activity and Phenolic Compounds in 10 Selected Herbs from Vrujci Spa, Serbia. Ind. Crop. Prod. 2014, 52, 519–527. [Google Scholar] [CrossRef]
- Yu, Y.; Li, H.; Zhang, B.; Wang, J.; Shi, X.; Huang, J.; Yang, J.; Zhang, Y.; Deng, Z. Nutritional and Functional Components of Mulberry Leaves from Different Varieties: Evaluation of Their Potential as Food Materials. Int. J. Food Prop. 2018, 21, 1495–1507. [Google Scholar] [CrossRef]
- Radojković, M.; Jokic, S.; Vidovic, S. Determination of Optimal Extraction Parameters of Mulberry Leaves Using Response Surface Methodology (RSM). Application of Innovative Techniques of the Extraction of Bioactive Components from by-Products of Plant Origin View Project Novel Extracts and Bioactive Compounds from under-Utilized Resources for High-Value Applications (BioUtilize) View Project. Rom. Biotechnol. Lett. 2012, 17, 7295–7308. [Google Scholar]
- Panyatip, P.; Padumanonda, T.; Yongram, C.; Kasikorn, T.; Sungthong, B.; Puthongking, P. Impact of Tea Processing on Tryptophan, Melatonin, Phenolic and Flavonoid Contents in Mulberry (Morus Alba L.) Leaves: Quantitative Analysis by LC-MS/MS. Molecules 2022, 27, 4979. [Google Scholar] [CrossRef] [PubMed]
- Agulló-Chazarra, L.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A.; Micol, V.; Herranz-López, M.; Barrajón-Catalán, E. Sweet Cherry Byproducts Processed by Green Extraction Techniques as a Source of Bioactive Compounds with Antiaging Properties. Antioxidants 2020, 9, 418. [Google Scholar] [CrossRef]
- Dulyanska, Y.; Cruz-Lopes, L.; Esteves, B.; Ferreira, J.V.; Domingos, I.; Lima, M.J.; Correia, P.M.R.; Ferreira, M.; Fragata, A.; Barroca, M.J.; et al. Evaluation of the Antioxidant Activity of Extracts Obtained Form Cherry Seeds. J. Hyg. Eng. Des. 2022, 40, 221–226. [Google Scholar]
- Alexandre, E.M.C.; Araújo, P.; Duarte, M.F.; de Freitas, V.; Pintado, M.; Saraiva, J.A. Experimental Design, Modeling, and Optimization of High-Pressure-Assisted Extraction of Bioactive Compounds from Pomegranate Peel. Food Bioprocess Technol. 2017, 10, 886–900. [Google Scholar] [CrossRef]
- Himel, M.A.R.; Ahmed, T.; Hossain, M.A.; Moazzem, M.S. Response Surface Optimization to Extract Antioxidants from Freeze-Dried Seeds and Peel of Pomegranate (Punica Granatum L.). Biomass Convers Biorefin 2022, 1, 1–16. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Gilbert-López, B.; Mendiola, J.A.; Quirantes-Piné, R.; Segura-Carretero, A.; Ibáñez, E. Optimization of Microwave-Assisted Extraction and Pressurized Liquid Extraction of Phenolic Compounds from Moringa Oleifera Leaves by Multiresponse Surface Methodology. Electrophoresis 2016, 37, 1938–1946. [Google Scholar] [CrossRef]
- Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Solvent Effects on the Antioxidant Capacity of Lipophilic and Hydrophilic Antioxidants Measured by CUPRAC, ABTS/Persulphate and FRAP Methods. Talanta 2010, 81, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant Mechanism of Tea Polyphenols and Its Impact on Health Benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, Mechanism, and Applications of Phenolic Antioxidants in Foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Hunyadi, A. The Mechanism(s) of Action of Antioxidants: From Scavenging Reactive Oxygen/Nitrogen Species to Redox Signaling and the Generation of Bioactive Secondary Metabolites. Med. Res. Rev. 2019, 39, 2505–2533. [Google Scholar] [CrossRef]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of Antioxidant Potency of Commonly Consumed Polyphenol-Rich Beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Zhang, D.; Chu, L.; Liu, Y.; Wang, A.; Ji, B.; Wu, W.; Zhou, F.; Wei, Y.; Cheng, Q.; Cai, S.; et al. Analysis of the Antioxidant Capacities of Flavonoids under Different Spectrophotometric Assays Using Cyclic Voltammetry and Density Functional Theory. J. Agric. Food Chem. 2011, 59, 10277–10285. [Google Scholar] [CrossRef]
- Kelley, D.; Adkins, Y.; Laugero, K. A Review of the Health Benefits of Cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef]
- Hooman, N.; Mojab, F.; Nickavar, B.; Pouryousefi-Kermani, P. Diuretic Effect of Powdered Cerasus Avium (Cherry) Tails on Healthy Volunteers—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/19783515/ (accessed on 17 January 2023).
- Lim, S.; Choi, C.-I. Pharmacological Properties of Morus Nigra L. (Black Mulberry) as A Promising Nutraceutical Resource. Nutrients 2019, 11, 437. [Google Scholar] [CrossRef]
- Ghorbani, A.; Hooshmand, S. Protective Effects of Morus Nigra and Its Phytochemicals against Hepatotoxicity: A Review of Preclinical Studies. Pharmacology 2021, 106, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Eghbali, S.; Askari, S.F.; Avan, R.; Sahebkar, A. Therapeutic Effects of Punica Granatum (Pomegranate): An Updated Review of Clinical Trials. J. Nutr. Metab. 2021, 2021, 97162. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Escarpa, A.; González, M.C. Approach to the Content of Total Extractable Phenolic Compounds from Different Food Samples by Comparison of Chromatographic and Spectrophotometric Methods. Anal. Chim. Acta 2001, 427, 119–127. [Google Scholar] [CrossRef]
- Aires, A.; Dias, C.; Carvalho, R.; Saavedra, M.J. Analysis of Glycosylated Flavonoids Extracted from Sweet-Cherry Stems, as Antibacterial Agents against Pathogenic Escherichia Coli Isolates. Acta Biochim. Pol. 2017, 64, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Radojković, M.; Zeković, Z.; Mašković, P.; Vidović, S.; Mandić, A.; Mišan, A.; Đurović, S. Biological Activities and Chemical Composition of Morus Leaves Extracts Obtained by Maceration and Supercritical Fluid Extraction. J. Supercrit. Fluids 2016, 117, 50–58. [Google Scholar] [CrossRef]
- Kaderides, K.; Kyriakoudi, A.; Mourtzinos, I.; Goula, A.M. Potential of Pomegranate Peel Extract as a Natural Additive in Foods. Trends Food Sci. Technol. 2021, 115, 380–390. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Mantegna, S.; Cravotto, G.; Perego, P. Extraction of Phenolics from Vitis Vinifera Wastes Using Non-Conventional Techniques. J. Food Eng. 2010, 100, 50–55. [Google Scholar] [CrossRef]
- Jarić, A.M.; Šeremet, D.; Cebin, A.V.; Jokić, S.; Komes, D. The Multiple-Response Modeling of Heat-Assisted, Microwave-Assisted and Subcritical Water Extraction on Selected Phenolics from Traditional Plant Species Teucrium Montanum. Prep. Biochem. Biotechnol. 2021, 52, 809–822. [Google Scholar] [CrossRef]
- Rodrigues, L.G.G.; Mazzutti, S.; Siddique, I.; da Silva, M.; Vitali, L.; Ferreira, S.R.S. Subcritical Water Extraction and Microwave-Assisted Extraction Applied for the Recovery of Bioactive Components from Chaya (Cnidoscolus Aconitifolius Mill.). J Supercrit Fluids 2020, 165, 104976. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).