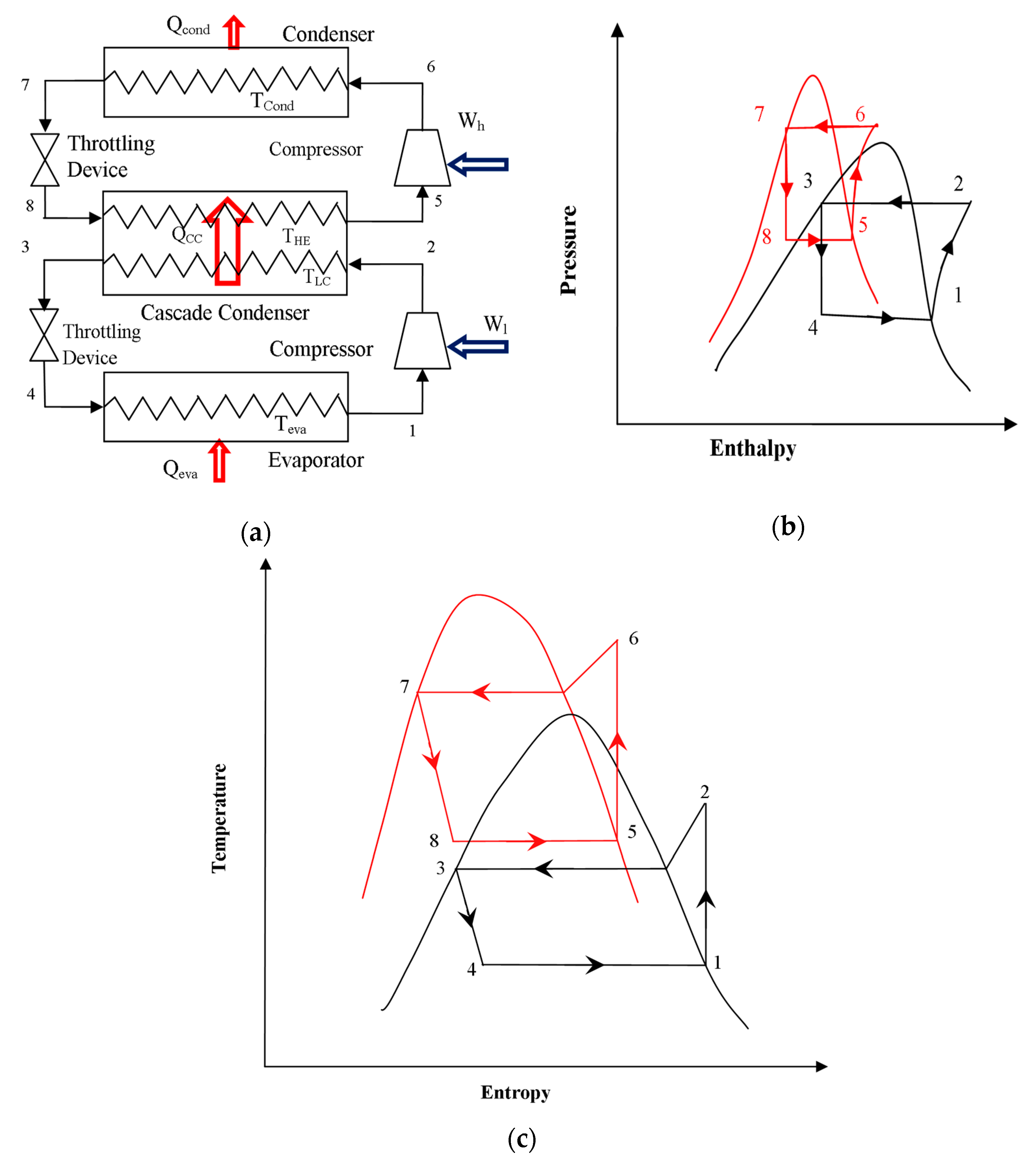
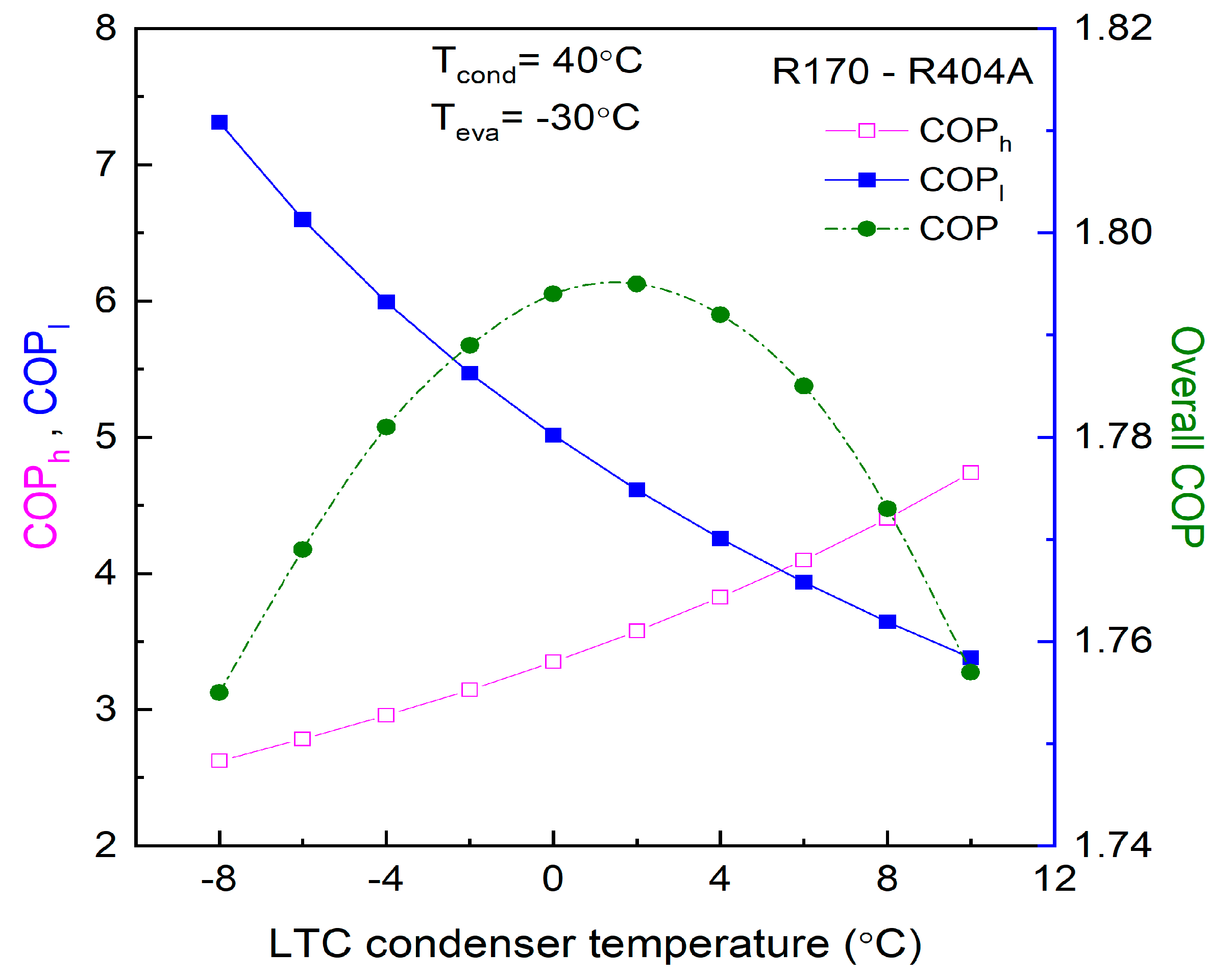
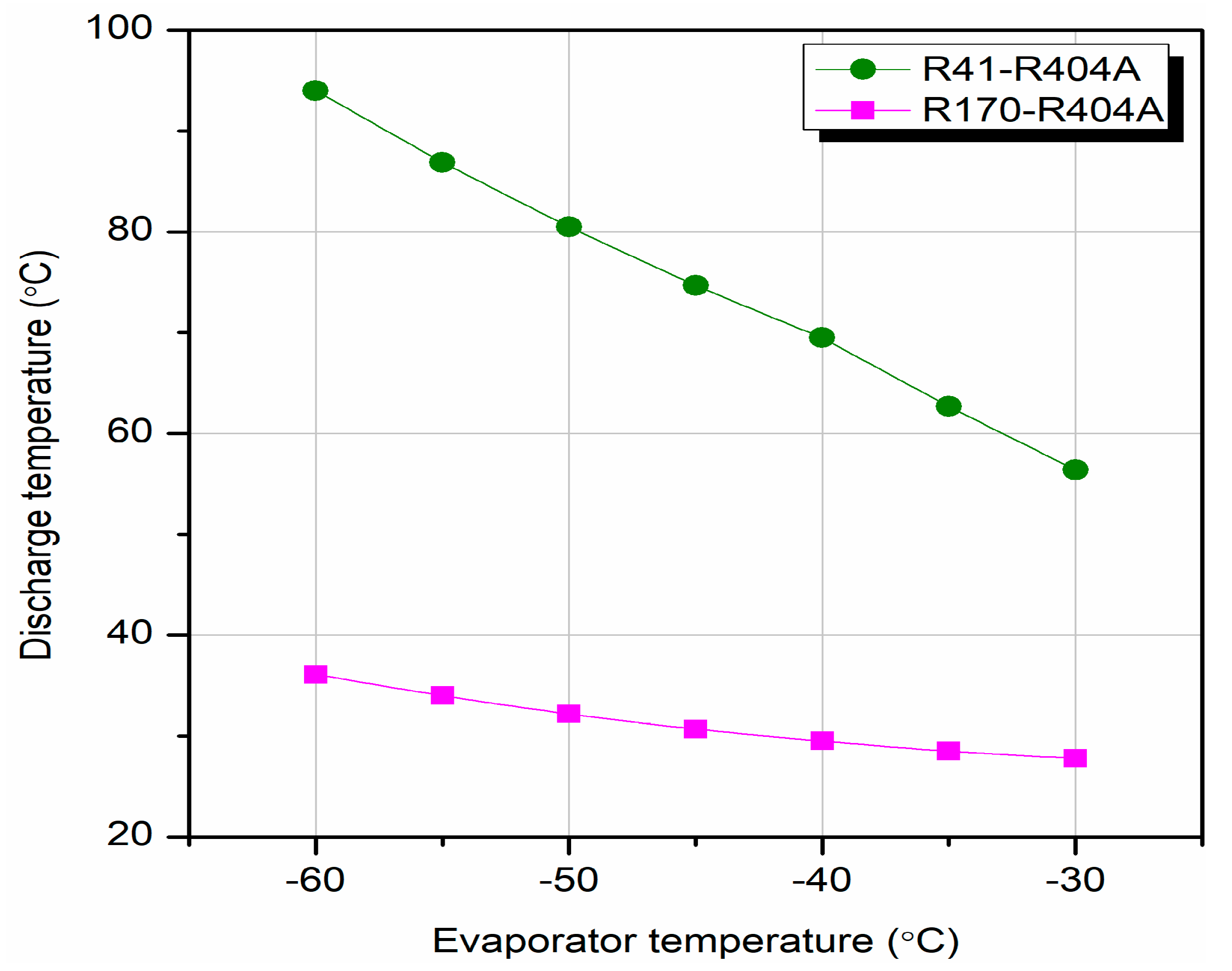
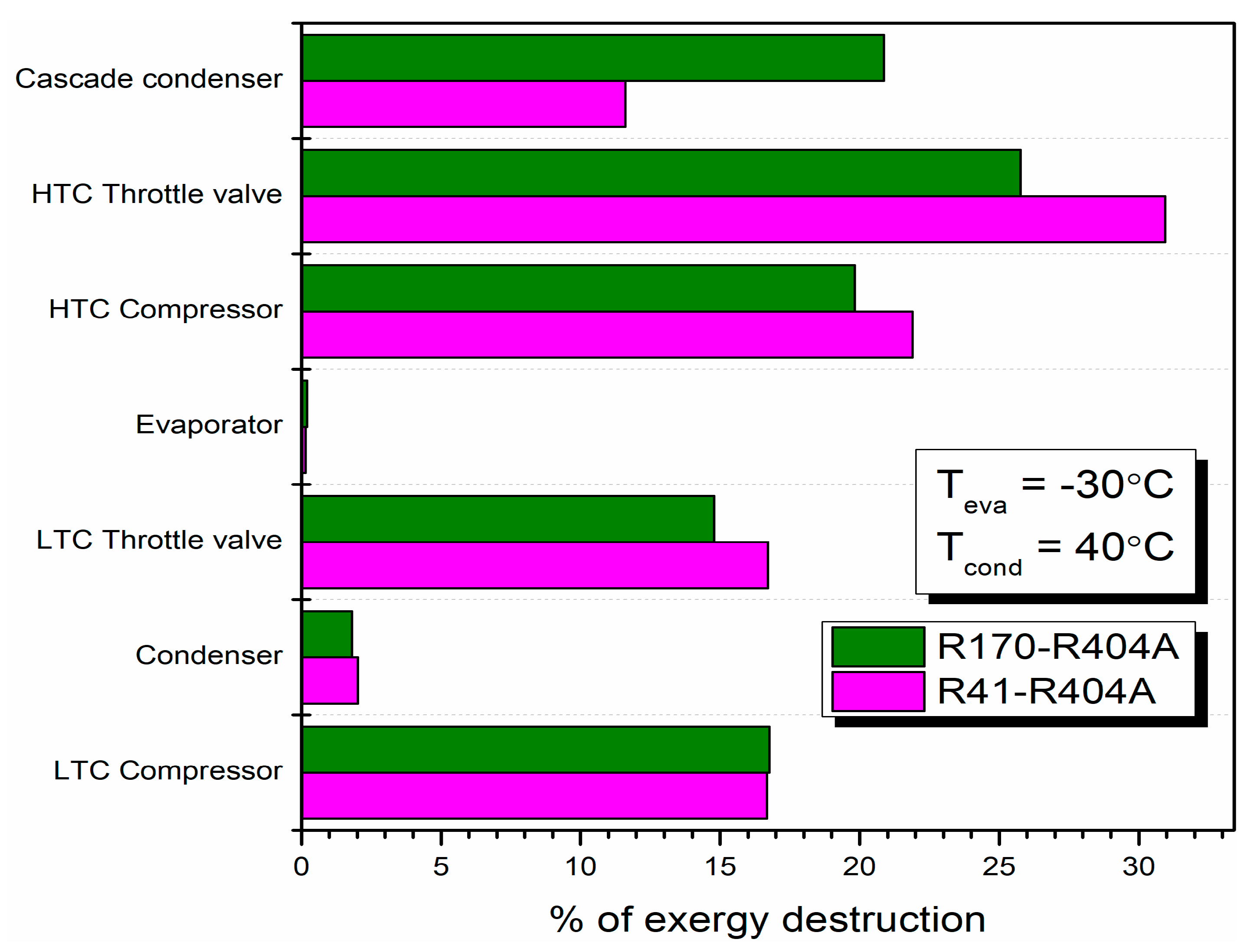
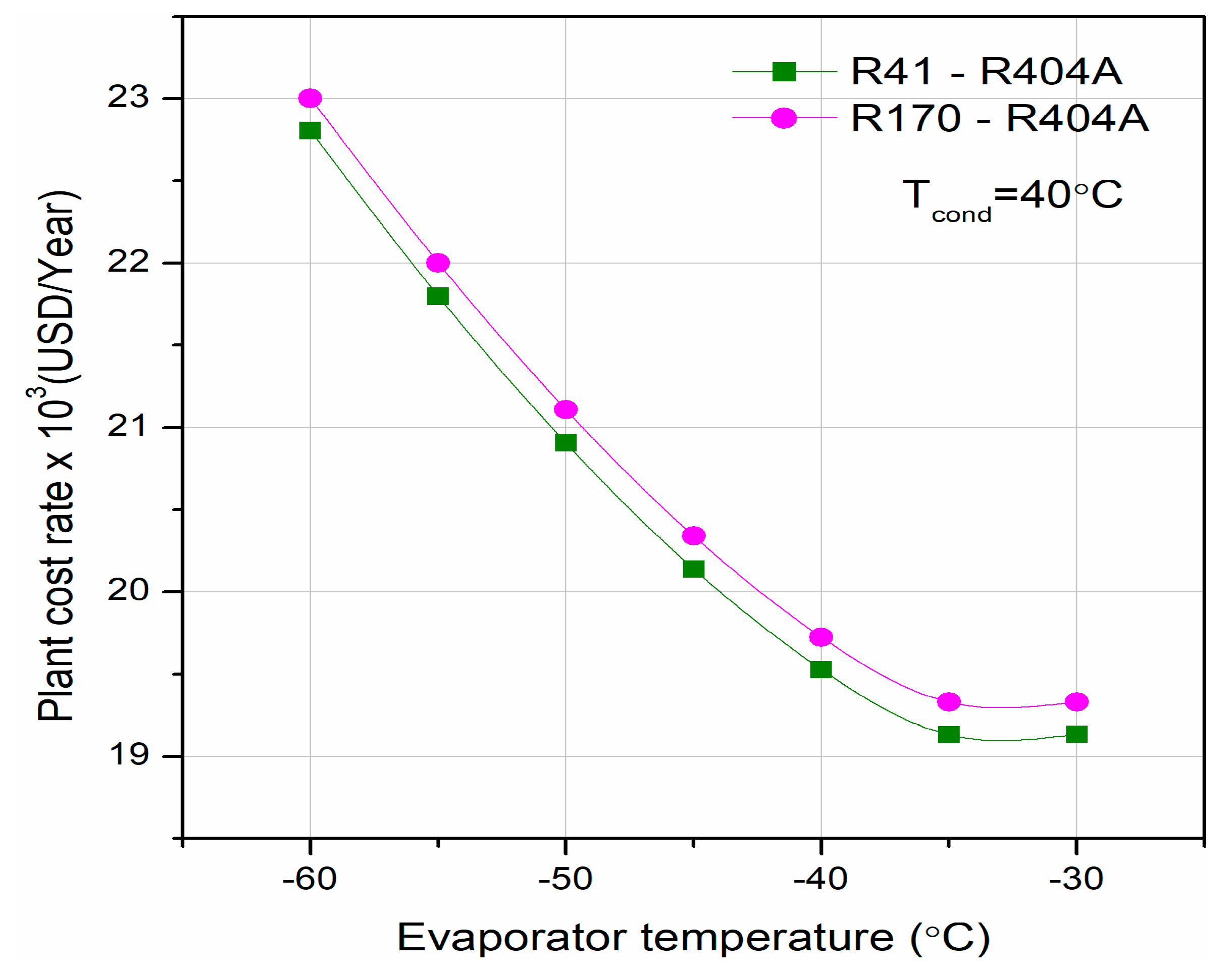
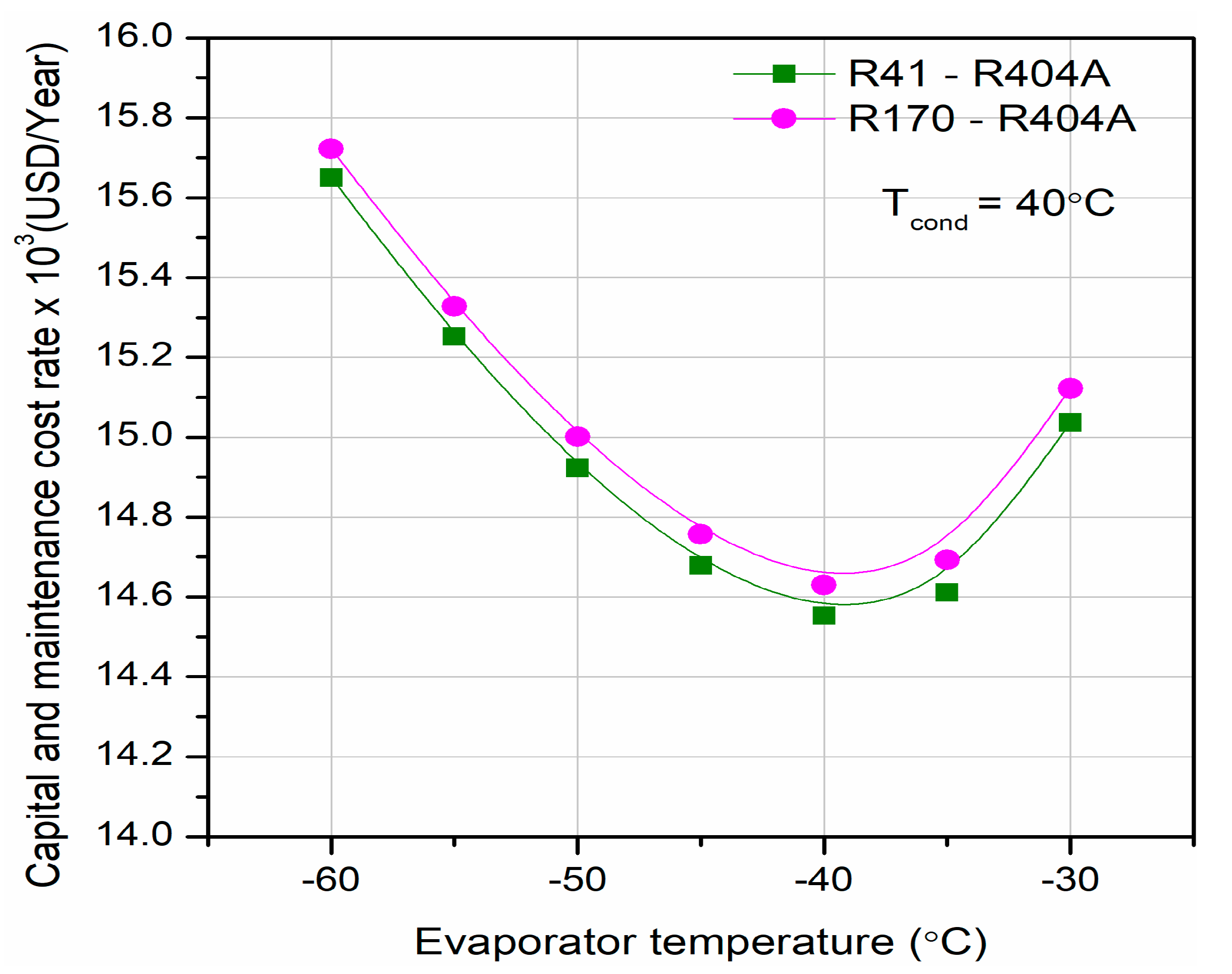
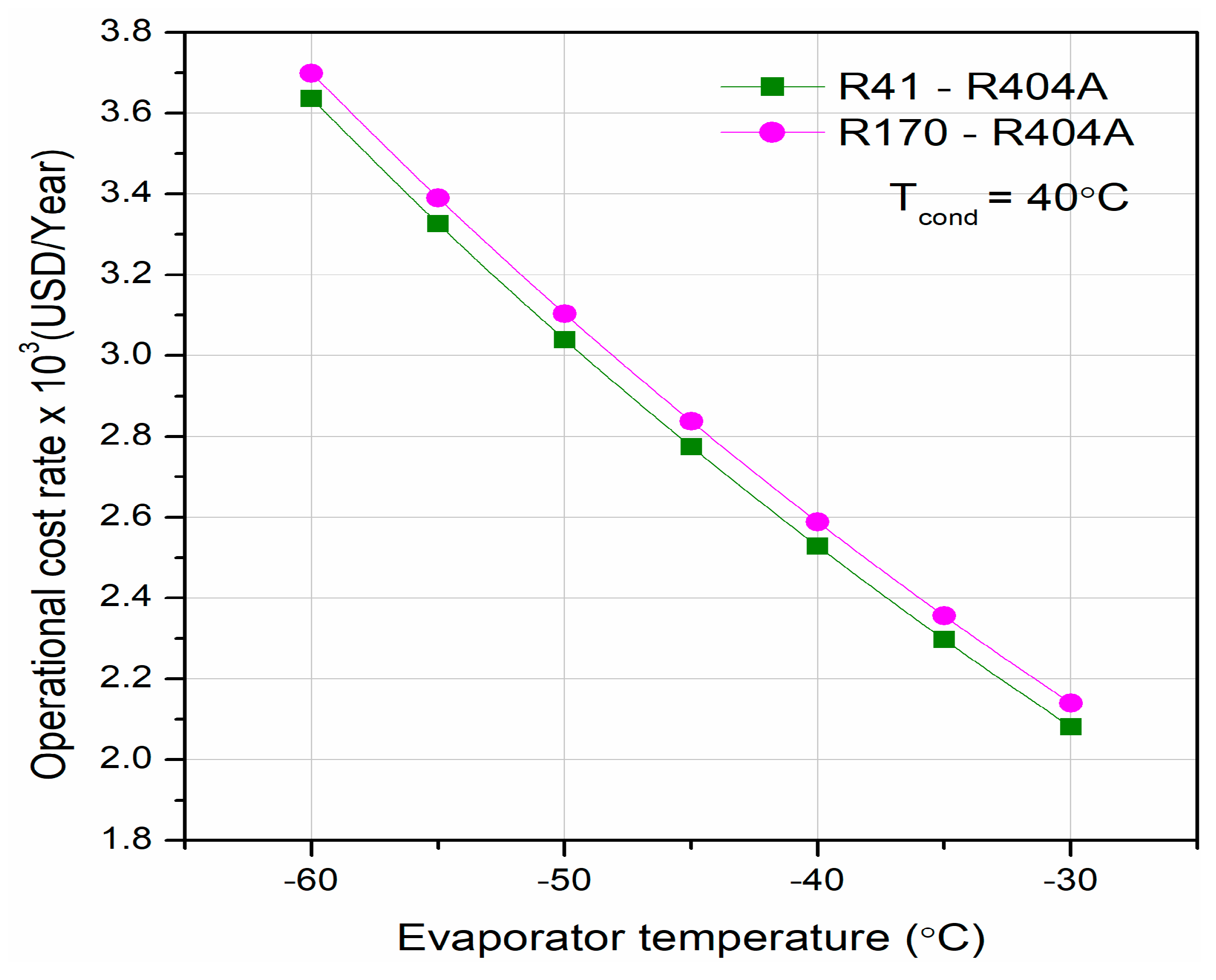
Different researchers have made numerous studies on CRSs. Lee et al. [
3] performed a thermodynamic analysis on a CRS using the R744–R717 refrigerant combination to determine the optimal condensing temperature (
) in a cascade condenser (CC) to maximize the COP and minimize the exergy loss. They noted a rise in the optimal condensing temperature of the cascade condenser (
) with
,
, and
. Hoşöz [
4] carried out a comparative experimental study between two refrigeration system configurations: single stage and a CRS using R134a as the refrigerant. The author noted a COP of 3.5 and 1.5 for the single-stage VCR and CRS, respectively, while keeping the refrigeration capacity fixed at 500 W. Nicola et al. [
5] carried out a simulation to evaluate the performance of a CRS using ammonia as the high-temperature refrigerant, blends of carbon dioxide, and four different HFCs, namely, R23, R41, R125, and R32, as low-temperature refrigerants. They concluded that the use of the HFC/carbon dioxide blends for low-temperature applications could be an attractive option. Niu and Zhang [
6] experimented on a CRS using blend of R290 and R744 in the low-temperature circuit (LTC) to replace R13 and R290 in the high-temperature cycle. They noted a higher COP and refrigeration effect with the new mixture compared to those using R13 as refrigerant. Ouadha et al. [
7] performed a comparison between CRS and two-stage refrigeration using natural refrigerants, namely, carbon dioxide (R744), ammonia (R717), and propane (R290). They observed slightly lower power consumption in the low-pressure compressor of the cascade system compared to the two-stage system. However, nearly 13% higher power consumption was noted in the high-temperature compressor of the cascade system compared to that of the two-stage system. Sun et al. [
8] used two refrigerant combinations in their numerical work on a CRS and found better performance using the R41–R404A refrigerant pair. They noted the maximum exergetic efficiencies of the R41/R404A and R23/R404A CRSs to be 44.38% and 42.98%, respectively. Dopazo and Fernández-Seara [
9] also experimentally investigated a CRS with the natural refrigerant combination R744–R717 to determine the optimal condenser temperature of the low-temperature circuit condenser. Dopazo et al. [
10] theoretically simulated a CRS using natural refrigerant pair R744–R717 and experienced a decrease in the COP with the increase in
and
. They also mentioned that the use of carbon dioxide was advantageous, as it reduced the flammability of the hydrocarbons. Rezayan and Behbahaninia [
11] conducted thermoeconomic optimization on a CRS using CO
2–NH
3. They investigated the effect of different operational parameters on the system cost rates. They noted the optimum annualized cost of the system to be USD 109242, which was 9.34% lower than the cost of the base case system. Colorado et al. [
12] carried out a thermodynamic analysis of a CRS for simultaneous cooling and heating using ammonia (NH
3), R134a, butane, and propane in the low-temperature cycle (LTC) and carbon dioxide (CO
2) in the high-temperature cycle (HTC) as refrigerants to find out the optimum performance and optimum design parameters. They found up to 7.3% improvement in the COP using butane in the LTC compared to that with NH
3–CO
2. Messineo and Panno [
13] performed a thermodynamic analysis of a two-stage CRS to replace synthetic refrigerants with natural refrigerants. They obtained a maximum COP of 1.71 using natural refrigerant pair R744–R717 at evaporator and condenser temperatures of −35 °C and 35 °C, respectively. Aminyavari et al. [
14] performed thermoeconomic optimization on a 50 kW cooling capacity CRS. They observed that a 24% increase in exergetic efficiency led to a 164% increase in the total cost rate. Ust and Karakurt [
15] performed an exergetic analysis of a CRS using different refrigerant couples, namely, R23–R290, R23–R404A, R23–R507A, and R23–R717. They noted a decrease in the rate of exergy destruction with the increase in
and a rise in the rate of exergy destruction with the rise in
and
. Kasi [
16] carried out a numerical analysis to analyze the energetic performance of a CRS separately using R-23, R508B, and R170 in the LTC and R134a, R290, R404A, R407C, and R410A in the LTC. They observed the best performances using refrigerant pair R170–R134a and worst performances using refrigerant pair R404A–R508B among all the investigated refrigerant pairs. Rawat and Pratihar [
17] thermodynamically analyzed a CRS using N
2O in the LTC and five different refrigerants, namely, R717, R290, R1290, R134a, and an azeotropic mixture R507A in the HTC. Gholamian et al. [
18] exergetically simulated a CO
2/NH
3-based CRS to determine the magnitude and location of the exergy destructions. The authors of the study determined through a conventional exergy analysis that the condenser of the NH
3 cycle, as well as the throttling valve and compressor of the CO
2 cycle, experienced the most significant exergy destruction. However, the results of an advanced exergy analysis indicated that improvements were needed in the throttling valve, compressor, and cascade condenser of the CO
2 cycle. In fact, the authors found that improving the performances of these components could yield a 63% improved cycle performance. Patel et al. [
19] performed a comparative thermoeconomic analysis and optimization of a CRS using refrigerant pairs NH
3/CO
2 and C
3H
8/CO
2. They concluded that the C
3H
8/CO
2 pair offered 5.33% less cost with 6.42% more exergy destruction compared to the NH
3/CO
2 pair. Roy and Mandal [
20] presented a numerical investigation on a CRS and recommended R161 as an alternative to R404A in the high-temperature cycle. Adebayo et al. [
21] performed a comparative thermodynamic analysis on a CRS using CO
2 in the LTC and four different refrigerants, namely, NH
3, R717, HFE7000, and HFE7100 in the HTC. Aktemur and Öztürk [
22] thermodynamically simulated a CRS using low GWP refrigerant R41 in the LTC and R1243zf, R423A, R601, R601A, R1233zd (E), and RE170 in the HTC. They noted the worst performances in terms of COP and exergy efficiency using R41–R423A. On the other hand, they noted the maximum COP and exergy efficiency to be 1.210 and 37.18%, respectively, using R41–RE170. In another study, Aktemur et al. [
23] studied the effect of
,
, and ΔT on the system’s thermodynamic performances using three low-GWP refrigerant pairs, namely, R41–R601, R41-cyclopentane, and R41–R602A. They noted the best thermodynamic performances using R41–R601, which exerted maximum exergetic efficiencies of 43.10%. However, they noted a very high compressors’ discharge temperature over 120 °C for all three refrigerant pairs. Zhang et al. [
24] conducted an experimental investigation on a CRS using R1270/CO
2 as the refrigerant combination and noted a rise in the COP and cooling capacity with the increase in T
HE. Chen et al. [
25] investigated the influence of the subcooling degree in the LTC of a NH
3/CO
2 CRS to find out the thermodynamic performances of the system. They reported 4.58% improvement in the COP and 4.4% improvement in the exergy efficiency of the modified system compared to the conventional CRS when the subcooling degree was kept fixed to 10 °C. Sun and Wang [
26] simulated a modified CRS for industrial application using the R1150/R717 refrigerant pair to replace the three-stage CRS using R1150/R41/R717 as the refrigerant combinations. Faruque et al. [
27] simulated a CRS using Trans-2-butane, Toluene, Cyclopentane, and Cis-2-butane as refrigerants and thermodynamically analyzed the system. They noted the maximum COP and exergy efficiency using the Trans-2-butane/Toluene refrigerant combination. Cabello et al. [
28] experimentally analyzed the energy performance of a CRS using four alternative refrigerant pairs, namely, R290/R744, R1270/R744, R600a/R744, and R1234ze(E)/R744 and compared the results with the system using R134a/R744. They also carried out an environmental analysis and noted less CO
2 emission using all four refrigerant pairs. Deymi-Dashtebayaz et al. [
29] presented an energy–exergoeconomic–environmental analysis on a CRS using six pairs of low-GWP refrigerants including R41–R161, R41–R1234yf, R41–R1234ze, R744–R161, R744–R1234yf, and R744–R1234ze and reported R41–R161 and R41–R1234ze as the best refrigerant pairs in terms of the COP/exergy efficiency and total cost rate, respectively. Soni et al. [
30] used CO
2 as a high-temperature cycle refrigerant and used different low-temperature refrigerants for the low-temperature cycle for the simulation of a CRS for ultra-low-temperature applications.