Human Cartilage Biomechanics: Experimental and Theoretical Approaches towards the Identification of Mechanical Properties in Healthy and Osteoarthritic Conditions
Abstract
1. Introduction
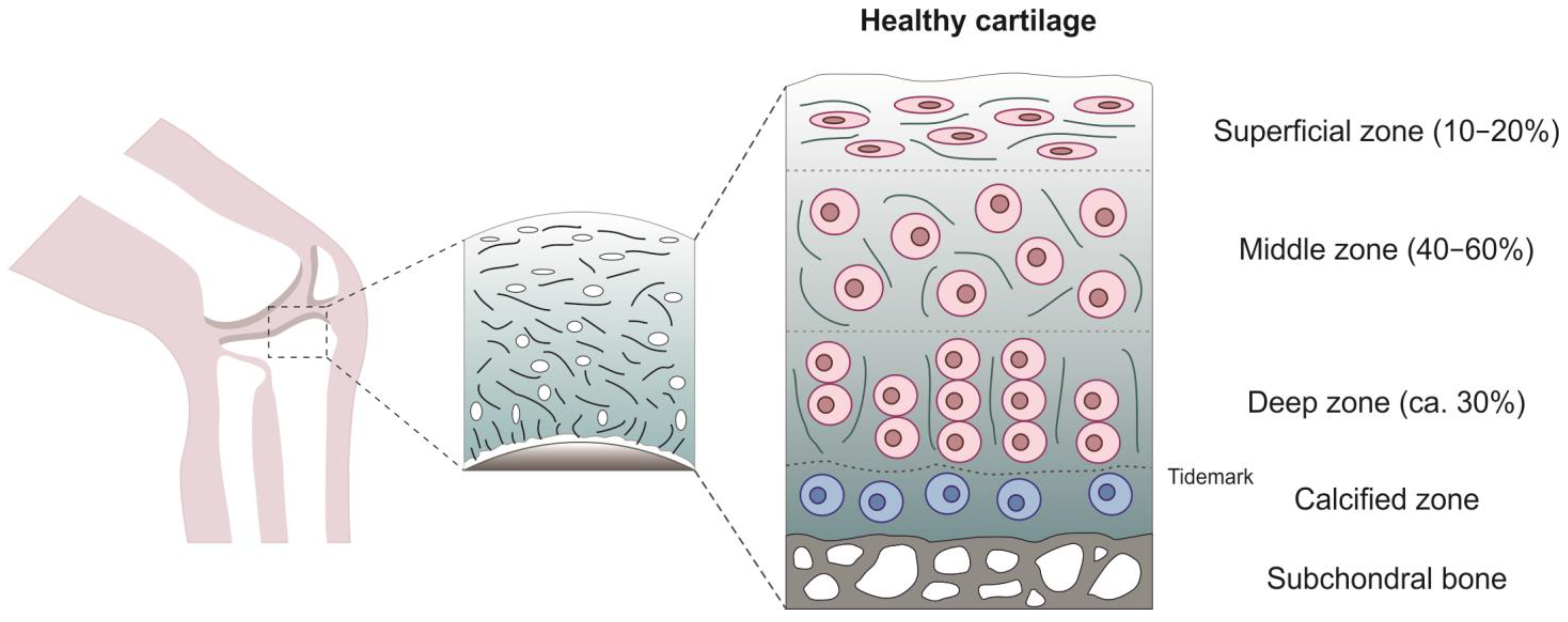
2. Articular Cartilage
3. AC Pathological Changes in Osteoarthritis
4. Experimental Methods for the Mechanical Characterization of AC
4.1. Atomic Force Microscopy Investigation
4.2. Compression Tests
4.3. Indentation Tests
4.4. Tensile Tests
4.5. Friction Tests
5. Biomechanics of Human Cartilage
5.1. Influence of the Site
5.2. Influence of the Depth
5.3. Age
5.4. Human vs. Animal
5.5. Tribological Properties
6. Influence of Osteoarthritis on Cartilage Biomechanics
7. Constitutive Modeling of Articular Cartilage
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, J.M.; Wise, B.C.; Bonnevie, E.D.; Mauck, R.L. A Systematic Review and Guide to Mechanical Testing for Articular Cartilage Tissue Engineering. Tissue Eng. Part C Methods 2019, 25, 593–608. [Google Scholar] [CrossRef]
- Mahmood, H.; Eckold, D.; Stead, I.; Shepherd, D.E.T.; Espino, D.M.; Dearn, K.D. A Method for the Assessment of the Coefficient of Friction of Articular Cartilage and a Replacement Biomaterial. J. Mech. Behav. Biomed. Mater. 2020, 103, 103580. [Google Scholar] [CrossRef]
- Olivotto, E.; Trisolino, G.; Belluzzi, E.; Lazzaro, A.; Strazzari, A.; Pozzuoli, A.; Cigolotti, A.; Ruggieri, P.; Evangelista, A.; Ometto, F.; et al. Macroscopic Synovial Inflammation Correlates with Symptoms and Cartilage Lesions in Patients Undergoing Arthroscopic Partial Meniscectomy: A Clinical Study. J. Clin. Med. 2022, 11, 4330. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.T.; Kulvaranon, M.L.; Cutcliffe, H.C.; Utturkar, G.M.; Smith, W.A.R.; Spritzer, C.E.; Guilak, F.; DeFrate, L.E. Obesity Alters the in Vivo Mechanical Response and Biochemical Properties of Cartilage as Measured by MRI. Arthritis Res. Ther. 2018, 20, 232. [Google Scholar] [CrossRef] [PubMed]
- Widmyer, M.R.; Utturkar, G.M.; Leddy, H.A.; Coleman, J.L.; Spritzer, C.E.; Moorman, C.T.; DeFrate, L.E.; Guilak, F. High Body Mass Index Is Associated with Increased Diurnal Strains in the Articular Cartilage of the Knee. Arthritis Rheum. 2013, 65, 2615–2622. [Google Scholar] [CrossRef]
- Li, Y.; Wei, X.; Zhou, J.; Wei, L. The Age-Related Changes in Cartilage and Osteoarthritis. Biomed Res. Int. 2013, 2013, 916530. [Google Scholar] [CrossRef] [PubMed]
- Global Bouden of Disease Global Bouden of Disease Study 2019 (GBD 2019) Results. Osteoarthritis–Level 3 Cause. 2020. Available online: https://www.healthdata.org/results/gbd_summaries/2019/osteoarthritis-level-3-cause (accessed on 1 December 2022).
- Masson, A.O.; Krawetz, R.J. Understanding Cartilage Protection in OA and Injury: A Spectrum of Possibilities. BMC Musculoskelet. Disord. 2020, 21, 432. [Google Scholar] [CrossRef]
- Seidenstuecker, M.; Watrinet, J.; Bernstein, A.; Suedkamp, N.P.; Latorre, S.H.; Maks, A.; Mayr, H.O. Viscoelasticity and Histology of the Human Cartilage in Healthy and Degenerated Conditions of the Knee. J. Orthop. Surg. Res. 2019, 14, 256. [Google Scholar] [CrossRef]
- Kleeman, R.U.; Krocker, D.; Cedrano, A.; Tuischer, J.; Duda, G.N. Altered Cartilage Mechanics and Histology in Knee Osteoarthritis: Relation to Clinical Assessment (ICRS Grade). Osteoarthr. Cartil. 2005, 13, 958–963. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Ojanen, S.; Mohammadi, A.; Finnilä, M.A.; Joukainen, A.; Kröger, H.; Saarakkala, S.; Korhonen, R.K.; Tanska, P. Elastic, Viscoelastic and Fibril-Reinforced Poroelastic Material Properties of Healthy and Osteoarthritic Human Tibial Cartilage. Ann. Biomed. Eng. 2019, 47, 953–966. [Google Scholar] [CrossRef]
- Ihnatouski, M.; Pauk, J.; Karev, D.; Karev, B. AFM-Based Method for Measurement of Normal and Osteoarthritic Human Articular Cartilage Surface Roughness. Materials 2020, 13, 2302. [Google Scholar] [CrossRef]
- Martínez-Moreno, D.; Jiménez, G.; Gálvez-Martín, P.; Rus, G.; Marchal, J.A. Cartilage Biomechanics: A Key Factor for Osteoarthritis Regenerative Medicine. Biochim. Biophys. Acta—Mol. Basis Dis. 2019, 1865, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Mostakhdemin, M.; Nand, A.; Ramezani, M. Articular and Artificial Cartilage, Characteristics, Properties and Testing Approaches—A Review. Polymers 2021, 13, 2000. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Devi, D.; Mandal, B.B. Tissue-Engineered Cartilage: The Crossroads of Biomaterials, Cells and Stimulating Factors. Macromol. Biosci. 2015, 15, 153–182. [Google Scholar] [CrossRef]
- Rojas-Murillo, J.A.; Simental-Mendía, M.A.; Moncada-Saucedo, N.K.; Delgado-Gonzalez, P.; Islas, J.F.; Roacho-Pérez, J.A.; Garza-Treviño, E.N. Physical, Mechanical, and Biological Properties of Fibrin Scaffolds for Cartilage Repair. Int. J. Mol. Sci. 2022, 23, 9879. [Google Scholar] [CrossRef]
- Li, M.; Sun, D.; Zhang, J.; Wang, Y.; Wei, Q.; Wang, Y. Application and Development of 3D Bioprinting in Cartilage Tissue Engineering. Biomater. Sci. 2022, 10, 5430–5458. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Wang, J.; Fu, Y.; Pan, H.; He, H.; Gan, Q.; Liu, C. Smart Biomaterials for Articular Cartilage Repair and Regeneration. Adv. Funct. Mater. 2023, 33, 2212561. [Google Scholar] [CrossRef]
- Barbon, S.; Contran, M.; Stocco, E.; Todros, S.; Macchi, V.; De Caro, R.; Porzionato, A. Enhanced Biomechanical Properties of Polyvinyl Alcohol-Based Hybrid Scaffolds for Cartilage Tissue Engineering. Processes 2021, 9, 730. [Google Scholar] [CrossRef]
- Todros, S.; Barbon, S.; Stocco, E.; Favaron, M.; Macchi, V.; De Caro, R.; Porzionato, A.; Pavan, P.G. Time-Dependent Mechanical Behavior of Partially Oxidized Polyvinyl Alcohol Hydrogels for Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2022, 125, 104966. [Google Scholar] [CrossRef]
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic Science of Articular Cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef]
- Gilbert, S.J.; Bonnet, C.S.; Blain, E.J. Mechanical Cues: Bidirectional Reciprocity in the Extracellular Matrix Drives Mechano-Signalling in Articular Cartilage. Int. J. Mol. Sci. 2021, 22, 13595. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sport. Health Multidiscip. Approach 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Lu, X.L.; Mow, V.C. Biomechanics of Articular Cartilage and Determination of Material Properties. Med. Sci. Sport. Exerc. 2008, 40, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Statham, P.; Jones, E.; Jennings, L.M.; Fermor, H.L. Reproducing the Biomechanical Environment of the Chondrocyte for Cartilage Tissue Engineering. Tissue Eng. Part B Rev. 2022, 28, 405–420. [Google Scholar] [CrossRef]
- Killen, M.-C.; Charalambous, C.P. Advances in Cartilage Restoration Techniques. In Advances in Medical and Surgical Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 71–83. [Google Scholar]
- Yuan, X.; Yang, S. Primary Cilia and Intraflagellar Transport Proteins in Bone and Cartilage. J. Dent. Res. 2016, 95, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Jagga, S.; Lee, S.-S.; Nam, J.-S. Interplay between Cartilage and Subchondral Bone Contributing to Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 2013, 14, 19805–19830. [Google Scholar] [CrossRef] [PubMed]
- Pesesse, L.; Sanchez, C.; Henrotin, Y. Osteochondral Plate Angiogenesis: A New Treatment Target in Osteoarthritis. Jt. Bone Spine 2011, 78, 144–149. [Google Scholar] [CrossRef]
- Goldring, S.R.; Goldring, M.B. Changes in the Osteochondral Unit during Osteoarthritis: Structure, Function and Cartilage–Bone Crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef]
- Han, E.; Chen, S.S.; Klisch, S.M.; Sah, R.L. Contribution of Proteoglycan Osmotic Swelling Pressure to the Compressive Properties of Articular Cartilage. Biophys. J. 2011, 101, 916–924. [Google Scholar] [CrossRef]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage Diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016, 2, 16072. [Google Scholar] [CrossRef]
- Belluzzi, E.; Macchi, V.; Fontanella, C.; Carniel, E.; Olivotto, E.; Filardo, G.; Sarasin, G.; Porzionato, A.; Granzotto, M.; Pozzuoli, A.; et al. Infrapatellar Fat Pad Gene Expression and Protein Production in Patients with and without Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 6016. [Google Scholar] [CrossRef] [PubMed]
- Favero, M.; El-Hadi, H.; Belluzzi, E.; Granzotto, M.; Porzionato, A.; Sarasin, G.; Rambaldo, A.; Iacobellis, C.; Cigolotti, A.; Fontanella, C.G.; et al. Infrapatellar Fat Pad Features in Osteoarthritis: A Histopathological and Molecular Study. Rheumatology 2017, 56, 1784–1793. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial Inflammation in Osteoarthritis Progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef]
- Englund, M.; Guermazi, A.; Lohmander, S.L. The Role of the Meniscus in Knee Osteoarthritis: A Cause or Consequence? Radiol. Clin. N. Am. 2009, 47, 703–712. [Google Scholar] [CrossRef]
- Belluzzi, E.; El Hadi, H.; Granzotto, M.; Rossato, M.; Ramonda, R.; Macchi, V.; De Caro, R.; Vettor, R.; Favero, M. Systemic and Local Adipose Tissue in Knee Osteoarthritis. J. Cell. Physiol. 2017, 232, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Sowers, M.F. Epidemiology of Risk Factors for Osteoarthritis: Systemic Factors. Curr. Opin. Rheumatol. 2001, 13, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, E.; Belluzzi, E.; Pozzuoli, A.; Cigolotti, A.; Scioni, M.; Goldring, S.R.; Goldring, M.B.; Ruggieri, P.; Ramonda, R.; Grigolo, B.; et al. Do Synovial Inflammation and Meniscal Degeneration Impact Clinical Outcomes of Patients Undergoing Arthroscopic Partial Meniscectomy? A Histological Study. Int. J. Mol. Sci. 2022, 23, 3903. [Google Scholar] [CrossRef]
- Ghouri, A.; Conaghan, P.G. Update on Novel Pharmacological Therapies for Osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2019, 11, 1759720X1986449. [Google Scholar] [CrossRef]
- Belluzzi, E.; Stocco, E.; Pozzuoli, A.; Granzotto, M.; Porzionato, A.; Vettor, R.; De Caro, R.; Ruggieri, P.; Ramonda, R.; Rossato, M.; et al. Contribution of Infrapatellar Fat Pad and Synovial Membrane to Knee Osteoarthritis Pain. Biomed Res. Int. 2019, 2019, 6390182. [Google Scholar] [CrossRef]
- Yu, H.; Huang, T.; Lu, W.W.; Tong, L.; Chen, D. Osteoarthritis Pain. Int. J. Mol. Sci. 2022, 23, 4642. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Su, G.; Hou, Y.; Chen, S.; Lin, D. Cartilage Degradation in Osteoarthritis: A Process of Osteochondral Remodeling Resembles the Endochondral Ossification in Growth Plate? Med. Hypotheses 2018, 121, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Torzilli, P.A.; Allen, S.N. Effect of Articular Surface Compression on Cartilage Extracellular Matrix Deformation. J. Biomech. Eng. 2022, 144, 091007. [Google Scholar] [CrossRef]
- Goldring, M.B. Articular Cartilage Degradation in Osteoarthritis. HSS J. 2012, 8, 7–9. [Google Scholar] [CrossRef]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.-J. Osteoarthritis: Toward a Comprehensive Understanding of Pathological Mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef]
- Singh, P.; Marcu, K.B.; Goldring, M.B.; Otero, M. Phenotypic Instability of Chondrocytes in Osteoarthritis: On a Path to Hypertrophy. Ann. N. Y. Acad. Sci. 2019, 1442, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Ali, M.H.; Wydra, F.; Li, X.; Hamilton, J.L.; An, H.S.; Cs-Szabo, G.; Andrews, S.; Moric, M.; Xiao, G.; et al. Characterization of Degenerative Human Facet Joints and Facet Joint Capsular Tissues. Osteoarthr. Cartil. 2015, 23, 2242–2251. [Google Scholar] [CrossRef]
- Liu, C.; Wang, B.; Xiao, L.; Li, Y.; Xu, L.; Zhao, Z.; Zhang, L. Protective Effects of the Pericellular Matrix of Chondrocyte on Articular Cartilage against the Development of Osteoarthritis. Histol. Histopathol. 2018, 33, 757–764. [Google Scholar] [CrossRef]
- Maldonado, M.; Nam, J. The Role of Changes in Extracellular Matrix of Cartilage in the Presence of Inflammation on the Pathology of Osteoarthritis. Biomed Res. Int. 2013, 2013, 284873. [Google Scholar] [CrossRef]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral Bone in Osteoarthritis: Insight into Risk Factors and Microstructural Changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, T.; Amado, I.N.; O’Brien, F.J.; Kennedy, O.D. The Role of Mechanobiology in Bone and Cartilage Model Systems in Characterizing Initiation and Progression of Osteoarthritis. APL Bioeng. 2022, 6, 011501. [Google Scholar] [CrossRef]
- Wilusz, R.E.; Zauscher, S.; Guilak, F. Micromechanical Mapping of Early Osteoarthritic Changes in the Pericellular Matrix of Human Articular Cartilage. Osteoarthr. Cartil. 2013, 21, 1895–1903. [Google Scholar] [CrossRef]
- Zimmerman, B.K.; Nims, R.J.; Chen, A.; Hung, C.T.; Ateshian, G.A. Direct Osmotic Pressure Measurements in Articular Cartilage Demonstrate Nonideal and Concentration-Dependent Phenomena. J. Biomech. Eng. 2021, 143, 041007. [Google Scholar] [CrossRef] [PubMed]
- Huttu, M.R.J.; Puhakka, J.; Mäkelä, J.T.A.; Takakubo, Y.; Tiitu, V.; Saarakkala, S.; Konttinen, Y.T.; Kiviranta, I.; Korhonen, R.K. Cell-Tissue Interactions in Osteoarthritic Human Hip Joint Articular Cartilage. Connect. Tissue Res. 2014, 55, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Lakin, B.A.; Ellis, D.J.; Shelofsky, J.S.; Freedman, J.D.; Grinstaff, M.W.; Snyder, B.D. Contrast-Enhanced CT Facilitates Rapid, Non-Destructive Assessment of Cartilage and Bone Properties of the Human Metacarpal. Osteoarthr. Cartil. 2015, 23, 2158–2166. [Google Scholar] [CrossRef]
- Nissinen, M.T.; Hänninen, N.; Prakash, M.; Mäkelä, J.T.A.; Nissi, M.J.; Töyräs, J.; Nieminen, M.T.; Korhonen, R.K.; Tanska, P. Functional and Structural Properties of Human Patellar Articular Cartilage in Osteoarthritis. J. Biomech. 2021, 126, 110634. [Google Scholar] [CrossRef]
- Padilla-Martinez, J.P.; Lewis, W.; Ortega-Martinez, A.; Franco, W. Intrinsic Fluorescence and Mechanical Testing of Articular Cartilage in Human Patients with Osteoarthritis. J. Biophotonics 2018, 11, e201600269. [Google Scholar] [CrossRef]
- Peters, A.E.; Akhtar, R.; Comerford, E.J.; Bates, K.T. The Effect of Ageing and Osteoarthritis on the Mechanical Properties of Cartilage and Bone in the Human Knee Joint. Sci. Rep. 2018, 8, 5931. [Google Scholar] [CrossRef]
- Dourthe, B.; Nickmanesh, R.; Wilson, D.R.; D’Agostino, P.; Patwa, A.N.; Grinstaff, M.W.; Snyder, B.D.; Vereecke, E. Assessment of Healthy Trapeziometacarpal Cartilage Properties Using Indentation Testing and Contrast-Enhanced Computed Tomography. Clin. Biomech. 2019, 61, 181–189. [Google Scholar] [CrossRef]
- Fischenich, K.M.; Wahlquist, J.A.; Wilmoth, R.L.; Cai, L.; Neu, C.P.; Ferguson, V.L. Human Articular Cartilage Is Orthotropic Where Microstructure, Micromechanics, and Chemistry Vary with Depth and Split-Line Orientation. Osteoarthr. Cartil. 2020, 28, 1362–1372. [Google Scholar] [CrossRef]
- Kempson, G.E. Age-Related Changes in the Tensile Properties of Human Articular Cartilage: A Comparative Study between the Femoral Head of the Hip Joint and the Talus of the Ankle Joint. BBA—Gen. Subj. 1991, 1075, 223–230. [Google Scholar] [CrossRef]
- Temple-Wong, M.M.; Bae, W.C.; Chen, M.Q.; Bugbee, W.D.; Amiel, D.; Coutts, R.D.; Lotz, M.; Sah, R.L. Biomechanical, Structural, and Biochemical Indices of Degenerative and Osteoarthritic Deterioration of Adult Human Articular Cartilage of the Femoral Condyle. Osteoarthr. Cartil. 2009, 17, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Boschetti, F.; Peretti, G.M. Tensile and Compressive Properties of Healthy and Osteoarthritic Human Articular Cartilage. Biorheology 2008, 45, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Bader, D.L.; Kempson, G.E.; Egan, J.; Gilbey, W.; Barrett, A.J. The Effects of Selective Matrix Degradation on the Short-Term Compressive Properties of Adult Human Articular Cartilage. BBA—Gen. Subj. 1992, 1116, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, J.T.A.; Huttu, M.R.J.; Korhonen, R.K. Structure-Function Relationships in Osteoarthritic Human Hip Joint Articular Cartilage. Osteoarthr. Cartil. 2012, 20, 1268–1277. [Google Scholar] [CrossRef]
- Nissi, M.J.; Rieppo, J.; Töyräs, J.; Laasanen, M.S.; Kiviranta, I.; Nieminen, M.T.; Jurvelin, J.S. Estimation of Mechanical Properties of Articular Cartilage with MRI—DGEMRIC, T2 and T1 Imaging in Different Species with Variable Stages of Maturation. Osteoarthr. Cartil. 2007, 15, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.L.; Kersh, M.E.; Walsh, N.C.; Ackland, D.C.; de Steiger, R.N.; Pandy, M.G. Mechanical Properties of Normal and Osteoarthritic Human Articular Cartilage. J. Mech. Behav. Biomed. Mater. 2016, 61, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Burgin, L.V.; Edelsten, L.; Aspden, R.M. The Mechanical and Material Properties of Elderly Human Articular Cartilage Subject to Impact and Slow Loading. Med. Eng. Phys. 2014, 36, 226–232. [Google Scholar] [CrossRef]
- Amarouch, M.Y.; El Hilaly, J.; Mazouzi, D. AFM and FluidFM Technologies: Recent Applications in Molecular and Cellular Biology. Scanning 2018, 2018, 7801274. [Google Scholar] [CrossRef] [PubMed]
- Kienle, S.; Boettcher, K.; Wiegleb, L.; Urban, J.; Burgkart, R.; Lieleg, O.; Hugel, T. Comparison of Friction and Wear of Articular Cartilage on Different Length Scales. J. Biomech. 2015, 48, 3052–3058. [Google Scholar] [CrossRef] [PubMed]
- Desrochers, J.; Amrein, M.W.; Matyas, J.R. Microscale Surface Friction of Articular Cartilage in Early Osteoarthritis. J. Mech. Behav. Biomed. Mater. 2013, 25, 11–22. [Google Scholar] [CrossRef]
- Braet; Seynaeve; De Zanger; Wisse Imaging Surface and Submembranous Structures with the Atomic Force Microscope: A Study on Living Cancer Cells, Fibroblasts and Macrophages. J. Microsc. 1998, 190, 328–338. [CrossRef]
- Park, S.; Hung, C.T.; Ateshian, G.A. Mechanical Response of Bovine Articular Cartilage under Dynamic Unconfined Compression Loading at Physiological Stress Levels. Osteoarthr. Cartil. 2004, 12, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Stolz, M.; Raiteri, R.; Daniels, A.U.; VanLandingham, M.R.; Baschong, W.; Aebi, U. Dynamic Elastic Modulus of Porcine Articular Cartilage Determined at Two Different Levels of Tissue Organization by Indentation-Type Atomic Force Microscopy. Biophys. J. 2004, 86, 3269–3283. [Google Scholar] [CrossRef] [PubMed]
- Berardo, A.; Pugno, N.M. A Model for Hierarchical Anisotropic Friction, Adhesion and Wear. Tribol. Int. 2020, 152, 106549. [Google Scholar] [CrossRef]
- Berardo, A.; Costagliola, G.; Ghio, S.; Boscardin, M.; Bosia, F.; Pugno, N.M. An Experimental-Numerical Study of the Adhesive Static and Dynamic Friction of Micro-Patterned Soft Polymer Surfaces. Mater. Des. 2019, 181, 107930. [Google Scholar] [CrossRef]
- Spagni, A.; Berardo, A.; Marchetto, D.; Gualtieri, E.; Pugno, N.M.; Valeri, S. Friction of Rough Surfaces on Ice: Experiments and Modeling. Wear 2016, 368–369, 258–266. [Google Scholar] [CrossRef]
- Lakin, B.A.; Grasso, D.J.; Shah, S.S.; Stewart, R.C.; Bansal, P.N.; Freedman, J.D.; Grinstaff, M.W.; Snyder, B.D. Cationic Agent Contrast-Enhanced Computed Tomography Imaging of Cartilage Correlates with the Compressive Modulus and Coefficient of Friction. Osteoarthr. Cartil. 2013, 21, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Katta, J.; Stapleton, T.; Ingham, E.; Jin, Z.M.; Fisher, J. The Effect of Glycosaminoglycan Depletion on the Friction and Deformation of Articular Cartilage. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2008, 222, 1–11. [Google Scholar] [CrossRef]
- Krishnan, R.; Kopacz, M.; Ateshian, G.A. Experimental Verification of the Role of Interstitial Fluid Pressurization in Cartilage Lubrication. J. Orthop. Res. 2004, 22, 565–570. [Google Scholar] [CrossRef]
- Forster, H.; Fisher, J. The Influence of Loading Time and Lubricant on the Friction of Articular Cartilage. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1996, 210, 109–118. [Google Scholar] [CrossRef]
- Ateshian, G.A. The Role of Interstitial Fluid Pressurization in Articular Cartilage Lubrication. J. Biomech. 2009, 42, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Caligaris, M.; Ateshian, G.A. Effects of Sustained Interstitial Fluid Pressurization under Migrating Contact Area, and Boundary Lubrication by Synovial Fluid, on Cartilage Friction. Osteoarthr. Cartil. 2008, 16, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, B.K.; Bonnevie, E.D.; Park, M.; Zhou, Y.; Wang, L.; Burris, D.L.; Lu, X.L. Role of Interstitial Fluid Pressurization in TMJ Lubrication. J. Dent. Res. 2015, 94, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Lakin, B.A.; Patel, H.; Stok, K.S.; Snyder, B.D.; Grinstaff, M.W. Contrast-Enhanced Computed Tomography Imaging Using a Cationic Contrast Agent Correlates with the Equilibrium Modulus of Mouse Tibial Plateau Cartilage. Osteoarthr. Cartil. 2014, 22, S345–S346. [Google Scholar] [CrossRef]
- Wahlquist, J.A.; DelRio, F.W.; Randolph, M.A.; Aziz, A.H.; Heveran, C.M.; Bryant, S.J.; Neu, C.P.; Ferguson, V.L. Indentation Mapping Revealed Poroelastic, but Not Viscoelastic, Properties Spanning Native Zonal Articular Cartilage. Acta Biomater. 2017, 64, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Huang, Y.; Xiao, Y.; Yang, X.; Lin, H.; Feng, G.; Zhu, X.; Zhang, X. Viscoelasticity in Natural Tissues and Engineered Scaffolds for Tissue Reconstruction. Acta Biomater. 2019, 97, 74–92. [Google Scholar] [CrossRef] [PubMed]
- Nia, H.T.; Gauci, S.J.; Azadi, M.; Hung, H.-H.; Frank, E.; Fosang, A.J.; Ortiz, C.; Grodzinsky, A.J. High-Bandwidth AFM-Based Rheology Is a Sensitive Indicator of Early Cartilage Aggrecan Degradation Relevant to Mouse Models of Osteoarthritis. J. Biomech. 2015, 48, 162–165. [Google Scholar] [CrossRef]
- Temple, M.M.; Bae, W.C.; Chen, M.Q.; Lotz, M.; Amiel, D.; Coutts, R.D.; Sah, R.L. Age- and Site-Associated Biomechanical Weakening of Human Articular Cartilage of the Femoral Condyle. Osteoarthr. Cartil. 2007, 15, 1042–1052. [Google Scholar] [CrossRef]
- Chahine, N.O.; Blanchette, C.; Thomas, C.B.; Lu, J.; Haudenschild, D.; Loots, G.G. Effect of Age and Cytoskeletal Elements on the Indentation-Dependent Mechanical Properties of Chondrocytes. PLoS ONE 2013, 8, e0061651. [Google Scholar] [CrossRef]
- Espinosa, M.G.; Otarola, G.A.; Hu, J.C.; Athanasiou, K.A. Vibrometry as a Noncontact Alternative to Dynamic and Viscoelastic Mechanical Testing in Cartilage. J. R. Soc. Interface 2021, 18, 20210765. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Berni, M.; Marchiori, G.; Cassiolas, G.; Muttini, A.; Barboni, B.; Martini, L.; Fini, M.; Lopomo, N.F.; Marcacci, M.; et al. Evaluation of Cartilage Biomechanics and Knee Joint Microenvironment after Different Cell-Based Treatments in a Sheep Model of Early Osteoarthritis. Int. Orthop. 2021, 45, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Cutcliffe, H.C.; DeFrate, L.E. Comparison of Cartilage Mechanical Properties Measured During Creep and Recovery. Sci. Rep. 2020, 10, 1547. [Google Scholar] [CrossRef]
- Reuter, T.; Hurschler, C. Comparison of Biphasic Material Properties of Equine Articular Cartilage Estimated from Stress Relaxation and Creep Indentation Tests. Curr. Dir. Biomed. Eng. 2021, 7, 363–366. [Google Scholar] [CrossRef]
- Kotelsky, A.; Woo, C.W.; Delgadillo, L.F.; Richards, M.S.; Buckley, M.R. An Alternative Method to Characterize the Quasi-Static, Nonlinear Material Properties of Murine Articular Cartilage. J. Biomech. Eng. 2018, 140, 011007. [Google Scholar] [CrossRef]
- Hu, K.; Radhakrishnan, P.; Patel, R.V.; Mao, J.J. Regional Structural and Viscoelastic Properties of Fibrocartilage upon Dynamic Nanoindentation of the Articular Condyle. J. Struct. Biol. 2001, 136, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Cutcliffe, H.C.; Kottamasu, P.K.; McNulty, A.L.; Goode, A.P.; Spritzer, C.E.; DeFrate, L.E. Mechanical Metrics May Show Improved Ability to Predict Osteoarthritis Compared to T1rho Mapping. J. Biomech. 2021, 129, 110771. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.J.; Noori-Dokht, H.; Karnik, S.; Alyafei, N.; Joukar, A.; Trippel, S.B.; Wagner, D.R. Anisotropic Properties of Articular Cartilage in an Accelerated in Vitro Wear Test. J. Mech. Behav. Biomed. Mater. 2020, 109, 103834. [Google Scholar] [CrossRef]
- Williams, J.L.; Vani, J.N.; Eick, J.D.; Petersen, E.C.; Schmidt, T.L. Shear Strength of the Physis Varies with Anatomic Location and Is a Function of Modulus, Inclination, and Thickness. J. Orthop. Res. 1999, 17, 214–222. [Google Scholar] [CrossRef]
- Cao, L.; Youn, I.; Guilak, F.; Setton, L.A. Compressive Properties of Mouse Articular Cartilage Determined in a Novel Micro-Indentation Test Method and Biphasic Finite. J. Biomech. Eng. 2006, 128, 766–771. [Google Scholar] [CrossRef]
- Boettcher, K.; Kienle, S.; Nachtsheim, J.; Burgkart, R.; Hugel, T.; Lieleg, O. The Structure and Mechanical Properties of Articular Cartilage Are Highly Resilient towards Transient Dehydration. Acta Biomater. 2016, 29, 180–187. [Google Scholar] [CrossRef]
- Patel, R.V.; Mao, J.J. Microstructural and Elastic Properties of the Extracellular Matrices of the Superficial Zone of Neonatal Articular Cartilage by Atomic Force Microscopy. Front. Biosci. 2003, 8, 18–25. [Google Scholar] [CrossRef]
- Katta, J.; Jin, Z.; Ingham, E.; Fisher, J. Biotribology of Articular Cartilage—A Review of the Recent Advances. Med. Eng. Phys. 2008, 30, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Link, J.M.; Salinas, E.Y.; Hu, J.C.; Athanasiou, K.A. The Tribology of Cartilage: Mechanisms, Experimental Techniques, and Relevance to Translational Tissue Engineering. Clin. Biomech. 2020, 79, 104880. [Google Scholar] [CrossRef]
- Moore, A.C.; Burris, D.L. Tribological and Material Properties for Cartilage of and throughout the Bovine Stifle: Support for the Altered Joint Kinematics Hypothesis of Osteoarthritis. Osteoarthr. Cartil. 2015, 23, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Feeney, E.; Guan, Y.; Cook, S.G.; Gourdon, D.; Bonassar, L.J.; Putnam, D. Boundary Mode Lubrication of Articular Cartilage with a Biomimetic Diblock Copolymer. Proc. Natl. Acad. Sci. USA 2019, 116, 12437–12441. [Google Scholar] [CrossRef]
- Middendorf, J.M.; Griffin, D.J.; Shortkroff, S.; Dugopolski, C.; Kennedy, S.; Siemiatkoski, J.; Cohen, I.; Bonassar, L.J. Mechanical Properties and Structure-Function Relationships of Human Chondrocyte-Seeded Cartilage Constructs after in Vitro Culture. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2017, 35, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Su, Æ.Y.; Wang, Æ.J. Influence of Dynamic Load on Friction Behavior of Human Articular Cartilage, Stainless Steel and Polyvinyl Alcohol Hydrogel as Artificial Cartilage. J. Mater. Sci. Mater. Med. 2010, 21, 147–154. [Google Scholar] [CrossRef]
- Li, F.; Wang, A.; Wang, C. Analysis of Friction between Articular Cartilage and Polyvinyl Alcohol Hydrogel Artificial Cartilage. J. Mater. Sci. Mater. Med. 2016, 27, 87. [Google Scholar] [CrossRef]
- Brand, R.A. Joint Contact Stress: A Reasonable Surrogate for Biological Processes? Iowa Orthop. J. 2005, 25, 82–94. [Google Scholar]
- Felson, D.T. Osteoarthritis: New Insights. Part 1: The Disease and Its Risk Factors. Ann. Intern. Med. 2000, 133, 635. [Google Scholar] [CrossRef]
- Caligaris, M.; Canal, C.E.; Ahmad, C.S.; Gardner, T.R.; Ateshian, G.A. Investigation of the Frictional Response of Osteo-arthritic Human Tibiofemoral Joints and the Potential Beneficial Tribological Effect of Healthy Synovial Fluid. Osteoarthr. Cartil. 2009, 17, 1327–1332. [Google Scholar] [CrossRef]
- Mow, V.C.; Kuei, S.C.; Lai, W.M.; Armstrong, C.G. Biphasic Creep and Stress Relaxation of Articular Cartilage in Compression: Theory and Experiments. J. Biomech. Eng. 1980, 102, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.; van Donkelaar, C.C.; Huyghe, J.M. A Comparison Between Mechano-Electrochemical and Biphasic Swelling Theories for Soft Hydrated Tissues. J. Biomech. Eng. 2005, 127, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Bachrach, N.M.; Mow, V.C.; Guilak, F. Incompressibility of the Solid Matrix of Articular Cartilage under High Hydrostatic Pressures. J. Biomech. 1998, 31, 445–451. [Google Scholar] [CrossRef]
- DiSilvestro, M.R.; Suh, J.K. A Cross-Validation of the Biphasic Poroviscoelastic Model of Articular Cartilage in Unconfined Compression, Indentation, and Confined Compression. J. Biomech. 2001, 34, 519–525. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, M.R.; Zhu, Q.; Suh, J.K. Biphasic Poroviscoelastic Simulation of the Unconfined Compression of Articular Cartilage: II--Effect of Variable Strain Rates. J. Biomech. Eng. 2001, 123, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Mow, V.C.; Ateshian, G.A. The Role of Flow-Independent Viscoelasticity in the Biphasic Tensile and Compressive Responses of Articular Cartilage. J. Biomech. Eng. 2001, 123, 410–417. [Google Scholar] [CrossRef]
- Mansour, J.M. Biomechanics of Cartilage. Kinesiol. Mech. Pathomech. Hum. Mov. 2003, 2, 66–75. [Google Scholar]
- Mansour, J.M.; Mow, V.C. The Permeability of Articular Cartilage under Compressive Strain and at High Pressures. J. Bone Jt. Surg. Am. 1976, 58, 509–516. [Google Scholar] [CrossRef]
- Berteau, J.-P.; Oyen, M.; Shefelbine, S.J. Permeability and Shear Modulus of Articular Cartilage in Growing Mice. Biomech. Model. Mechanobiol. 2016, 15, 205–212. [Google Scholar] [CrossRef]
- Maroudas, A.; Bullough, P. Permeability of Articular Cartilage. Nature 1968, 219, 1260–1261. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.M.; Hou, J.S.; Mow, V.C. A Triphasic Theory for the Swelling and Deformation Behaviors of Articular Cartilage. J. Biomech. Eng. 1991, 113, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Huyghe, J.M.; Janssen, J.D. Quadriphasic Mechanics of Swelling Incompressible Porous Media. Int. J. Eng. Sci. 1997, 35, 793–802. [Google Scholar] [CrossRef]
- Klika, V.; Gaffney, E.A.; Chen, Y.C.; Brown, C.P. An Overview of Multiphase Cartilage Mechanical Modelling and Its Role in Understanding Function and Pathology. J. Mech. Behav. Biomed. Mater. 2016, 62, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Donzelli, P.S.; Spilker, R.L.; Ateshian, G.A.; Mow, V.C. Contact Analysis of Biphasic Transversely Isotropic Cartilage Layers and Correlations with Tissue Failure. J. Biomech. 1999, 32, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Speirs, A.D.; Beaulé, P.E.; Ferguson, S.J.; Frei, H. Stress Distribution and Consolidation in Cartilage Constituents Is Influenced by Cyclic Loading and Osteoarthritic Degeneration. J. Biomech. 2014, 47, 2348–2353. [Google Scholar] [CrossRef]
- Ateshian, G.A.; Wang, H.; Lai, W.M. The Role of Interstitial Fluid Pressurization and Surface Porosities on the Boundary Friction of Articular Cartilage. J. Tribol. 1998, 120, 241–248. [Google Scholar] [CrossRef]
- Graindorge, S.; Ferrandez, W.; Jin, Z.; Ingham, E.; Grant, C.; Twigg, P.; Fisher, J. Biphasic Surface Amorphous Layer Lubrication of Articular Cartilage. Med. Eng. Phys. 2005, 27, 836–844. [Google Scholar] [CrossRef]
- Pawaskar, S.S.; Jin, Z.M.; Fisher, J. Modelling of Fluid Support inside Articular Cartilage during Sliding. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2007, 221, 165–174. [Google Scholar] [CrossRef]
- Taylor, Z.A.; Miller, K. Constitutive Modeling of Cartilaginous Tissues: A Review. J. Appl. Biomech. 2006, 22, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.; Soulhat, J.; Shirazi-Adl, A.; Hunziker, E.B.; Buschmann, M.D. Unconfined Compression of Articular Cartilage: Nonlinear Behavior and Comparison With a Fibril-Reinforced Biphasic Model. J. Biomech. Eng. 2000, 122, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Li, L.P.; Buschmann, M.D.; Shirazi-Adl, A. A Fibril Reinforced Nonhomogeneous Poroelastic Model for Articular Cartilage: Inhomogeneous Response in Unconfined Compression. J. Biomech. 2000, 33, 1533–1541. [Google Scholar] [CrossRef]
- Li, L.P.; Shirazi-Adl, A.; Buschmann, M.D. Alterations in Mechanical Behaviour of Articular Cartilage Due to Changes in Depth Varying Material Properties--a Nonhomogeneous Poroelastic Model Study. Comput. Methods Biomech. Biomed. Eng. 2002, 5, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Soulhat, J.; Buschmann, M.; Shirazi-Adl, A. Nonlinear Analysis of Cartilage in Unconfined Ramp Compression Using a Fibril Reinforced Poroelastic Model. Clin. Biomech. 1999, 14, 673–682. [Google Scholar] [CrossRef]
- Korhonen, R.K.; Laasanen, M.S.; Töyräs, J.; Lappalainen, R.; Helminen, H.J.; Jurvelin, J.S. Fibril Reinforced Poroelastic Model Predicts Specifically Mechanical Behavior of Normal, Proteoglycan Depleted and Collagen Degraded Articular Cartilage. J. Biomech. 2003, 36, 1373–1379. [Google Scholar] [CrossRef]
- Wu, J.Z.; Herzog, W.; Epstein, M. Modelling of Location- and Time-Dependent Deformation of Chondrocytes during Cartilage Loading. J. Biomech. 1999, 32, 563–572. [Google Scholar] [CrossRef]
- Federico, S.; Grillo, A.; La Rosa, G.; Giaquinta, G.; Herzog, W. A Transversely Isotropic, Transversely Homogeneous Microstructural-Statistical Model of Articular Cartilage. J. Biomech. 2005, 38, 2008–2018. [Google Scholar] [CrossRef]
- Baaijens, F.P.T.; Trickey, W.R.; Laursen, T.A.; Guilak, F. Large Deformation Finite Element Analysis of Micropipette Aspiration to Determine the Mechanical Properties of the Chondrocyte. Ann. Biomed. Eng. 2005, 33, 494–501. [Google Scholar] [CrossRef]
- Arduino, A.; Pettenuzzo, S.; Berardo, A.; Salomoni, V.A.; Majorana, C.; Carniel, E.L. A Continuum-Tensegrity Computational Model for Chondrocyte Biomechanics in AFM Indentation and Micropipette Aspiration. Ann. Biomed. Eng. 2022, 50, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Agoram, B.; Barocas, V.H. Coupled Macroscopic and Microscopic Scale Modeling of Fibrillar Tissues and Tissue Equivalents. J. Biomech. Eng. 2001, 123, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Manzano, S.; Armengol, M.; Price, A.J.; Hulley, P.A.; SGill, H.; Doblaré, M.; Hamdy Doweidar, M. Inhomogeneous Response of Articular Cartilage: A Three-Dimensional Multiphasic Heterogeneous Study. PLoS ONE 2016, 11, e0157967. [Google Scholar] [CrossRef] [PubMed]


| Point of View | Healthy Tissue | OA Tissue |
|---|---|---|
| Macroscopic level | Full thickness tissue Intact articular surface | Thinned tissue Eroded articular surface Patchy proteoglycan staining Increased calcified cartilage |
| Joint space narrowing | ||
| Cellular level | ||
| Chondrocytes | Quiescent cells | Hypertrophic-like phenotype Increased catabolism |
| Proinflammatory proteins production | ||
| Production of extracellular matrix-degrading proteinases | ||
| ECM features | ||
| ECM Proteolysis | No | Yes |
| Proteoglycan content | High | Loss (proteoglycan degradation) |
| Collagen content | Type II and type IX collagen | Type II collagen denaturation and degradation Type X collagen production |
| Glycosaminoglycans | Synthesis of chondroitin sulphate, keratan sulphate and hyaluronic acid | Loss |
| Mechanical Test | Ref. | Harvesting Site | Total N of Donors | Healthy/OA | Age of the Donors (y/o) | Protocol |
|---|---|---|---|---|---|---|
| AFM | [12] | femoral head | 50 (F:M = 25:25) | OA | 40–65 | N/A |
| [55] | medial and lateral condyle | 8 (F:M = 6:2) | OA | 53–83 | 15 mm/s | |
| confined compression | [56] | femoral condyle | 4 (F:M = 2:2) | H | 65.7 | SR 1 displacement ramp at 0.25 um/s (60 min) |
| plane-ended indentation | [11] | tibia | 7 (F:M = 1:6) | H and OA | 68–79 | 4 ramps of SR, 5% strain each, (100%/s and 15 min of relaxation time) |
| [57] | femoral head | 16 | OA | N/A | 4 ramps of SR, 5% strain each, (100%/s and 15 min of relaxation time) | |
| [58] | metacarpal joint | 12 (F:M = 6:6) | H | 47–80 | 4 ramps of SR, 5% strain each, (2.5%/s and 5 min of relaxation time) | |
| [59] | patellar | 6 F:M = 1:5 | OA | 68–79 | 3 ramps of SR, 5% strain each, (100%/s and 15 min of relaxation time) | |
| [60] | medial and lateral condyle | 6 (F:M = 3:3) | OA | 54–83 | 2 mm/s | |
| [61] | knee joints cartilage + subcondral bone and trabecular bone | 12 (F:M = 4:8) | H and OA | 31–88 | 500 nm amplitude (110 Hz) | |
| spherical indentation | [62] | trapeziometacarpal joint | 16 (F:M = 10:6) | H | 66–101 | 0.1 mm at 0.5 mm/s, (relaxation time 10 s) |
| [63] | lateral condyle | 4 (F:M = 2:2) | H | 55–61 | 12.5 um (1, 4 and 8 um/s) | |
| [9] | tibial plateau | 25 (F:M = 2:1) 13 (F:M = 2:1) | OA H | 72 | 0.3 mm at 0.1 mm/s, (10 s relaxation time) | |
| tensile | [64] | femoral head and talus | N/A | H | 7–90 | 0.08 mm/s |
| [65] | lateral and medial condyles | 31 | H | 30 ± 2 48 ± 1 70 ± 3 | 0.08 mm/s | |
| tensile and unconfined compression | [66] | femoral head | 3 1 | OA H | 85 ± 8 76 | compression: 6 ramps of SR, 3% strain each, (0.2%/s and relaxation time 20 min. Tension: 4 ramps of SR, 2% each (0.2%/s and relaxation time 200–250 min) |
| unconfined compression | [67] | femoral condyle and femoral head | N/A | H | 36–86 | N/A |
| [10] | tibia plateaus | 21 (F:M = 15:6) | OA | N/A | up to 25% strain, (relaxation time 60–120 min) | |
| [68] | femoral head | 9 | OA | N/A | 4 ramps of SR, 5% strain each, (100%/s and 15 min of relaxation time) | |
| [69] | patellar | 12 | H | 24–78 | 1 ramp of SR, 10% strain, (2 mm/s and 40 min of relaxation time) | |
| [70] | medial and lateral condyle and tibial plateau | 10 (F:M = 6:4) 3 (F:M = 2:1) | OA H | 69.7 ± 9.3 59.1 ± 7.2 | strain 30% with 20%/s | |
| [71] | femoral head | 14 (F:M = 6:8) | H | 63–89 | 100%/min up to 0.15 MPa |
| Harvesting Site | Ref. | IM (MPa) 1 | HA or Eeq (MPa) 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Healthy | OA | Healthy | OA | ||||||
| E | M | A | E | M | A | ||||
| femoral head | [12] | 1.7 | 1.3 | 1.2 | 1.2 | N/A | N/A | ||
| [57] | N/A | Mean E0 2 [0.1–8] Mean E” 46 [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124] | N/A | 0.4 [0.02–1] | |||||
| [68] | N/A | Ef0 OA: 0.59 ± 0.48 Ef″ OA: 0.61 ± 0.61 Enf OA: 0.23 ± 0.22 | N/A | N/A | |||||
| [71] | 1.60 ± 0.51 to 2.47 ± 0.49 | N/A | N/A | N/A | |||||
| condyles | [56] | N/A | N/A | 0.499 ± 0.208 to 1.597 ± 0.455 | N/A | ||||
| tibia plateau | [10] | N/A | N/A | N/A | 0.50 ± 0.14 | 0.37 ± 0.13 | 0.28 ± 0.12 | ||
| [11] | 6.87 ± 2.57 | 3.69 ± 2.07 | 1.67 ± 1.08 | 1.19 ± 0.56 | 0.42 ± 0.25 | 0.21 ± 0.15 | |||
| [9] | 3.43 ± 0.36 | 2.09 ± 0.18 | N/A | N/A | |||||
| patellar joint | [69] | 4.47 ± 2.22 | N/A | 0.53 ± 0.25 | |||||
| metacarpal | [62] | MC1: 1.64 ± 1.86 trapezium: 0.99 ± 1.26 | N/A | N/A | N/A | ||||
| [58] | N/A | N/A | 0.5–4 | N/A | |||||
| Other measurements 3 | |||||||||
| Harvesting site | Ref. | Shear storage modulus G’ | |||||||
| Grade 0 | Grade 0–1 | Grade 1–3 | Grade 0–4 | ||||||
| Age < 45 y/o | 45 < age < 55 | 55 < age < 75 | Age > 75 | ||||||
| condyles | [61] | 0.90 ± 0.55 to 1.30 ± 0.65 | 0.41 ± 0.54 to 0.96 ± 0.50 | 0.14 ± 0.31 to 0.55 ± 0.45 | 0.15 ± 0.09 to 0.40 ± 0.34 | ||||
| Sample Source | Ref. | Harvesting Site | Mechanical Tests | IM (MPa) | HA or Eeq (MPa) | Other Findings |
|---|---|---|---|---|---|---|
| Bovine | [93] | humeral head | tensile to unconfined compression | N/A | (f [Hz]) Emin–Emax | N/A |
| (0.1) 14.6 ± 6.9– 48.54 ± 17.0 | ||||||
| (1) 1.16.1 ± 5.2– 65.7 ± 15.8 | ||||||
| (10) 24.2 ± 6.6– 61.7 ± 13.3 | ||||||
| (40) 28.7 ± 7.8– 60.9 ± 13.4 | ||||||
| [94] | femoral condyle, trochlear groove and patella | unconfined compression, indentation and vibrometry | condyle: 1.4 ± 0.5 patella: 1.5 ± 0.5 trochlea: 1.45 ± 0.3 | condyle: 0.48 ± 0.12, patella: 0.35 ± 0.05, trochlea: 0.28 ± 0.02 | (Edyn) condyle: 27.4 ± 14.3 patella: 46.7 ± 11.0 trochlea: 56.4 ± 29.9 | |
| [101] | femoral condyles | wear | N/A | N/A | COF 1 (long) 0.265 ± 0.033 COF (trans) 0.247 ± 0.034 | |
| [102] | tibiae | shear | N/A | N/A | shear modulus 3.16 ± 1.01 tangent modulus 3.29 (1.02) (uncompressed and compressed regions) | |
| [69] | patella | unconfined compression | N/A | 0.61 ± 0.18 | N/A | |
| Equine | [97] | medial anterior condyles | indentation | N/A | 0.6–0.9 (1) 0.7–0.9 (2) | k (mm4/Ns) 0.004–0.019 (1) k (mm4/Ns) 0.008–0.014 (2) |
| Murine | [98] | femoral condyles | custom made test | N/A | 6.4 | ν (-) 0.25 |
| [103] | tibial plateau | indentation | N/A | 2 ± 0.3 | ν (-) 0.2 | |
| Ovine | [104] | patellofemoral grooves | indentation | 0.9 ± 0.8 | N/A | N/A |
| [73] | hinderleg | AFM | N/A | N/A | COF 2 (ddH2O) 0.3–0.33 COF (154 mM NaCl) 0.4–0.55 COF (2M NaCl) 0.6 COF (synovial fluid) 0.5–0.6 | |
| [95] | condyles | Indentation | H 12.3 ± 4.8 OA 2.7 ± 1.7 | N/A | N/A | |
| Porcine | [96] | femurs and tibiae | Confined compression | N/A | tibia: 1.2 ± 0.5 femur: 0.4 ± 0.2 | N/A |
| [100] | condyles | Confined compression | N/A | tibia: H 0.5 ± 0.1 OA 0.4 ±0.2 | N/A | |
| femur: H 0.4 ± 0.1 OA 0.2 ± 0.1 | ||||||
| [69] | patella | Unconfined compression | N/A | 0.85 ± 0.25 | N/A | |
| [87] | mandibular condyle | Custom-built micro-tribometer and indentation | N/A | 0.25 ± 0.06 | COF 3, 0.025 ± 0.004 anterior-posterior sliding direction 0.028 ± 0.004 latero-medial sliding direction | |
| Rabbit | [99] | mandibular condyles | AFM | 2.3 ± 0.3 (AM region) 1.0 ± 0.1 (PL region) | N/A | N/A |
| [105] | mandibular condyles | AFM | neonatal 0.95 ± 0.15 to 1.02 ± 0.22 | N/A | N/A | |
| Dog | [74] | Femoral condyles | AFM | N/A | N/A | COF 4, H: 0.15 ± 0.06 (for every load) OA: 0.22 ± 0.09 (load 0.5 µN) to 0.13 ± 0.10 (load 5 µN) |
| Biomechanical Changes | Healthy AC | OA AC |
|---|---|---|
| Instantaneous and equilibrium elastic properties | Differences between anatomical sites and cartilage zones | Reduced with influence of the OA grade |
| Tribological properties | Low COF and efficient lubrication | COF increased at the microscale, no significant variations at the macroscale, reduced lubrication |
| Smooth articular joint surfaces | Increased superficial roughness | |
| Fibril network properties | Possible variations with cartilage depth due to different organizations | Decrease in the initial fibril network modulus from early to advanced OA, proportional to the PG content. |
| Permeability | Mainly governed by the physiological porosity and compressive load | Altered and increased, promoting a higher load to be transmitted to the solid cartilage component |
| Elastic properties of the subchondral bone | Increased with increasing age | Increased with increasing OA grade |
| Elastic properties of the trabecular bone | N/A | Not influenced by OA grade |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belluzzi, E.; Todros, S.; Pozzuoli, A.; Ruggieri, P.; Carniel, E.L.; Berardo, A. Human Cartilage Biomechanics: Experimental and Theoretical Approaches towards the Identification of Mechanical Properties in Healthy and Osteoarthritic Conditions. Processes 2023, 11, 1014. https://doi.org/10.3390/pr11041014
Belluzzi E, Todros S, Pozzuoli A, Ruggieri P, Carniel EL, Berardo A. Human Cartilage Biomechanics: Experimental and Theoretical Approaches towards the Identification of Mechanical Properties in Healthy and Osteoarthritic Conditions. Processes. 2023; 11(4):1014. https://doi.org/10.3390/pr11041014
Chicago/Turabian StyleBelluzzi, Elisa, Silvia Todros, Assunta Pozzuoli, Pietro Ruggieri, Emanuele Luigi Carniel, and Alice Berardo. 2023. "Human Cartilage Biomechanics: Experimental and Theoretical Approaches towards the Identification of Mechanical Properties in Healthy and Osteoarthritic Conditions" Processes 11, no. 4: 1014. https://doi.org/10.3390/pr11041014
APA StyleBelluzzi, E., Todros, S., Pozzuoli, A., Ruggieri, P., Carniel, E. L., & Berardo, A. (2023). Human Cartilage Biomechanics: Experimental and Theoretical Approaches towards the Identification of Mechanical Properties in Healthy and Osteoarthritic Conditions. Processes, 11(4), 1014. https://doi.org/10.3390/pr11041014











