Research on the Characteristics and Kinetics of the Pyrolysis Process and Products Generation of Jimsar (China) Oil Shale Using TG-FTIR
Abstract
1. Introduction
2. Experimental Section
2.1. Material
2.2. TG-FTIR Experiments
2.3. Kinetic Analysis of Oil Shale Pyrolysis
2.4. Kinetic and Thermodynamic Analysis of Gaseous Product Generation
2.4.1. Kinetic Analysis of Gaseous Products
2.4.2. Thermodynamic Analysis of Gaseous Products
3. Results and Discussion
3.1. Thermogravimetric Analysis
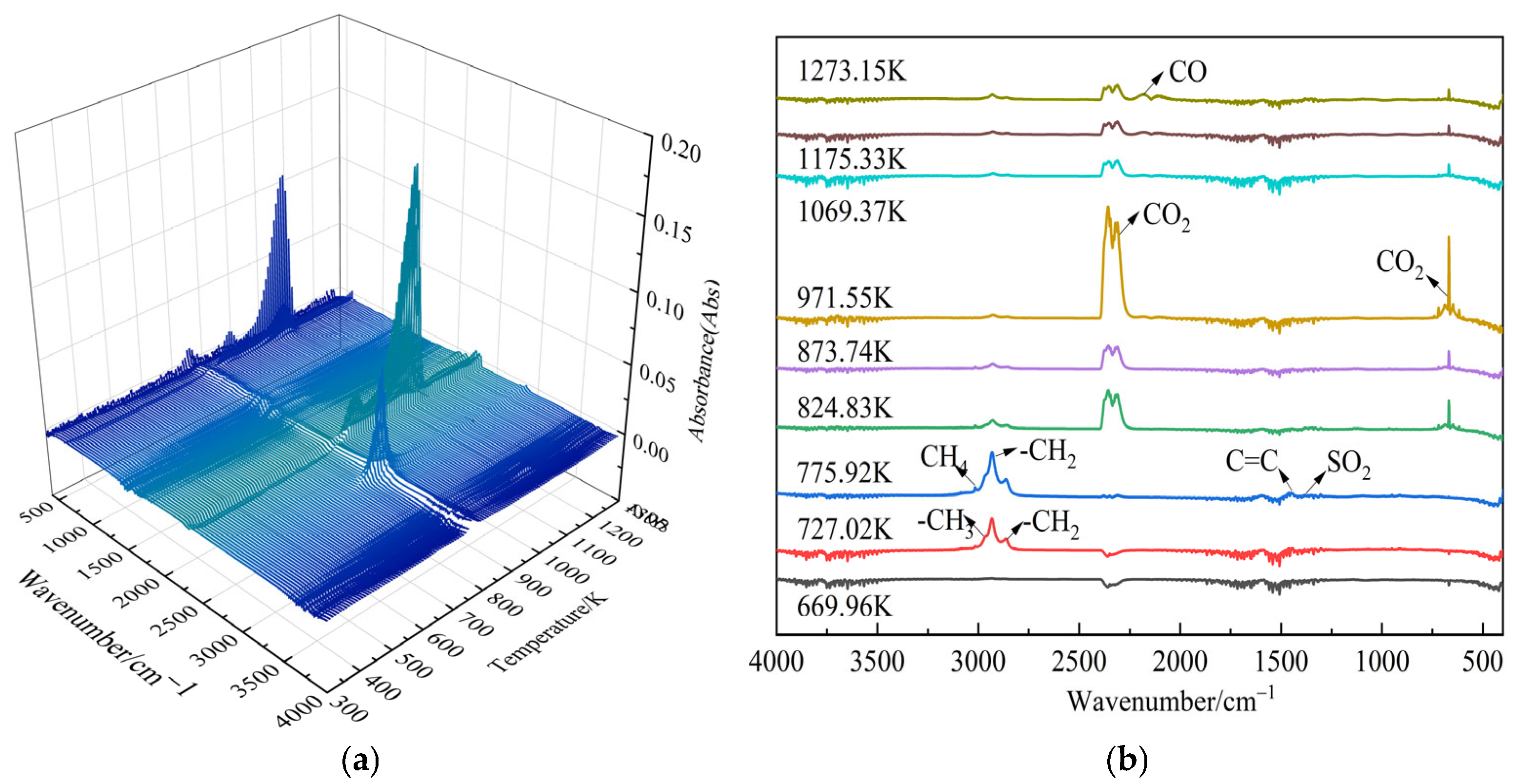
3.2. Infrared Spectroscopy of Gaseous Products
3.2.1. Gram–Schmidt Curves
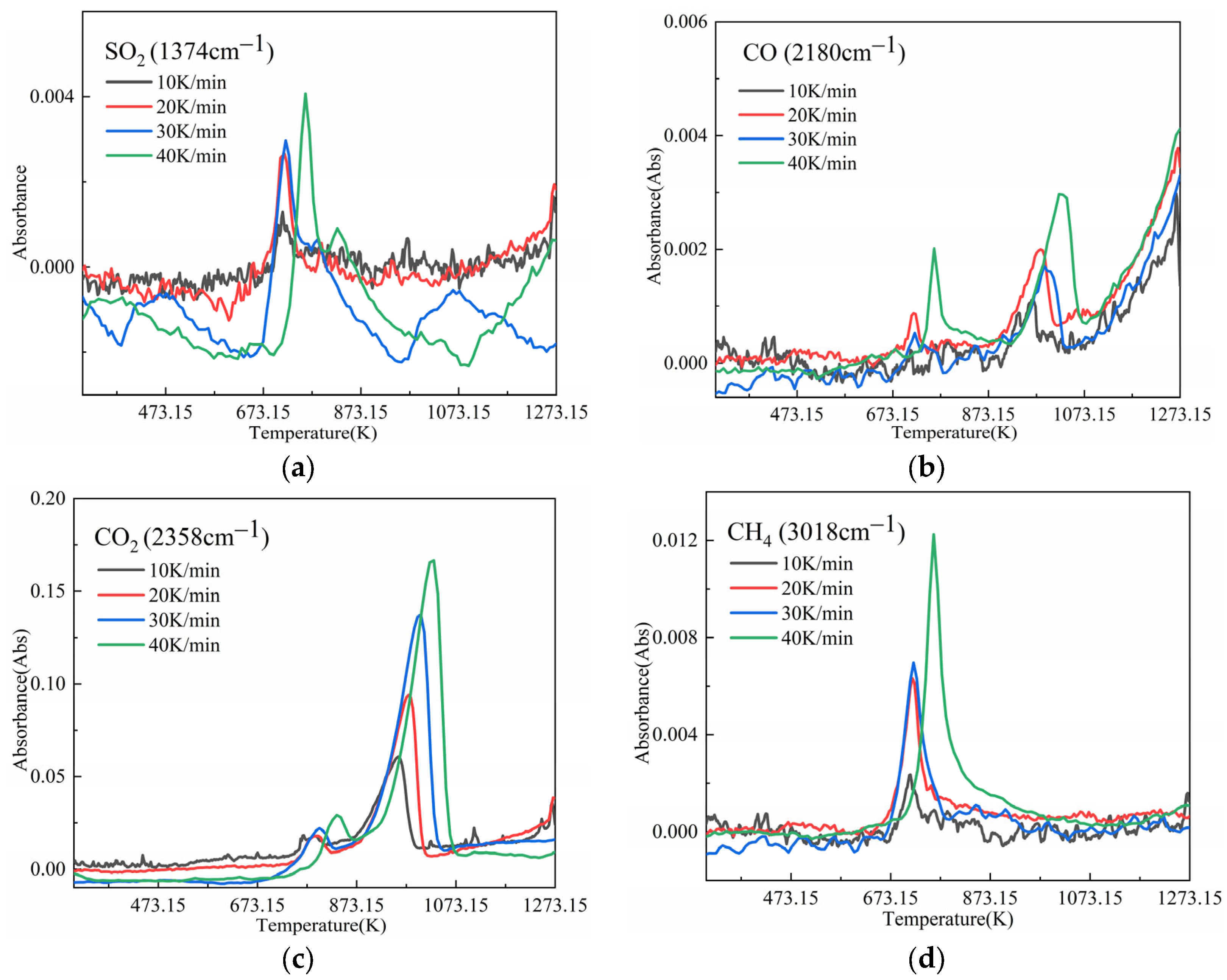
3.2.2. Gaseous Product Analysis
3.3. Kinetic Analysis of Oil Shale Pyrolysis
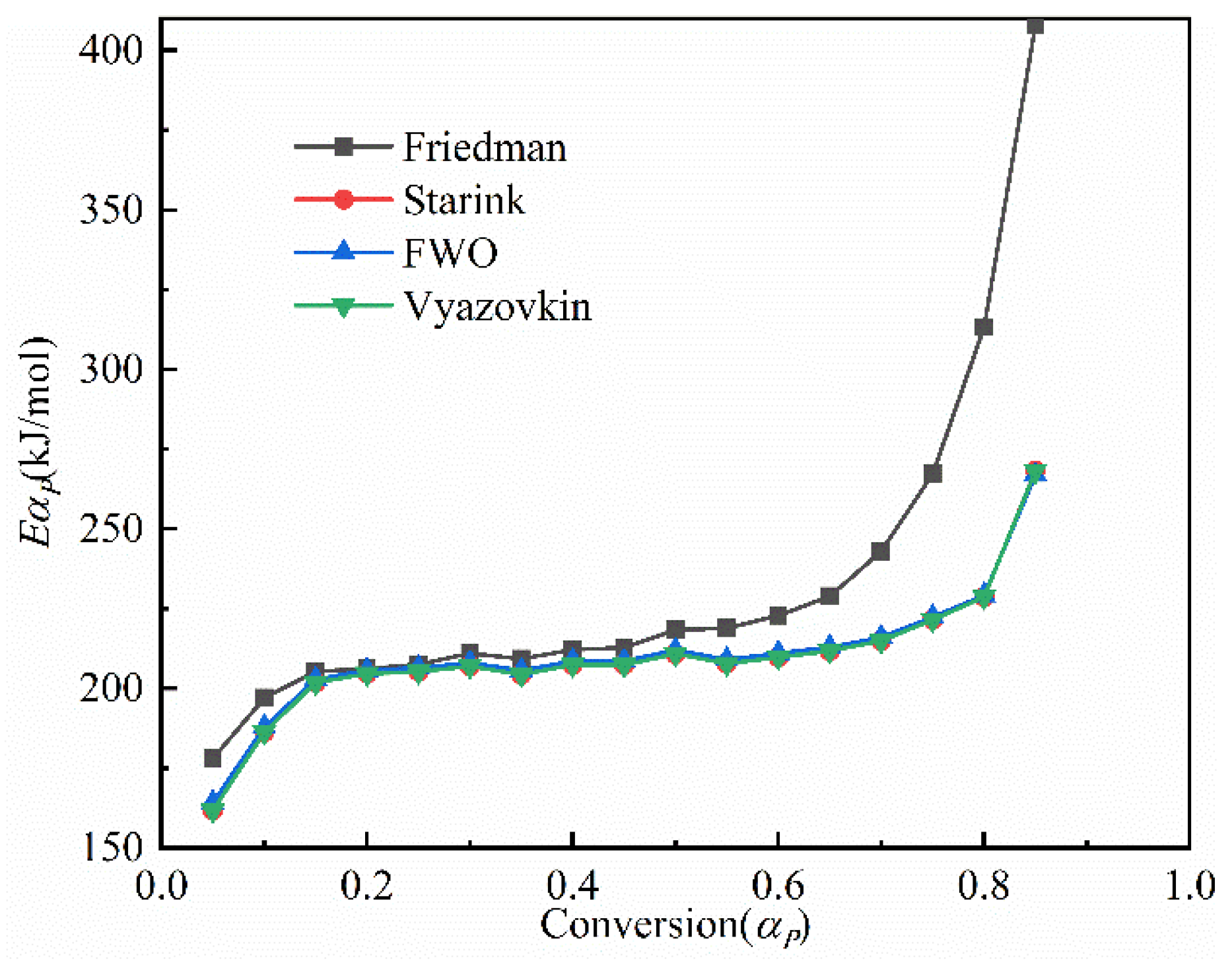
3.3.1. The Calculation of the Activation Energy
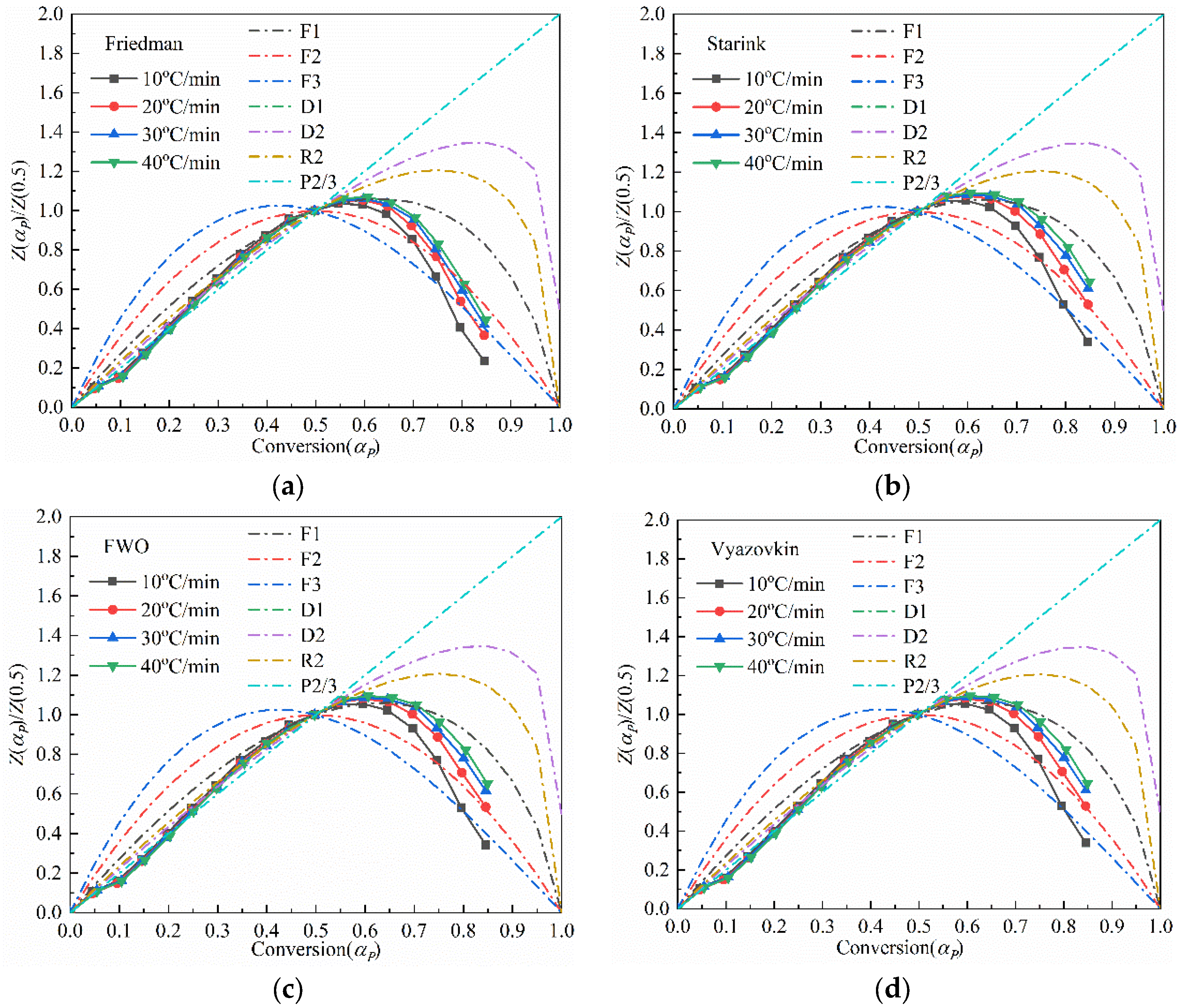
3.3.2. The Evaluation of the Reaction Mechanisms
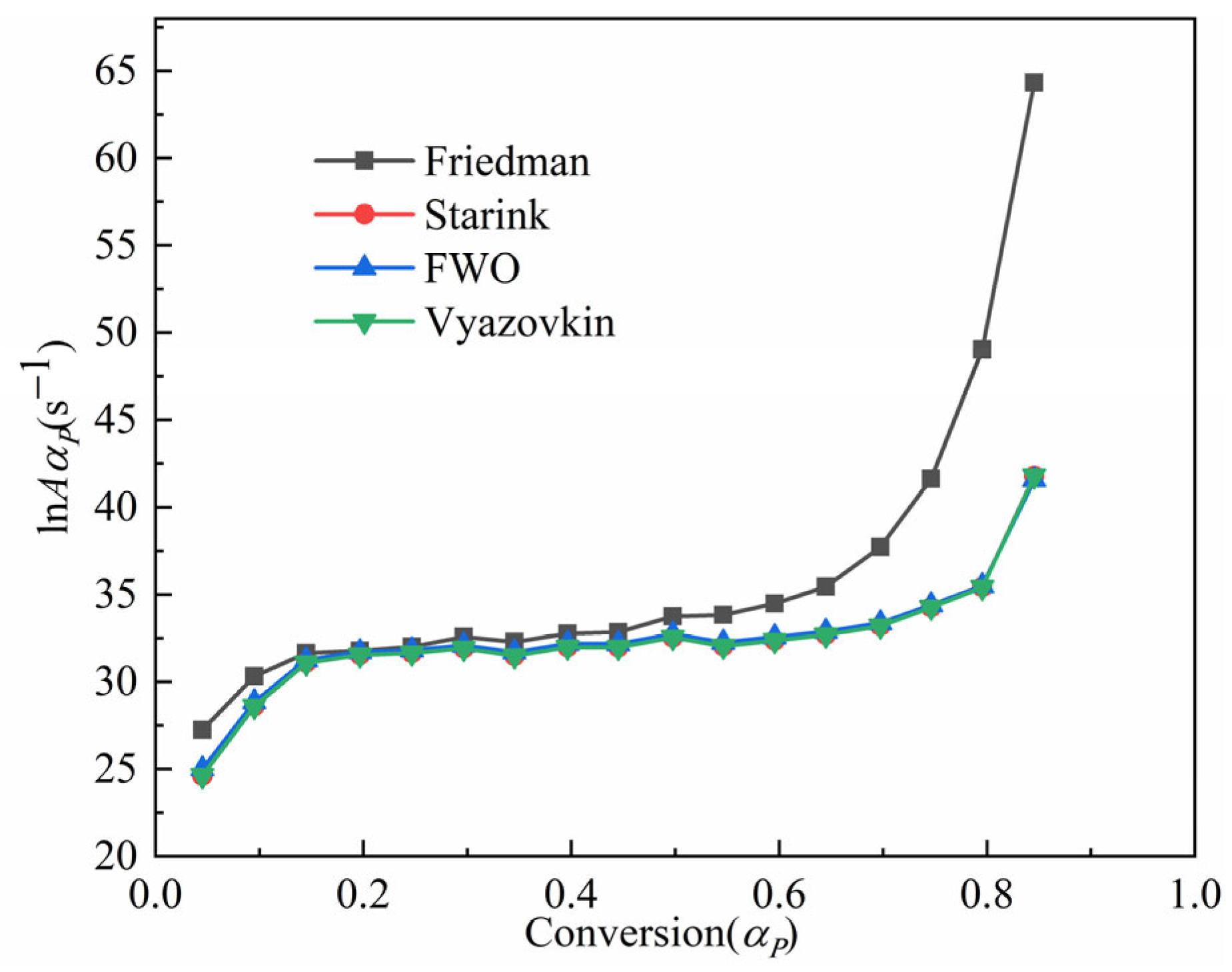
3.3.3. The Calculation of the Pre-Exponential Factor
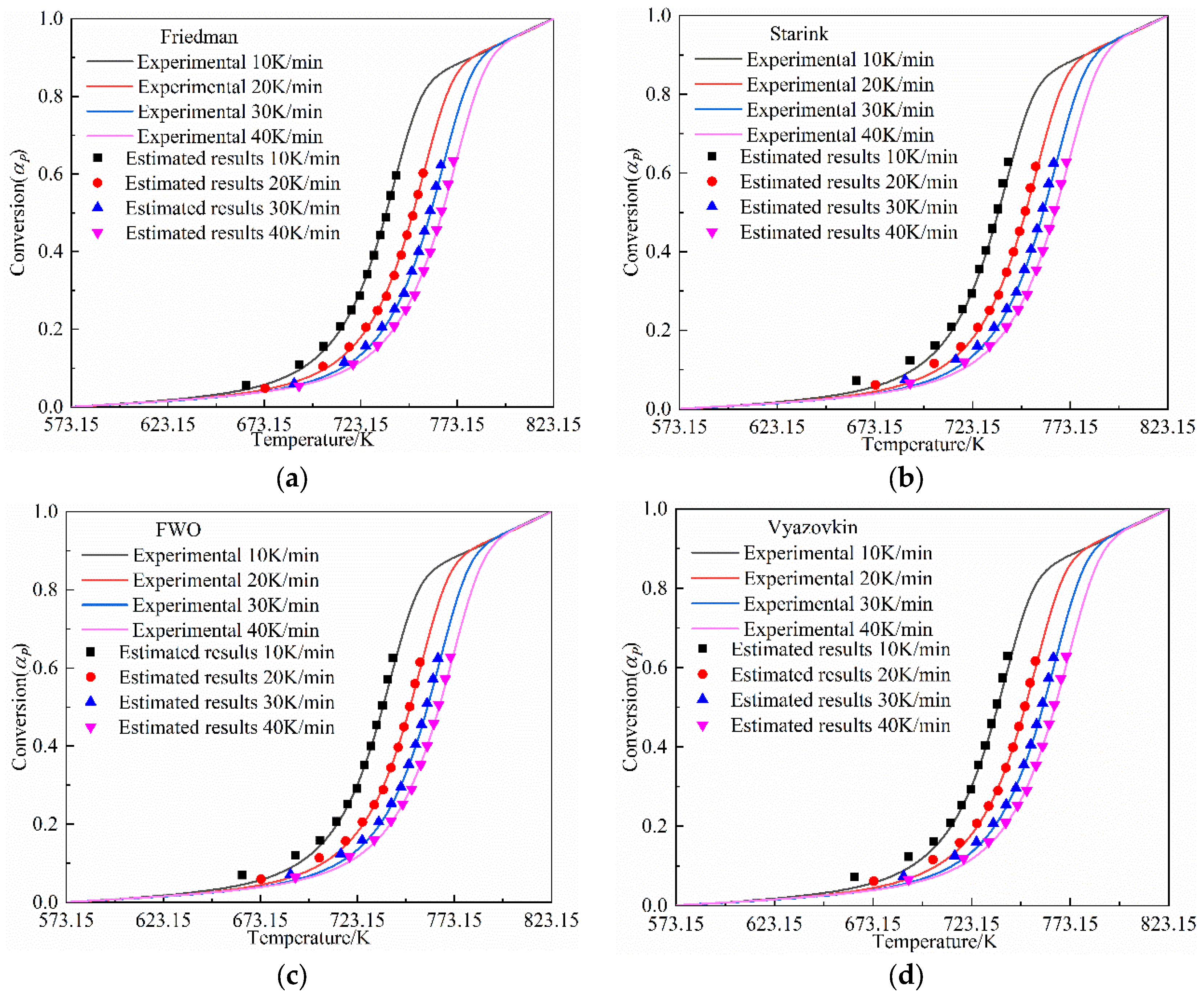
3.3.4. The Verification of the Kinetic Parameters
3.4. Kinetic Analysis of Gaseous Product Generation
3.4.1. Kinetic Analysis
3.4.2. Thermodynamic Parameters Calculation
4. Conclusions
- (1)
- The pyrolysis of the Jimsar oil shale was a multi-step process with a complex reaction mechanism. The isoconversional kinetic studies showed that the pyrolysis of the Jimsar oil shale had different reaction models in different conversion rate ranges: ① = 0–0.2: the reaction model was mainly in the power law (). ② = 0.2–0.6: the reaction model was mainly a phase interfacial reaction (). ③ = 0.6–1: the reaction model was mainly a chemical reaction between the F1 and F2 models ().
- (2)
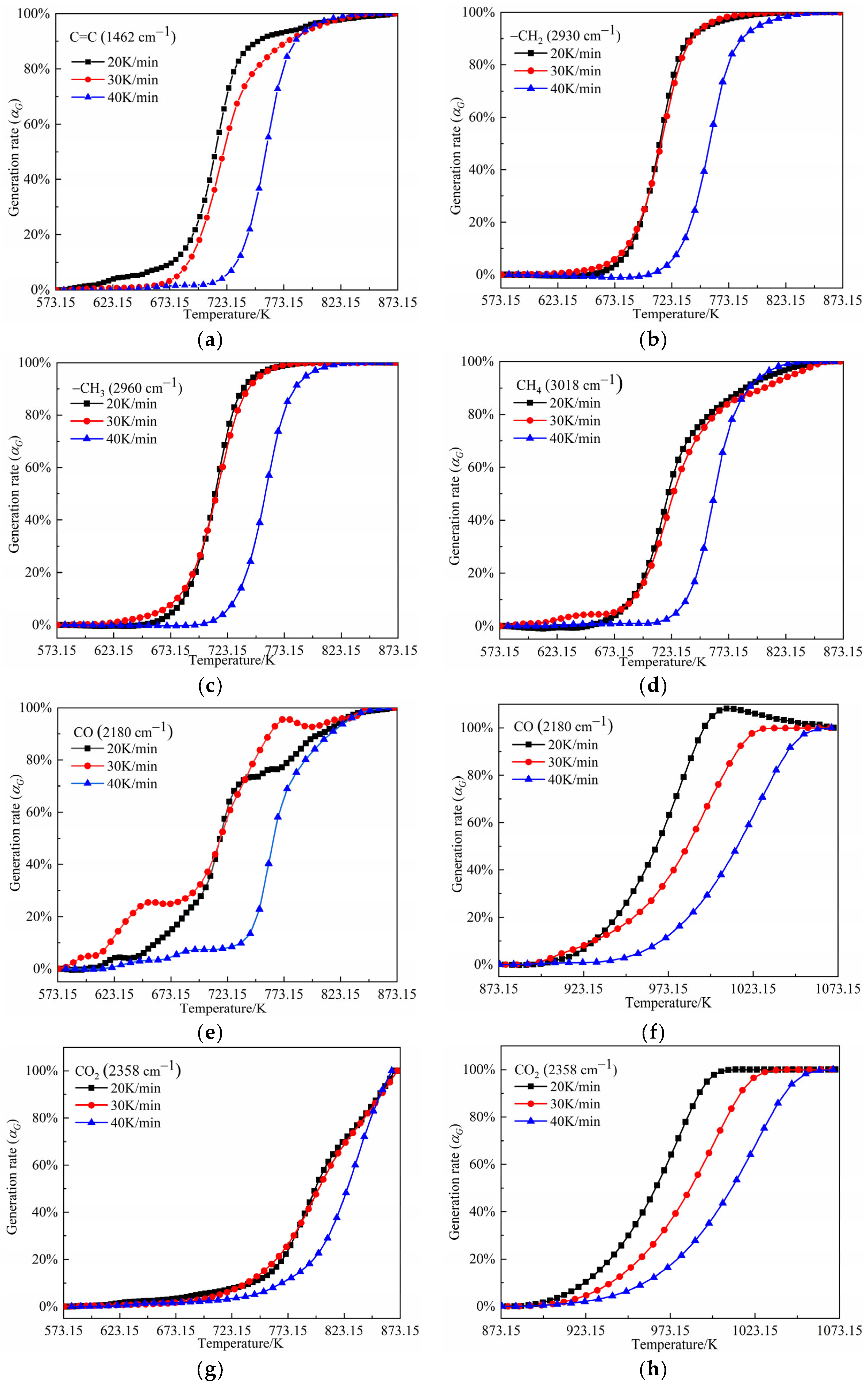
- In the temperature range of 573.15–873.15 K, the gaseous products released from the oil shale pyrolysis were mainly composed of small-molecule compounds (CO2, SO2, CO, and CH4), aliphatic (–CH2, –CH3), aromatic (C=C), and O–H functional groups. In addition, shale oils composed of –CH2, –CH3, and C=C were mainly generated in the temperature range of 673.15–823.15 K. The generation models of C=C, –CH2, –CH3, CH4, CO, and CO2 are F2, F2, F2, F3, F3, and F2, respectively. In addition, the generation activation energies and pre-exponential factors of the above components decreased first and then increased with the increase in the heating rate.
- (3)
- In the temperature range of 873.15–1073.15 K, the gaseous products were mainly composed of CO and CO2. The generation models of CO and CO2 were both D3 diffusion models. In addition, the activation energies of CO and CO2 formation in the higher temperature range of 873.15–1073.15 K were larger than those in the temperature range of 573.15–873.15 K.
- (4)
- The relative values and variation in the enthalpy, Gibbs free energy, and entropy of the components evolved at different heating rates and temperature ranges corresponded with those of the activation energy.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Taheri-Shakib, J.; Kantzas, A. A comprehensive review of microwave application on the oil shale: Prospects for shale oil production. Fuel 2021, 305, 121519. [Google Scholar] [CrossRef]
- Wang, L.; Yang, D.; Kang, Z.Q. Evolution of permeability and mesostructure of oil shale exposed to high-temperature water vapor. Fuel 2021, 290, 119786. [Google Scholar] [CrossRef]
- Moine, E.C.; Groune, K.; El Hamidi, A.; Khachani, M.; Halim, M.; Arsalane, S. Multistep process kinetics of the non-isothermal pyrolysis of Moroccan Rif oil shale. Energy 2016, 115, 931–941. [Google Scholar] [CrossRef]
- He, L.; Ma, Y.; Yue, C.; Li, S.; Tang, X. The heating performance and kinetic behaviour of oil shale during microwave pyrolysis. Energy 2022, 244, 123021. [Google Scholar] [CrossRef]
- Xu, T.; Huang, X.M. Study on combustion mechanism of asphalt binder by using TG-FTIR technique. Fuel 2010, 89, 2185–2190. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Mehmood, M.A.; Liu, C.G.; Tawab, A.; Bai, F.W.; Sakdaronnarong, C.; Xu, J.; Rahimuddin, S.A.; Gull, M. Bioenergy potential of Wolffia arrhiza appraised through pyrolysis, kinetics, thermodynamics parameters and TG-FTIR-MS study of the evolved gases. Bioresour. Technol. 2018, 253, 297–303. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, H.; Hou, X.; Qiu, R.; Chen, Z. Pyrolytic behavior and kinetic of wood sawdust at isothermal and non-isothermal conditions. Renew. Energy 2019, 142, 284–294. [Google Scholar] [CrossRef]
- Li, S.; Ma, X.; Liu, G.; Guo, M. A TG–FTIR investigation to the co-pyrolysis of oil shale with coal. J. Anal. Appl. Pyrolysis 2016, 120, 540–548. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, X.; Han, X.; Liu, J. A TG–FTIR investigation to the catalytic effect of mineral matrix in oil shale on the pyrolysis and combustion of kerogen. Fuel 2013, 104, 307–317. [Google Scholar] [CrossRef]
- You, Y.; Han, X.; Wang, X.; Jiang, X. Evolution of gas and shale oil during oil shale kerogen pyrolysis based on structural characteristics. J. Anal. Appl. Pyrolysis 2019, 138, 203–210. [Google Scholar] [CrossRef]
- Pan, L.; Lu, H.; Dai, F.; Pei, S.; Wu, Q.; Huang, J. Numerical study of the flow, heat transfer and pyrolysis process in the gas full circulation oil shale retort. Oil Shale 2021, 38, 317–337. [Google Scholar] [CrossRef]
- Chi, M.; Xu, X.; Cui, D.; Zhang, H.; Wang, Q. A TG-FTIR investigation and kinetic analysis of oil shale kerogen pyrolysis using the distributed activation energy model. Oil Shale 2016, 33, 228–247. [Google Scholar] [CrossRef]
- Bai, F.T.; Liu, Y.M.; Lai, C.; Sun, Y.H.; Wang, J.X.; Sun, P.C.; Xue, L.F.; Zhao, J.M.; Guo, M.Y. Thermal Degradations and Processes of Four Kerogens via Thermogravimetric-Fourier-Transform Infrared: Pyrolysis Performances, Products, and Kinetics. Energy Fuels 2020, 34, 2969–2979. [Google Scholar] [CrossRef]
- Baruah, B.; Tiwari, P. Compositional and kinetic study of thermal degradation of kerogen using TG-FTIR, NMR, and microscopic study. AIChE J. 2022, 68, e17396. [Google Scholar] [CrossRef]
- Huss, E.B.; Burnham, A.K. Gas evolution during pyrolysis of various Colorado oil shales. Fuel 1982, 61, 1188–1196. [Google Scholar] [CrossRef]
- Suuberg, E.; Sherman, J.; Lilly, W. Product evolution during rapid pyrolysis of Green River Formation oil shale. Fuel 1987, 66, 1176–1184. [Google Scholar] [CrossRef]
- Campbell, J.H.; Koskinas, G.J.; Gallegos, G.; Gregg, M. Gas evolution during oil shale pyrolysis. 1. Nonisothermal rate measurements. Fuel 1980, 59, 718–726. [Google Scholar] [CrossRef]
- Campbell, J.H.; Gallegos, G.; Gregg, M. Gas evolution during oil shale pyrolysis. 2. Kinetic and stoichiometric analysis. Fuel 1980, 59, 727–732. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Perez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Vyazovkin, S. Chapter 13—Isoconversional Kinetics. In Handbook of Thermal Analysis and Calorimetry; Elsevier Science B.V.: Amsterdam, The Netherlands, 2008; Volume 5, pp. 503–538. [Google Scholar]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Flynn, J.; Wall, L. General Treatment of the Thermogravimetry of Polymers. J. Res. Natl. Bur. Stand A Phys. Chem. 1966, 70A, 487–523. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Dollimore, D. Linear and Nonlinear Procedures in Isoconv-ersional Computations of the Activation Energy of Nonisothermal Reactions in Solids. J. Chem. Inf. Model. 1996, 36, 42–45. [Google Scholar]
- Hu, J.; Danish, M.; Lou, Z.; Zhou, P.; Zhu, N.; Yuan, H.; Qian, P.S. Effectiveness of wind turbine blades waste combined with the sewage sludge for enriched carbon preparation through the co-pyrolysis processes. J. Clean. Prod. 2018, 174, 780787. [Google Scholar] [CrossRef]
- Jayaraman, K.; Kok, M.V.; Gokalp, I. Thermogravimetric and mass spectrometric (TG-MS) analysis and kinetics of coal-biomass blends. Renew. Energy 2017, 101, 293–300. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Tariq, R.; Hameed, Z.; Ali, I.; Naqvi, M.; Chen, W.-H.; Ceylan, S.; Rashid, H.; Ahmad, J.; Taqvi, S.A.; et al. Pyrolysis of high ash sewage sludge: Kinetics and thermodynamic analysis using Coats-Redfern method. Renew. Energy 2019, 131, 854–860. [Google Scholar] [CrossRef]
- Huang, L.M.; Liu, J.Y.; He, Y.; Sun, S.Y.; Chen, J.C.; Sun, J.; Chang, K.L.; Kuo, J.H.; Ning, X.A. Thermodynamics and kinetics parameters of co-combustion between sewage sludge and water hyacinth in CO2/O−2 atmosphere as biomass to solid biofuel. Bioresour. Technol. 2016, 218, 631–642. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, Y.S.; Kim, S.H. Investigation of Thermodynamic Parameters in the Thermal Decomposition of Plastic Waste-Waste Lube Oil Compounds. Environ. Sci. Technol. 2010, 44, 5313–5317. [Google Scholar] [CrossRef]
- Olugbenga, A.G.; Yahya, M.D.; Garba, M.U. Utilizing the Decomposition of Onelga Oil Shale to Fix Kinetics Parameter and Heat Energy for Pyrolyser-Reactor. Int. Rev. Mech. Eng. 2021, 15, 106. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Zhang, H.; Xu, X.; Yang, Q.; Wang, P. The simulation study on application of the FG-DVC model to the pyrolysis of Huadian oil shale of China at different heating rates. Oil Shale 2016, 33, 111–124. [Google Scholar] [CrossRef]
- Pan, L.W.; Dai, F.Q.; Li, G.Q.; Liu, S. A TGA/DTA-MS investigation to the influence of process conditions on the pyrolysis of Jimsar oil shale. Energy 2015, 86, 749–757. [Google Scholar] [CrossRef]
- Pan, L.; Dai, F.; Huang, J.; Liu, S.; Li, G. Study of the effect of mineral matters on the thermal decomposition of Jimsar oil shale using TG–MS. Thermochim. Acta 2016, 31, 627–629. [Google Scholar] [CrossRef]
- Qian, Y.A.; Zhan, J.H.; Lai, D.G.; Li, M.Y.; Liu, X.X.; Xu, G.W. Primary understanding of non-isothermal pyrolysis behavior for oil shale kerogen using reactive molecular dynamics simulation. Int. J. Hydrogen Energy 2016, 41, 12093–12100. [Google Scholar] [CrossRef]
- Qu, B.Y.; Li, A.M.; Qu, Y.; Wang, T.; Zhang, Y.; Wang, X.; Gao, Y.; Fu, W.; Ji, G.Z. Kinetic analysis of waste tire pyrolysis with metal oxide and zeolitic catalysts. J. Anal. Appl. Pyrolysis 2020, 152, 104949. [Google Scholar] [CrossRef]
- Mishra, G.; Bhaskar, T. Non isothermal model free kinetics for pyrolysis of rice straw. Bioresour. Technol. 2014, 169, 614–621. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, F.; Bai, C.; Wen, L.; Zou, C. Thermal behavior and kinetics of the pyrolysis of the coal used in the COREX process. J. Anal. Appl. Pyrolysis 2013, 104, 660–666. [Google Scholar] [CrossRef]
- Chen, H.; Li, B.; Zhang, B. Decomposition of pyrite and the interaction of pyrite with coal organic matrix in pyrolysis and hydropyrolysis. Fuel 2000, 79, 1627–1631. [Google Scholar] [CrossRef]
- Lu, H.; Pan, L.; Guo, Y.; Dai, F.; Pei, S.; Huang, J.; Liu, S. The effect of pyrolysis conditions on the composition of Chinese Jimsar shale oil using FT-IR, 1H-NMR and 13C-NMR techniques. Oil Shale 2022, 39, 37–60. [Google Scholar] [CrossRef]
- Pan, L.; Dai, F.; Pei, S.; Huang, J.; Liu, S. Influence of particle size and temperature on the yield and composition of products from the pyrolysis of Jimsar (China) oil shale. J. Anal. Appl. Pyrolysis 2021, 157, 105211. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Solid-state kinetic models: Basics and mathematical fundamentals. J. Phys. Chem. B 2006, 110, 17315. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Niu, S.L.; Lu, C.M.; Cheng, S.Q. Comparative evaluation of thermal degradation for biodiesels derived from various feedstocks through transesterification. Energy Convers. Manag. 2015, 98, 81–88. [Google Scholar] [CrossRef]
- Turmanova, S.C.; Genieva, S.D.; Dimitrova, A.S.; Vlaev, L.T. Non-isothermal degradation kinetics of filled with rise husk ash polypropene composites. eXPRESS Polym. Lett. 2008, 2, 133–146. [Google Scholar] [CrossRef]





| Proximate analysis | Moisture (ad) | 0.50 |
| Volatile matter (ad) | 17.48 | |
| Fixed carbon (ad) | 0.90 | |
| Ash (ad) | 81.12 | |
| Ultimate analysis | Cd | 11.48 |
| Hd | 1.68 | |
| Od | 8.35 | |
| Nd | 0.605 | |
| Sd | 0.51 |
| β/(K/min) | Ts/K | Tmax/K | D/10−10/(min·K3) | ||
|---|---|---|---|---|---|
| 10 | 729.82 | 1.27 | 737.48 | 709.82 | 1.37 |
| 20 | 746.15 | 1.89 | 749.48 | 709.82 | 1.92 |
| 30 | 756.15 | 2.11 | 760.15 | 703.15 | 2.09 |
| 40 | 765.15 | 2.30 | 772.48 | 693.82 | 2.23 |
| Component | Heating Rates (K/min) | Generation Model | 573.15–873.15 (K) | Generation Model | 873.15–1073.15 (K) | ||||
|---|---|---|---|---|---|---|---|---|---|
| (kJ/mol) | R2 | (1/s) | E (kJ/mol) | R2 | (1/s) | ||||
| CO | 20 | ) | 173.85 | 0.961 | 29.35 | D3 | 679.19 | 0.998 | 81.96 |
| 30 | ) | 102.92 | 0.944 | 15.59 | 498.61 | 0.998 | 58.42 | ||
| 40 | D3 | 336.78 | 0.998 | 50.31 | 592.33 | 0.997 | 68.08 | ||
| CO2 | 20 | ) | 189.40 | 0.988 | 28.20 | D3 | 589.71 | 0.999 | 70.78 |
| 30 | ) | 122.40 | 0.995 | 17.57 | 541.82 | 0.999 | 63.67 | ||
| 40 | ) | 301.57 | 0.995 | 42.75 | 526.59 | 0.999 | 60.48 | ||
| Component | Generation Model | Heating Rates (K/min) | 573.15–873.15 (K) | ||
|---|---|---|---|---|---|
| (kJ/mol) | R2 | (1/s) | |||
| C=C | ) | 20 | 253.22 | 0.978 | 42.95 |
| 30 | 217.16 | 0.962 | 36.50 | ||
| 40 | 400.34 | 0.999 | 64.82 | ||
| –CH2 | ) | 20 | 342.06 | 0.998 | 58.27 |
| 30 | 307.16 | 0.993 | 52.64 | ||
| 40 | 378.58 | 0.999 | 61.37 | ||
| –CH3 | ) | 20 | 342.64 | 0.995 | 58.43 |
| 30 | 284.39 | 0.988 | 48.76 | ||
| 40 | 390.12 | 0.999 | 63.26 | ||
| CH4 | ) | 20 | 266.18 | 0.983 | 44.90 |
| 30 | 237.90 | 0.976 | 40.21 | ||
| 40 | 530.83 | 0.996 | 85.92 | ||
| Component | Generation Model | Heating Rates (K/min) | 573.15–873.15 (K) | Generation Model | 873.15–1073.15 (K) | ||||
|---|---|---|---|---|---|---|---|---|---|
| (kJ/mol) | (kJ/mol) | (kJ/mol) | (kJ/mol) | (J/mol) | (kJ/mol) | ||||
| CO | F3 | 20 | 126.59 | 133.78 | −0.014 | D3 | 673.30 | 374.81 | 0.42 |
| D1 | 30 | 99.20 | 156.04 | −0.13 | 492.65 | 331.26 | 0.23 | ||
| D3 | 40 | 332.73 | 254.22 | 0.16 | 586.10 | 357.48 | 0.31 | ||
| CO2 | F2 | 20 | 185.09 | 197.24 | −0.023 | D3 | 583.85 | 352.62 | 0.33 |
| F1 | 30 | 118.04 | 176.71 | −0.11 | 535.76 | 339.97 | 0.27 | ||
| D2 | 40 | 296.90 | 242.47 | 0.097 | 520.31 | 337.71 | 0.24 | ||
| Component | Generation Model | Heating Rates (K/min) | 573.15–873.15 (K) | ||
|---|---|---|---|---|---|
| (kJ/mol) | (kJ/mol) | (kJ/mol) | |||
| C=C | F2 | 20 | 249.57 | 205.36 | 0.10 |
| 30 | 213.45 | 192.55 | 0.05 | ||
| 40 | 396.30 | 259.32 | 0.28 | ||
| –CH2 | F2 | 20 | 338.37 | 237.34 | 0.23 |
| 30 | 303.45 | 222.74 | 0.18 | ||
| 40 | 374.54 | 251.49 | 0.25 | ||
| –CH3 | F2 | 20 | 338.95 | 237.33 | 0.23 |
| 30 | 280.68 | 214.35 | 0.15 | ||
| 40 | 386.08 | 255.39 | 0.27 | ||
| CH4 | F3 | 20 | 262.49 | 210.72 | 0.12 |
| 30 | 234.19 | 199.55 | 0.08 | ||
| 40 | 526.79 | 304.51 | 0.46 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Pan, L.; Chen, P.; Liu, T. Research on the Characteristics and Kinetics of the Pyrolysis Process and Products Generation of Jimsar (China) Oil Shale Using TG-FTIR. Processes 2023, 11, 1535. https://doi.org/10.3390/pr11051535
Lu H, Pan L, Chen P, Liu T. Research on the Characteristics and Kinetics of the Pyrolysis Process and Products Generation of Jimsar (China) Oil Shale Using TG-FTIR. Processes. 2023; 11(5):1535. https://doi.org/10.3390/pr11051535
Chicago/Turabian StyleLu, Hao, Luwei Pan, Pingan Chen, and Ting Liu. 2023. "Research on the Characteristics and Kinetics of the Pyrolysis Process and Products Generation of Jimsar (China) Oil Shale Using TG-FTIR" Processes 11, no. 5: 1535. https://doi.org/10.3390/pr11051535
APA StyleLu, H., Pan, L., Chen, P., & Liu, T. (2023). Research on the Characteristics and Kinetics of the Pyrolysis Process and Products Generation of Jimsar (China) Oil Shale Using TG-FTIR. Processes, 11(5), 1535. https://doi.org/10.3390/pr11051535






