Abstract
Ionic liquids are molten salts that possess excellent chemical and thermal stability. Due to their inherent qualities in green chemistry, ionic liquids have been identified as potential substitutes for traditional organic solvents. These useful physical and chemical properties lead to some promising applications in fields such as building polymer engineering alternative materials and renewable energy technologies. Although they are classified as green solvents, these new solvents exist in a high-temperature environment, which is related to thermochemical reactivity and safety; there are few related studies. To analyze the possible high-temperature application environment of ionic liquids in the future, we analyzed the new ionic liquid 1-Benzyl-3-methylimidILlium bis (trifluoromethylsulfonyl) imide ([BZMIM][TF2N]), which lacks thermal analysis basis. This study used thermogravimetric analysis as the basis of the reaction model. We calculated the thermal hazard, kinetics, and parameter analysis of the reaction characterized by experimental thermal analysis data. The reaction model can be used to construct the actual temperature change calculation. The results show that [BZMIM][TF2N] will enter a runaway reaction when the temperature exceeds 270 °C. When operating [BZMIM][TF2N] at high temperatures, attention should be paid to the possibility of thermal hazards caused by its self-decomposition reaction.
1. Introduction
Solvents play a key role in synthesizing and extracting organic and inorganic products. Their by-products, such as volatile organic compounds (VOCs), may cause serious damage to human and animal health and environmental threats due to their high volatility and toxicity. In view of this global problem, support for replacing fossil-based solvents with green solvents mainly stems from the increasing demand from governments and people for clean technologies that are less harmful to the environment. This trend mainly involves developing and implementing chemical products and processes to reduce or eliminate the use or production of substances harmful to human health and the environment.
Science, technology, and scholars strive to continuously find environmental protection alternatives, such as green solvents ionic liquids (ILs), to replace fossil solvents. Their use and development has led to the production or improvement of many different synthesis and extraction processes for ionic liquids of industrial interest. In the past decade, various ionic liquids that conform to most green chemical principles have been introduced to the market and begun to attract more attention in the synthesis and extraction processes. From the perspective of market prospects, there are obstacles to continuously reducing ionic liquids’ prices. The ionic liquids’ output must increase and its competitiveness gradually improve relative to traditional solvents.
Ionic liquids, also known as low-temperature molten salts, are salts that appear liquid at normal temperature and are generally composed of organic cations and inorganic or organic anions. Ionic liquids, as ionic compounds, have a low melting point mainly because of the asymmetry of some substituents in their structures, which means that the ions do not stack into crystals regularly. The combination of different anions and cations determines ionic liquids’ chemical, physical, biological, and thermal stability. It has a wide range of applications in materials, electrochemistry, physics, synthesis, biology, engineering, chemistry, and other fields [1,2,3]. In recent years, it has been applied to traditional building materials. The conclusion is that ionic liquid composite phase change materials with good thermal insulation effect can be obtained after adding, which can reduce the temperature difference between day and night in the building and, finally, achieve the purpose of green energy saving [4,5,6].
On the other hand, in construction and decoration, the building materials and furniture used are the main sources of indoor air pollutants. VOCs emitted from dry and wet building materials mainly come from organic solvents, adhesives, and caulking agents used in the manufacturing or decorating building materials; these pollutants lead to indoor air quality degradation, pollution, and indirectly negative effects on human health. Ionic liquids can be applied to the separation and recovery of VOCs. The principle is that they can be designed by combining anions and cations. When applied to nano-porous materials, they have large specific surface areas and space effects, achieving an excellent adsorption effect [7,8,9]. Ionic liquids have especially good application prospects in the field of gas sensors. Using ionic liquids’ physical and chemical gas-sensing properties to develop new gas-sensing materials can lay an important foundation for sensor technology. The above factors meet China’s requirements and expectations for green building performance; thus, they have great research value and market demand.
However, while imidazole ionic liquids are generally considered as harmless green solvents, they ignore the potential safety hazards related to their use, storage, and transportation, resulting in uncertainty in safety production. For example, in 2013, when ionic liquids were prepared in the laboratory, an explosion accident occurred due to improper operation. Imidazole ionic liquid is not flammable; however, the gas produced by decomposition near the fire source or at high temperature can mix with a small amount of water vapor, causing combustion accidents. At present, the risk of combustion and explosion has not been considered in the application technology of imidazole ionic liquids; thus, it is of great significance to study the thermal stability and decomposition mechanism of imidazole ionic liquids for intrinsically safe production and application [10,11,12].
Because of the infinite combination of anions and cations, syntheses and evaluations of new ionic liquids are published and patented daily. It is necessary to constantly update these new solvents’ progress, analysis, and organization. Chemically modifying imidazole cations by functionalizing different parts can affect the absorption and selectivity of different gases [13]. For example, bis (trifluoromethylsulfonyl) imine [NTF2-], the alkyl side chain in imidazole cation, is functionalized to change the non-polar nano-region, which may affect the gas solubility of analytes dissolved in non-polar [14].
The rapidly expanding field of ionic liquids research has led to a plethora of new solvents being developed and patented. This constant progress necessitates regular updates and organization of information pertaining to the synthesis, evaluation, and properties of novel ILs. Customizing imidazole cations through chemical modifications allows the tailoring of absorption and selectivity for various gases [7]. For instance, by functionalizing the alkyl side chain in imidazole cations with bis (trifluoromethylsulfonyl) imine [NTf2-], the non-polar nano-region can be altered, potentially influencing the solubility of analytes in non-polar environments [14].
Furthermore, the vast array of potential anion and cation combinations in ionic liquids offers opportunities for fine-tuning their properties to suit specific applications. This versatility can lead to the development of innovative solutions across a broad spectrum of industries, including energy, pharmaceuticals, and agriculture. As the demand for sustainable and eco-friendly alternatives to traditional solvents continues to grow, the exploration and optimization of ionic liquids become increasingly relevant.
Moreover, customizing ionic liquids can extend their applicability to emerging fields, such as carbon capture and storage. Their tunable properties can be harnessed for efficient separation and sequestration of greenhouse gases. In this context, research into the design and synthesis of ILs with enhanced absorption capacities and selectivity’s for CO2 and other greenhouse gases could significantly contribute to combating climate change.
In conclusion, the ongoing development of new ionic liquids and their expanding range of applications highlight the importance of staying informed about advancements in the field. By understanding the potential of these versatile solvents, researchers and industries can develop innovative, eco-friendly solutions that address critical global challenges. However, evaluating the safety aspects and potential hazards associated with ionic liquids is crucial to ensuring their responsible implementation across various sectors. Through continuous research, development, and risk assessment, ionic liquids can promote a greener and more sustainable future.
This paper will discuss the reaction modes of emerging ionic liquids in their processes and their relationship with safety. The harmfulness of ILs can be seen from the analysis of their thermal decomposition characteristics. Ionic liquid materials may cause decomposition reaction by self-reaction. Therefore, the factors that cause harm under high temperatures can avoid the subsequent anti-response hazard by controlling the temperature [15,16]. Although the thermal safety of several IL compounds has been discussed in the existing literature, market demand has led to the introduction of new IL compounds without relevant safety analysis; thus, their harmfulness needs to be studied in advance. In addition to safety concerns, the study of reactivity parameters could improve process efficiency and energy-saving measures for temperature control. However, there is a lack of research on the perfect reactivity of new IL initiators in the literature. Due to the limitation of instruments, the reaction characteristics of emerging ILs were discussed comprehensively and the safety evaluation model was improved. The reaction mechanism of emerging ILs was completed. In this study, a new IL initiator, 1-Benzyl-3-methylimidILlium bis (trifluoromethylsulfonyl) imide ([BZMIM][TF2N]) (Figure 1), was used to evaluate and improve the kinetic parameters of thermal reaction degradation by the analytical reaction mode method.
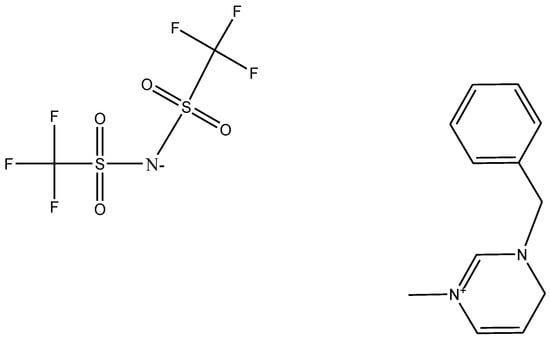
Figure 1.
The material structure of 1-Benzyl-3-methylimidILlium bis (trifluoromethylsulfonyl) imide.
2. Materials and Methods
2.1. Sample
In the experimental portion, a composition containing 98 mass percentage of [BZMIM][TF2N] was purchased from the Wako Pure Chemical Industries, Ltd. (Taipei, Taiwan). The sample volume required by the experimental instrument was roughly 5.0 mg. In addition, due to the thermal sensitivity of IL and to avoid self-decomposition, [BZMIM][TF2N] must be refrigerated below 4.0 °C.
2.2. Thermogravimetric Analyzer (TGA)
The principle of TGA made by Mettler Toledo was that under the isothermal or non-isothermal heating environment created by the instrument through the heating furnace. The mass changes of samples caused by a series of phenomena, such as phase change, water precipitation or decomposition reaction, were recorded; the overall reaction trend could be deduced. Based on mass change data, the transformation, kinetics, and reaction mechanism could be evaluated with numerical analysis. In this study, the test procedure was heated from 25.0 to 600.0 °C at certain heating rates of 0.5, 1.0, 2.0, 4.0, and 8.0 °C min−1 [17,18], respectively. The flow of nitrogen gas (50 mL min−1) was maintained throughout the experimental procedure. The mass change data of samples was recorded through the programmed control system microbalance built in the instrument.
3. The Modified Reaction Mechanism and the Subsequent Kinetic Calculation Mode
3.1. Kinetic Model of Different Reaction Stage
TG can establish different reaction stages by demonstrating the mass loss trend in different reaction intervals. To supplement and enrich [BZMIM][TF2N]’s thermal hazard assessment model, this study recognized the reaction model by TG and change in f (α) to make the calculated results approach the actual reaction model as much as possible. The model used for fitting [19] uses the reaction progress (α) as the basic variable to point out the reaction process from beginning to end; it then analyzes the reaction rate (dα/dt) and reaction mechanism through other parameters Ea and A. The evaluation process is calculated by non-linear fitting based on mass loss (TG) and specific mass loss rate (DTG) [20].
The different stages of reaction [21,22] can be represented as:
3.2. Extending from the Analysis of Material Dynamics to the Thermal Balance of the System
The establishment of the reaction kinetics model is an essential basis for the analysis of runaway reactions. The import of the supplement to the reaction mode improves the follow-up calculations. The temperature change in the self-decomposition heat of a large number of substances under the influence of the container system is numerically simulated; the calculation by the modified reaction mode then ensures that the phenomenon of rapid temperature rise in a short time can be amended in actual operation. The simulated situation can be expressed as Equation (3):
where is the density, Cp is the specific heat capacity, λ is the thermal conductivity coefficient, T is the temperature, and W is the heat generation due to reaction.
The basic assumption of boundary conditions is shown as follows:
where X is the normal to the surface of an object, subscripts w and e denote the surface and environment, respectively, and U prompts the heat transfer coefficient.
3.3. Safety Operation and Related Evaluation Parameters of Actual Manufacturing Process
The reaction rate is always the key factor of chemical process efficiency. However, for safety reasons, the reaction rate still has its limitations. Process deviation, such as improper operation, leads to the process temperature exceeding the allowable limit, while the rapid increase in reaction rate drives a large amount of heat to be released with the increase in temperature, which eventually leads to the damage, leakage, and even fire and explosion of process equipment. In the typical thermal runaway scenario of the above chemical process, if the initial heat can be effectively ventilated or cooled by the process personnel or system and the appropriate response can be performed quickly, the process can be adjusted to normal operation. Adequate response time also means that even if heat accumulation is formed at the initial stage, hidden dangers can still be eliminated early under safety measures. Therefore, the parameters related to reaction process and time efficiency are of great significance in normal operation and emergency response, which can be characterized by estimating the time required for substances to reach the maximum reaction rate (TMRad and TMRiso).
The reaction rate indicates the time interval required for the decomposition and predicts the consumption state and intensity of the reaction. If the reaction rate is at a high level when the process safety measures are missing, a thermal runaway situation will occur. Therefore, influencing the reaction rate is closely related to the actual temperature controlled on cooling; an appropriate time interval can be established for adjusting the process and emergency response. Kinetic models are obtained by determining the reaction mode from TG, combined with the numerical methods used to assess the time required for substances to reach the maximum reaction rates (TMRad).
Instead of using expensive and complicated adiabatic experiments, numerical calculations are used to analyze the change in reaction over time to actual process conditions. Kossoy and Akhmetshin [23] discussed the calculation of TMRad, which examined the relationship between reaction rate and hazardous properties. Wang et al. [24] using thermal safety software [25] to calculate the corresponding kinetic and reaction hazard parameters. The kinetic model of Netzsch Group [26] can obtain the reaction progress (α) from TGA data and carry out non-linear fitting to predict the hazard parameters related to the reaction rate under adiabatic conditions.
3.4. Thermal Stability on Storage Condition
TG is used to determine the reaction mode and characterize the α, while a dynamic model close to the real reaction mode is obtained by non-linear fitting. Finally, the reactivity hazard of substances in the actual state is calculated by numerical simulation. Considering the interaction between material self-reaction and the external environment, the temperature stability in actual state is calculated. The actual test [27] has a great demand for sample quantity and experiment time [28]. In this study, the changes of actual material temperature parameters were calculated by kinetic and system heat exchange models [29,30,31,32]:
where Cp is the sample heat capacity, λ is the thermal conductivity, ρ is the density of [BZMIM][TF2N], x is the package radius, and g is a geometry factor that varies by the type of packaging. ΔHd and dα/dt are described by TGA and kinetics models [28,32,33], respectively.
To ascertain the influence of different temperature environments on reactants, initial ambient temperature when the substance produces a specified internal temperature rise due to self-reaction within a specific time (TCL) can be used to appraise the reaction hazard.
4. Results and Discussion
As shown in the Figure 2, the experimental data curves are divided into TG and dTG signal curves. The mass loss shown in the experimental curve shows that AIBA has two reaction stages, which lead to different intervals of material consumption. The reaction kinetics was derived on this basis and the multi-stage model fitted the experimental curves. The calculated values are compared with the actual experimental results and the fitting degree is gradually optimized. The evaluation results are beneficial to determine the process’ operation mode and temperature control. Reaction parameters, such as reaction rate and activation energy, can be used to verify actual operation and as a source for estimating the whole reaction system model.
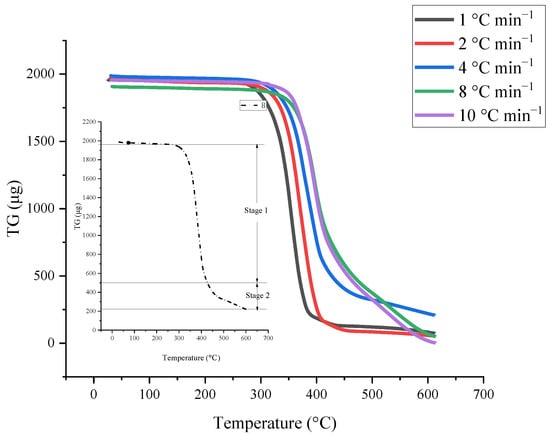
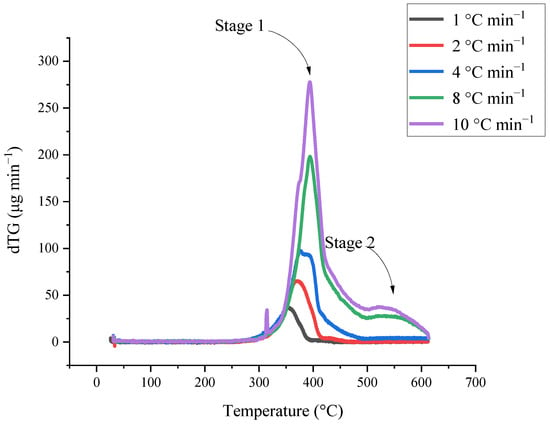
Figure 2.
Mass loss and mass loss rate of [BZMIM][TF2N] by TGA results with different heating rates at 1.0, 2.0, 4.0, 8.0, and 10.0 °C min−1.
The basic thermal hazard parameters were attained from TG experiments that [BZMIM][TF2N] had two reaction stages; the thermogravimetric data were used to evaluate the reaction mode by non-linear fitting. The kinetic analysis results are shown in Figure 3 and Figure 4; these results exhibited the comparison between the relevant kinetic parameters of the reaction equation and the experimental data of [BZMIM][TF2N]. The simulated numerical results show two nth decomposition reactions of [BZMIM][TF2N] in Table 1. Consideration should be paid to the kinetic parameters in Table 2 under operating conditions, which can be used as a reference for process operating conditions. Incorrect process parameters may accelerate the reaction of [BZMIM][TF2N] and lead to the possibility of out-of-control reaction.
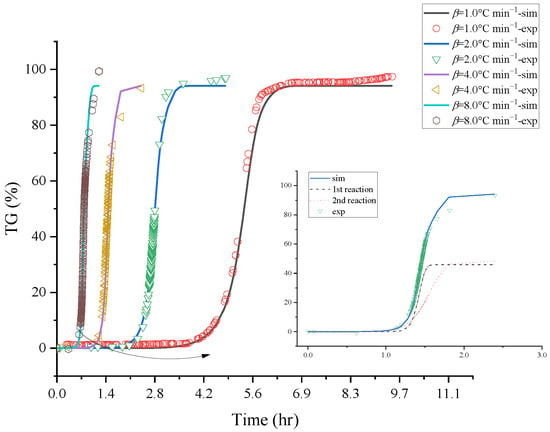
Figure 3.
Comparisons of [BZMIM][TF2N]’s mass loss (top) and mass loss rate (bottom) vs. time curves at heating rates of 1.0, 2.0, 4.0, and 8.0 °C min−1 by experiments and simulations.
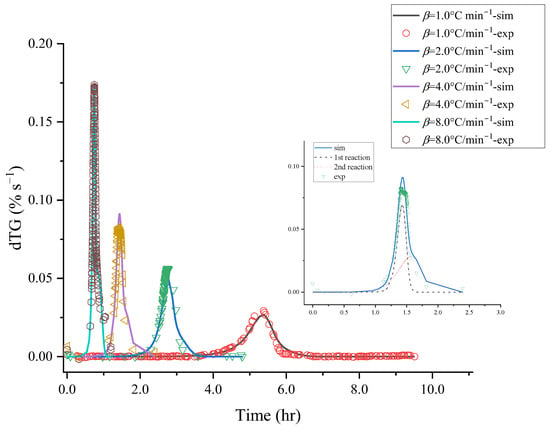
Figure 4.
Comparisons of [BZMIM][TF2N]’s mass loss (top) and mass loss rate (bottom) vs. time curves at heating rates of 1.0, 2.0, 4.0, and 8.0 °C min−1 by experiments and simulations.

Table 1.
Thermokinetics parameters evaluation for [BZMIM][TF2N] of the heating rates of 0.5, 1.0, 2.0, 4.0, and 8.0 °C min−1.

Table 2.
Physical parameters of [BZMIM][TF2N].
The study of thermodynamic parameters and runaway response evaluation for [BZMIM][TF2N] based on the TG experiment and kinetic model fitting has provided valuable insights into its thermal stability during storage and transportation. Simulations were conducted for two packaging scenarios: 25.0 g boxes and 50.0 g barrels. The temperature rise of [BZMIM][TF2N] in these containers, resulting from self-reaction at various ambient temperatures, was estimated. It is worth noting that the actual storage method for [BZMIM][TF2N] typically involves using an additional plastic bag within a fiberboard outer container, which might explain the discrepancies between the simulated and real-life results due to incomplete contact heat exchange.
Based on the TG experiment at 4.0 °C min−1 heating rate and the corresponding fitting kinetic model, the thermodynamic parameters were calculated by numerical analysis. The subsequent runaway response evaluation was carried out. This study simulated the storage of [BZMIM][TF2N] in a 25.0 g box and 50.0 g barrel. It estimated the temperature rises of [BZMIM][TF2N] in the two types of packages due to self-reaction at different ambient temperatures. The results of the calculation are given in Figure 5 and Figure 6. According to Kossoy [33], IL (as [BZMIM][TF2N]) is usually pre-packaged with an extra plastic bag and put into an outer container made of fiberboard. The incomplete contact heat exchange in the container in this storage mode may be the reason why the simulated results are not completely consistent with the actual results. The container-related parameters are shown in Table 2.
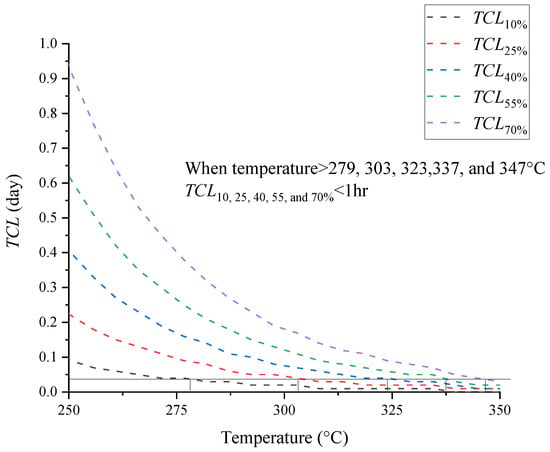
Figure 5.
Simulation results of [BZMIM][TF2N] for TCL with the heating rate of 4.0 °C min−1.
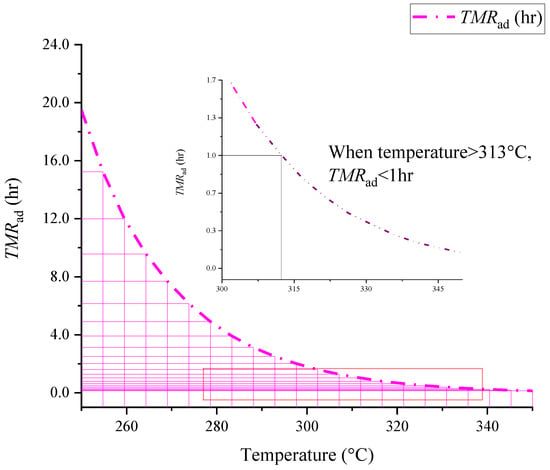
Figure 6.
Evaluation of TMRad values of [BZMIM][TF2N] at different initial temperatures under adiabatic conditions.
Figure 5 presents runaway reaction mode results for [BZMIM][TF2N]. When temperature > 279, 303, 323, 337, and 347 °C, the TCL on consumed 10, 25, 40, 55, and 70% of [BZMIM][TF2N] < 1hr, respectively, including TCL available under temperature changes based on heat exchange evaluation. The TCL results showed that 70% of [BZMIM][TF2N] was consumed after 1 h at 92.0 °C, which indicated that the thermal stability of [BZMIM][TF2N] would decrease when the operating environment exceeded 92.0 °C. [BZMIM][TF2N] had high hazardous characteristics in storage and transportation. In addition to the adjustment measures for temperature during storage and transportation of [BZMIM][TF2N], the containers in different states contribute slightly to the variation in thermal hazard parameters. These findings highlight the highly hazardous characteristics of [BZMIM][TF2N] during storage and transportation.
After the construction of reaction kinetics, the change in reaction rate can be analyzed and predicted. Figure 6 shows the results for TMRad calculation at different initial temperatures. [BZMIM][TF2N] has a long TMRad at <279.0 °C; however, when temperature exceeds this value, the TMRad will be less than 1 hr. Therefore, in the temperature range of 279.0 °C, the stability of [BZMIM][TF2N] to slight temperature changes will decrease, affecting the overall reaction time; this results in runaway reaction. The shortening of the reaction period may not leave enough time for safe operation in actual process. Therefore, attention must be paid to controlling and monitoring the temperature in this interval to ensure the elimination of process deviation and emergency measures.
Figure 7 and Figure 8 present the calculation results of runaway reactions. Compared with low temperatures, the time required for self-reaction to cause runaway reaction temperature to rise in the higher ambient temperature environment was shorter. With the increase in ambient temperature, the self-exothermic temperature rises exponentially. The initial temperature of the runaway reaction caused by [BZMIM][TF2N] in box and barrel was 270.0 °C. The difference in the ambient temperatures values which can prompt the runaway reaction under different packages is the basis for the actual operating temperature. Decomposition response measures for [BZMIM][TF2N] should be evaluated at low environmental conditions to reduce the cooling failure scenario of runaway response and alert storage. Considering the potential risks associated with [BZMIM][TF2N], it is crucial to implement adequate temperature control measures during storage and transportation. Moreover, understanding the influence of container types on the variation in thermal hazard parameters can help inform the design of safer storage and transportation methods.
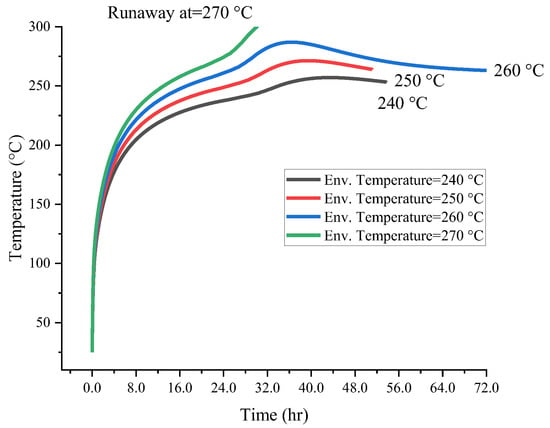
Figure 7.
Simulation results of critical temperature for [BZMIM][TF2N] with 25.0 g box packages.
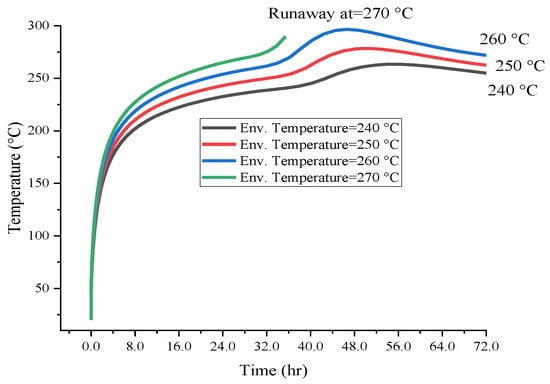
Figure 8.
Simulation results of critical temperature for [BZMIM][TF2N] with 50.0 g drum packages.
In conclusion, the evaluation of the thermal stability and runaway response of ionic liquids like [BZMIM][TF2N] is essential for ensuring their safe handling and minimizing potential hazards. By identifying the critical temperature thresholds and accounting for container-related factors, researchers and industry professionals can develop strategies to reduce risks and improve safety measures in storage and transportation environments.
5. Conclusions
The thermogravimetric loss curve of TG can be used to discriminate [BZMIM][TF2N] two-stage reaction, while the whole thermogravimetric data can be used as reaction process data for kinetic analysis with the autocatalytic numerical model. The kinetic model provides a basis for calculating the runaway reaction of [BZMIM][TF2N]. The phased evaluation program uses the small-scale TGA experiment to compute the thermodynamic parameters of [BZMIM][TF2N], such as TMRad, and TCL, step-by-step under actual process conditions, which brings a more economical and convenient mode.
The thermal runaway temperature curves of [BZMIM][TF2N] packed in a 25.0 g box and 50.0 g barrel under storage conditions are simulated numerically. When the ambient temperatures of [BZMIM][TF2N] in boxes and barrels exceed 270.0 °C, the demand for heat dissipation efficiency of the system also increases; in this situation, the proper response of cooling systems to high-temperature environments is critical.
The thermogravimetric loss curve obtained from TG analysis can effectively differentiate the two-stage [BZMIM][TF2N] reaction. Utilizing the complete thermogravimetric data, the autocatalytic numerical model allows for a comprehensive kinetic analysis of the reaction process. This kinetic model serves as a foundation for evaluating the runaway reaction potential of [BZMIM][TF2N]. The phased evaluation approach enables the computation of thermodynamic parameters, such as TMRad and TCL, for [BZMIM][TF2N] under actual process conditions using small-scale TGA experiments, resulting in a more cost-effective and convenient method.
Numerical simulations of the thermal runaway temperature curves for [BZMIM][TF2N] stored in 25.0 g boxes and 50.0 g barrels have been performed. When the ambient temperatures for [BZMIM][TF2N] in boxes and barrels exceed 270.0 °C, the heat dissipation efficiency requirements for the system increase significantly. Therefore, it is crucial for cooling systems to respond adequately to high-temperature environments to prevent thermal runaway.
To ensure safe handling and storage of [BZMIM][TF2N], proper monitoring of ambient temperatures and the implementation of efficient cooling systems are essential. This will help maintain the thermal stability of the ionic liquid and minimize the risk of runaway reactions. In addition, further research into the packaging materials and designs for [BZMIM][TF2N] could contribute to improved safety measures and the prevention of hazardous incidents.
In summary, applying TG analysis and kinetic models enables a more in-depth understanding of the thermal stability and runaway reaction potential of ionic liquids such as [BZMIM][TF2N]. By identifying critical temperature thresholds and evaluating the effectiveness of cooling systems, researchers and industry professionals can develop strategies to enhance safety and mitigate risks during storage and transportation.
Author Contributions
L.-C.H.: Conceptualization, Methodology, Writing—Review and Editing, Data curation, Writing—Original draft preparation. To guarantee the safety design, N.-H.P.: Visualization, Investigation, Writing—Review and Editing. Thermal equilibrium, which estimated the TMRad, TCL and runaway for safe temperatures and emergency responses, are described and fixed. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to the technical support from National Yunlin University of Science and Technology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liaw, H.J.; Liou, Y.R.; Liu, P.H.; Chen, H.Y.; Shu, C.M. Increased flammability hazard when ionic liquid [C6mim][Cl] is exposed to high temperatures. J. Hazard. Mater. 2019, 367, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pan, L.; Wang, L.; Zou, J.J. Review on synthesis and properties of high-energy-density liquid fuels: Hydrocarbons, nanofluids and energetic ionic liquids. Chem. Eng. Sci. 2018, 180, 95–125. [Google Scholar] [CrossRef]
- Ogunlaja, A.S.; Alade, O.S. Catalysed oxidation of quinoline in model fuel and the selective extraction of quinoline-N-oxide with imidazoline-based ionic liquids. Egypt. J. Pet. 2018, 27, 159–168. [Google Scholar] [CrossRef]
- Durak, O.; Zeeshan, M.; Habib, N.; Gulbalkan, H.C.; Alsuhile, A.A.A.M.; Caglayan, H.P.; Kurtoğlu-Öztulum, S.F.; Zhao, Y.; Haslak, Z.P.; Uzun, A.; et al. Composites of porous materials with ionic liquids: Synthesis, characterization, applications, and beyond. Microporous Mesoporous Mater. 2022, 332, 111703. [Google Scholar] [CrossRef]
- Barrulas, R.V.; Zanatta, M.; Casimiro, T.; Corvo, M.C. Advanced porous materials from poly(ionic liquid)s: Challenges, applications and opportunities. Chem. Eng. J. 2021, 411, 128528. [Google Scholar] [CrossRef]
- He, D.; Liang, R.; Zhao, J.; Liu, Z.; Lu, Z.; Sun, G. Effect of ionic liquids in compatibility with PCE and cement paste containing clay. Constr. Build. Mater. 2020, 264, 120265. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Shah, K.; Atkin, R.; Moghtaderi, B. Physicochemical interactions of ionic liquids with coal; the viability of ionic liquids for pre-treatments in coal liquefaction. Fuel 2015, 143, 244–252. [Google Scholar] [CrossRef]
- Maton, C.; De Vos, N.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef]
- Schneider, S.; Hawkins, T.; Ahmed, Y.; Deplazes, S.; Mills, J. Ionic Liquid Fuels for Chemical Propulsion. In Ionic Liquids: Science and Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2012; Volume 1117, pp. 1–25. [Google Scholar]
- Domańska, U.; Karpińska, M.; Wlazło, M. Bis(trifluoromethylsulfonyl)imide, or dicyanamide-based ionic liquids in the liquid–liquid extraction of hex-1-ene from hexane and cyclohexene from cyclohexane. J. Chem. Thermodyn. 2017, 105, 375–384. [Google Scholar] [CrossRef]
- Jalili, A.H.; Mehdizadeh, A.; Ahmadi, A.N.; Zoghi, A.T.; Shokouhi, M. Solubility behavior of CO2 and H2S in 1-benzyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ionic liquid. J. Chem. Thermodyn. 2022, 167, 106721. [Google Scholar] [CrossRef]
- Liu, S.H.; Lin, W.C.; Xia, H.; Hou, H.Y.; Shu, C.M. Combustion of 1-butylimidazolium nitrate via DSC, TG, VSP2, FTIR, and GC/MS: An approach for thermal hazard, property and prediction assessment. Process Saf. Environ. Prot. 2018, 116, 603–614. [Google Scholar] [CrossRef]
- Lin, W.C.; Yu, W.L.; Liu, S.H.; Huang, S.Y.; Hou, H.Y.; Shu, C.M. Thermal hazard analysis and combustion characteristics of four imidazolium nitrate ionic liquids. J. Therm. Anal. Calorim. 2018, 133, 683–693. [Google Scholar] [CrossRef]
- Liu, S.H.; Shu, C.M. Advanced technology of thermal decomposition for AMBN and ABVN by DSC and VSP2. J. Therm. Anal. Calorim. 2015, 121, 533–540. [Google Scholar] [CrossRef]
- Liu, S.H.; Hou, H.Y.; Shu, C.M. Thermal hazard evaluation of the autocatalytic reaction of benzoyl peroxide using DSC and TAM III. Thermochim. Acta 2015, 605, 68–76. [Google Scholar] [CrossRef]
- Kossoy, A.A.; Sheinman, I.Y. Evaluating Thermal Explosion Hazard by Using Kinetics-Based Simulation Approach. Process Saf. Environ. Prot. 2004, 82, 421–430. [Google Scholar] [CrossRef]
- Kossoy, A.A.; Benin, A.I.; Akhmetshin, Y.G. An advanced approach to reactivity rating. J. Hazard. Mater. 2005, 118, 9–17. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Somma, I.D.; Sanchirico, R. Kinetic and safety assessment for salicylic acid nitration by nitric acid/acetic acid system. J. Hazard. Mater. 2006, 134, 1–7. [Google Scholar] [CrossRef]
- Gonzales, N.O.; Levin, M.E.; Zimmerman, L.W. The reactivity of sodium borohydride with various species as characterized by adiabatic calorimetry. J. Hazard. Mater. 2007, 142, 639–646. [Google Scholar] [CrossRef]
- Kossoy, A.A.; Akhmetshin, Y.G. Identification of kinetic models for the assessment of reaction hazards. Process Saf. Prog. 2007, 26, 209–220. [Google Scholar] [CrossRef]
- Wang, S.Y.; Kossoy, A.A.; Yao, Y.D.; Chen, L.P.; Chen, W.H. Kinetics-based simulation approach to evaluate thermal hazards of benzaldehyde oxime by DSC tests. Thermochim. Acta 2017, 655, 319–325. [Google Scholar] [CrossRef]
- ChemInform Saint Petersburg (CISP) Ltd. Thermal Safety Software. 2022. Available online: http://www.cisp.spb.ru (accessed on 5 May 2022).
- Opfermann, J. Kinetic analysis using multivariate non-linear regression. I. Basic concepts. J. Therm. Anal. Calorim. 2000, 60, 641–658. [Google Scholar] [CrossRef]
- United Nations. Recommendations on the Transport of Dangerous Goods: Manual of Tests and Criteria, 6th ed.; United Nations Publications: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Kossoy, A.A.; Sheinman, I.Y. Comparative analysis of the methods for SADT determination. J. Hazard. Mater. 2007, 142, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Li, X.R.; Koseki, H. SADT prediction of autocatalytic material using isothermal calorimetry analysis. Thermochim. Acta 2005, 431, 113–116. [Google Scholar] [CrossRef]
- Malow, M.; Wehrstedt, K.D. Prediction of the self-accelerating decomposition temperature (SADT) for liquid organic peroxides from differential scanning calorimetry (DSC) measurements. J. Hazard. Mater. 2005, 120, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Steensma, M.; Schuurman, P.; Malow, M.; Krause, U.; Wehrstedt, K.D. Evaluation of the validity of the UN SADT H.4 test for solid organic peroxides and self-reactive substances. J. Hazard. Mater. 2005, 117, 89–102. [Google Scholar] [CrossRef]
- Lv, J.; Chen, L.; Chen, W.; Gao, H.; Peng, M. Kinetic analysis and self-accelerating decomposition temperature (SADT) of dicumyl peroxide. Thermochim. Acta 2013, 571, 60–63. [Google Scholar] [CrossRef]
- Kossoy, A.A.; Belokhvostov, V.M.; Koludarova, E.Y. Thermal decomposition of AIBN: Part D: Verification of simulation method for SADT determination based on AIBN benchmark. Thermochim. Acta 2015, 621, 36–43. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).