Abstract
Type 2 diabetes mellitus (T2DM) is the metabolic disease with the highest morbidity rates worldwide. The condition is characterized by hyperglycemia, insulin resistance, hyperlipidemia, and chronic inflammation, among other detrimental conditions. These decrease the efficiency of the immune system, leading to an increase in the susceptibility to bacterial infections. Maintaining an optimal blood glucose level is crucial in relation to the treatment of T2DM, because if the level of this carbohydrate is lowered, the risk of infections can be reduced. Currently, this is achieved using synthetic drug treatments that seek to moderately inhibit digestive enzymes (e.g., α-amylase and α-glucosidase), such as acarbose, voglibose, miglitol, etc. However, the use of these compounds also generates unwanted side effects such as nausea, diarrhea, stomach aches and a loss of appetite. Therefore, there is an increasing demand to find effective and safe alternatives for treating T2DM, such as herbal treatments. As a result, there has been a search for possible drugs from plants with both antidiabetic and antibacterial activity. This study presents a review of the molecular and cellular mechanisms of T2DM, secondary effects of the disease such as bacterial infections, and general comprehension of synthetic and natural product treatments to help patients.
1. Introduction
Type 2 diabetes mellitus (T2DM) is one of the most widespread and important metabolic diseases nowadays in Mexico (Figure 1A, [1]) and worldwide [2]. Despite being non-contagious, it can be considered a pandemic, inflicting enormous physiological and psychological strain on patients and extreme costs on public and private healthcare systems, particularly for the treatment of complications and provision of preventive pharmaceuticals (Figure 1B, [3]). Estimates suggest that more than 600 million patients will be diagnosed with T2DM by the year 2035, painting a grim picture of the future [4] (Figure 1C, [5]). Therefore, there is an urgent need for substantial and sustainable improvements in all aspects of T2DM management, from diagnosis to treatment and, hopefully, to prevention. Importantly, T2DM is one of the five diseases with the highest morbidity rates worldwide [6]. In Mexico, for example, T2DM cases and mortality are rising particularly fast, which is at least partially based on the increasingly urban lifestyle of the population [7] (Figure 1D, [4]). This change of lifestyle correlates with a pronounced increase in carbohydrate consumption, which is estimated to comprise 61% of the average diet [8]. T2DM is the most common cause of death in women and the second most common cause of death in men in Mexico [7,9]. The denominator of T2DM-related pathologies is a persistent state of hyperglycemia, which upsets and dysregulates nutrient metabolism, mostly via impaired insulin signaling processes [10]. Therefore, maintaining physiologically normal glucose levels in the blood of patients is one of the chief priorities in the treatment of T2DM. It is important to highlight that although diabetes is an irreversible condition, a lack of control of this condition leads to a decrease in the patient’s lifespan and quality of life, in addition to the development of diseases such as peripheral arterial disease, stroke, ischemic heart disease, heart failure, and chronic kidney disease, increasing the risk of death [11,12,13].
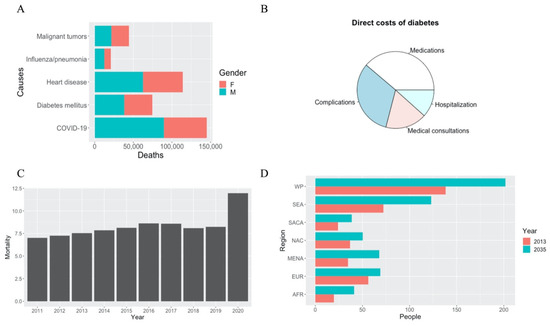
Figure 1.
Overview of data related to T2DM. (A) Number of deaths due to the five primary causes in the January–June 2021 period in Mexico, according to INEGI (Instituto Nacional de Estadística y Geografía). Orange portions of bars indicate female deaths and green indicate male deaths. (B) Pie chart representing the main direct costs of diabetes, where medications are the biggest portion, according to Bello-Chavolla et al., 2017. (C) Evolution of the mortality rate of DM from 2011–2020 per 10,000 inhabitants, according to INEGI (D) Projections of diabetes cases by 2035 compared to cases in 2013, according to Guariguata et al., 2014. WP: Western Pacific; EUR: Europe; AFR: Africa; MENA: Middle East and North Africa; NAC: North America and Caribbean; SACA: South and Central America; SEA: South-East Asia.
Nowadays, many patients with T2DM have access to synthetic drugs that can control carbohydrate homeostasis. One of the strategies for achieving this goal is a moderate downregulation of digestive enzymes (e.g., α-amylase and α-glucosidase). In addition to prescribing synthetic drugs, this can be achieved using acknowledged traditional medicine approaches with the help of natural extracts. Extracts from plants have been useful for treating T2DM [14,15,16,17]. The latter strategy represents an interesting alternative, with increased accessibility in certain regions and reduced side effects for the patient who is under treatment. Treatments often include ways to combat bacterial infections that can occur as a secondary pathology in diabetic patients (see Section 3). An important example is diabetic foot infections, which can originate due to hyperglycemic damage to blood vessels and nerves in the foot [18]. Often, standard antibiotic therapies are the method of choice for the treatment of these infections [19].
In this review, we first provide a summary of synthetic drugs and their uses, before moving on to the treatment of bacterial infections in T2DM patients and continuing with an overview on T2DM-relevant compounds isolated from medical plants. Finally, we propose some new directions for effectively utilizing medical plant extracts as an interesting alternative for the treatment of T2DM.
2. Brief Overview of Strategies to Combat T2DM
In this section, we give a short overview of established and commonly prescribed pharmaceuticals for the treatment of T2DM (Figure 2 and Table 1). For a detailed description of compounds for the treatment of T2DM and their modes of action, the reader is referred to several excellent reviews on this topic [20,21,22].
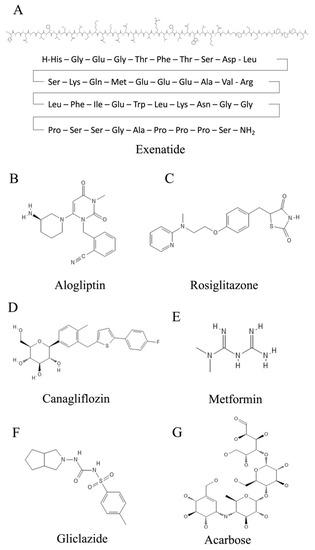
Figure 2.
Chemical structures of compounds used for the treatment of T2DM. (A) Exenatide. (B) Alogliptin. (C) Rosiglitazone. (D) Canagliflozin. (E) Metformin. (F) Gliclazide. (G) Acarbose.
One strategy to treat T2DM is to enhance both the production and release of insulin from pancreatic β cells by modulating signaling via the GLP1 (glucagon-like-peptide 1) system [23,24]. GLP1 is a peptide hormone that is formed in the intestine. GLP1 interacts with its receptor, GLPR1 (glucagon-like-peptide receptor 1), which is located on the β cells of the pancreas and on the neurons of the brain. In addition, by increasing insulin levels, the amount of the insulin antagonist glucagon circulating in the blood is decreased. A further effect mediated by GLP1 signaling is reduced food intake [25]. Consequently, agonists of GLP1R are attractive compounds for treating T2DM-associated complications. Among these are drugs such as dulaglutide, exenatide (Figure 2A and Table 1) and liraglutide. Dulaglutide is a recombinant DNA-produced analog of GLP1 (7-37) that is covalently connected to each Fc arm of human IgG [26]. Exenatide has a natural origin, as it was first identified in the saliva of the North American Gila monster (Heloderma suspectum), a venomous lizard, although it is now manufactured biotechnologically [27]. A new strategy is the use of dual GIP (glucose-dependent insulinotropic polypeptide)/GLP-1 receptor agonists such as tirzepatide, which has recently been approved by the European Medicines Agency [28]. This approach seems to be promising, as the treatments demonstrate improved efficacy, i.e., better reduction of glycated hemoglobin (HbA1c) and body weight than the exclusive use of GLP-1 analogues such as dulaglutide and semaglutide [29].
GLP1 is subject to degradation by the enzyme dipeptidyl-peptidase 4 (DPP4) [30]. In addition to several other complications, T2DM patients present elevated DPP4 levels. In addition to maintaining higher levels of detrimental glucose, disease phenotypes of hepatocytes are manifested, e.g., fibrosis or even apoptosis [24]. The gliptins are competitive inhibitors of DPP4 and can, therefore, counteract the detrimental action of this proteolytic enzyme. Among these are compounds such as alogliptin (Figure 2B and Table 1) and linagliptin, in addition to a few others [31].
Another way to combat T2DM is to enhance the sensitivity of the body to respond to insulin. Here, thiazolidinedione insulin sensitizers play an important role [32]. Two established members of this family are pioglitazone and rosiglitazone (Figure 2C and Table 1), which are both capable of strongly activating the nuclear receptor peroxisome proliferator-activated receptor gamma (PPARγ) [33]. This receptor has various important roles in the differentiation and function of adipocytes. Ligand binding to PPARγ leads to the stimulation of adipokine production and processing and increases the sensitivity of target cells to insulin, among other functions [34,35]. This process is accompanied by a decrease in free fatty acids in the blood plasma. Both pioglitazone and rosiglitazone have additional mechanisms of action [36]. Pioglitazone has been shown to decrease gluconeogenesis in the liver and reduce the quantity of glucose and glycated hemoglobin in the bloodstream [37]. In addition to its insulin-sensitizing function, rosiglitazone shows anti-inflammatory effects [38]. This is due to an increase in the NF-κB inhibitor (IκB), which targets the molecular signal nuclear factor kappa-B (NF-κB), thereby reducing the inflammatory response.
The Na-dependent glucose transporter SGLT2 is necessary for the reabsorption of glucose in the kidney’s proximal tubule epithelium [39]. As such, it is also an attractive target for pharmacological intervention for the treatment of T2DM. The inhibition of SGLT2 by gliflozins (e.g., canagliflozin (Figure 2D and Table 1) and dapagliflozin) results in the elevated excretion of excess glucose, in addition to reducing both body weight and risks of complications in the cardiovascular system [40,41].
Metformin is one of the oldest pharmacological substances for lowering glucose levels in plasma. It was already described in 1922 [42,43]. The biguanide metformin (Figure 2E and Table 1) has several modes of action that help patients suffering from T2DM and obesity. For example, it inhibits the metabolic pathway of gluconeogenesis in the liver, mostly by inhibiting mitochondrial glycerol-3-phosphate dehydrogenase [44,45]. Furthermore, it is proposed to down-regulate the mitochondrial respiratory chain via binding to complex I and to activate the metabolic regulator kinase AMP-activated protein kinase (AMPK) [46]. Although it is the first line of treatment against T2DM and obesity, its exact molecular mechanisms remain to be elucidated.
There are also ways to increase the secretion of insulin by the β cells of the pancreas. The sulfonylureas (e.g., gliclazide (Figure 2F and Table 1)) offer relevant treatment options in this regard [47,48]. They block the potassium channels of the β cells. The subsequent cell depolarization leads to an influx of calcium ions, which elicits exocytosis of insulin-containing vesicles from the β cells. This helps to lower the glucose levels in the blood.
In 1996, the Bayer group introduced acarbose as an intestinal drug. This compound originates from soil bacteria (Actinoplanes utahensis) [49]. Acarbose (C25H43NO18) is a tetrasaccharide-derived metabolite that is constituted by valienamine bonded via nitrogen to isomaltotriose (Figure 2G) [50]. This is a drug used in the treatment of T2DM, and it plays a fundamental role as a mixed non-competitive inhibitor of α-amylase and competitive inhibitor of α-glucosidase in delaying the complete release of glucose for absorption into the bloodstream, meaning glucose will not appear in the digestive system but will appear completely in the distal parts of the intestine [51,52]. Said enzymes participate in the decomposition of carbohydrates or the hydrolysis of oligosaccharides (sucrose and glucose), that is, the inhibitor slows down the metabolism with the aim that the foods consumed with a high sugar content are more slowly assimilated for people with diabetes [53]. In other words, acarbose blocks the ability of the previously mentioned digestive enzymes to break down starch and carbohydrates in the gastrointestinal tract.
Acarbose is one of the most widely used drugs because it is an affordable compound. However, acarbose is not currently the most widely used inhibitor by the medical community due to its gastrointestinal side effects (i.e., flatulence and abdominal discomfort). The doses of its application range from 25 mg to 200 mg, with 100 mg being the average dose of its oral administration three times a day after food ingestion [54].
Lastly, there are further options to pharmacologically target α-glycoside hydrolases in the intestine using iminosugars such as the drug miglitol [55]. Mechanistically, the conjugate acid mimics the positive charge characteristic of the transition state for the enzymatic hydrolysis of the glucoside bond [56]. One route to synthesize iminosugars is via chemical conversion of 1,2-azidoacetates [57]. This approach allows the production of potential antidiabetic drugs such as novel piperidine derivatives [58].

Table 1.
Several pharmacological compounds for the treatment of T2DM.
Table 1.
Several pharmacological compounds for the treatment of T2DM.
| Compound | Molecular Weight | Description of Function | References |
|---|---|---|---|
| Alogliptin | 339.39 g/mol | A highly selective DPP4 inhibitor, it results in the prolonged effect of incretin GLP1 and, thus, increases insulin secretion and inhibits glucagon secretion, helping to lower blood glucose and to achieve improved glycemic control in T2DM patients. | [59] |
| Rosiglitazone | 357.43 g/mol | Through activation of PPARγ, it has primary effects on adipose tissue and decreases insulin resistance by reducing hepatic triglycerides, decreasing visceral fat mass, and increasing subcutaneous fat mass. | [36] |
| Canagliflozin | 444.52 g/mol | As a competitive, reversible, and highly selective SGLT2 inhibitor, it leads to a reduction in glucose reabsorption from primary urine. The induced glucosuria results in optimized glycemic control as well as an energy deficit, which translates into a body weight reduction. | [60] |
| Metformin | 129.16 g/mol | Exact mechanisms remain elusive. It acts as an antihyperglycemic agent, possibly through a decrease in hepatic glucose production partially caused by an interaction with the mitochondrial respiratory chain complex I. | [61] |
| Gliclazide | 323.41 g/mol | Belongs to the group of sulfonylureas that stimulate basal and meal-stimulated insulin secretion through binding to the B cell receptor SUR1, a subunit of an ATP sensitive potassium channel, which, when blocked, leads to an increase in insulin secretion. | [62,63] |
| Exenatide | 4186.63 g/mol | A GLP1R agonist, it acts to increase glucose-dependent insulin secretion from B cells, suppress glucagon secretion, and delay gastric emptying, and it leads to a reduction in calorie intake and body weight. | [64,65] |
3. The Treatment of Bacterial Infections in T2DM Patients
Individuals with T2DM are at a higher risk of developing infectious diseases. The main reasons for this are an impaired immune system, a hyperglycemic environment, and other associated factors [66]. In addition, there is a relationship between diabetes and bacterial infections, since bacterial infections such as malignant otitis externa, periodontitis, emphysematous pyelonephritis, and emphysematous cholecystitis are much more frequent in diabetic patients. These can be more serious in diabetics than in non-diabetics [67]. These infections are usually the first manifestation of unrecognized long-standing diabetes [68]. The most common bacteria are streptococci, pneumococci, and enterobacteria, and several reasons can explain this correlation [69,70,71]. In T2DM patients, more glucose is available that can be used by invading bacteria as a source of metabolic energy, which boosts the proliferation rate. Perhaps even more pronounced is the link between a strongly activated and compromised immune system and bacterial infections in T2DM patients [66]. The end products of choline degradation by Firmicutes, Actinobacteria, and Proteobacteria have been associated with the development of cardiovascular disease and diabetes as products that favor the development of harmful oxidative stress [72].
In addition to the medical complications seen in patients suffering from T2DM, an elevated risk of contracting bacterial infections is observed, with Klebsiella pneumoniae and Escherichia coli being regarded as the most common ones [73]. The most frequent site of infection is the urinary tract (UTIs, urinary tract infections) [74,75,76,77] (Figure 3), leading to acute pyelonephritis and asymptomatic bacteriuria, among other complications. In 2005, Brown et al. identified further risk factors for infections of the urinary tract, such as age and additional conditions (e.g., primarily diabetic cystopathy and nephropathy) [78]. The main risk factors for UTIs in T2DM are inadequate glycemic control, duration of T2DM, diabetic microangiopathy, impaired leukocyte function, recurrent vaginitis, and anatomical and functional abnormalities of the urinary tract [79,80].
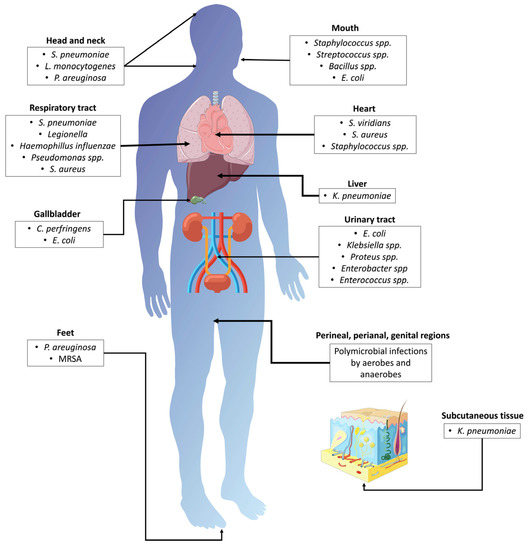
Figure 3.
Bacterial infections associated with T2DM. MRSA: methicillin-resistant Staphylococcus aureus; S. pneumoniae: Streptococcus pneumoniae; L. monocytogenes: Listeria monocytogenes; E. coli: Escherichia coli; S. aureus: Staphylococcus aureus; S. viridians: Streptococcus viridians; K. pneumoniae: Klebsiella pneumoniae; C. perfringens: Clostridium perfringens; P. aeruginosa: Pseudomonas aeruginosa.
Several reasons can explain this correlation. In T2DM patients, there is a clearly documented link between a strongly activated and compromised immune system and bacterial infections in T2DM patients [76,81]. Here, various aspects can be distinguished [82]. First, the rheological attributes of blood cells are significantly altered in many T2DM patients [83]. For example, blood viscosity is increased, leading to a multitude of adverse effects, such as limited oxygen concentrations in the microenvironment of immune cells. Therefore, only a limited defense can be mounted against intruding bacteria, increasing the chance of severe bacterial infections. Another example is the lowered blood pH that is often seen in patients suffering from hyperglycemia [84]. This effect is mainly caused by diabetic ketoacidosis and can result in a decreased blood pH below the physiological value of approximately 7.3. Further biochemical alterations that might favor infections in T2DM patients are an impairment of the pentose phosphate pathway, which is participating in the antioxidant defense by synthesizing the reduction equivalent NADPH [85], and the limited functionality of the Na+/K+ ATPase [86], which regulates a plethora of cellular functions.
Diabetes-associated immunodeficiency is also a predisposing factor for pneumococcal and Haemophilus influenzae meningitis, which can cause bacterial meningitis, leading to an altered mental status and increased mortality [68]. Cefotaxime/ceftriaxone plus amoxicillin/ampicillin/penicillin G are the standard antibiotics used in these cases [87,88].
Foot infections are a serious and common complication of DM. High blood sugar levels can damage the nerves and blood vessels in the feet, leading to poor circulation and decreased sensation [89]. This can make it difficult to detect injuries, such as cuts or blisters, which can then become infected and potentially lead to more serious complications [89]. In fact, foot infections are one of the most common reasons for hospitalization among people with diabetes [89]. If left untreated, they can lead to serious complications such as osteomyelitis (infection of the bone), gangrene, and even amputation. In severe cases, foot infections can also lead to sepsis (a potentially life-threatening infection that spreads throughout the body) [90]. Staphylococcus aureus and Staphylococcus epidermidis are isolated from around 60% of all infected ulcers. Enterococci, streptococci, and enterobacteria are less frequent, and 15% of infected ulcers contain strictly anaerobic bacteria [90]. Therefore, it is important for people with diabetes to take proper care of their feet, including daily washing and inspection, wearing appropriate footwear, and seeking prompt medical attention for any injuries or signs of infection [89]. This can help prevent foot infections and reduce the risk of serious complications.
The three most serious head and neck infections in diabetic persons are invasive external otitis (IEO), rhinocerebral mucormycosis, and periodontitis [66]. IEO, also known as malignant otitis externa, is a serious infection that can affect the ear canal and skull base. It is most commonly seen in elderly patients with poorly controlled diabetes, although it can also occur in individuals with weakened immune systems or those with chronic ear infections [91]. Rhinocerebral mucormycosis is another serious infection that can affect the head and neck region [92]. It is a rare but life-threatening fungal infection that can affect the sinuses, brain, and other organs. It is a rare, opportunistic and invasive infection caused by fungi of the class Zygomycetes [92]. Periodontitis is more common in persons with T2DM and is considered the sixth most common complication of DM [93]. This condition initiates or disseminates insulin resistance, thus affecting glycemic control. Poor glycemic control has been associated with a greater incidence and progression of gingivitis and periodontitis [94]. This pathogenesis is mainly caused by Porphyromonas gingivalis, which acts as a critical agent by altering host immune homeostasis [95]. Lipopolysaccharides, proteases, fimbriae, and other virulence factors are among the strategies used by P. gingivalis to promote bacterial colonization and facilitate the growth of the surrounding microbial community [95]. These virulence factors modulate various host immune components by evading bacterial clearance or inducing an inflammatory environment [96].
Fournier gangrene is a type of necrotizing fasciitis that affects the male genitalia and surrounding areas. The most common bacteria that cause this condition are E. coli, Klebsiella spp., Proteus spp., and Peptostreptococcus spp. [97]. However, it is not uncommon for the infection to be polymicrobial, involving several different types of bacteria such as Clostridium, aerobic or anaerobic streptococci, and Bacteroides [67]. Approximately 70% of patients with Fournier gangrene have DM, which is a risk factor for developing this condition. The infection typically starts in the scrotum but can extend to involve the penis, perineum, and abdominal wall [75]. It is important to note that despite the severity of the infection, the testicles are usually spared from the disease [67]. Early diagnosis and aggressive treatment are crucial to preventing the spread of the infection and reducing mortality rates. Treatment involves surgical debridement to remove the infected tissue, along with broad-spectrum antibiotics to target the bacterial infection [98]. Patients with Fournier gangrene often require hospitalization and intensive care management [99].
Respiratory tract infections are responsible for many medical appointments by persons with T2DM [79]. The most frequent respiratory infections associated with DM are caused by Streptococcus pneumoniae and the influenza virus, and persons with DM also have a high possibility of being infected with Mycobacterium tuberculosis. Table 2 gives an overview of bacterial pathogens that are associated with T2DM.
Some of the drugs described in the previous section have been investigated in terms of whether they have beneficial functions for treating bacterial infections in T2DM patients. Only metformin is reported to have clear effects on reducing the pathophysiology of infectious disease mediated by bacteria [100,101]. In a mouse model, metformin administration reduced infections mediated by bacteria such as Staphylococcus aureus and Pseudomonas aeruginosa [102]. Specifically, P. aeruginosa growth is inhibited by metformin in an epithelial cell line of the lungs (Calu-3) [103]. Furthermore, metformin administration also reduces the risk of infections by Mycobacterium tuberculosis [104]. For other commonly used drugs to combat T2DM, either no data are available at the time of writing or they have no effects [82].

Table 2.
Associated pathogenesis of bacterial infections in T2DM.
Table 2.
Associated pathogenesis of bacterial infections in T2DM.
| Disease | Microorganism | Symptoms | Conventional Treatment | References |
|---|---|---|---|---|
| Head and neck infections | Streptococcus pneumoniae and Listeria monocytogenes Pseudomonas aeruginosa | Bacterial meningitis Malignant otitis externa | Cefotaxime/ceftriaxone plus amoxicillin/ampicillin/penicillin G. Long-term monotherapy with oral ciprofloxacin. | [68,105,106] |
| Periodontitis | Staphylococcus spp., Streptococcus spp., Bacillus spp., E. coli | Inflammation in the periodontal tissues is stimulated by the long-term presence of subgingival biofilm, suppuration from periodontal pockets, and tooth loss | Oral and periodontal health (intensive periodontal treatment) and glycemic control. | [107] |
| Respiratory infections | Streptococcus pneumonia, Legionella spp., Haemophilus influenza, Pseudomonas spp., Staphylococcus aureus | Community-acquired pneumonia/hospital-acquired pneumonia | Initial outpatient treatment: Combination therapy with amoxicillin/clavulanic acid/cephalosporin and macrolide/doxycycline or monotherapy with respiratory fluoroquinolone. Severe in-patient pneumonia: β lactam + macrolide or β lactam + fluoroquinolone. | [108,109,110] |
| Infective endocarditis | Streptococcus viridans, Staphylococcus aureus and Enterococcus species | Acute heart failure, stroke, atrioventricular block, septic shock, and cardiogenic shock | Ampicillin with flucloxacillin, oxacillin with gentamicin, or vancomycin with gentamicin and rifampicin. | [111,112] |
| Emphysematous cholecystitis | Clostridium perfringens and E. coli | Biliary tract infection | Surgical removal of the gallbladder and broad-spectrum antimicrobial therapy. | [113] |
| Liver | Klebsiella pneumoniae | Pyogenic liver abscess | Combined antibiotic therapy with carbapenems. | [114,115] |
| Urinary tract | E. coli, Klebsiella spp., Proteus spp., Enterobacter spp., and Enterococci | Cystitis, pyelonephritis, severe urosepsis, renal abscesses and renal papillary necrosis | Ertapenem, nitrofurantoin, ciprofloxacin, ofloxacin, trimethoprim–sulfamethoxazole, cefuroxime, and gentamicin. | [116,117,118] |
| Foot infections in diabetes | Pseudomonas aeruginosa, MRSA | Foot ulcer | β-lactamase inhibitor-amoxicillin/clavulanate; trimethoprim–sulfamethoxazole; carbapenem; aminoglycoside, colistin, and fluoroquinolone; amputation | [119] |
| Fournier’s gangrene | Polymicrobial infections by aerobes and anaerobes | Necrotizing fasciitis of the perineal, genital, or perianal regions | Broad-spectrum antibiotics and surgical debridement. | [120,121] |
| Necrotizing fasciitis | Klebsiella pneumoniae | Extensive necrosis of subcutaneous tissue and fascia | Surgery, oxacillin, and a third-generation cephalosporin. | [122] |
MRSA: methicillin-resistant Staphylococcus aureus.
Compounds from plants can yield valuable results in the treatment of bacterial infections, such as juice extracted from cranberries [123]. It was found that active compounds in this juice are potent inhibitors of bacterial adherence to cells in the urogenital epithelium. The use of plants as a complementary treatment for diabetes and the bacteria that occur in T2DM is supported by several studies. Plants present different bioactive effects, such as anti-inflammatory, antioxidant, bactericidal, and fungicidal activity [124]. In the next section, we discuss the use of plants that can help to treat T2DM and associated infections.
4. Overview of Medical Plant Compounds for the Treatment of T2DM
As previously described, hyperglycemia secondary to T2DM is a condition that can alter the immune system through multiple pathways. In addition, the wounds in these patients have the characteristic that they can take more time to heal, are difficult to manage, and can cause ulcers, infections, and even amputations if not suitably treated [125,126,127]. If hyperglycemia is controlled, the susceptibility of these patients to generate infections should decrease [82,127,128]. Currently, the treatment for infections is pharmacological; however, the use of antibiotics can generate adverse effects, such as hepatotoxicity and nephrotoxicity [124]. In addition, bacteria are known to generate multidrug resistance over time; therefore, non-pharmacological alternatives are sought for the treatment of infections in diabetic patients. Metabolites such as polyphenols, flavonoids and alkaloids act as important antimicrobial agents due to their efficiency in treating bacterial infections and preventing resistance [129,130].
This work suggests the use of plants in two different ways. The first one is to use plants as a direct factor, which means to use certain ingredients as antibacterial agents, and the second one is to use plants as an indirect factor, which means to use hypoglycemic plant ingredients that will decrease the susceptibility to infections. In this way, plants become a non-pharmacological alternative for treating infections in patients with T2DM.
4.1. Mechanism of Action of Natural Products in the Treatment of T2DM and Associated Infections
4.1.1. Antibacterial Activity of Plants
As outlined in Section 3, infections in patients with diabetes are rather common, especially stemming from bacteria such as Proteus vulgaris, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pyogenes, and Proteus mirabilis. To evaluate alternative treatments against infections, ethanolic extracts of shrubs such as Brachylaena ilicifolia and Brachylaena elliptica were tested against the bacteria mentioned before under laboratory conditions. The results showed that phytochemicals such as proanthocyanidins, alkaloids, flavonoids, and polyphenols and B. ilicifolia extracts inhibited the growth of Pseudomonas aeruginosa, thus serving as potent antibacterial agents [131].
Urinary tract infections are a common part of these conditions, and the use of antibiotics has caused bacterial resistance. This is why urine samples were taken from volunteers with urinary tract infections (UTIs) to isolate multidrug-resistant Escherichia coli bacteria. Subsequently, the synergistic effect of the antibacterial activity of the aqueous and methanolic extracts of Prunella vulgaris, commonly known as the “heal all” or “self-heal” plant, was evaluated with the following antibiotics: cefixime, ciprofloxacin, tobramycin and ofloxacin. The results that were significant were those of the cefixime + P. vulgaris aqueous extract group, which had a synergistic effect, and the ciprofloxacin + P. vulgaris (aqueous and ethanolic extract) group, which had no synergistic effect. This means that concomitant antibiotic use with Prunella vulgaris aqueous extract serves to generate a synergistic effect against multidrug-resistant bacteria [132].
As mentioned above, cranberry is commonly used as a prophylactic treatment against UTIs. To discover its biological functioning, a clinical study was carried out using “NutriCan” capsules in healthy men and women. The results showed a higher decrease in bacterial adhesion in men than in women, which was correlated with a time-dependent increase in the Tamm–Horsfall protein (THP). The THP is a high-mannose glycoprotein (from the innate immune system), which is of vital importance because on the surface of the type 1 fimbriated uropathogenic Escherichia coli (UPEC), there is uroplakin that is anchored with the conserved mannose moieties of the THP. This prevents attachment to uroepithelial cells, which are found in the nephron of the kidney, especially tubular cells in the region of the loop of Henle, thus preventing infections [133].
Another condition secondary to UTIs is cystitis (bladder inflammation). In a clinical study in which women with uncomplicated cystitis were evaluated, it was shown that the use of trimethoprim–sulfamethoxazole with green tea had a synergistic effect on decreasing the prevalence of cystitis. The antibacterial activity of green tea is attributed to its phytochemicals, such as polyphenols and catechins [134].
In infections, we can find those of the nosocomial type (hospital-acquired infections), and Klebsiella pneumoniae is part of those most commonly found, being mainly present in blood, urinary and respiratory infections. The antibacterial activity against Klebsiella pneumoniae of glycolic extracts from Juglans regia L., which is commonly known as Persian walnut or English walnut, Pfaffia paniculata K., which is commonly known as ginseng, and Rosmarius officinalis L., which is commonly known as rosemary, was evaluated. J. regia and R. officinalis contain monoterpenes that can change the membrane permeability and kill the bacteria, whereas the antibacterial effect of P. paniculata is attributed to pfameric acid, which is a triterpene [135].
Currently, many microorganisms have become multidrug-resistant (MDR) (at least to three different antibiotics). The effects of methanolic extracts of the following plants, Oxalis corniculata, Cinnamomum tamala, Ageratina adenophora, and Artemisa vulgaris, which are commonly known as creeping woodsorrel, Indian Cassia, crofton weed, and mugwort, respectively, were tested against different MDR bacteria. The plants that showed antibacterial activity against E. coli were O. corniculate and A. vulgaris; against S. aureus were C. tamala, A. adenophora and A. vulgaris; and against S. typhi were A. adenophora and O. corniculate. The only plant that showed antibacterial activity against C. koseri and K. pneumoniae was O. curniculate. This activity was attributed to phytochemicals such as terpenoids, alkaloids, tannins, and flavonoids [136].
As part of the other nosocomial infections, Staphylococcus aureus is also part of the main ones. The antibacterial effect of Arbutus pavarii, known locally in Libya as Schmar, methanolic extracts and fractions (ethyl acetate, hexane, chloroform, butanol) was evaluated against methicillin-resistant S. aureus. The methicillin-resistant Staphylococcus aureus were isolated from a nasal swab from a student from Putra University of Malaysia, the MRSA KCCM 12255 was obtained from the Korean Culture Center of Microorganisms and the MRSA ATCC was obtained from the American Type Culture Collection. Various phytochemicals involved in antibacterial activity were identified, such as gallic acid, catechins, epigallocatechin gallate, epicatechin gallate, quercetin, flavonoids, phenolic acids and arbutin. Catechins damage the membrane of bacteria by causing a potassium leak, which causes a rupture of the membrane and the death of the bacteria; other phytochemicals can block the synthesis of amino acids [137].
4.1.2. Hypoglycemic and Hypolipidemic Plants
An indirect way to decrease susceptibility to infections in patients with T2DM is to decrease hyperglycemia in general to maintain the proper functioning of the immune system [82]. It has been seen that curcumin-enriched yogurt with insulin in diabetic Wistar rats induced by streptozotocin (STZ) maintained normal blood glucose levels and decreased oxidative stress and dyslipidemia. These effects are due to the increased translocation of GLUT-4 via the phosphorylation of AKT. Curcumin per se has the ability to scavenge ROS, to increase the activity of delta-aminolevulinic dehydratase, superoxide dismutase (SOD) and catalase (CAT) as well as to decrease thiobarbituric acid reactive substances (TBARS) [138].
The ethanolic extract from leaves of Avicennia marina, commonly known as gray mangrove plant, has proved to be a suitable hypoglycemic agent in a diabetic Swiss Webster mouse model induced by streptozotocin (STZ), in addition to acting as an antioxidant agent, increasing CAT and glutathione (GSH) levels, as well as decreasing toxins such as H2O2, malondialdehyde (MDA) and nitric oxide (NO) and protecting organs such as the kidney and liver in order to avoid comorbidities [139]. Another study used the same plants, Avicennia marina and Rhizophora mucronata, and the aqueous extract (also from leaves) in a diabetic Wistar rat model induced by STZ. They proved to be useful hypoglycemic agents stimulating insulin secretion, which led to comments that phytochemicals such as flavonoids from the plants could prevent pancreatic β-cell apoptosis. In addition, these plants decreased MDA and lipid peroxidation, and both have the ability to be antioxidants per se [140].
Another effect that is sought in the use of plants against T2DM is the hypolipidemic effect, in addition to the hypoglycemic one. The hydroalcoholic extracts of Eryngium caucasicum roots showed this effect in a diabetic Wistar rat model induced by STZ and nicotinamide (NA), raising serum insulin levels and decreasing blood glucose. It is worth mentioning that carotenes and flavonoids could be responsible for reducing oxidative stress. This treatment also led to a decreased homeostasis model assessment (HOMA), which is a method for measuring fasting insulin and glucose in blood and is useful in determining insulin resistance and the functioning of pancreatic β-cells. Saponins suppress cholesterol absorption while increasing bile secretion, alkaloids inhibit cholesterol synthesis, and both natural products cause the elevation of high-density lipoproteins (HDL) and the decrease of very-low-density lipoproteins (VLDL) and low-density lipoproteins (LDL). E. caucasicum also demonstrated hepatoprotection by decreasing serum glutamic pyruvic transaminase (SGPT) and glutamic oxaloacetic transaminase (SGOT), therefore protecting the structural integrity of the liver. The insulin increase can inhibit lipase, which means that processes such as the stimulation of fatty acids in phospholipids for later cholesterol and plasma release are slowed down [141].
T2DM can also be combated using beverages from plants that are consumed worldwide, such as coffee. In a diabetic Wistar rat model (STZ + high fat diet), Coffea arabica aqueous extract proved to be a hypolipidemic and hypoglycemic agent. It also ameliorated insulin resistance. These effects are attributed to chlorogenic acid present in Coffea arabica, which has been reported to decrease adipocyte numbers in the abdominal area. It can modulate transport proteins that consequently decrease renal lipid peroxidation, renal triglycerides, and oxidative stress. The chlorogenic acid promotes the expression of antioxidant proteins by modifying the mRNA levels of antioxidant genes encoding glutathione peroxidase (GPx), catalase (CAT) and superoxide dismutase (SOD) [142].
There are other types of plants that are not commonly used; however, they exert antidiabetic effects, such as Datura stramonium L., commonly known as Jimsonweed or thorn apple. The hydro-methanolic root extract of this plant has improved glucose metabolism through insulin secretion, inhibiting α-amylase and α-glucosidase, blocking gluconeogenesis, protecting pancreatic β cells from inflammation and oxidative stress, improving glucose transporters GLUT-2 and GLUT-4 through flavonoids and polyphenols, and also reducing serum triglyceride, as was tested in a Swiss albino diabetic mice induced by STZ [143].
There are ornamental plants that have medicinal uses, such as Enhydra fluctuans, commonly known as Harkuch or Helencha. Aqueous alcohol Enhydra fluctuans extracts administered to Long Evans rats in which diabetic conditions were induced by STZ exerted anti-dyslipidemic and hypoglycemic effects by improving insulin sensitivity and the utilization of glucose at the peripheral level, decreasing glycogenolysis, decreasing fasting blood glucose, decreasing the ratio of TG/HDL-cholesterol, and inhibiting protein carbonylation and lipid peroxidation [144].
Fruit plants, in addition to serving as food, can also be effective antidiabetic agents when other parts of the plant, such as mango leaves, are utilized. Hydro-alcoholic extracts of Mangifera indica L. leaves exerted hypoglycemic effects via polyphenols that inhibited postprandial fat utilization and glucose, inhibited alpha-glucosidase, increased insulin sensitivity, blocked pancreatic lipase activities, induced GLUT-4 and decreased LDL levels in a Swiss albino diabetic mouse model, which was experimentally induced via administration of alloxan monohydrate [145].
This work proposes the use of plants as a complementary treatment for T2DM (Figure 4). As described above, the plants that can be used range from the best known (e.g., consumed in beverages such as coffee) to the not so commonly known plants. The antidiabetic effects of plants are attributed to the presence of phytochemicals such as polyphenols, flavonoids, catechins, and alkaloids, among others. Plants can be antidiabetic in two ways, acting as direct agents, lowering blood lipids and glucose, acting as antioxidant agents, or increasing antioxidant enzymes, increasing insulin sensitivity, improving glucose transport by transporters such as GLUT-4 or decreasing lipid peroxidation, or they may act as indirect agents, attacking the infections to which people with T2DM are more susceptible, thereby indirectly preserving the immune system and blocking hyperglycemia. Table 3 shows the different types of plants that can be used directly or indirectly to treat infections, their origin, and their mechanism of action.
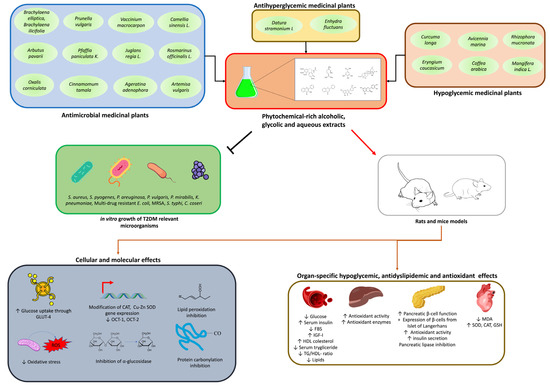
Figure 4.
Alternative herbal treatments for T2DM, alongside the effects found in the literature. AUC: acute uncomplicated cystitis; THP: Tamm–Horsfall protein; FBS: fasting blood glucose; GLUT-4: glucose transporter type 4; GSH: glutathione; HDL: high-density lipoprotein; IGF-I: insulin-like growth factor I; LDL: low-density lipoprotein; MDA: malondialdehyde; NA: nicotinamide; OCT-1: organic cation transporter; MRSA: methicillin-resistant Staphylococcus aureus; T2DM: Type 2 diabetes mellitus; CAT: catalase; TG: triglycerides; S. aureus: Staphylococcus aureus; S. pyogenes: Streptococcus pyogenes; P. aeruginosa: Pseudomonas aeruginosa; P. vulgaris: Proteus vulgaris; P. mirabilis: Proteus mirabilis; K. pneumoniae: Klebsiella pneumoniae.

Table 3.
Alternative herbal treatments for T2DM.
5. Conclusions
Changes in the lifestyle of the population have increased chronic diseases worldwide. T2DM is a multifactorial metabolic disease, and new risk factors for it have been identified. The denominator of T2DM-related pathologies is a persistent state of hyperglycemia and dysregulation of other metabolites, such as lipids. Current drugs for treatment disclose various mechanisms of action, including enzymatic inhibitors, blockers of receptors, and stimulants of the release of insulin, among others. Infections are a common and severe sequel to diabetes and increase both morbidity and mortality in patients. The hyperglycemic environment causes immune system dysfunction in diabetic individuals. There are numerous synthetic compounds that help to control blood glucose levels and secondary infections. Examples of resistance to both antibiotics and insulin have been documented. Since ancient times, natural products have been utilized to exert therapeutic effects, and their structures can imitate or block the action of natural mediators in the human body. Recently, more attention has been given to validating natural compounds that can be added for the management of the hyperglycemic state. Diabetes is a metabolic disease that does not have a cure and is an irreversible condition, which is why more research in this field is urgently needed to understand the cellular and molecular mechanisms of its pathology and to propose new and effective ways to manage it. Future medications should be capable of providing multifaceted improvements to high blood glucose levels and to better control secondary infections attributed to immune system depression. However, even the most effective advances in therapy will not prove to be effective if people are unable to adapt to more healthy lifestyles. Overall, the traditional treatment of bacterial infections is the use of antibiotics; however, this can cause adverse effects and long-term bacterial resistance. In this work, effective alternatives have been presented to treat T2DM using medical plants. T2DM-associated infections can be indirectly prevented by reducing the state of hyperglycemia and, therefore, decreasing the susceptibility to infections using antidiabetic plants, and directly by utilizing antibacterial plants against bacterial infections.
Author Contributions
Conceptualization, methodology, and writing—original draft preparation, C.Q.S., E.M.M.-M., R.P.-S., J.H.E.-L., B.L.-S. and M.A.T.-V.; visualization, C.Q.S., M.A.T.-V., B.L.-S. and J.S.L.-O.; supervision, E.M.M.-M., R.P.-S. and C.Q.S.; project administration, E.M.M.-M., C.Q.S. and R.P.-S.; All authors have read and agreed to the published version of the manuscript.
Funding
Consejo Nacional de Ciencia y Tecnología (CONACYT) granted a scholarship to Mario Adrián Tienda-Vázquez (CVU: 858269) and Brenda Luna-Sosa (CVU: 861786).
Data Availability Statement
Not applicable.
Acknowledgments
Consejo Nacional de Ciencia y Tecnología (CONACYT) granted a scholarship to Mario Adrián Tienda-Vázquez (CVU: 858269) and Brenda Luna-Sosa (CVU: 861786). CONACYT is thankfully acknowledged for support under the Sistema Nacional de Investigadores (SNI) program, as awarded to Elda M. Melchor-Martínez (CVU: 230784), Christian Quintus Scheckhuber (CVU: 743687), Roberto Parra-Saldívar (CVU: 35753) and Joel H. Elizondo-Luévano (CVU: 1418935). The authors appreciate the support from Tecnologico de Monterrey for the literature services. We thank Freepik.com, pixabay.com, SciDraw.com, PNGWing.com and Dreamstime for providing visual resources for the figures.
Conflicts of Interest
The authors declare no conflict of interest.
References
- INEGI. Comunicado de Prensa Núm. 24/22. Available online: https://www.inegi.org.mx/contenidos/saladeprensa/boletines/2022/dr/dr2021.pdf (accessed on 20 January 2023).
- Reed, J.; Bain, S.; Kanamarlapudi, V. A Review of Current Trends with Type 2 Diabetes Epidemiology, Aetiology, Pathogenesis, Treatments and Future Perspectives. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 3567–3602. [Google Scholar] [CrossRef] [PubMed]
- Bello-Chavolla, O.Y.; Rojas-Martinez, R.; Aguilar-Salinas, C.A.; Hernández-Avila, M. Epidemiology of Diabetes Mellitus in Mexico. Nutr. Rev. 2017, 75, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global Estimates of Diabetes Prevalence for 2013 and Projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- INEGI. Comunicado De Prensa Núm. 645/21. Available online: https://www.inegi.org.mx/contenidos/saladeprensa/aproposito/2021/EAP_Diabetes2021.pdf (accessed on 20 January 2023).
- Kharroubi, A.T. Diabetes Mellitus: The Epidemic of the Century. World J. Diabetes 2015, 6, 850. [Google Scholar] [CrossRef] [PubMed]
- Bello-Chavolla, O.Y.; Bahena-López, J.P.; Vargas-Vázquez, A.; Antonio-Villa, N.E.; Márquez-Salinas, A.; Fermín-Martínez, C.A.; Rojas, R.; Mehta, R.; Cruz-Bautista, I.; Hernández-Jiménez, S.; et al. Clinical Characterization of Data-Driven Diabetes Subgroups in Mexicans Using a Reproducible Machine Learning Approach. BMJ Open Diabetes Res. Care 2020, 8, e001550. [Google Scholar] [CrossRef]
- Barquera, S.; Schillinger, D.; Aguilar-Salinas, C.A.; Schenker, M.; Rodríguez, L.A.; Hernández-Alcaraz, C.; Sepúlveda-Amor, J. Collaborative Research and Actions on Both Sides of the US-Mexico Border to Counteract Type 2 Diabetes in People of Mexican Origin. Global. Health 2018, 14, 84. [Google Scholar] [CrossRef]
- Secretaria de Salud. Información Para La Rendición de Cuentas. Available online: http://www.salud.gob.mx/unidades/evaluacion/saludmex2002/saludmexico2002.pdf (accessed on 20 January 2023).
- Thompson, A.; Kanamarlapudi, V. Agonist-Induced Internalisation of the Glucagon-like Peptide-1 Receptor Is Mediated by the Gαq Pathway. Biochem. Pharmacol. 2015, 93, 72–84. [Google Scholar] [CrossRef]
- Zareini, B.; Blanche, P.; D’Souza, M.; Elmegaard Malik, M.; Nørgaard, C.H.; Selmer, C.; Gislason, G.; Kristensen, S.L.; Køber, L.; Torp-Pedersen, C.; et al. Type 2 Diabetes Mellitus and Impact of Heart Failure on Prognosis Compared to Other Cardiovascular Diseases. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e006260. [Google Scholar] [CrossRef] [PubMed]
- Kushner, P.R.; Cavender, M.A.; Mende, C.W. Role of Primary Care Clinicians in the Management of Patients with Type 2 Diabetes and Cardiorenal Diseases. Clin. Diabetes 2022, 40, 401–412. [Google Scholar] [CrossRef]
- Viigimaa, M.; Sachinidis, A.; Toumpourleka, M.; Koutsampasopoulos, K.; Alliksoo, S.; Titma, T. Macrovascular Complications of Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 110–116. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Heinrich, M. Mexican Plants with Hypoglycaemic Effect Used in the Treatment of Diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef]
- Jacob, B.; Narendhirakannan, R.T. Role of Medicinal Plants in the Management of Diabetes Mellitus: A Review. 3 Biotech 2019, 9, 4. [Google Scholar] [CrossRef]
- Alam, S.; Sarker, M.M.R.; Sultana, T.N.; Chowdhury, M.N.R.; Rashid, M.A.; Chaity, N.I.; Zhao, C.; Xiao, J.; Hafez, E.E.; Khan, S.A.; et al. Antidiabetic Phytochemicals From Medicinal Plants: Prospective Candidates for New Drug Discovery and Development. Front. Endocrinol. 2022, 13, 800714. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B. Roles of Alkaloids from Medicinal Plants in the Management of Diabetes Mellitus. J. Chem. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Macdonald, K.E.; Boeckh, S.; Stacey, H.J.; Jones, J.D. The Microbiology of Diabetic Foot Infections: A Meta-Analysis. BMC Infect. Dis. 2021, 21, 770. [Google Scholar] [CrossRef] [PubMed]
- Barwell, N.D.; Devers, M.C.; Kennon, B.; Hopkinson, H.E.; McDougall, C.; Young, M.J.; Robertson, H.M.A.; Stang, D.; Dancer, S.J.; Seaton, A.; et al. Diabetic Foot Infection: Antibiotic Therapy and Good Practice Recommendations. Int. J. Clin. Pract. 2017, 71, e13006. [Google Scholar] [CrossRef] [PubMed]
- Dowarah, J.; Singh, V.P. Anti-Diabetic Drugs Recent Approaches and Advancements. Bioorg. Med. Chem. 2020, 28, 115263. [Google Scholar] [CrossRef] [PubMed]
- Gloyn, A.L.; Drucker, D.J. Precision Medicine in the Management of Type 2 Diabetes. Lancet. Diabetes Endocrinol. 2018, 6, 891–900. [Google Scholar] [CrossRef]
- Xie, F.; Chan, J.C.N.; Ma, R.C.W. Precision Medicine in Diabetes Prevention, Classification and Management. J. Diabetes Investig. 2018, 9, 998–1015. [Google Scholar] [CrossRef]
- Gimeno, R.E.; Briere, D.A.; Seeley, R.J. Leveraging the Gut to Treat Metabolic Disease. Cell Metab. 2020, 31, 679–698. [Google Scholar] [CrossRef]
- Ferguson, D.; Finck, B.N. Emerging Therapeutic Approaches for the Treatment of NAFLD and Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2021, 17, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Chen, L.; Yang, W.; Xie, A.M. GLP-1 Suppresses Feeding Behaviors and Modulates Neuronal Electrophysiological Properties in Multiple Brain Regions. Front. Mol. Neurosci. 2021, 14, 793004. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Dulaglutide: A Review in Type 2 Diabetes. Drugs 2020, 80, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Triplitt, C.; Chiquette, E. Exenatide: From the Gila Monster to the Pharmacy. J. Am. Pharm. Assoc. 2006, 46, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Dual GIP/GLP-1 Receptor Agonists: New Advances for Treating Type-2 Diabetes. Ann. Endocrinol. 2023, 84, 316–321. [Google Scholar] [CrossRef]
- Scheen, A.J.; Radermecker, R.P.; Paquot, N. Focus on tirzepatide, a dual unimolecular GIP-GLP-1 receptor agonist in type 2 diabetes. Rev. Med. Suisse 2022, 18, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Ahrén, B. Glucose-Lowering Action through Targeting Islet Dysfunction in Type 2 Diabetes: Focus on Dipeptidyl Peptidase-4 Inhibition. J. Diabetes Investig. 2021, 12, 1128–1135. [Google Scholar] [CrossRef]
- Deacon, C.F. Dipeptidyl Peptidase 4 Inhibitors in the Treatment of Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2020, 16, 642–653. [Google Scholar] [CrossRef]
- Mastrototaro, L.; Roden, M. Insulin Resistance and Insulin Sensitizing Agents. Metabolism 2021, 125, 154892. [Google Scholar] [CrossRef]
- Wang, S.; Dougherty, E.J.; Danner, R.L. PPARγ Signaling and Emerging Opportunities for Improved Therapeutics. Pharmacol. Res. 2016, 111, 76–85. [Google Scholar] [CrossRef]
- Muise, E.S.; Azzolina, B.; Kuo, D.W.; El-Sherbeini, M.; Tan, Y.; Yuan, X.; Mu, J.; Thompson, J.R.; Berger, J.P.; Wong, K.K. Adipose Fibroblast Growth Factor 21 Is Up-Regulated by Peroxisome Proliferator-Activated Receptor Gamma and Altered Metabolic States. Mol. Pharmacol. 2008, 74, 403–412. [Google Scholar] [CrossRef]
- Astapova, O.; Leff, T. Adiponectin and PPARγ: Cooperative and Interdependent Actions of Two Key Regulators of Metabolism. Vitam. Horm. 2012, 90, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E. Thiazolidinediones: The Forgotten Diabetes Medications. Curr. Diab. Rep. 2019, 19, 151. [Google Scholar] [CrossRef]
- Smith, U. Pioglitazone: Mechanism of Action. Int. J. Clin. Pract. Suppl. 2001, 121, 13–18. [Google Scholar]
- Siebert, A.; Goren, I.; Pfeilschifter, J.; Frank, S. Anti-Inflammatory Effects of Rosiglitazone in Obesity-Impaired Wound Healing Depend on Adipocyte Differentiation. PLoS ONE 2016, 11, e0168562. [Google Scholar] [CrossRef]
- Ghezzi, C.; Loo, D.D.F.; Wright, E.M. Physiology of Renal Glucose Handling via SGLT1, SGLT2 and GLUT2. Diabetologia 2018, 61, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Tentolouris, A.; Vlachakis, P.; Tzeravini, E.; Eleftheriadou, I.; Tentolouris, N. SGLT2 Inhibitors: A Review of Their Antidiabetic and Cardioprotective Effects. Int. J. Environ. Res. Public Health 2019, 16, 2965. [Google Scholar] [CrossRef]
- Cowie, M.R.; Fisher, M. SGLT2 Inhibitors: Mechanisms of Cardiovascular Benefit beyond Glycaemic Control. Nat. Rev. Cardiol. 2020, 17, 761–772. [Google Scholar] [CrossRef]
- Lamoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef]
- Pernicova, I.; Korbonits, M. Metformin--Mode of Action and Clinical Implications for Diabetes and Cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; MacDonald, M.J.; et al. Metformin Suppresses Gluconeogenesis by Inhibiting Mitochondrial Glycerophosphate Dehydrogenase. Nature 2014, 510, 542–546. [Google Scholar] [CrossRef]
- LaMoia, T.E.; Butrico, G.M.; Kalpage, H.A.; Goedeke, L.; Hubbard, B.T.; Vatner, D.F.; Gaspar, R.C.; Zhang, X.M.; Cline, G.W.; Nakahara, K.; et al. Metformin, Phenformin, and Galegine Inhibit Complex IV Activity and Reduce Glycerol-Derived Gluconeogenesis. Proc. Natl. Acad. Sci. USA 2022, 119, e2122287119. [Google Scholar] [CrossRef]
- Rena, G.; Pearson, E.R.; Sakamoto, K. Molecular Mechanism of Action of Metformin: Old or New Insights? Diabetologia 2013, 56, 1898–1906. [Google Scholar] [CrossRef]
- Lv, W.; Wang, X.; Xu, Q.; Lu, W. Mechanisms and Characteristics of Sulfonylureas and Glinides. Curr. Top. Med. Chem. 2020, 20, 37–56. [Google Scholar] [CrossRef]
- Nichols, C.G. Personalized Therapeutics for KATP-Dependent Pathologies. Annu. Rev. Pharmacol. Toxicol. 2022, 63, 541–563. [Google Scholar] [CrossRef]
- Schwientek, P.; Szczepanowski, R.; Rückert, C.; Kalinowski, J.; Klein, A.; Selber, K.; Wehmeier, U.F.; Stoye, J.; Pühler, A. The Complete Genome Sequence of the Acarbose Producer Actinoplanes Sp. SE50/110. BMC Genom. 2012, 13, 112. [Google Scholar] [CrossRef]
- Furman, B.L. Acarbose. Ref. Modul. Biomed. Sci. 2017, 2007, 1–3. [Google Scholar] [CrossRef]
- Van de Laar, F.A.; Lucassen, P.L.B.J.; Akkermans, R.P.; Van de Lisdonk, E.H.; Rutten, G.E.H.M.; Van Weel, C. Alpha-Glucosidase Inhibitors for Type 2 Diabetes Mellitus. Cochrane Database Syst. Rev. 2005, 2009, CD003639. [Google Scholar] [CrossRef]
- Tanoeyadi, S.; Tsunoda, T.; Ito, T.; Philmus, B.; Mahmud, T. Acarbose May Function as a Competitive Exclusion Agent for the Producing Bacteria. ACS Chem. Biol. 2023, 18, 367–376. [Google Scholar] [CrossRef]
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef]
- Harrison, D.E.; Strong, R.; Alavez, S.; Astle, C.M.; DiGiovanni, J.; Fernandez, E.; Flurkey, K.; Garratt, M.; Gelfond, J.A.L.; Javors, M.A.; et al. Acarbose Improves Health and Lifespan in Aging HET3 Mice. Aging Cell 2019, 18, e12898. [Google Scholar] [CrossRef]
- Gloster, T.M.; Davies, G.J. Glycosidase Inhibition: Assessing Mimicry of the Transition State. Org. Biomol. Chem. 2010, 8, 305–320. [Google Scholar] [CrossRef]
- Tseng, P.-S.; Ande, C.; Moremen, K.W.; Crich, D. Influence of Side Chain Conformation on the Activity of Glycosidase Inhibitors. Angew. Chem. Int. Ed. Engl. 2023, 62, e202217809. [Google Scholar] [CrossRef]
- Chennaiah, A.; Bhowmick, S.; Vankar, Y.D. Conversion of Glycals into Vicinal-1{,}2-Diazides and 1,2-(or 2,1)-Azidoacetates Using Hypervalent Iodine Reagents and Me3SiN3. Application in the Synthesis of N-Glycopeptides, Pseudo-Trisaccharides and an Iminosugar. RSC Adv. 2017, 7, 41755–41762. [Google Scholar] [CrossRef]
- Sharma, R.; Soman, S.S. Design and Synthesis of Sulfonamide Derivatives of Pyrrolidine and Piperidine as Anti-Diabetic Agents. Eur. J. Med. Chem. 2015, 90, 342–350. [Google Scholar] [CrossRef]
- Kaku, K.; Kisanuki, K.; Shibata, M.; Oohira, T. Benefit-Risk Assessment of Alogliptin for the Treatment of Type 2 Diabetes Mellitus. Drug Saf. 2019, 42, 1311–1327. [Google Scholar] [CrossRef]
- Haas, B.; Eckstein, N.; Pfeifer, V.; Mayer, P.; Hass, M.D.S. Efficacy, Safety and Regulatory Status of SGLT2 Inhibitors: Focus on Canagliflozin. Nutr. Diabetes 2014, 4, e143. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the Glucoregulatory Mechanisms of Metformin in Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef]
- Mikov, M.; Đanić, M.; Pavlović, N.; Stanimirov, B.; Goločorbin-Kon, S.; Stankov, K.; Al-Salami, H. Potential Applications of Gliclazide in Treating Type 1 Diabetes Mellitus: Formulation with Bile Acids and Probiotics. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 269–280. [Google Scholar] [CrossRef]
- Szeto, V.; Chen, N.; Sun, H.; Feng, Z. The Role of KATP Channels in Cerebral Ischemic Stroke and Diabetes. Acta Pharmacol. Sin. 2018, 39, 683–694. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 Receptor Agonists in the Treatment of Type 2 Diabetes—State-of-the-Art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef]
- Bridges, A.; Bistas, K.G.; Jacobs, T.F. Exenatide. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Casqueiro, J.; Casqueiro, J.; Alves, C. Infections in Patients with Diabetes Mellitus: A Review of Pathogenesis. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. S1), S27–S36. [Google Scholar] [CrossRef] [PubMed]
- Calvet, H.M.; Yoshikawa, T.T. Infections in Diabetes. Infect. Dis. Clin. N. Am. 2001, 15, 407–421. [Google Scholar] [CrossRef] [PubMed]
- van Veen, K.E.B.; Brouwer, M.C.; van der Ende, A.; van de Beek, D. Bacterial Meningitis in Diabetes Patients: A Population-Based Prospective Study. Sci. Rep. 2016, 6, 36996. [Google Scholar] [CrossRef]
- Thomsen, R.W.; Hundborg, H.H.; Lervang, H.-H.; Johnsen, S.P.; Schønheyder, H.C.; Sørensen, H.T. Risk of Community-Acquired Pneumococcal Bacteremia in Patients with Diabetes: A Population-Based Case-Control Study. Diabetes Care 2004, 27, 1143–1147. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.W.; Hundborg, H.H.; Lervang, H.-H.; Johnsen, S.P.; Schønheyder, H.C.; Sørensen, H.T. Diabetes Mellitus as a Risk and Prognostic Factor for Community-Acquired Bacteremia Due to Enterobacteria: A 10-Year, Population-Based Study among Adults. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005, 40, 628–631. [Google Scholar] [CrossRef]
- Thomsen, R.W.; Riis, A.H.; Kjeldsen, S.; Schønheyder, H.C. Impact of Diabetes and Poor Glycaemic Control on Risk of Bacteraemia with Haemolytic Streptococci Groups A, B, and G. J. Infect. 2011, 63, 8–16. [Google Scholar] [CrossRef]
- Palau-Rodriguez, M.; Tulipani, S.; Isabel Queipo-Ortuño, M.; Urpi-Sarda, M.; Tinahones, F.J.; Andres-Lacueva, C. Metabolomic Insights into the Intricate Gut Microbial-Host Interaction in the Development of Obesity and Type 2 Diabetes. Front. Microbiol. 2015, 6, 1151. [Google Scholar] [CrossRef] [PubMed]
- Ribera, M.C.; Pascual, R.; Orozco, D.; Pérez Barba, C.; Pedrera, V.; Gil, V. Incidence and Risk Factors Associated with Urinary Tract Infection in Diabetic Patients with and without Asymptomatic Bacteriuria. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 389–393. [Google Scholar] [CrossRef]
- Patterson, J.E.; Andriole, V.T. Bacterial Urinary Tract Infections in Diabetes. Infect. Dis. Clin. N. Am. 1997, 11, 735–750. [Google Scholar] [CrossRef]
- Joshi, N.; Caputo, G.M.; Weitekamp, M.R.; Karchmer, A.W. Infections in Patients with Diabetes Mellitus. N. Engl. J. Med. 1999, 341, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.R.; Hux, J.E. Quantifying the Risk of Infectious Diseases for People with Diabetes. Diabetes Care 2003, 26, 510–513. [Google Scholar] [CrossRef]
- Boyko, E.J.; Fihn, S.D.; Scholes, D.; Abraham, L.; Monsey, B. Risk of Urinary Tract Infection and Asymptomatic Bacteriuria among Diabetic and Nondiabetic Postmenopausal Women. Am. J. Epidemiol. 2005, 161, 503–510. [Google Scholar] [CrossRef]
- Brown, J.S.; Wessells, H.; Chancellor, M.B.; Howards, S.S.; Stamm, W.E.; Stapleton, A.E.; Steers, W.D.; Van Den Eeden, S.K.; McVary, K.T. Urologic Complications of Diabetes. Diabetes Care 2005, 28, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Weerarathna, T.; McCarthy, J.S.; Davis, T.M.E. Common Infections in Diabetes: Pathogenesis, Management and Relationship to Glycaemic Control. Diabetes Metab. Res. Rev. 2007, 23, 3–13. [Google Scholar] [CrossRef]
- Chen, S.L.; Jackson, S.L.; Boyko, E.J. Diabetes Mellitus and Urinary Tract Infection: Epidemiology, Pathogenesis and Proposed Studies in Animal Models. J. Urol. 2009, 182, S51–S56. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.M.A.J.; Gorter, K.J.; Hak, E.; Goudzwaard, W.L.; Schellevis, F.G.; Hoepelman, A.I.M.; Rutten, G.E.H.M. Increased Risk of Common Infections in Patients with Type 1 and Type 2 Diabetes Mellitus. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005, 41, 281–288. [Google Scholar] [CrossRef]
- Chávez-Reyes, J.; Escárcega-González, C.E.; Chavira-Suárez, E.; León-Buitimea, A.; Vázquez-León, P.; Morones-Ramírez, J.R.; Villalón, C.M.; Quintanar-Stephano, A.; Marichal-Cancino, B.A. Susceptibility for Some Infectious Diseases in Patients with Diabetes: The Key Role of Glycemia. Front. Public Health 2021, 9, 559595. [Google Scholar] [CrossRef]
- Melkumyants, A.M.; Balashov, S.A. Effect of Blood Viscocity on Arterial Flow Induced Dilator Response. Cardiovasc. Res. 1990, 24, 165–168. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; Kitabchi, A.E. Diabetic Ketoacidosis: Risk Factors and Management Strategies. Treat. Endocrinol. 2003, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Mason, P.J.; Vulliamy, T.J. Glucose-6-Phosphate Dehydrogenase Deficiency. Baillieres. Best Pract. Res. Clin. Haematol. 2000, 13, 21–38. [Google Scholar] [CrossRef]
- Zadhoush, F.; Sadeghi, M.; Pourfarzam, M. Biochemical Changes in Blood of Type 2 Diabetes with and without Metabolic Syndrome and Their Association with Metabolic Syndrome Components. J. Res. Med. Sci. 2015, 20, 763–770. [Google Scholar] [CrossRef]
- Pomar, V.; de Benito, N.; Mauri, A.; Coll, P.; Gurguí, M.; Domingo, P. Characteristics and Outcome of Spontaneous Bacterial Meningitis in Patients with Diabetes Mellitus. BMC Infect. Dis. 2020, 20, 292. [Google Scholar] [CrossRef]
- van de Beek, D.; Cabellos, C.; Dzupova, O.; Esposito, S.; Klein, M.; Kloek, A.T.; Leib, S.L.; Mourvillier, B.; Ostergaard, C.; Pagliano, P.; et al. ESCMID Guideline: Diagnosis and Treatment of Acute Bacterial Meningitis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2016, 22 (Suppl. S3), S37–S62. [Google Scholar] [CrossRef]
- Joseph, W.S.; Lipsky, B.A. Medical Therapy of Diabetic Foot Infections. J. Am. Podiatr. Med. Assoc. 2010, 100, 395–400. [Google Scholar] [CrossRef]
- Nicolau, D.P.; Stein, G.E. Therapeutic Options for Diabetic Foot Infections: A Review with an Emphasis on Tissue Penetration Characteristics. J. Am. Podiatr. Med. Assoc. 2010, 100, 52–63. [Google Scholar] [CrossRef]
- Carfrae, M.J.; Kesser, B.W. Malignant Otitis Externa. Otolaryngol. Clin. N. Am. 2008, 41, 537–549. [Google Scholar] [CrossRef]
- Artal, R.; Agreda, B.; Serrano, E.; Alfonso, J.I.; Vallés, H. Rhinocerebral mucormycosis: Report on eight cases. Acta Otorrinolaringol. Esp. 2010, 61, 301–305. [Google Scholar] [CrossRef]
- Alves, C.; Andion, J.; Brandão, M.; Menezes, R. Pathogenic aspects of the periodontal disease associated to diabetes mellitus. Arq. Bras. Endocrinol. Metabol. 2007, 51, 1050–1057. [Google Scholar] [CrossRef]
- Simpson, T.C.; Needleman, I.; Wild, S.H.; Moles, D.R.; Mills, E.J. Treatment of Periodontal Disease for Glycaemic Control in People with Diabetes. Cochrane Database Syst. Rev. 2010, 5, CD004714. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Kopra, E.; Pietiäinen, M.; Lehto, M.; Zaric, S.; Paju, S.; Salminen, A. Periodontitis and Cardiometabolic Disorders: The Role of Lipopolysaccharide and Endotoxemia. Periodontol. 2000 2022, 89, 19–40. [Google Scholar] [CrossRef]
- Janket, S.-J.; Javaheri, H.; Ackerson, L.K.; Ayilavarapu, S.; Meurman, J.H. Oral Infections, Metabolic Inflammation, Genetics, and Cardiometabolic Diseases. J. Dent. Res. 2015, 94, 119S–127S. [Google Scholar] [CrossRef]
- Montoya Chinchilla, R.; Izquierdo Morejon, E.; Nicolae Pietricicâ, B.; Pellicer Franco, E.; Aguayo Albasini, J.L.; Miñana López, B. Fournier’s gangrene. Descriptive analysis of 20 cases and literature review. Actas Urol. Esp. 2009, 33, 873–880. [Google Scholar] [CrossRef]
- Tran, H.A.; Hart, A.M. Fournier’s Gangrene. Intern. Med. J. 2006, 36, 200–201. [Google Scholar] [CrossRef]
- Kobayashi, S. Fournier’s Gangrene. Am. J. Surg. 2008, 195, 257–258. [Google Scholar] [CrossRef]
- Mendy, A.; Gopal, R.; Alcorn, J.F.; Forno, E. Reduced Mortality from Lower Respiratory Tract Disease in Adult Diabetic Patients Treated with Metformin. Respirology 2019, 24, 646–651. [Google Scholar] [CrossRef]
- Shih, C.J.; Wu, Y.L.; Chao, P.W.; Kuo, S.C.; Yang, C.Y.; Li, S.Y.; Ou, S.M.; Chen, Y.T. Association between Use of Oral Anti-Diabetic Drugs and the Risk of Sepsis: A Nested Case-Control Study. Sci. Rep. 2015, 5, 15260. [Google Scholar] [CrossRef]
- Garnett, J.P.; Baker, E.H.; Naik, S.; Lindsay, J.A.; Knight, G.M.; Gill, S.; Tregoning, J.S.; Baines, D.L. Metformin Reduces Airway Glucose Permeability and Hyperglycaemia-Induced Staphylococcus Aureus Load Independently of Effects on Blood Glucose. Thorax 2013, 68, 835–845. [Google Scholar] [CrossRef]
- Gill, S.K.; Hui, K.; Farne, H.; Garnett, J.P.; Baines, D.L.; Moore, L.S.P.; Holmes, A.H.; Filloux, A.; Tregoning, J.S. Increased Airway Glucose Increases Airway Bacterial Load in Hyperglycaemia. Sci. Rep. 2016, 6, 27636. [Google Scholar] [CrossRef]
- Pan, S.W.; Yen, Y.F.; Kou, Y.R.; Chuang, P.H.; Su, V.Y.F.; Feng, J.Y.; Chan, Y.J.; Su, W.J. The Risk of TB in Patients With Type 2 Diabetes Initiating Metformin vs Sulfonylurea Treatment. Chest 2018, 153, 1347–1357. [Google Scholar] [CrossRef]
- Carlton, D.A.; Perez, E.E.; Smouha, E.E. Malignant External Otitis: The Shifting Treatment Paradigm. Am. J. Otolaryngol. 2018, 39, 41–45. [Google Scholar] [CrossRef]
- Yang, T.-H.; Xirasagar, S.; Cheng, Y.-F.; Wu, C.-S.; Kao, Y.-W.; Shia, B.-C.; Lin, H.-C. Malignant Otitis Externa Is Associated with Diabetes: A Population-Based Case-Control Study. Ann. Otol. Rhinol. Laryngol. 2020, 129, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-T.; Chen, T.H.-H.; Wang, P.-E.; Lai, H.; Lo, M.-T.; Chen, P.Y.-C.; Chiu, S.Y.-H. A Population-Based Study on the Association between Type 2 Diabetes and Periodontal Disease in 12,123 Middle-Aged Taiwanese (KCIS No. 21). J. Clin. Periodontol. 2009, 36, 372–379. [Google Scholar] [CrossRef]
- Akbar, D.H. Bacterial Pneumonia: Comparison between Diabetics and Non-Diabetics. Acta Diabetol. 2001, 38, 77–82. [Google Scholar] [CrossRef]
- Brunetti, V.C.; Ayele, H.T.; Yu, O.H.Y.; Ernst, P.; Filion, K.B. Type 2 Diabetes Mellitus and Risk of Community-Acquired Pneumonia: A Systematic Review and Meta-Analysis of Observational Studies. CMAJ Open 2021, 9, E62–E70. [Google Scholar] [CrossRef] [PubMed]
- Di Yacovo, S.; Garcia-Vidal, C.; Viasus, D.; Adamuz, J.; Oriol, I.; Gili, F.; Vilarrasa, N.; García-Somoza, M.D.; Dorca, J.; Carratalà, J. Clinical Features, Etiology, and Outcomes of Community-Acquired Pneumonia in Patients with Diabetes Mellitus. Medicine 2013, 92, 42–50. [Google Scholar] [CrossRef]
- Movahed, M.R.; Hashemzadeh, M.; Jamal, M.M. Increased Prevalence of Infectious Endocarditis in Patients with Type II Diabetes Mellitus. J. Diabetes Complicat. 2007, 21, 403–406. [Google Scholar] [CrossRef]
- Abe, T.; Eyituoyo, H.O.; De Allie, G.; Olanipekun, T.; Effoe, V.S.; Olaosebikan, K.; Mather, P. Clinical Outcomes in Patients with Native Valve Infective Endocarditis and Diabetes Mellitus. World J. Cardiol. 2021, 13, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Abengowe, C.U.; McManamon, P.J. Acute Emphysematous Cholecystitis. Can. Med. Assoc. J. 1974, 111, 1112–1114. [Google Scholar]
- Thomsen, R.W.; Jepsen, P.; Sørensen, H.T. Diabetes Mellitus and Pyogenic Liver Abscess: Risk and Prognosis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2007, 44, 1194–1201. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.; Wu, S.; Peng, J. A Comparison of Pyogenic Liver Abscess in Patients with or without Diabetes: A Retrospective Study of 246 Cases. BMC Gastroenterol. 2018, 18, 144. [Google Scholar] [CrossRef]
- Mnif, M.F.; Kamoun, M.; Kacem, F.H.; Bouaziz, Z.; Charfi, N.; Mnif, F.; Naceur, B.B.; Rekik, N.; Abid, M. Complicated Urinary Tract Infections Associated with Diabetes Mellitus: Pathogenesis, Diagnosis and Management. Indian J. Endocrinol. Metab. 2013, 17, 442–445. [Google Scholar] [CrossRef]
- Nitzan, O.; Elias, M.; Chazan, B.; Saliba, W. Urinary Tract Infections in Patients with Type 2 Diabetes Mellitus: Review of Prevalence, Diagnosis, and Management. Diabetes. Metab. Syndr. Obes. 2015, 8, 129–136. [Google Scholar] [CrossRef]
- Geerlings, S.E.; Meiland, R.; van Lith, E.C.; Brouwer, E.C.; Gaastra, W.; Hoepelman, A.I.M. Adherence of Type 1-Fimbriated Escherichia Coli to Uroepithelial Cells: More in Diabetic Women than in Control Subjects. Diabetes Care 2002, 25, 1405–1409. [Google Scholar] [CrossRef]
- Nagendra, L.; Boro, H.; Mannar, V. Bacterial Infections in Diabetes. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Thwaini, A.; Khan, A.; Malik, A.; Cherian, J.; Barua, J.; Shergill, I.; Mammen, K. Fournier’s Gangrene and Its Emergency Management. Postgrad. Med. J. 2006, 82, 516–519. [Google Scholar] [CrossRef]
- Yaghan, R.J.; Al-Jaberi, T.M.; Bani-Hani, I. Fournier’s Gangrene: Changing Face of the Disease. Dis. Colon Rectum 2000, 43, 1300–1308. [Google Scholar] [CrossRef]
- Cheng, N.-C.; Tai, H.-C.; Chang, S.-C.; Chang, C.-H.; Lai, H.-S. Necrotizing Fasciitis in Patients with Diabetes Mellitus: Clinical Characteristics and Risk Factors for Mortality. BMC Infect. Dis. 2015, 15, 417. [Google Scholar] [CrossRef]
- Raz, R.; Chazan, B.; Dan, M. Cranberry Juice and Urinary Tract Infection. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2004, 38, 1413–1419. [Google Scholar] [CrossRef]
- Tienda-Vázquez, M.A.; Morreeuw, Z.P.; Sosa-Hernández, J.E.; Cardador-Martínez, A.; Sabath, E.; Melchor-Martínez, E.M.; Iqbal, H.M.N.; Parra-Saldívar, R. Nephroprotective Plants: A Review on the Use in Pre-Renal and Post-Renal Diseases. Plants 2022, 11, 818. [Google Scholar] [CrossRef]
- Pitocco, D.; Spanu, T.; Di Leo, M.; Vitiello, R.; Rizzi, A.; Tartaglione, L.; Fiori, B.; Caputo, S.; Tinelli, G.; Zaccardi, F.; et al. Diabetic Foot Infections: A Comprehensive Overview. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 26–37. [Google Scholar] [CrossRef]
- Brem, H.; Tomic-canic, M. Cellular and Molecular Basis of Wound Healing in Diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Fiayyaz, F.; Sabir, S.; Khurshid, M. Diabetes-Associated Infections: Development of Antimicrobial Resistance and Possible Treatment Strategies. Arch. Microbiol. 2020, 202, 953–965. [Google Scholar] [CrossRef]
- Ferlita, S.; Yegiazaryan, A.; Noori, N.; Lal, G.; Nguyen, T.; To, K.; Venketaraman, V. Type 2 Diabetes Mellitus and Altered Immune System Leading to Susceptibility to Pathogens, Especially Mycobacterium Tuberculosis. J. Clin. Med. 2019, 8, 2219. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Medicinal Plants and Their Effects on Diabetic Wound Healing. Vet. World 2019, 12, 653–663. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Pinto, D.C.G.A.; Cunha, Â.; Silva, H. Halophytes as Medicinal Plants against Human Infectious Diseases. Appl. Sci. 2022, 12, 7493. [Google Scholar] [CrossRef]
- Sagbo, I.J.; Afolayan, A.J.; Bradley, G. Antioxidant, Antibacterial and Phytochemical Properties of Two Medicinal Plants against the Wound Infecting Bacteria. Asian Pac. J. Trop. Biomed. 2017, 7, 817–825. [Google Scholar] [CrossRef]
- Komal, S.; Kazmi, S.A.J.; Khan, J.A.; Gilani, M.M. Antimicrobial Activity of Prunella Vulgaris Extracts against Multi-Drug Resistant Escherichia Coli from Patients of Urinary Tract Infection. Pak. J. Med. Sci. 2018, 34, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Scharf, B.; Sendker, J.; Dobrindt, U.; Hensel, A. Influence of Cranberry Extract on Tamm-Horsfall Protein in Human Urine and Its Antiadhesive Activity against Uropathogenic Escherichia Coli. Planta Med. 2019, 85, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Kheirabadi, Z.; Mehrabani, M.; Sarafzadeh, F.; Dabaghzadeh, F.; Ahmadinia, N. Green Tea as an Adjunctive Therapy for Treatment of Acute Uncomplicated Cystitis in Women: A Randomized Clinical Trial. Complement. Ther. Clin. Pract. 2019, 34, 13–16. [Google Scholar] [CrossRef]
- De Paula Ramos, L.; Da Rocha Santos, C.E.; Camargo Reis Mello, D.; Nishiama Theodoro, L.; De Oliveira, F.E.; Back Brito, G.N.; Campos Junqueira, J.; Cardoso Jorge, A.O.; Dias De Oliveira, L. Klebsiella Pneumoniae Planktonic and Biofilm Reduction by Different Plant Extracts: In Vitro Study. Sci. World J. 2016, 2016, 3521413. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In Vitro Antimicrobial Activity of Some Medicinal Plants against Human Pathogenic Bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef]
- Buzgaia, N.; Awin, T.; Elabbar, F.; Abdusalam, K.; Lee, S.Y. Antibacterial Activity of Arbutus Pavarii Pamp against Methicillin-Resistant Staphylococcus Aureus (MRSA) and UHPLC-MS/MS Profile of the Bioactive Fraction. Plants 2020, 9, 1539. [Google Scholar] [CrossRef]
- Gutierres, V.O.; Assis, R.P.; Arcaro, C.A.; Oliveira, J.O.; Lima, T.F.O.; Beretta, A.L.R.Z.; Costa, P.I.; Baviera, A.M.; Brunetti, I.L. Curcumin Improves the Effect of a Reduced Insulin Dose on Glycemic Control and Oxidative Stress in Streptozotocin-Diabetic Rats. Phyther. Res. 2019, 33, 976–988. [Google Scholar] [CrossRef]
- Okla, M.K.; Alamri, S.A.; Alatar, A.A.; Hegazy, A.K.; Al-Ghamdi, A.A.; Ajarem, J.S.; Faisal, M.; Abdel-Salam, E.M.; Ali, H.M.; Salem, M.Z.M.; et al. Antioxidant, Hypoglycemic, and Neurobehavioral Effects of a Leaf Extract of Avicennia Marina on Autoimmune Diabetic Mice. Evid.-Based Complement. Altern. Med. 2019, 2019, 1263260. [Google Scholar] [CrossRef]
- Zeid, I.E.M.E.A.; Al-Jaghthmi, O.H.A.; Heba, H.M. Augmentation of Insulin Secretion Induced by Rhizophora Mucronata and Avicennia Marina Extracts in Streptozotocin-Induced Diabetic Rats. Int. J. Pharm. Res. Allied Sci. 2019, 8, 14–22. [Google Scholar]
- Afshari, M.; Mohammadshahi, M.; Malayeri, A.R.; Zaheri, L. Antidiabetic, Hepato-Protective and Hypolipidemic Effects of Eryngium Caucasicum Extract in Streptozotocin-Nicotinamide Induced Type 2 Diabetes in Male Rats. Iraq Med. J. 2019, 3, 11–16. [Google Scholar]
- Boonphang, O.; Ontawong, A.; Pasachan, T.; Phatsara, M.; Duangjai, A.; Amornlerdpison, D.; Jinakote, M.; Srimaroeng, C. Antidiabetic and Renoprotective Effects of Coffea Arabica Pulp Aqueous Extract through Preserving Organic Cation Transport System Mediated Oxidative Stress Pathway in Experimental Type 2 Diabetic Rats. Molecules 2021, 26, 1907. [Google Scholar] [CrossRef]
- Belayneh, Y.M.; Birhanu, Z.; Birru, E.M.; Getenet, G. Evaluation of in Vivo Antidiabetic, Antidyslipidemic, and in Vitro Antioxidant Activities of Hydromethanolic Root Extract of Datura Stramonium L. (Solanaceae). J. Exp. Pharmacol. 2019, 11, 29–38. [Google Scholar] [CrossRef]
- Hasan, M.N.; Sabrin, F.; Rokeya, B.; Khan, M.S.H.; Ahmed, M.U.; Matondo, A.; Billah, M.M.; Akter, S. Glucose and Lipid Lowering Effects of Enhydra Fluctuans Extract in Cadmium Treated Normal and Type-2 Diabetic Model Rats. BMC Complement. Altern. Med. 2019, 19, 278. [Google Scholar] [CrossRef]
- Saleem, M.; Tanvir, M.; Akhtar, M.F.; Iqbal, M.; Saleem, A. Antidiabetic Potential of Mangifera indica L. cv. Anwar Ratol Leaves: Medicinal Application of Food Wastes. Medicina 2019, 55, 353. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).