Abstract
Orange essential oil (OEO) is mainly composed of D-Limonene and other oxygenated compounds that contribute to the orange flavor and aroma. However, D-Limonene is unstable in the presence of heat, light, and water, affecting the quality of the OEO. Therefore, the objective of this study was to fractionate OEO by distillation, both molecular and fractionated (hybrid), producing a D-Limonene-rich fraction. The OEO was characterized by physicochemical tests and gas chromatography combined with mass spectrometry (GC–MS). The fractionation of the OEO was carried out by molecular distillation and fractional distillation following, in both cases, a factorial design (23) with central points, considering the D-Limonene percentage in the distillate and the residue as a response variable. According to the physicochemical characterization, the predominant optical isomer was dextrorotatory, where D-Limonene is the main component of OEO (92.584%). For molecular distillation, the D-Limonene content was reduced to 47.964% in the residue or deterpenated fraction, while for fractional distillation, it was 86.779%. For this study, molecular distillation was considered a non-thermal process (use of low temperatures) that promoted the efficient recovery of oxygenated compounds. In contrast, fractional distillation favored the recovery of D-Limonene in the light fraction.
1. Introduction
Citrus is one of the most widely grown fruits worldwide, especially oranges, of which 47.24 million tons were produced in the period 2020–2021 [1]. In addition to fresh consumption, oranges are an important raw material in the food industry for the production of juices and the extraction of essential oils [2]. The orange essential oil (OEO) is found in the dispersed cavities of the peel (representing between 1 and 3% of the fresh weight); it is extracted from the flavedo, which is rich in oleiferous glands [3,4]. The OEO has properties such as antioxidant, anticarcinogenic, anti-inflammatory, cardioprotective, neuroprotective, antibacterial, and antifungal [5]. Meanwhile, the most frequent industrial applications of OEO are in the perfume or food industries, where it is recognized as GRAS (generally recognized as safe) and is often used as a flavoring agent for beverages, ice cream, and other food products. In addition, a significant amount of these oils is used to manufacture toilet soaps, cosmetics, and other household care products [6,7]. In general, the annual global production of citrus essential oil is approximately 16,000 tons, and the cost is about $14,000/ton on the international market [8]. The OEO consists of hundreds of compounds (volatile and non-volatile), including terpenic hydrocarbons and oxygenated compounds. Of the terpenes group, D-Limonene represents approximately 95% of the oil. However, limonene is chemically unstable, i.e., it is prone to structural rearrangement in the presence of air, light, heat, and water, which results in undesirable flavor characteristics in OEO [9,10,11]. However, the D-Limonene is widely used as flavoring, a cleansing agent, and as insecticide; it also has antimicrobial and antibacterial properties. It can be used as a biodegradable solvent, in adhesives, in pharmaceutical processes, and in chemical synthesis [12]. Therefore, D-Limonene is also considered a molecule of commercial interest.
On the other hand, oxygenated compounds include aldehydes, ketones, esters, alcohols, and acids, which constitute the fraction that gives the characteristic flavor of citrus fruits; however, they only represent about 5% by weight [9]. The reduction in terpenes is essential for the utilization of minority compounds (oxygenated) with high added value and to exploit the D-Limonene-enriched fraction (deterpenation).
The traditional method of recovering the essential oil from the peel is by cold-pressing [6]. After pressing, solvent extraction (hydroalcoholic mixture) is often used to extract the compounds of interest from OEO. However, this technique has a low extraction yield and requires large amounts of solvent [3]. On the other hand, molecular distillation or short-path distillation is a technology developed for the separation of thermosensitive liquid mixtures or those with high boiling points. Vacuum application in the distillation space helps liquid molecules evaporate easily without being affected by the negative impacts of heat on the evaporator surface [13]. Therefore, utilizing this technology helps prevent thermal damage to the OEO. Several reports indicate that molecular distillation has been successfully employed to concentrate and refine certain essential oils, such as orange [10], rosemary [14], oregano [15], mandarin, and lemon [16].
Another distillation technology used in the essential oil industry is fractional vacuum distillation [17]. This method is based on differences in volatility, whereas mass transfer occurs by contact between a vapor phase and a liquid phase at the same temperature and pressure. Separation efficiency depends on the type of packing, diameter, and packing height. In this case, applying a vacuum reduces the boiling temperature of the compounds, thus increasing volatility and favoring separation. In addition, the vacuum also delays the degradation of thermolabile compounds [18,19]. The antecedent of this technique has been described in the fractionation of orange and green mandarin essential oils [2,20,21,22]. However, in some cases, the essential oil spends a long time in the heat source at high temperatures, affecting the thermolabile compounds.
Among the innovative technologies, a little explored alternative is represented by hybrid distillation equipment due to its characteristics such as high vacuum and low residence time in contact with the heat source. In this context, fractional distillation equipment with a Wiped Film Evaporator represent a potential option to fractionate citrus oils without compromising their integrity. To our knowledge, this is the first report using this type of technology to fractionate OEO. Therefore, the objective of the present investigation was to evaluate the fractionation (generating two fractions: one rich in D-Limonene and the other rich in oxygenated compounds) of orange essential oil by molecular distillation and using a hybrid fractional distillation prototype coupled with a Wiped Film Evaporator to compare the two separation technologies.
2. Materials and Methods
2.1. Raw Material
Orange (Citrus sinensis) essential oil was provided by Frutech International Corporation SA de CV. The OEO was placed in an amber bottle and stored under refrigeration until use.
2.2. Physicochemical Properties of Orange Essential Oil
The development of the physicochemical methodologies was based on French standards and ISO standards reported by Torres, (2013) [23].
2.2.1. Specific Gravity and Refractive Index
The specific gravity and refractive index were determined in a densimeter (4500 M, Anton Paar GmbH, Graz, Austria) coupled to a refractometer (RXA 170, Anton Paar GmbH, Graz, Austria). The OEO analysis was performed with 3 mL at 20 °C.
2.2.2. Optical Rotation
The determination of the optical rotation of OEO was carried out with a digital polarimeter (AP-300, ATAGOTM, Bellevue, WA, USA).
2.2.3. Aldehyde Percentage
The percentage of total aldehydes was determined by using the ISO1279-1996—determination of carbonyl compound content with slight modifications. Briefly, a sample of 4 g of OEO was added to a hydroxylamine hydrochloride ethanolic solution (30 mL) previously titrated. The hydrochloride was titrated with KOH/CH3OH (0.1 M) to a pH of approximately 3.4. The mixture was stirred for 1 min and then maintained at a standstill for 30 min. After this time, the solution was titrated again with KOH/CH3OH (0.1 M) to a pH of 3.4. The KOH/CH3OH was added with a 25 mL volumetric burette, and the pH was measured with a potentiometer (HI 110, HANNA®, Woonsocket, RI, USA).
2.2.4. Miscibility in Ethanol
The test was based on the gradual addition of ethanol (96%) to a test tube with OEO at 20 °C according to the NF ISO 875. The mixture was homogenized until dissolution was observed.
2.3. Identification and Quantification of Compounds by Gas Chromatography–Mass Spectrometry
The identification and quantification of compounds of OEO were performed by gas chromatography (7890A, Agilent Technologies, Santa Clara, CA, USA) coupled with a single quadrupole mass spectrometer (5975C, Agilent Technologies, Santa Clara, CA, USA). Separation was performed on a HP-5 MS (30 m × 0.25 mm × 0.25 m) capillary column. The GC oven temperature was controlled at 75 °C for 1 min with the heating rate from 2.5 °C/min to 130 °C for 2 min, and finally from 25 °C/min to 260 °C for 5 min. Helium gas was used as carrier gas at a constant flow rate of 0.9 mL/min, the split ratio was 1:250 and the injected volume 0.2 μL. Parameters for MS analysis 5975C were with EI ion source, electron energy 70 eV, temperature of interface 260 °C, m/z = 50–550, amu. Identification of compounds was carried out by comparing their mass spectra with those of Wiley 275 library. The relative percentage of the components was calculated from GC–MS peak areas [23].
2.4. Fractionation of Orange Essential Oil
2.4.1. Molecular Distillation
Fractionation of the OEO was carried out in a molecular distiller (ICL-04A Short Path Distillation, InCon Process Systems, St. Charles, IL, USA), schematized in Figure 1. The distillation body of the equipment consists of an evaporator with a 0.04 m2 surface area. The evaporator is constructed as a jacketed pipe. For heating, oil is circulated in the jacket. In the center of the evaporator, there is a coil condenser with a contact area of 0.02 m2. The residue and distillate receptors are connected at the bottom of the evaporator. The heating surface is separated from the condensing surface by 0.03 m. The feed to the distiller was regulated by a peristaltic pump (77201-60, Master-flexTM, Gelsenkirchen, Germany). The evaporator and condenser temperatures were controlled by an oil recirculator (SE-Z, Julabo, Seelbach, Germany) and a chiller (7306A11B, PolyScience, Niles, IL, USA), respectively. The vacuum system consisted of a vacuum pump and a diffusion pump.
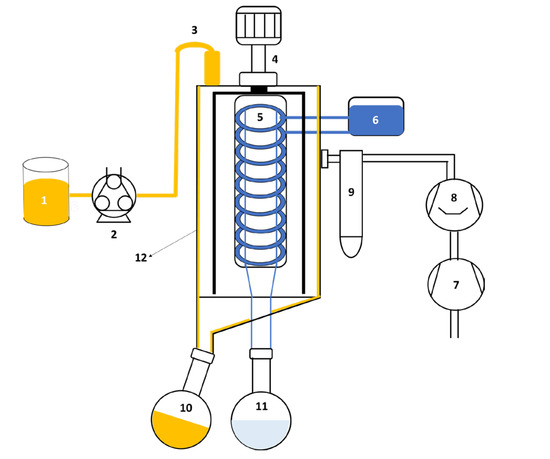
Figure 1.
Simplified schematic of the molecular distillation unit: (1) essential oil tank; (2) peristaltic pump; (3) feed; (4) motor; (5) condenser; (6) condenser cooling system; (7) vacuum pump; (8) diffusion pump; (9) trap vacuum; (10) residue collector; (11) distillate collector; and (12) evaporator surface.
The evaluation of OEO fractionation was performed with an experimental design 23 with 5 centrals points; the factors studied were pressure (1.5–2.0 mmHg), temperature (30–35 °C) and feed flow (10–12 g/min), while the rotational speed was constant to 300 rpm, and the temperature of was kept at 0 °C. The response variable was the relative area percentage of limonene in the distillate and the residue. Limonene and other characteristic oxygenated compounds were determined as described in Section 2.3.
2.4.2. Fractional Distillation with a Wiped Film Evaporator
Fractional distillation of OEO was performed in a hybrid prototype with a wiped film evaporator (ICL-04WR, InCon Process Systems, St. Charles, IL, USA), schematized in Figure 2. The equipment consists of a wiper film evaporator with a surface area of 0.04 m2. The evaporator is constructed as a jacketed pipe. For heating, oil is circulated in the jacket. In the internal part it has three parallel rollers in contact with the evaporator wall controlled by a rotor.
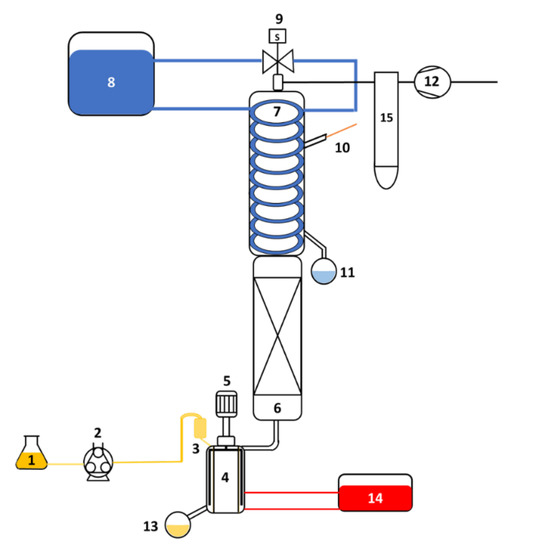
Figure 2.
Simplified schematic of the fractional distillation equipment: (1) essential oil tank; (2) peristaltic pump; (3) feed; (4) evaporator; (5) motor; (6) packed column; (7) condenser; (8) condenser cooling system; (9) solenoid valve; (10) thermometer; (11) distillate collector; (12) vacuum pump; (13) residue collector; (14) heating system; and (15) trap vacuum.
In addition, the system has a packed column (with rashing rings) with a height of 0.6 m and a diameter of 0.28 m; the thermal isolation of the column is provided by a double vacuum wall, while a condensate trap prevents vapors from reaching the vacuum pump (Rv3, Edwards, Burgess Hill, UK). A solenoid valve and a peristaltic pump (77201-60, MasterflexTM, Gelsenkirchen, Germany) control the reflux ratio and feed rate. A recirculator and a chiller were used to control the temperature in the evaporator and condenser.
To evaluate the separation process in this equipment, an experimental design 23 with 4 central points was carried out, where the factors studied were temperature (60–80 °C), feed (4–10 g/min), and reflux ratio (0–1). The response variable was the relative area percentage of limonene in the distillate and residue. Limonene quantification in both fractions was performed by GC–MS, as described in Section 2.3.
2.5. Statistical Analysis
According to the experimental design, the separation process was performed under different conditions, and the corresponding experiments were carried out. Statistical analyses were performed using Design-Expert 11 software (Stat-Ease Inc., Minneapolis, MN, USA). Data were analyzed by analyses of variance test (one-way ANOVA). The statistical significance of the variable was determined at a probability level of 5% (p ≤ 0.05). Regression equations were used to determine if the variables and levels affected the percentage of the compounds studied in both distillation techniques. The variables studied in the molecular distillation were feed (9.5–11.5 g/min), pressure (1.5–2 mmHg), and temperature (30–35 °C), while the variables for fractional distillation were temperature (60–80 °C), feed (4–10 g/min), and reflux ratio (0–1).
3. Results and Discussion
3.1. Physicochemical Characterization
Physicochemical tests such as specific gravity, refractive index, optical rotation, aldehyde percentage, and solubility are important indicators of the OEO quality. These tests are adopted by various regulatory institutions such as the French Standards Association (AFNOR) or the International Organization for Standardization (ISO) to detect adulteration in essential oils [24]. The values obtained from the physicochemical characterization of the OEO are presented in Table 1. In addition to weight, specific gravity can be used to determine the quality and purity of essential oil; their values range between 0.696 and 1.88, although most are less than 1, which implies that essential oils are lighter than water and consequently have low water-solubility or none at all [25]; such is the case of this oil that has a value of 0.846 and is soluble in ethanol. The refractive index represents a characteristic physical constant of oil, usually ranging from 1.450 to 1.590 [24]. Therefore, the value obtained is within this range. The refractive index in essential oils is an average weighting of the refractive indices of the components of the mixture [26]. Since OEO is mainly composed of D-Limonene (Table 2) it will have a refractive index close to that of D-Limonene (1.472) as shown by Torres-Alvarez et al. 2016 [27]. Consequently, the refractive index is useful as a qualitative measure of the purity of essential oil [25].
The optical rotation of an oil is the angle through which the plane of polarization is rotated when polarized light passes through it. Optically active substances are termed dextrorotatory (D or +) when they rotate plane-polarized light to the right, while laevorotatory (L or -) substances rotate it to the left [28]. For the OEO analyzed, the direction of rotation is dextrorotatory with an angle of 96.93, similar to that reported by Javed et al. 2014 for C. sinensis var. Mousami [29]. On the other hand, among the oxygenated compounds, aldehydes have been found to have the greatest influence on the flavor quality of citrus oils [30]. In this study, the aldehyde percentage obtained was 0.857, this value is close to the range reported (0.9–1.7%) by Muhoho Njoroge et al. 2005 [31] for different orange varieties. Regarding other commercial orange essential oils, Table 1 shows a comparison between the parameters obtained from the oil provided by Frutech and the values reported in the specification sheet for the oil market by PRAAN naturals® [32] and NHR ESSENTIAL OILS ORGANIC [33]. The results indicate that the quality of the oil used is similar to that of the commercial oils referenced.

Table 1.
Physicochemical characterization of OEO and comparison with other reference values.
Table 1.
Physicochemical characterization of OEO and comparison with other reference values.
| Physicochemical Analysis | This Investigation | PRAAN Naturals® | NHR ESSENTIAL OILS ORGANIC | Javed et al., 2014 [29] |
|---|---|---|---|---|
| Specific gravity | 0.846 ± 0.0005 | 0.845–0.849 | 0.828–0.855 | 0.842 |
| Refractive index | 1.468 ± 0.0021 | 1.472–1.474 | 1.460–1.490 | 1.471 |
| Optical rotation (°) | +96.93 ± 0.87 | +94–+99 | +90–+100 | +91 |
| Aldehyde percentage | 0.857 ± 0.160 | 0.5–2.5% | NR | NR |
| Ethanol miscibility | 1:1 (ethanol 95%:OEO) | Soluble | NR | Soluble |
1:1 represents the volume proportion of ethanol and OEO; NR: value not reported.

Table 2.
Retention time, identified compounds, and relative percentage of orange essential oil.
Table 2.
Retention time, identified compounds, and relative percentage of orange essential oil.
| Retention Time (min) | Identified Compound | Relative Percentage |
|---|---|---|
| 6.189 | α-Pinene | 0.444 |
| 6.573 | Camphene | 0.002 |
| 7.329 | β-Pinene | 1.130 |
| 7.809 | Octanal | 0.140 |
| 7.997 | α- Phellandrene | 0.026 |
| 8.369 | α-Terpinene | 0.047 |
| 8.971 | D-Limonene | 92.589 |
| 9.828 | γ-Terpinene | 0.013 |
| 10.845 | Terpinolene | 0.022 |
| 11.14 | Linalool | 0.465 |
| 11.305 | Nonanal | 0.020 |
| 13.336 | β-Citronellal | 0.026 |
| 14.516 | 1-Terpinen-4-ol | 0.001 |
| 15.082 | α-Terpineol | 0.031 |
| 15.642 | Decanal | 0.148 |
| 16.701 | Nerol (cis-geraniol) | 0.001 |
| 17.313 | Neral (cis-citral) | 0.033 |
| 17.885 | Geraniol (trans-geraniol) | 0.001 |
| 18.692 | Geranial (trans-citral) | 0.040 |
| 20.483 | Undecanal | 0.015 |
| 25.604 | Dodecanal | 0.024 |
| 26.687 | Valencene | 0.012 |
| - | Other unidentified compounds | 4.77 |
3.2. Orange Essential oil (OEO) Analysis
When gas chromatography is combined with mass spectrometry (GC–MS), the result is a powerful analytical tool useful to separate, identify, and quantify complex mixtures of chemicals [34], and this is the case with essential oils. The results of the identification and chemical composition analysis of OEO by GC–MS are presented in Table 2. With the mass detector, it was possible to identify 22 compounds representing 95.23% of the total. Among the characteristic compounds of OEO, hydrocarbon monoterpenes were found in the highest abundance, with D-Limonene being the major component (92.589%) followed by α and β-pinene (0.444 and 1.13%). In the case of oxygenated monoterpenes (alcohols), linalool (0.465%) was found in the highest quantity.
Two other groups of compounds contributing to the aromatic profile of OEO are aldehydes and sesquiterpenes, of which the main components were decanal (0.148%) and valencene (0.012%). Torres-Alvarez et al. 2016 [27] also characterized OEO provided by Frutech International Corporation, in which a total of 16 compounds were identified from an unconcentrated sample, and the main compound was D-Limonene (91.12%) with a percentage similar to that found in this work. On the other hand, linalool (0.65%), α-pinene (1.13%), and decanal (0.44%) did show some variation, while they did not report the presence of valencene. In other characterization studies, the characteristic compounds of OEO were reported according to the following values: D-Limonene 74.45–96.68%, linalool 0.17–1.54%, decanal 0.16–0.44% and valencene 0.01–0.47% [2,27,31,35,36,37,38,39,40]. The variations in the values can depend significantly on the extraction method. However, other factors that could have an influence are genetic variability, harvest time, geographical location, and growing conditions [19].
3.3. Results of Statistical Analysis
In the present study, the effects of three independent parameters (factors) were investigated for both molecular distillation and fractional distillation. The levels choice was based on the knowledge acquired previously. The evaluation in the molecular distillation of OEO fractionation was performed with a factorial design 23 with five central points, 13 experimental runs were required, while for fractional distillation there were only 12 experimental runs because only four central points were used.
3.3.1. Molecular Distillation
With respect to the distillate fraction, a colorless liquid was recovered in each experiment. The highest percentage of D-Limonene obtained was 92.688%, corresponding to a feed of 9.5 g/min, 1.5 mmHg, and 35 °C (Table 3). Under these described operating conditions, 206 g was recovered in the collector, while for other compounds of interest, linalool (0.427%) and decanal (0.083%) were found, and both percentages were lower than those reported in the feed (Table 2). Martins et al. 2013 [38] fractionated OEO by molecular distillation, and for the light fraction, they increased the D-Limonene percentage from 93.41 (in the feed) to 93.91, working with an evaporation temperature of 82.5 °C. The increase obtained represents a result four times higher than that reported in the present investigation. A possible cause of the variation in these results could be directly related to the operating conditions, since the difference in evaporator operating temperature between the two studies is approximately 48 °C.

Table 3.
Results of D-Limonene percentage according to factorial design of molecular distillation.
While the analysis of the residue (deterpenated fraction) showed that the D-Limonene percentage was reduced to 47.964%, as shown in Table 3, the experiment that yielded the lowest amount of D-Limonene also presented the highest percentages for the following oxygenated compounds, concerning the feed: linalool (3.887%), decanal (3.044%), and valencene (0.571%); these three compounds were increased by 8.3, 20.6, and 46.6-fold, respectively. In the case of the residue, its trade price is influenced by the valencene content [38].
The factorial design applied allowed the identification of the significant experimental factors influencing the D-Limonene percentage of the OEO. Statistical testing of the model was performed by using the analysis of variance (ANOVA). The ANOVA results on the factorial design for molecular distillation are shown in Table 4; p-values less than 0.05 indicated that the model terms were significant, in the case of distillate all factors were found to be significant (X1, X2, X3), and for the residue fraction, only pressure (X2), temperature (X3), as well as the interaction between X1X2 were significant.

Table 4.
ANOVA results for the experimental design of molecular distillation.
The coefficient of determination (R2) for the D-limonene percentage was 0.9664 and 0.8533 in the distillate and residue, respectively. Thus, it is inferred that the accuracy and general predictive ability of the quadratic polynomial regression model were adequate (since, for a good fit of the model, R2 should not be less than 80% [41]) and are represented by equations shown in Table 5.

Table 5.
Predictive models in molecular distillation (distillate and residue) for D-Limonene and its correlation coefficient.
The response surface of the D-Limonene percentage in distillate and residue are shown in Figure 3. The surface response suggests the interaction between factors X2 and X3, and it was observed that increasing the temperature and having a low pressure increased the percentage of D-Limonene (Figure 3a) for distillate fraction. In the case of Figure 3b, for the residue, what is desired is to minimize the D-Limonene percentage; therefore, to make this occur, it is also necessary to work with high temperatures and low pressures.
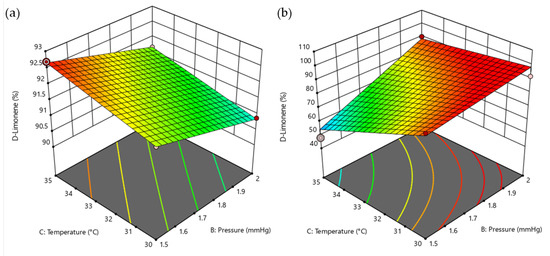
Figure 3.
Response-surface representations in molecular distillation. (a) Percentage of D-Limonene in distillate and (b) percentage of D-Limonene in residue.
The statistical analysis of this research contributes to the generation and expansion of knowledge on the fractionation of OEO by molecular distillation by evaluating a set of independent variables that had not been considered in other studies.
3.3.2. Fractional Distillation
The experimental matrix and the D-Limonene percentages obtained for fractional distillation (with a wiped film evaporator) in the light and heavy fractions are shown in Table 6. The D-Limonene was concentrated from 92.584% (from the feed) to 93.224%, higher than that obtained by molecular distillation. However, the mass percent from distillate (%mD) recovered was 71%, i.e., 178 g were collected; therefore, the %mD could be considered as another response variable in the experimental design to increase the amount of mass recovered in the distillate.

Table 6.
Results of D-Limonene percentage according to factorial design of fractional distillation.
On the other hand, for the deterpenated fraction, the lowest D-Limonene percentage obtained was 86.779%, which suggests that the separation efficiency was poor for the conditions studied, and it would be necessary to include even more stages in the distillation processing conditions to increase the degree of purity of the chemical species of interest in both fractions.
For example, Perini et al. 2017 [2] recovered 95% of the D-limonene feed in OEO to a fractionated distillation column with three stages, while for this work with a single stage, 66% was obtained. In addition to the number of stages, the residence time in the heat source could also influence the difference in percentages, because the hybrid equipment used in this research employs residence times in the order of seconds, while Perini et al. 2017 [2] operated the column for up to 45 min.
Concerning oxygenated compounds in the deterpenated fraction, linalool, decanal and valencene increased 3.1-, 5.8-, and 8.3-fold, respectively. These increases are lower than those obtained in molecular distillation. In consideration of the fact that D-Limonene recovery in the distillate was better for fractional distillation, the residual fraction still containing a high percentage of D-Limonene could be fed to the molecular distillation equipment where the separation of oxygenated compounds was more effective; thus, both types of distillation could complement each other and improve the purification of high-value compounds.
A series of probabilistic variables such as the square sum, mean square, Fisher distribution value, and p-value are shown in Table 7. The ANOVA table partitions the variability of D-Limonene (%) into separate pieces for each of the effects, then tests the statistical significance of each effect by comparing its mean square against an estimate of the experimental error. In the case of distillate, the ANOVA analysis demonstrated that the significant terms were as follows: temperature (X1), feed (X2), reflux ratio (X3), as well as the interaction between X1 X3 and X2 X3 (p < 0.05). On the other hand, in the residue fraction, only one effect has a p-value less than 0.05, indicating that it is significantly different from zero at a confidence level of 95.0%.

Table 7.
ANOVA results for the experimental design of fractional distillation.
Based on the ANOVA analysis and fitting the factors with the responses using the least squares method, the D-limonene percentage is obtained by equations shown in Table 8 as a function of the independent variables X1−X3. Moreover, the quality of the fit was expressed with the coefficient of the determination (R2) for distillate and residue. In the case of distillate, the R2 was 0.9691, that is, it described 96% of all the data. In the residue, it can be observed that only 76% of the variability of the data is explained, and while this value is less than 80%, which is necessary to be considered a good fit, we can assume that by having a significant factor in the response variable (D-Limonene percentage), this result still provides useful information, although the data points are located further away from the regression line.

Table 8.
Predictive models in fractional distillation (distillate and residue) for D-Limonene and its correlation coefficient.
The response surface provides a suggestion of the response that the variable of interest (D-Limonene) may have, concerning the interaction of the different factors. Figure 4a corresponds to the distillate fraction in the fractional distillation; it is desirable to maximize the %D-Limonene and for that to happen, the required combination of the temperature and feed factors is high and low, respectively. As for the residue fraction, it is desirable that the %D-Limonene decreases (Figure 4b); therefore, a high temperature in combination with low feed should be managed.
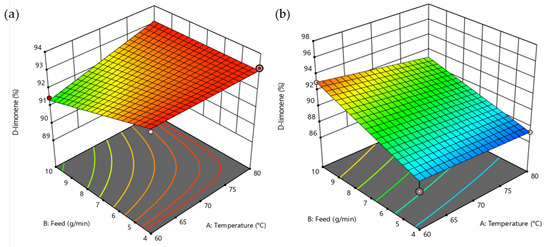
Figure 4.
Response-surface representations in fractional distillation. (a) D-Limonene (%) in distillate and (b) D-Limonene (%) in residue.
4. Conclusions
The molecular distiller and the fractionated distiller coupled to a wiped film evaporator are two pieces of equipment that can fractionate orange essential oil. However, molecular distillation can be considered practically a “non-thermal” process, since it has proven to be effective in concentrating oxygenated compounds (linalool, decanal, and valencene) at low temperatures (30–35 °C) in the heavy fraction.
In the molecular distillation, in both distillate and residue, the variables which demonstrated more influence on the D-Limonene separation were temperature and pressure, regardless of whether it was desired to maximize or minimize the D-Limonene percentage. However, in fractional distillation, the variables that maximized the D-Limonene percentage in the distillate fraction were temperature and feed, while for minimizing the response variable in the residue fraction, only feed was significant.
In addition, due to the experimental processing conditions, the thermal damage of bioactive compounds and pigments, which are also of special interest in the OEO, could be avoided. However, if the interest is to reduce the oxygenated compounds percentage in the light fraction, fractional distillation is an adequate option since the packed column obtains a rectified light fraction with a high D-Limonene content.
The residue obtained from molecular distillation has a potential use within the food industry as a beverage flavoring agent, while the distilled portion from the fractional distillation with high D-Limonene content can be used in the pesticide, pharmaceutical, and other industries. For future research, it is expected that both distillation techniques may be combined to increase the D-Limonene percentage compared with that obtained by individual equipment.
Author Contributions
Conceptualization, J.A.G.-F., E.A.-G. and G.M.G.-M.; Formal analysis, J.A.G.-F., E.A.-G., D.A.F.-M., M.G.-V. and G.M.G.-M.; Investigation, J.A.G.-F., E.A.-G., D.A.F.-M., R.I.C.-G., Á.S.-J., M.G.-V., L.G.T.-M. and G.M.G.-M.; Methodology, J.A.G.-F. and L.G.T.-M.; Project administration, J.A.G.-F., E.A.-G. and G.M.G.-M.; Resources, J.A.G.-F., E.A.-G. and G.M.G.-M.; Supervision, J.A.G.-F., E.A.-G. and G.M.G.-M.; Writing—original draft, D.A.F.-M. and M.G.-V.; Writing—review and editing, J.A.G.-F., E.A.-G., R.I.C.-G., Á.S.-J. and G.M.G.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, A.C. (CIATEJ), and Universidad de Guadalajara (UDG). Financial support for Lilia Guadalupe Torres-Martínez originated from Consejo Nacional de Ciencia y Tecnología (Conacyt) through scholarship 249359.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors would like to thank Frutech International Corporation S.A. de C.V.; Consejo Nacional de Ciencia y Tecnología, CONACYT, for scholarship No. 249359; Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, A.C. and Universidad de Guadalajara.
Conflicts of Interest
The authors declare no conflict of interest.
References
- USDA Foreign Agricultural Service. Citrus: World Markets and Trade. 2022. Available online: https://www.fas.usda.gov/data/citrus-world-markets-and-trade (accessed on 30 August 2022).
- Perini, J.F.; Silvestre, W.P.; Agostini, F.; Toss, D.; Pauletti, G.F. Fractioning of Orange (Citrus sinensis L.) essential oil using vacuum fractional distillation. Sep. Sci. Technol. 2017, 52, 1397–1403. [Google Scholar] [CrossRef]
- Gugo, G.; Di Giocamo, A. The Genus Citrus, 1st ed.; Taylor and Francis Group: London, UK, 2002; pp. 114–148. [Google Scholar]
- Golmohammadi, M.; Borghei, A.; Zenouzi, A.; Zenouzi, A. Optimization of essential oil extraction from orange peels using steam explosion. Heliyon 2018, 4, e00893. [Google Scholar] [CrossRef]
- Razola-Díaz, M.; Guerra-Hernández, E.; García-Villanova, B.; Verardo, V. Recent developments in extraction and encapsulation techniques of orange essential oil. Food Chem. 2021, 354, 129575. [Google Scholar] [CrossRef]
- Sahraoui, N.; Abert, M.; El, M.; Boutekedjiret, C.; Chemat, F. Valorization of citrus by-products using Microwave Steam Distillation (MSD). Innov. Food Sci. Emerg. Technol. 2011, 12, 163–170. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Biological Activities and Safety of Citrus spp. Essential Oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef]
- Deng, W.; Liu, K.; Cao, S.; Sun, J.; Zhong, B.; Chun, J. Chemical Composition, Antimicrobial, Antioxidant, and Antiproliferative Properties of Grapefruit Essential Oil Prepared by Molecular Distillation. Molecules 2020, 25, 217. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xu, Y.; Wang, X. Microencapsulation of sweet orange oil terpeneless using the orifice method. J. Food Eng. 2012, 110, 390–394. [Google Scholar] [CrossRef]
- Martinello, M.A.; Pagliero, C.L.; Allevi, C.A. Deterpenation of Orange Essential Oil by Molecular Distillation. Int. J. Eng. Trends Technol. 2016, 30, 161–165. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Ramos-Ibarra, J.; Arriola-Guevara, E.; Toriz, G.; Guatemala-Morales, G.; Corona-Gonzalez, R. Enzymatic extraction of limonene, limonin and other relevant compounds from Citrus sinensis (orange) and Citrus aurantiifolia (lime) by-products. Rev. Mex. Ing. Química 2021, 20, 1–13. [Google Scholar] [CrossRef]
- Ketenoglu, O. Molecular Distillation in the Extraction, Recovery, and Concentration of Food Molecules. In Innovative Food Processing Technologies a Comprehensive Review, 1st ed.; Knoerzer, K., Muthukumarappan, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 3, pp. 122–134. [Google Scholar]
- Mezza, G.N.; Borgarello, A.V.; Daguero, J.D.; Pramparo, M.C. Obtention of Rosemary Essential Oil Concentrates by Molecular Distillation and Free Radical Scavenging Capacity Analysis. Int. J. Food Eng. 2013, 9, 147–153. [Google Scholar] [CrossRef]
- Ruben, O.; Nepote, V.; Nelson, R. Antioxidant activity of fractions from oregano essential oils obtained by molecular distillation. Food Chem. 2014, 156, 212–219. [Google Scholar]
- Yang, F.; Zhang, H.; Tian, G.; Ren, W.; Li, J.; Xiao, H.; Zheng, J. Effects of Molecular Distillation on the Chemical Components, Cleaning, and Antibacterial Abilities of Four Different Citrus Oils. Front. Nutr. 2021, 8, 731724. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, W.P.; Livinalli, N.F.; Baldasso, C.; Tessaro, I. Pervaporation in the separation of essential oil components: A review. Trends Food Sci. Technol. 2019, 93, 42–52. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Rilo, F.; Agostini, F.; Toss, D.; Fernandes, F. Fractionation of rosemary (Rosmarinus officinalis L.) essential oil using vacuum fractional distillation. J. Food Sci. Technol. 2019, 56, 5422–5434. [Google Scholar] [CrossRef] [PubMed]
- Do, D.N.; Nguyen, D.P.; Phung, V.-D.; Le, X.-T.; Le, T.M.; Do, V.M.; Minh, B.Q.; Luu, X.C. Fractionating of Lemongrass (Cymbopogon citratus) Essential Oil by Vacuum Fractional Distillation. Processes 2021, 9, 593. [Google Scholar] [CrossRef]
- Stuart, G.; Lopes, D.; Oliveira, J. Deterpenation of Brazilian Orange Peel Oil by Vacuum Distillation. J. Am. Oil Chem. Soc. 2001, 78, 1041–1044. [Google Scholar] [CrossRef]
- Beneti, S.; Rosset, E.; Corazza, M.; Frizzo, C.; Di Luccio, M.; Oliveira, J.V. Fractionation of citronella (Cymbopogon winterianus) essential oil and concentrated orange oil phase by batch vacuum distillation. J. Food Eng. 2011, 102, 348–354. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Agostini, F.; Muniz, L.A.; Pauletti, G.F. Fractionating of green mandarin (Citrus deliciosa Tenore) essential oil by vacuum fractional distillation. J. Food Eng. 2016, 178, 90–94. [Google Scholar] [CrossRef]
- Torres, L. Evaluación de la Destilación Molecular y un Prototipo Híbrido (Destilación Fraccionada con Evaporador de Película Barrida) para la Purificación y Concentración de Compuestos Terpénicos del Aceite Esencial de Naranja (Citrus sinensis). Master’s Thesis, Universidad de Guadalajara, Guadalajara, Mexico, 2013. [Google Scholar]
- Can, K.; Buchbauer, G. Handbook of Essential Oils Science, Technology and Applications, 2nd ed.; Taylor and Francis Group: Boca Raton, FL, USA, 2016; pp. 165–229. [Google Scholar]
- Fakayode, O.A.; Abobi, K.E. Optimization of oil and pectin extraction from orange (Citrus sinensis) peels: A response surface approach. J. Anal. Sci. Technol. 2018, 9, 20. [Google Scholar] [CrossRef]
- Valarezo, E.; Ojeda-Riascos, S.; Cartuche, L.; Andrade-González, N.; González-Sanchez, I.; Meneses, M. Extraction and Study of the Essential Oil of Copal (Dacryodes peruviana), an Amazonian Fruit with the Highest Yield Worldwide. Plants 2020, 9, 1658. [Google Scholar] [CrossRef] [PubMed]
- Torres-Alvarez, C.; Núñez, A.; Rodríguez, J.; Castillo, S.; Leos-Rivas, C.; Báez-González, J. Chemical composition, antimicrobial, and antioxidant activities of orange essential oil and its concentrated oils. CyTA—J. Food 2016, 15, 129–135. [Google Scholar] [CrossRef]
- Clarke, S. Essential Chemistry for Aromatherapy, 2nd ed.; Churchill Livingstone Elsevier: London, UK, 2008; pp. 104–105. [Google Scholar]
- Javed, S.; Javaid, A.; Nawaz, S.; Saeed, M.; Mahmood, Z.; Siddiqui, S.; Ahmad, R. Phytochemistry, GC-MS Analysis, Antioxidant and Antimicrobial Potential of Essential Oil from Five Citrus Species. J. Agric. Sci. 2014, 6, 201–208. [Google Scholar] [CrossRef]
- Xu, B.; Sims, C.; Etxeberria, E.; Goodrich Schneider, R. Physicochemical and Sensory Properties of Cold Pressed Oils from Florida Hamlin and Valencia Oranges Affected by Huanglongbing. J. Food Sci. 2017, 82, 2158–2166. [Google Scholar] [CrossRef]
- Muhoho Njoroge, S.; Koaze, H.; Karanja, P.N.; Sawamura, M. Essential oil constituents of three varieties of Kenyan sweet oranges (Citrus sinensis). Flavour Fragance J. 2005, 20, 80–85. [Google Scholar] [CrossRef]
- Natural Sourcing. Organic Sweet Orange Essential Oil. 2022. Available online: http://www.praannaturals.com/downloads/specsheets/SPEC_Organic_Essential_Oil_Orange_CP_OEOORANGECPMXUS291.pdf (accessed on 2 September 2022).
- NHR Organic Oils. Organic Orange Essential Oil Sweet (Citrus sinensis). 2022. Available online: https://www.nhrorganicoils.com/uploads/20170505112247e_Orange_Spec.pdf (accessed on 2 September 2022).
- Madeiros, P. Gas Chromatography–Mass Spectrometry (GC–MS). In Encyclopedia of Geochemistry a Comprehensive Reference Source on the Chemistry of the Earth, 1st ed.; White, W., Ed.; Springer: Cham, Switzerland, 2018; pp. 530–535. [Google Scholar]
- Ayala, J.; Montero, G.; Campbell, H.; García, C.; Coronado, M.; León, J.; Sagaste, C.; Pérez, L. Extraction and Characterization of Orange Peel Essential Oil from Mexico and United States of America. J. Essent. Oil Bear. Plants 2017, 20, 897–914. [Google Scholar] [CrossRef]
- Farhat, A.; Fabiano-Tixier, A.-S.; El Maataoui, M.; Maingonnat, J.; Romdhane, M.; Chemat, F. Microwave steam diffusion for extraction of essential oil from orange peel: Kinetic data, extract’s global yield and mechanism. Food Chem. 2011, 125, 255–261. [Google Scholar] [CrossRef]
- Mitiku, S.; Sawamura, M.; Itoh, T.; Ukeda, H. Volatile components of peel cold-pressed oils of two cultivars of sweet orange (Citrus sinensis (L.) Osbeck) from Ethiopia. Flavour Fragance J. 2000, 15, 240–244. [Google Scholar] [CrossRef]
- Martins, P.F.; Medeiros, H.H.; Sbaite, P.; Wolf Maciel, M.R. Enrichment of oxyterpenes from orange oil by short path evaporation. Sep. Purif. Technol. 2013, 116, 385–390. [Google Scholar] [CrossRef]
- Qiao, Y.; Xie, B.J.; Zhang, Y.; Fan, G.; Yao, X.L.; Pan, S.Y. Characterization of aroma active compounds in fruit juice and peel oil of Jinchen sweet orange fruit (Citrus sinensis (L.) Osbeck) by GC-MS and GC-O. Molecules 2008, 13, 1333–1344. [Google Scholar] [CrossRef]
- Haypek, E.; Silva, L.; Batista, E.; Marques, D.; Meireles, M.; Meirelles, A. Recovery of aroma compounds from orange. Braz. J. Chem. Eng. 2000, 17, 705–712. [Google Scholar] [CrossRef]
- Gutiérrez, H.; De la Vara, R. Análisis y Diseño de Experimentos, 2nd ed.; McGraw-Hill Companies/Interamericana Editores S.A. de C.V.: Mexico City, Mexico, 2008; pp. 384–420. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).