Mechanism and Kinetics of Interaction of FLiNaK–CeF3 Melt with Water Vapors and Oxygen in the Air Atmosphere
Abstract
1. Introduction
2. Materials and Methods
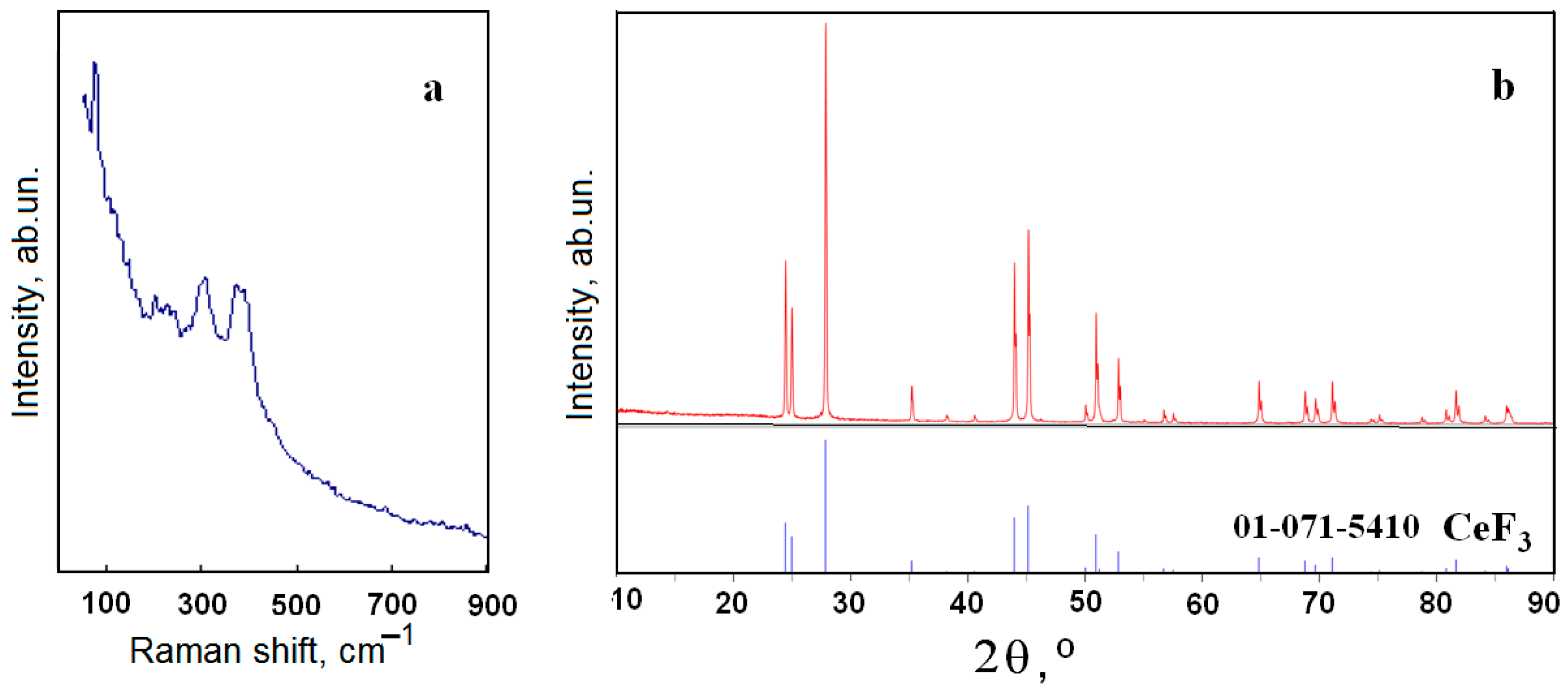
2.1. Reagents Preparation and Certification
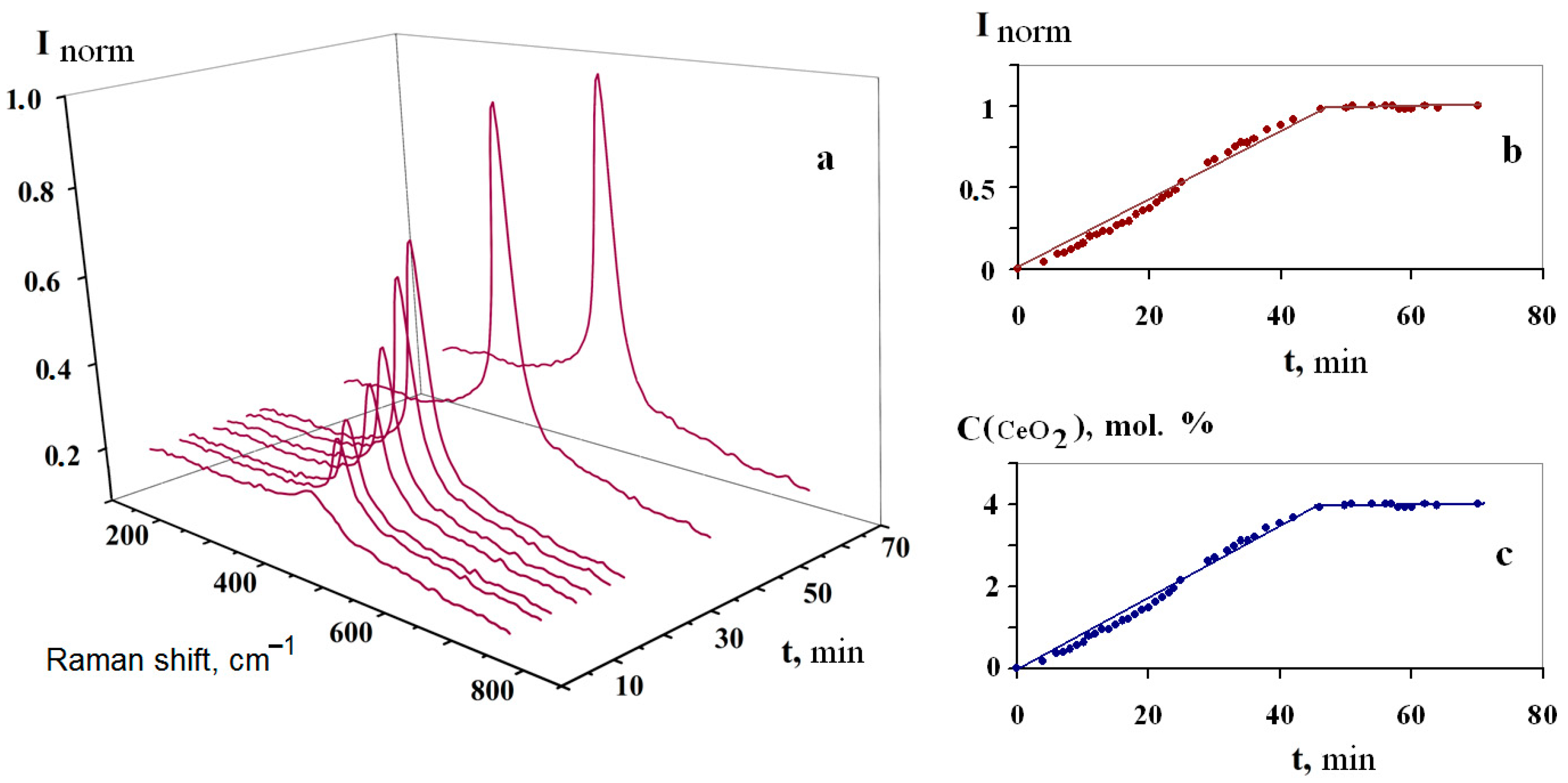
2.2. Technique for Raman Spectra Recording
3. Results and Discussion
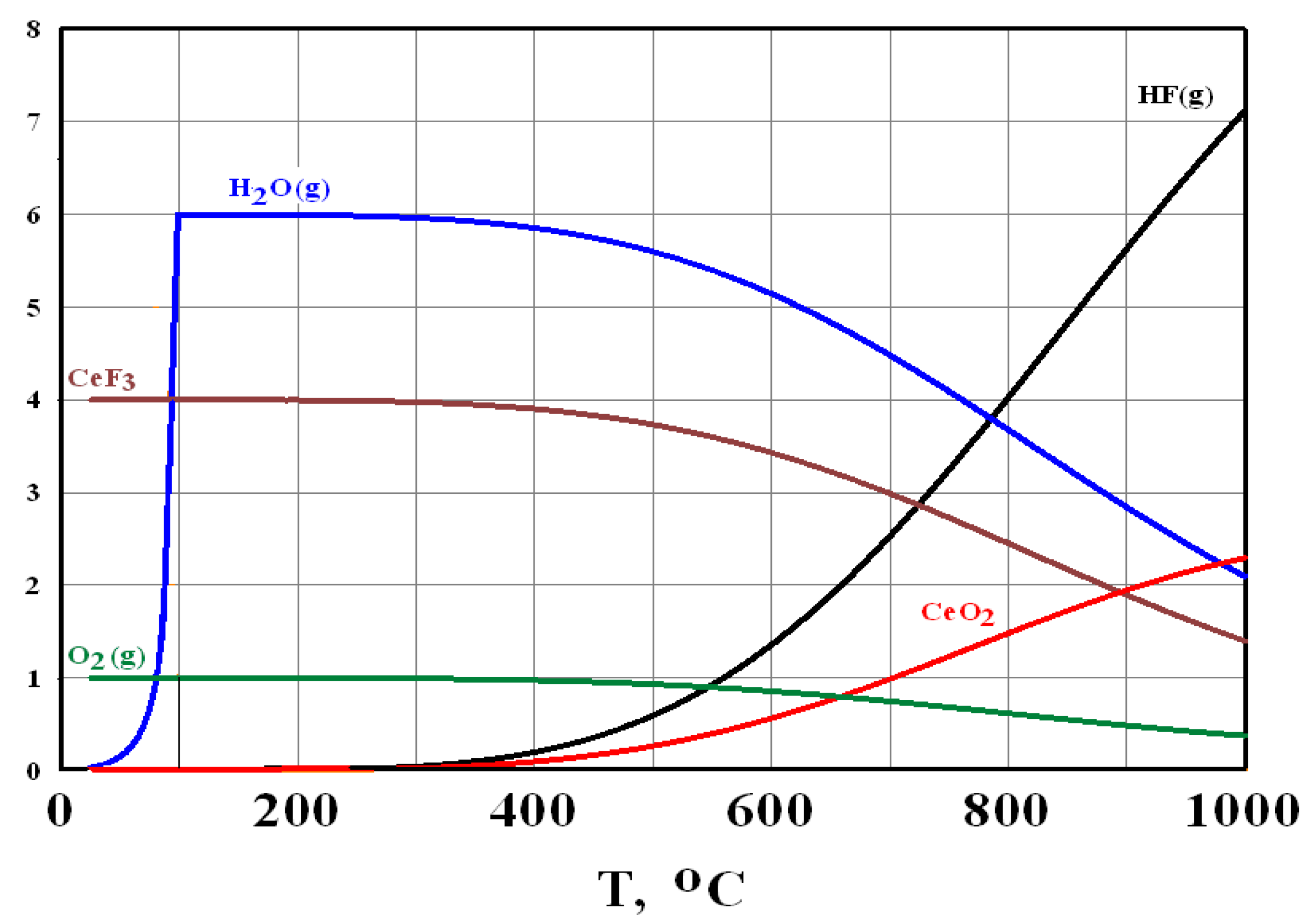
3.1. Mechanism of Interaction between FLiNaK–CeF3 Melts and the Components of the Air Atmosphere
| Reaction | ΔG, kJ | K | Log (K) |
|---|---|---|---|
CeF3 + H2O(g) = CeOF + 2HF(g)
| −134.173 | 3.399 × 106 | 6.531 |
2CeF3 + O2(g) = 2CeOF + 2F2(g)
| 472.360 | 1.015 × 10−23 | −22.994 |
2CeOF + H2O(g) = Ce2O3 + 2HF(g)
| 496.561 | 6.734 × 10−25 | −24.172 |
2CeOF + 2H2O(g) = 2CeO2 + H2(g) + 2HF(g)
| 456.433 | 6.049 × 10−23 | −22.218 |
4CeF3 + 3O2(g) = 2Ce2O3 + 6F2(g)
| 640.189 | 4.103 × 10−131 | −130.387 |
2CeOF + 2H2O(g) = 2CeO2 + 2H2(g) + F2(g)
| 1015.477 | 3.702 × 10−50 | −49.432 |
2Ce2O3 + O2(g) = 4CeO2
| −457.639 | 1.893 × 1022 | 22.277 |
2CeOF + O2(g) = 2CeO2 + F2(g)
| 638.095 | 8.683 × 10−32 | 31.061 |
CeF3 + O2(g) = CeO2 + 1.5F2(g)
| 555.227 | 9.387 × 10−28 | −27.027 |
4CeF3 + 6H2O(g) + O2(g) = 4CeO2 + 12HF(g)
| −1.208 | 1.145 × 100 | 0.059 |
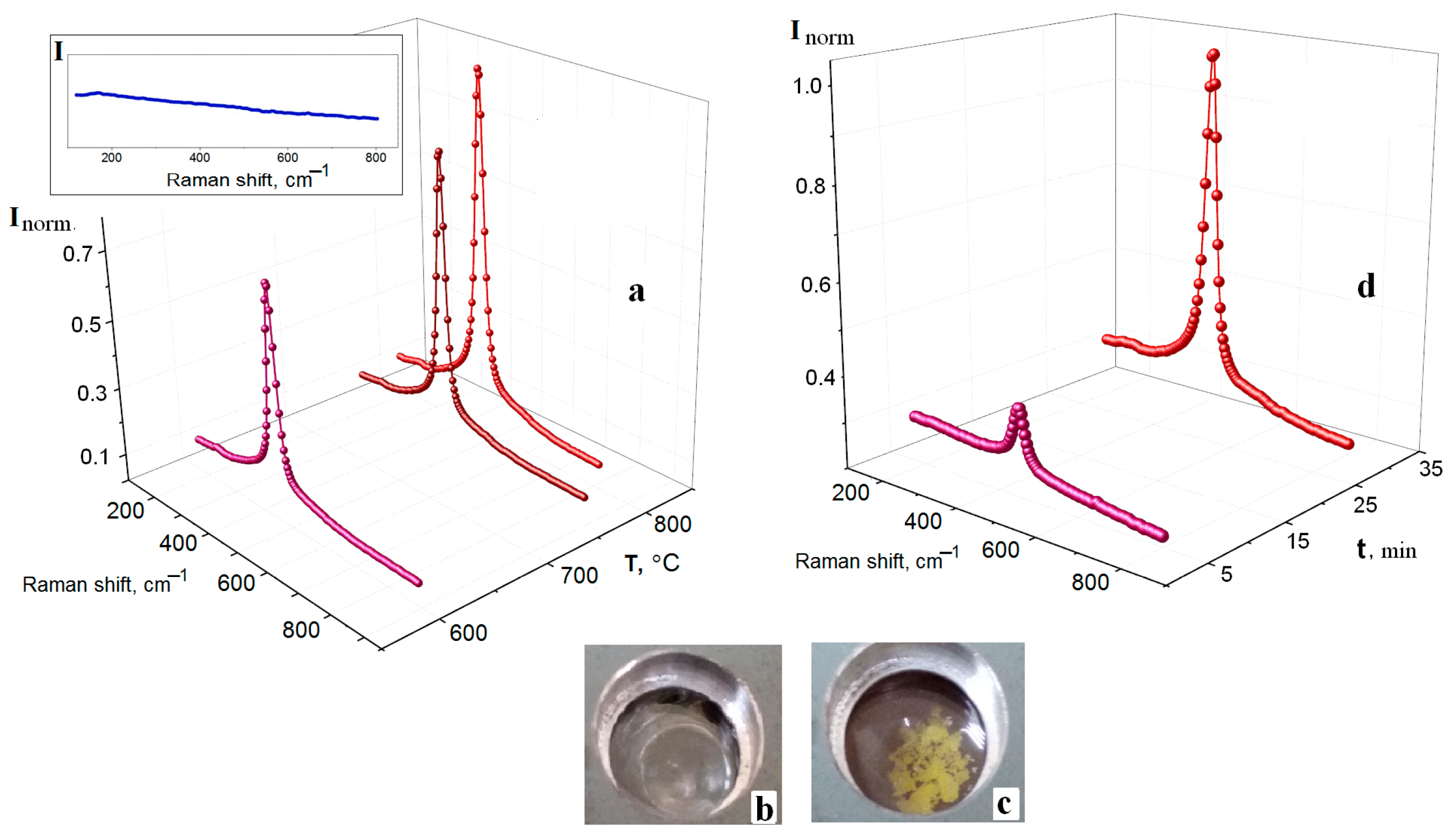
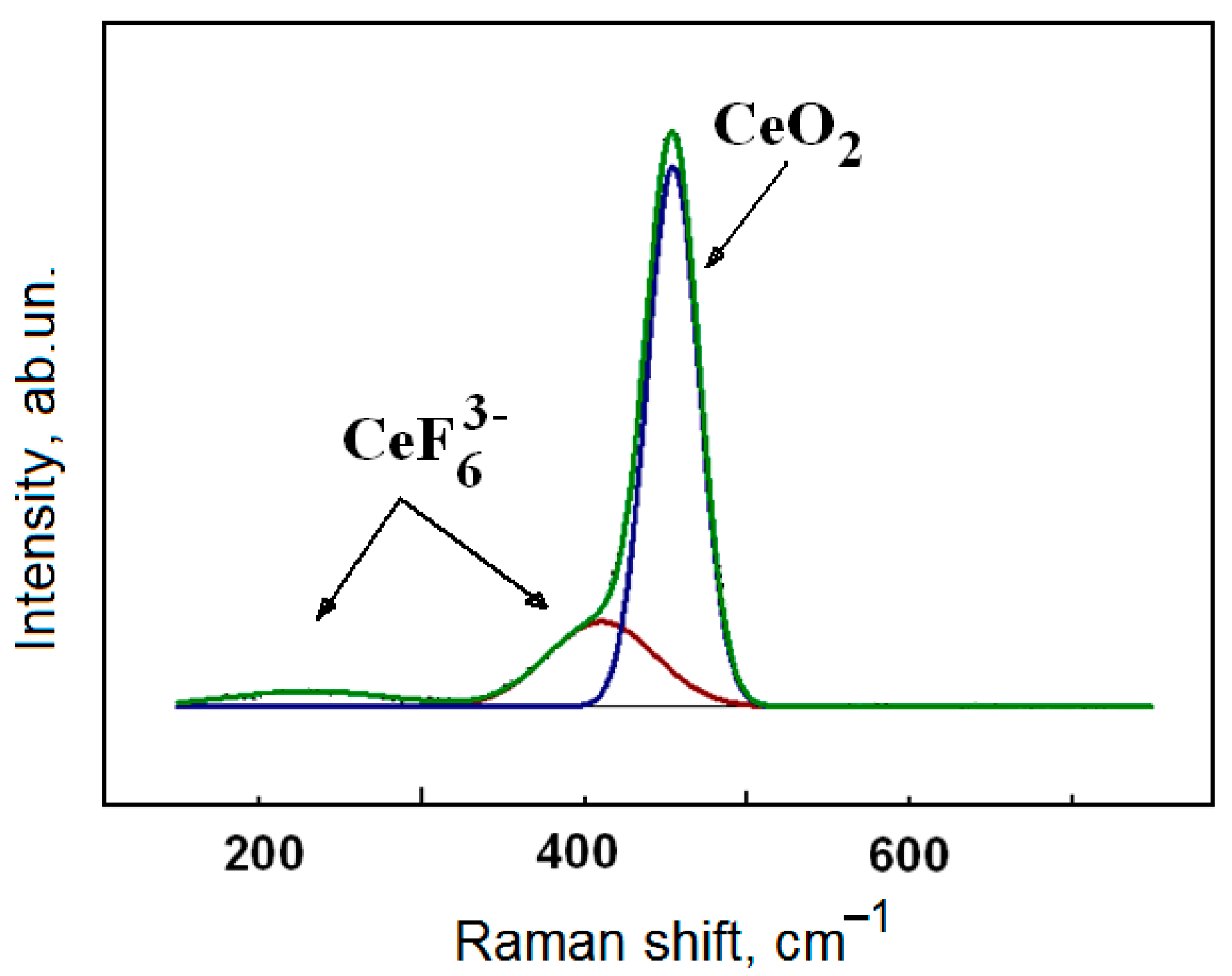
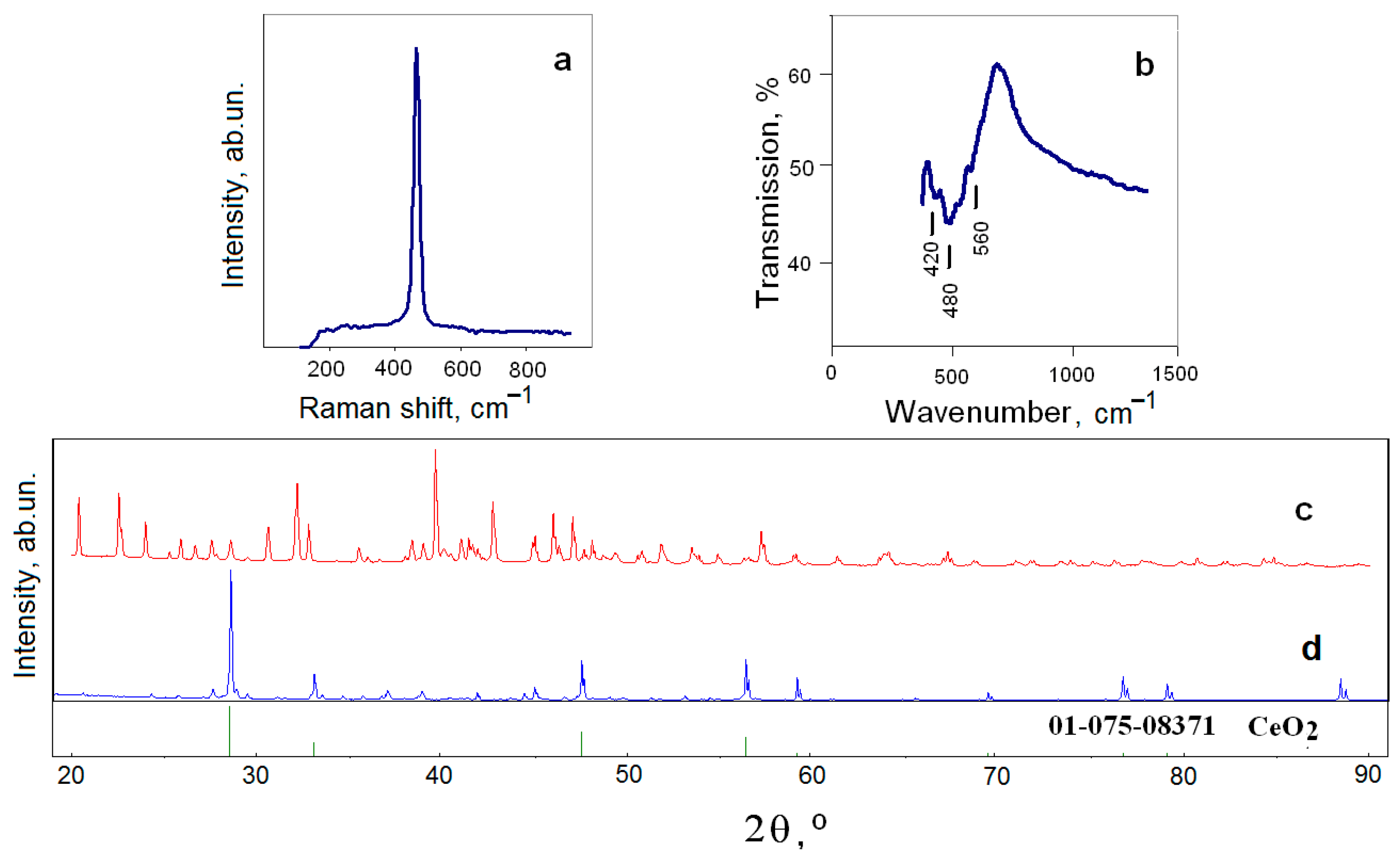
3.2. Kinetics of Interaction of FLiNaK–CeF3 Melts with Water Vapor and Oxygen in the Air Atmosphere
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenthal, M.W.; Kasten, P.R.; Briggs, R.B. Molten-salt reactors—History, status, and potential. Nucl. Appl. Technol. 1970, 8, 107–117. [Google Scholar] [CrossRef]
- Serp, J.; Allibert, M.; Beneš, O.; Delpech, S.; Feynberg, O.; Ghetta, V.; Heuer, D.; Holcomb, D.; Ignatiev, V.; Kloosterman, J.L.; et al. The molten salt reactor (MSR) in generation IV: Overview and perspectives. Prog. Nucl. Energy 2014, 77, 308–319. [Google Scholar] [CrossRef]
- Ignatiev, V.V.; Kormilitsyn, M.V.; Kormilitsyna, L.A.; Semchenkov, Y.M.; Fedorov, Y.S.; Feinberg, O.S.; Kryukov, O.V.; Khaperskaya, A.V. Molten-salt reactor for nuclear fuel cycle closure on all actinides. Sov. At. Energy 2019, 125, 279–283. [Google Scholar] [CrossRef]
- Yu, C.; Li, X.; Cai, X.; Zou, C.; Ma, Y.; Wu, J.; Han, J.; Chen, J. Minor actinide incineration and Th-U breeding in a small FLiNaK Molten Salt Fast Reactor. Ann. Nucl. Energy 2017, 99, 335–344. [Google Scholar] [CrossRef]
- Ponomarev, L.I.; Seregin, M.B.; Parshin, A.P.; Mel’Nikov, S.A.; Mikhalichenko, A.A.; Zagorets, L.P.; Manuilov, R.N.; Rzheutskii, A.A. Fuel salt for the molten-salt reactor. Sov. At. Energy 2013, 115, 5–10. [Google Scholar] [CrossRef]
- Beneš, O.; Konings, R. Actinide burner fuel: Potential compositions based on the thermodynamic evaluation of MF–PuF3 (M=Li, Na, K, Rb, Cs) and LaF3–PuF3 systems. J. Nucl. Mater. 2008, 377, 449–457. [Google Scholar] [CrossRef]
- Karfidov, E.A.; Zaikov, Y.P.; Nikitina, E.V.; Seliverstov, K.E.; Dub, A.V. High-Temperature Passivation of the Surface of Candidate Materials for MSR by Adding Oxygen Ions to FLiNaK Salt. Materials 2022, 15, 5174. [Google Scholar] [CrossRef]
- Karfidov, E.; Nikitina, E.; Erzhenkov, M.; Seliverstov, K.; Chernenky, P.; Mullabaev, A.; Tsvetov, V.; Mushnikov, P.; Karimov, K.; Molchanova, N.; et al. Corrosion Behavior of Candidate Functional Materials for Molten Salts Reactors in LiF–NaF–KF Containing Actinide Fluoride Imitators. Materials 2022, 15, 761. [Google Scholar] [CrossRef]
- Uhlíř, J. Chemical processing of liquid fuel. In Book Molten Salt Reactors and Thorium Energy; Dolan, T., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 209–230. [Google Scholar] [CrossRef]
- Pshenichny, R.N.; Omelchuk, A.A. Interaction of rare-earth oxides with binary molten mixtures of zirconium and alkali metal fluorides. Russ. J. Inorg. Chem. 2012, 57, 115–119. [Google Scholar] [CrossRef]
- Beneš, O.; Konings, R.J.M. Thermodynamic assessment of the LiF–CeF3–ThF4 system: Prediction of PuF3 concentration in a molten salt reactor fuel. J. Nucl. Mater. 2013, 435, 164–171. [Google Scholar] [CrossRef]
- Marsac, R.; Réal, F.; Banik, N.; Pédrot, M.; Pourret, O.; Vallet, V. Aqueous chemistry of Ce(iv): Estimations using actinide analogues. Dalton Trans. 2017, 46, 13553–13561. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Bae, S.; Park, T. Electrochemical and Spectroscopic Monitoring of Interactions of Oxide Ion with U (III) and Ln (III) (Ln = Nd, Ce, and La) in LiCl-KCl Melts. J. Electrochem. Soc. 2017, 164, H5068–H5073. [Google Scholar] [CrossRef]
- Inagaki, T.; Yoshimura, Y.; Kanda, Y.; Matsumoto, Y.; Minami, K. Development of CeF3 crystal for high-energy electromagnetic calorimetry. Nucl. Instrum. Methods Phys. Res. A 2000, 443, 126–135. [Google Scholar] [CrossRef]
- Yonezawa, S.; Jae-Ho, K.; Takashima, M. Pyrohydrolysis of rare-earth trifluorides in moist air. Solid State Sci. 2002, 4, 1481–1485. [Google Scholar] [CrossRef]
- Banks, C.V.; Burke, K.E.; O’Laughlin, J.W. The determination of fluorine in rare earth fluorides by high temperature hydrolysis. Anal. Chim. Acta 1958, 19, 239–243. [Google Scholar] [CrossRef]
- Zakiryanova, I.D. In situ Raman Spectroscopic Study of the Kinetics of the Chemical Dissolution of Ytterbium Oxide in YbCl3–KCl Melt. J. Appl. Spectrosc. 2021, 88, 755–760. [Google Scholar] [CrossRef]
- Zakiryanova, I.D. Kinetics of Dissolution of Gadolinium Oxide in GdCl3–KCl Chloride Melt According to Raman Spectroscopy Data. J. Appl. Spectrosc. 2022, 89, 443–449. [Google Scholar] [CrossRef]
- Zakir′yanova, I.D.; Arkhipov, P.A.; Zakir′yanov, D.O. Reaction Mechanism of Lead(II) Oxide With A PbCl2–CsCl Melt According to Raman Spectroscopic Data. J. Appl. Spectrosc. 2016, 82, 301–304. [Google Scholar] [CrossRef]
- Zakir′yanova, I.D.; Nikolaeva, E.V.; Bove, A.L. In Situ Investigation of the Mechanism of BaO Dissolution in Chloride Melts by Raman Spectroscopy. J. Appl. Spectrosc. 2015, 81, 919–923. [Google Scholar] [CrossRef]
- Arkhipov, P.A.; Zakir’Yanova, I.D.; Kholkina, A.S.; Korzun, I.V.; Khudorozhkova, A.O. Interaction between the CsCl–PbCl2(18.3–81.7 mol%)-PbO System Components. J. Electrochem. Soc. 2017, 164, H5322–H5326. [Google Scholar] [CrossRef]
- Kataev, A.; Tkacheva, O.; Zakiryanova, I.; Apisarov, A.; Dedyukhin, A.; Zaikov, Y. Interaction of B2O3 with molten KF-AlF3 and KF-NaF-AlF3. J. Mol. Liq. 2017, 231, 149–153. [Google Scholar] [CrossRef]
- Zong, G.; Zhang, Z.; Sun, J.; Xiao, J. Preparation of high-purity molten FLiNaK salt by the hydrofluorination process. J. Fluor. Chem. 2017, 197, 134–141. [Google Scholar] [CrossRef]
- Derek, J.; Toshinobu, Y.; Janz, J. Fusion Properties and Heat Capacities of the Eutectic LiF-NaF-KF Melt. J. Chem.Eng. Data 1982, 27, 366–367. [Google Scholar]
- Bauman, R.P.; Porto, S.P.S. Lattice Vibrations and Structure of Rare-Earth Fluorides. Phys. Rev. 1967, 161, 842–850. [Google Scholar] [CrossRef]
- Rimoldi, T.; Orsi, D.; Lagonegro, P.; Ghezzi, B.; Galli, C.; Rossi, F.; Salviati, G.; Cristofolini, L. CeF3-ZnO scintillating nanocomposite for self-lighted photodynamic therapy of cancer. J. Mater. Sci. Mater. Med. 2016, 27, 1–9. [Google Scholar] [CrossRef]
- Mushnikov, P.N.; Tkacheva, O.Y.; Kholkina, A.S.; Zaikov, Y.P.; Shishkin, V.Y.; Dub, A.V. Phase Diagram of the Quasibinary System LiF–NaF–KF–CeF3. At. Energy 2022, 131, 263–267. [Google Scholar] [CrossRef]
- Arkhipov, P.A.; Zakiryanova, I.D.; Kholkina, A.S.; Bausheva, A.V.; Khudorozhkova, A.O. Phase Equilibria and Interaction Between the CsCl–PbCl2–PbO System Components. Z. Naturforsch. 2015, 70, 851–858. [Google Scholar] [CrossRef]
- Frandsen, B.A.; Nickerson, S.D.; Clark, A.D.; Solano, A.; Baral, R.; Williams, J.; Neuefeind, J.; Memmott, M. The structure of molten FLiNaK. J. Nucl. Mater. 2020, 537, 152219. [Google Scholar] [CrossRef]
- Zakiryanov, D. The refined determination of the ion pair lifetimes in ionic liquids. Comput. Theor. Chem. 2022, 1210, 113646. [Google Scholar] [CrossRef]
- Kirillov, S. Dynamic criterion of the complex formation and the structure of molten halides: To the fortieth anniversary of the autocomplex model for the structure of melts. Russ. J. Electrochem. 2007, 43, 901–908. [Google Scholar] [CrossRef]
- Dracopoulos, V.; Papatheodorou, G.N. Isotropic and anisotropic Raman scattering from molten alkali-metal fluorides. Phys. Chem. Chem. Phys. 2000, 2, 2021–2025. [Google Scholar] [CrossRef]
- Dracopoulos, V.; Gilbert, B.; Papatheodorou, G.N. Vibrational modes and structure of lanthanide fluoride–potassium fluoride binary melts LnF3–KF (Ln=La, Ce, Nd, Sm, Dy, Yb). J. Chem. Soc. Faraday Trans. 1998, 94, 2601–2604. [Google Scholar] [CrossRef]
- Borresen, B.; Dracopoulos, V.; Photiadis, G.; Gilbertand, B.; Papatheodorou, G.N. Vibrational Modes and Structure of Rare Earth Fluorides and Bromides in Binary Melts: LnX3-KX (X= F, Br; Ln= Y, La, Ce, Nd, Sm, Gd, Dy, Yb). Proc. Electrochem. Soc. 1996, 1996–1997, 11–27. [Google Scholar] [CrossRef]
- Stefanidaki, E.; Photiadis, G.M.; Kontoyannis, C.G.; Vik, A.F.; Østvold, T. Oxide solubility and Raman spectra of NdF3–LiF–KF–MgF2–Nd2O3 melts. J. Chem. Soc. Dalton Trans. 2002, 11, 2302–2307. [Google Scholar] [CrossRef]
- Rollet, A.-L.; Rakhmatullin, A.; Bessada, C. Local Structure Analogy of Lanthanide Fluoride Molten Salts. Int. J. Thermophys. 2005, 26, 1115–1125. [Google Scholar] [CrossRef]
- Cui, R.; Wang, C. In situ high temperature Raman and DFT analysis of cerium fluoride and oxyfluoride structures in molten FLiNaK. J. Raman Spectrosc. 2021, 52, 1148–1154. [Google Scholar] [CrossRef]
- Anderson, A. The Raman Effect: Application; Marcel Dekker: New York, NY, USA, 1971; pp. 864–872. [Google Scholar]
- Maensiri, S.; Labuayai, S.; Laokul, P.; Klinkaewnarong, J.; Swatsitang, E. Structure and optical properties of CeO2 nanoparti-cles prepared by using lemongrass plant extract solution. Jpn. J. Appl. Phys. 2014, 53, 06JG14. [Google Scholar] [CrossRef]
- Cui, J.; Hope, G.A. Raman and Fluorescence Spectroscopy of CeO2, Er2O3, Nd2O3, Tm2O3, Yb2O3, La2O3, and Tb4O7. J. Spectr. 2015, 2015, 940172. [Google Scholar] [CrossRef]
- Heydari, H.; Gholivand, M. A novel high-performance supercapacitor based on high-quality CeO2/nitrogen-doped reduced graphene oxide nanocomposite. Appl. Phys. A 2017, 123, 187. [Google Scholar] [CrossRef]
- Kosacki, I.; Suzuki, T.; Anderson, H.U.; Colomban, P. Raman scattering and lattice defects in nanocrystalline CeO2 thin films. Solid State Ionics 2002, 149, 99–105. [Google Scholar] [CrossRef]
- Khan, S.B.; Faisal, M.; Rahman, M.M.; Akhtar, K.; Asiri, A.M.; Khan, A.; Alamry, K.A. Effect of particle size on the photocatalytic activity and sensing properties of CeO2 nanoparticles. Int. J. Electrochem. Sci. 2013, 8, 7284–7297. [Google Scholar]
- Gottlieb, M. Optical Properties of Lithium Fluoride in the Infrared. J. Opt. Soc. Am. 1960, 50, 343–349. [Google Scholar] [CrossRef]
- HSC Chemistry 9 [Software], Outokumpu Research Oy: Pori, Finland, 2007.
- Brandmüller, J.; Moser, H. Einführung in die Ramanspektroskopie; Dr. Dietrich Steinkopff Verlag: Darmstadt, Germany, 1962; pp. 320–330. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakiryanova, I.D.; Mushnikov, P.N.; Nikolaeva, E.V.; Zaikov, Y.P. Mechanism and Kinetics of Interaction of FLiNaK–CeF3 Melt with Water Vapors and Oxygen in the Air Atmosphere. Processes 2023, 11, 988. https://doi.org/10.3390/pr11040988
Zakiryanova ID, Mushnikov PN, Nikolaeva EV, Zaikov YP. Mechanism and Kinetics of Interaction of FLiNaK–CeF3 Melt with Water Vapors and Oxygen in the Air Atmosphere. Processes. 2023; 11(4):988. https://doi.org/10.3390/pr11040988
Chicago/Turabian StyleZakiryanova, Irina D., Petr N. Mushnikov, Elena V. Nikolaeva, and Yury P. Zaikov. 2023. "Mechanism and Kinetics of Interaction of FLiNaK–CeF3 Melt with Water Vapors and Oxygen in the Air Atmosphere" Processes 11, no. 4: 988. https://doi.org/10.3390/pr11040988
APA StyleZakiryanova, I. D., Mushnikov, P. N., Nikolaeva, E. V., & Zaikov, Y. P. (2023). Mechanism and Kinetics of Interaction of FLiNaK–CeF3 Melt with Water Vapors and Oxygen in the Air Atmosphere. Processes, 11(4), 988. https://doi.org/10.3390/pr11040988







