Nickel Oxide Nanoparticles on KIT-6: An Efficient Catalyst in Methane Combustion
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
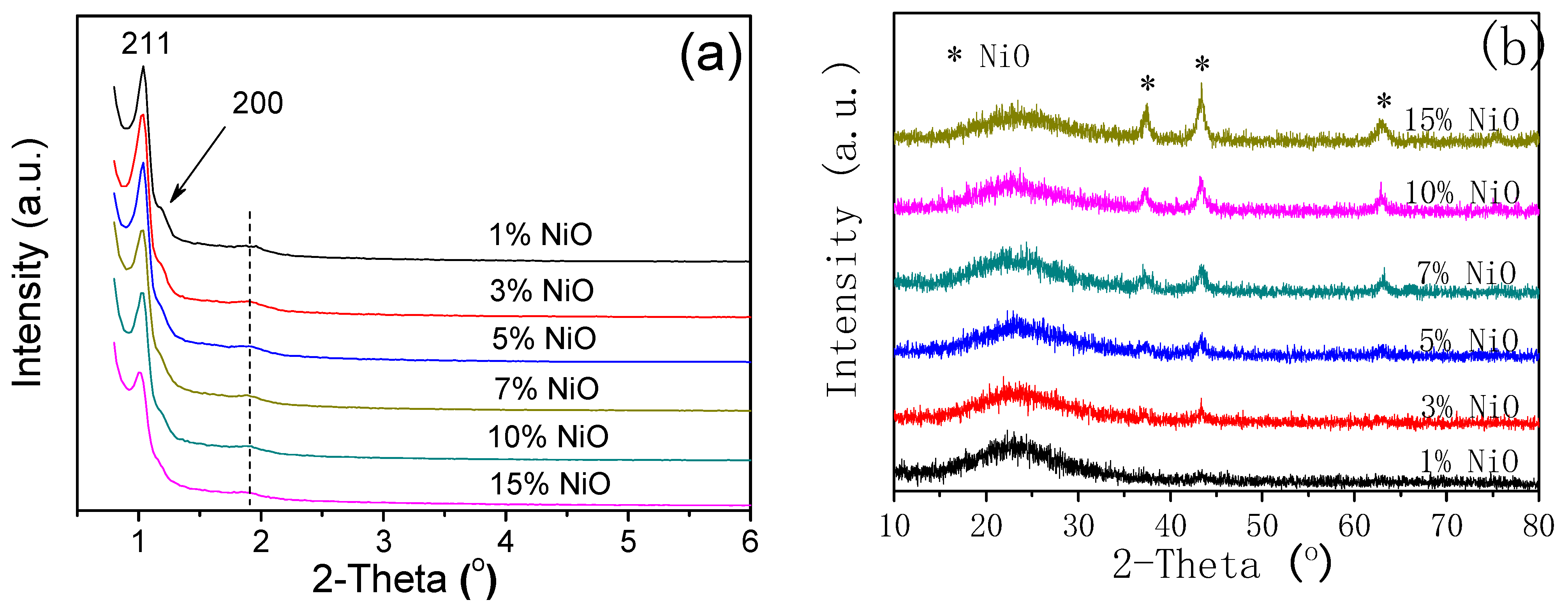
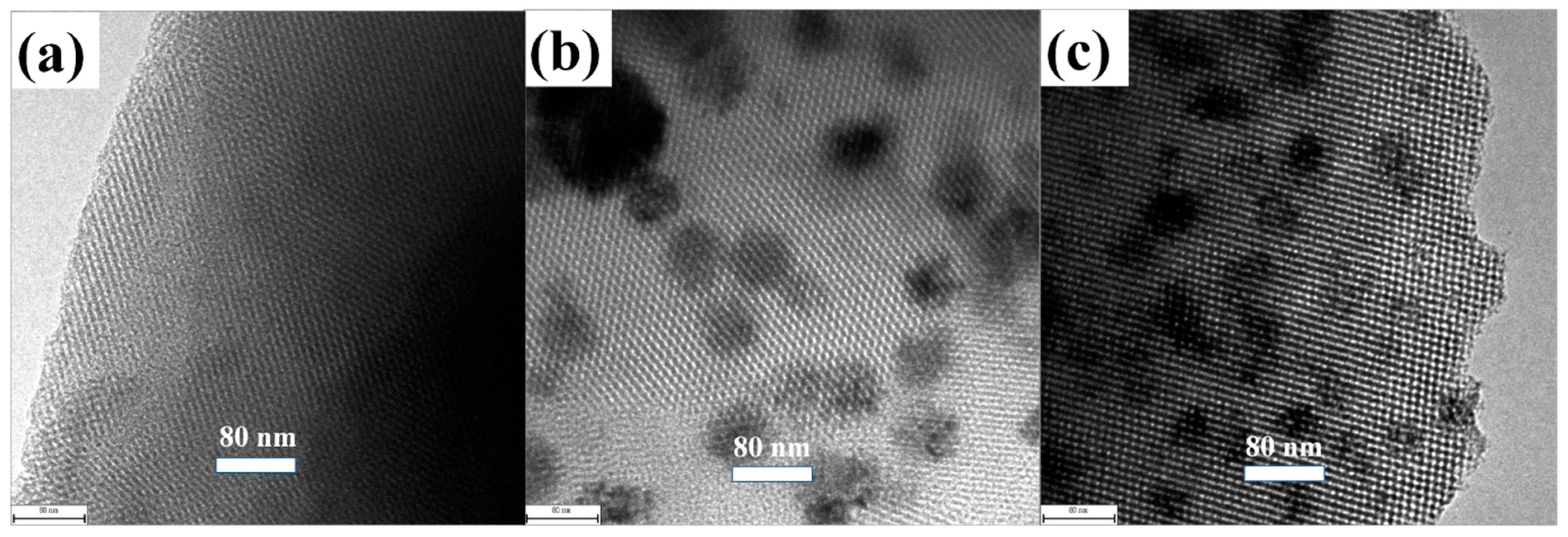
2.2. Characterization
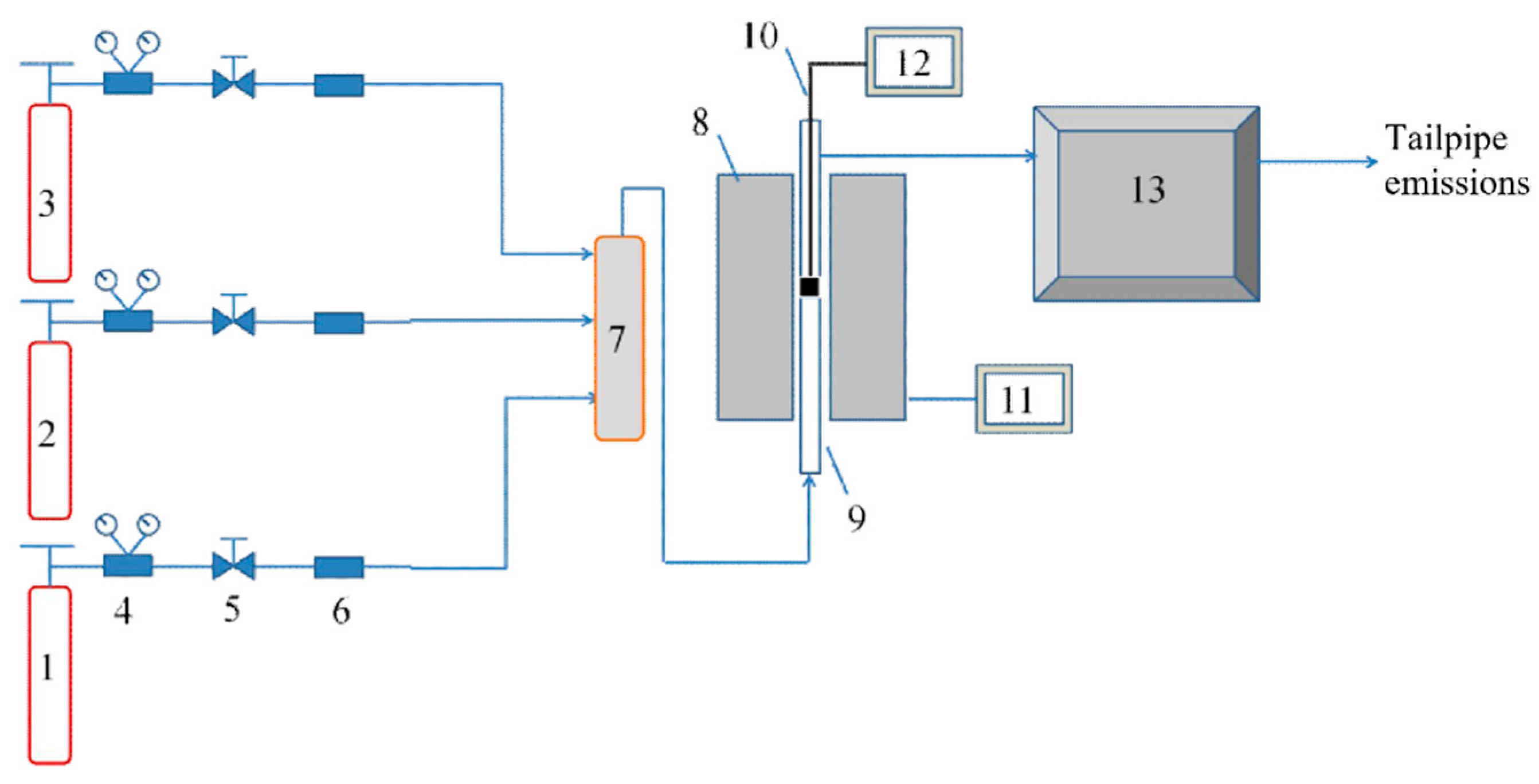
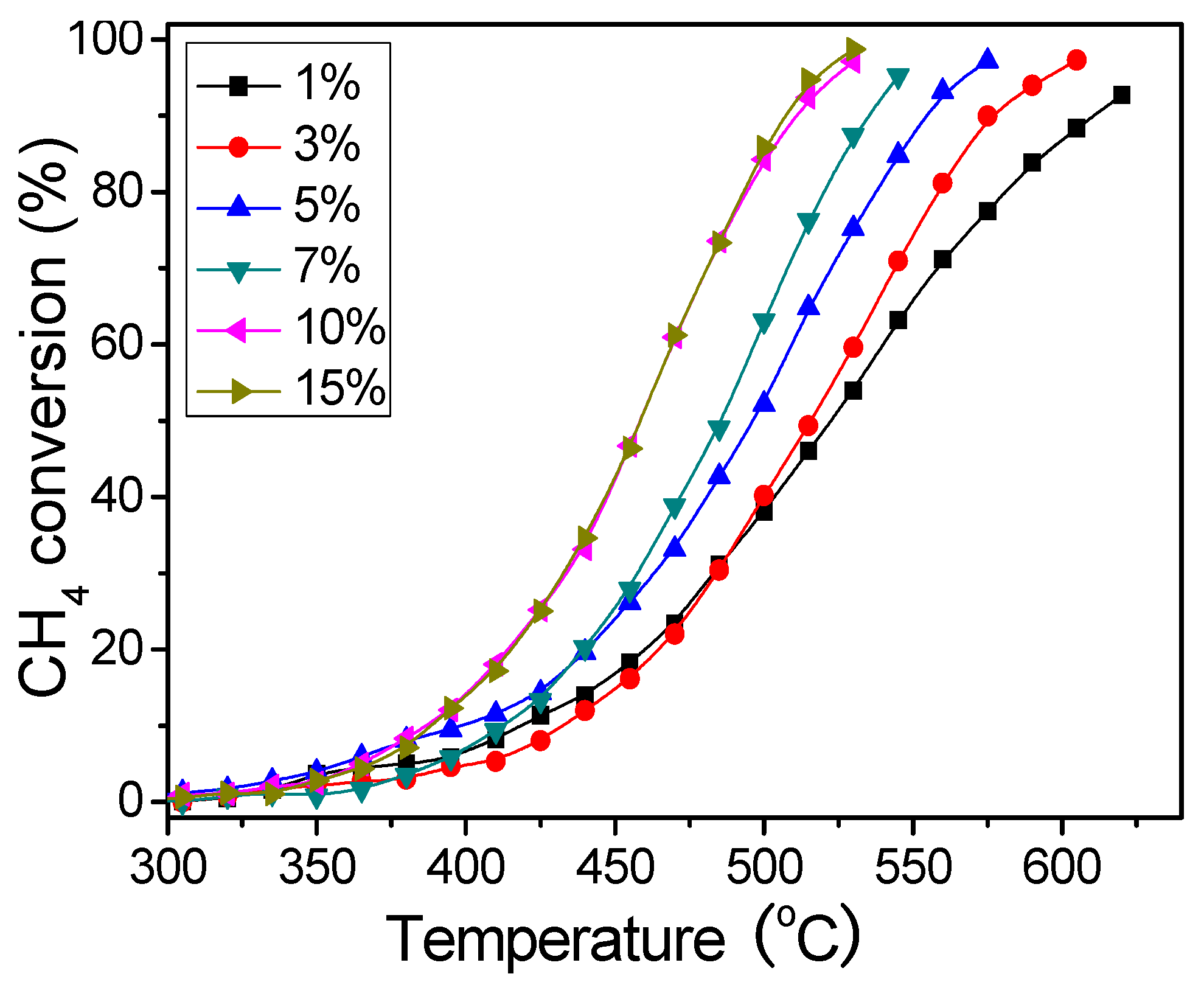
2.3. Tests of Catalytic Activity
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, A.; Austin, D.; Song, H. Investigations of thermochemical upgrading of biomass and its model compounds: Opportunities for methane utilization. Fuel 2019, 246, 443–453. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Guan, N.; Li, L. Methane activation and utilization: Current status and future challenges. Energy Technol. 2020, 8, 1900826. [Google Scholar] [CrossRef]
- He, L.; Fan, Y.; Bellettre, J.; Yue, J.; Luo, L. A review on catalytic methane combustion at low temperatures: Catalysts, mechanisms, reaction conditions and reactor designs. Renew. Sustain. Energy Rev. 2020, 119, 109589. [Google Scholar] [CrossRef]
- Neuberg, S.; Pennemann, H.; Shanmugam, V.; Zapf, R.; Kolb, G. Promoting effect of Rh on the activity and stability of Pt-based methane combustion catalyst in microreactors. Catal. Commun. 2021, 149, 106202. [Google Scholar] [CrossRef]
- Al-Fatesh, A.; Singh, S.K.; Kanade, G.S.; Atia, H.; Fakeeha, A.H.; Ibrahim, A.A.; El-Toni, A.M.; Labhasetwar, N.K. Rh promoted and ZrO2/Al2O3 supported Ni/Co based catalysts: High activity for CO2 reforming, steam–CO2 reforming and oxy–CO2 reforming of CH4. Int. J. Hydrog. Energy 2018, 43, 12069–12080. [Google Scholar] [CrossRef]
- Yang, W.; Kim, M.-Y.; Polo-Garzon, F.; Gong, J.; Jiang, X.; Huang, Z.; Chi, M.; Yu, X.; Wang, X.; Guo, Y. CH4 combustion over a commercial Pd/CeO2-ZrO2 three-way catalyst: Impact of thermal aging and sulfur exposure. Chem. Eng. J. 2023, 451, 138930. [Google Scholar] [CrossRef]
- Rezaei Shadegan, H.; Maghsoodi, S.; Ghanavati, B.; Shahbazi Kootenaei, A.; Azimi, A. Catalytic combustion of methane over La2BCoO6 perovskites containing Ni, Cu and Fe: Impact of B-sites on oxygen species and catalytic activity. React. Kinet. Mech. Catal. 2020, 131, 737–752. [Google Scholar] [CrossRef]
- Huang, F.; Tian, M.; Zhu, Y.; Wang, X.; Wang, A.; Li, L.; Lin, J.; Wang, J. Fe-substituted Ba-hexaaluminate with enhanced oxygen mobility for CO2 capture by chemical looping combustion of methane. J. Energy Chem. 2019, 29, 50–57. [Google Scholar] [CrossRef]
- Zhao, K.; Li, Z.; Bian, L. CO2 methanation and co-methanation of CO and CO2 over Mn-promoted Ni/Al2O3 catalysts. Front. Chem. Sci. Eng. 2016, 10, 273–280. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, S.; Zhang, L.; Chen, Z.; Wang, M.; Wang, S. A facile method to promote LaMnO3 perovskite catalyst for combustion of methane. Catal. Commun. 2017, 97, 88–92. [Google Scholar] [CrossRef]
- Fan, X.; Li, L.; Jing, F.; Li, J.; Chu, W. Effects of preparation methods on CoAlOx/CeO2 catalysts for methane catalytic combustion. Fuel 2018, 225, 588–595. [Google Scholar] [CrossRef]
- Rizamarhaiza, M.; Sufizar, A.; Hamimah Abdul, R.; Mohd Azham, A.; Mas Fawzi Mohd, A.; Hariati, T. Producing the Methane Conversion by Steam Methane Reforming Over SiO2-NiO Catalyst. J. Adv. Res. Fluid Mech. Therm. Sci. 2022, 99, 28–34. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.; Wang, J.; Li, Z.; Xu, J. Catalytic combustion of methane over a highly active and stable NiO/CeO2 catalyst. Front. Chem. Sci. Eng. 2020, 14, 534–545. [Google Scholar] [CrossRef]
- Chai, R.; Zhang, Z.; Chen, P.; Zhao, G.; Liu, Y.; Lu, Y. Ni-foam-structured NiO-MOx-Al2O3 (M = Ce or Mg) nanocomposite catalyst for high throughput catalytic partial oxidation of methane to syngas. Microporous Mesoporous Mater. 2017, 253, 123–128. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Ibrahim, A.A.; AlSharekh, A.M.; Alqahtani, F.S.; Kasim, S.O.; Fakeeha, A.H. Iron catalyst for decomposition of methane: Influence of Al/Si ratio support. Egypt. J. Pet. 2018, 27, 1221–1225. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, S.; Wang, S.; Zhao, Y.; Ma, X. Effect of the addition of Ce and Zr over a flower-like NiO-MgO (111) solid solution for CO2 reforming of methane. J. CO2 Util. 2018, 26, 123–132. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Fakeeha, A.H.; Ibrahim, A.A.; Khan, W.U.; Atia, H.; Eckelt, R.; Seshan, K.; Chowdhury, B. Decomposition of methane over alumina supported Fe and Ni–Fe bimetallic catalyst: Effect of preparation procedure and calcination temperature. J. Saudi Chem. Soc. 2018, 22, 239–247. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Arafat, Y.; Ibrahim, A.A.; Shaikh, H.; Atia, H.; Abasaeed, A.E.; Armbruster, U.; Al-Fatesh, A.S. Highly Selective Syngas/H2 Production via Partial Oxidation of CH4 Using (Ni, Co and Ni–Co)/ZrO2–Al2O3 Catalysts: Influence of Calcination Temperature. Processes 2019, 7, 141. [Google Scholar] [CrossRef]
- Fakeeha, A.; Ibrahim, A.A.; Aljuraywi, H.; Alqahtani, Y.; Alkhodair, A.; Alswaidan, S.; Abasaeed, A.E.; Kasim, S.O.; Mahmud, S.; Al-Fatesh, A.S. Hydrogen Production by Partial Oxidation Reforming of Methane over Ni Catalysts Supported on High and Low Surface Area Alumina and Zirconia. Processes 2020, 8, 499. [Google Scholar] [CrossRef]
- Bukhari, S.; Chin, C.; Setiabudi, H.; Vo, D.-V.N. Tailoring the properties and catalytic activities of Ni/SBA-15 via different TEOS/P123 mass ratios for CO2 reforming of CH4. J. Environ. Chem. Eng. 2017, 5, 3122–3128. [Google Scholar] [CrossRef]
- Lv, Y.; Xin, Z.; Meng, X.; Tao, M.; Bian, Z. Ni based catalyst supported on KIT-6 silica for CO methanation: Confinement effect of three dimensional channel on NiO and Ni particles. Microporous Mesoporous Mater. 2018, 262, 89–97. [Google Scholar] [CrossRef]
- Tao, M.; Meng, X.; Lv, Y.; Bian, Z.; Xin, Z. Effect of impregnation solvent on Ni dispersion and catalytic properties of Ni/SBA-15 for CO methanation reaction. Fuel 2016, 165, 289–297. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Akmal, Z.S. Direct decomposition of methane over SBA-15 supported Ni, Co and Fe based bimetallic catalysts. Appl. Surf. Sci. 2015, 330, 418–430. [Google Scholar] [CrossRef]
- Murthy, P.R.; Zhang, J.-C.; Li, W.-Z. Exceptionally stable sol-immobilization derived Pd/SBA-15 catalysts for methane combustion. Catal. Sci. Technol. 2021, 11, 3609–3618. [Google Scholar] [CrossRef]
- MERKACHE, R. 3D ordered mesoporous Fe-KIT-6 catalysts for methylcyclopentane (MCP). In Proceedings of the International Conference on Materials Science ICMS2018, Sétif, Algeria, 12–14 September 2018. [Google Scholar]
- Tang, C.; Huang, J.; Zhang, D.; Jiang, Q.; Zhou, G. CO2 utilization by dry reforming of CH4 over mesoporous Ni/KIT-6 catalyst. Int. J. Chem. React. Eng. 2021, 19, 1167–1178. [Google Scholar] [CrossRef]
- Mahfouz, R.; Estephane, J.; Gennequin, C.; Tidahy, L.; Aouad, S.; Abi-Aad, E. CO2 reforming of methane over Ni and/or Ru catalysts supported on mesoporous KIT-6: Effect of promotion with Ce. J. Environ. Chem. Eng. 2021, 9, 104662. [Google Scholar] [CrossRef]
- Kleitz, F.; Choi, S.H.; Ryoo, R. Cubic Ia3d large mesoporous silica: Synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Commun. 2003, 9, 2136–2137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Schmidt-Winkel, P.; Lukens, W.W.; Yang, P.; Margolese, D.I.; Lettow, J.S.; Ying, J.Y.; Stucky, G.D. Microemulsion Templating of Siliceous Mesostructured Cellular Foams with Well-Defined Ultralarge Mesopores. Chem. Mater. 2000, 12, 686–696. [Google Scholar] [CrossRef]
- Miao, Y.; Lu, G.; Liu, X.; Guo, Y.; Wang, Y.; Guo, Y. Effects of preparation procedure in sol–gel method on performance of MoO 3 /SiO 2 catalyst for liquid phase epoxidation of propylene with cumene hydroperoxide. J. Mol. Catal. A Chem. 2009, 306, 17–22. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Xue, B. Catalytic combustion of methane over M (Ni, Co, Cu) supported on ceria–magnesia. Fuel Process. Technol. 2009, 90, 652–656. [Google Scholar] [CrossRef]
- Thaicharoensutcharittham, S.; Meeyoo, V.; Kitiyanan, B.; Rangsunvigit, P.; Rirksomboon, T. Catalytic combustion of methane over NiO/Ce0.75Zr0.25O2 catalyst. Catal. Commun. 2009, 10, 673–677. [Google Scholar] [CrossRef]
- Zapata, B.; Valenzuela, M.A.; Palacios, J.; Torres-Garcia, E. Effect of Ca, Ce or K oxide addition on the activity of Ni/SiO2 catalysts for the methane decomposition reaction. Int. J. Hydrog. Energy 2010, 35, 12091–12097. [Google Scholar] [CrossRef]
- Nakamura, N.; Takahashi, R.; Sato, S.; Sodesawa, T.; Yoshida, S. Ni/SiO2 catalyst with hierarchical pore structure prepared by phase separation in sol–gel process. Phys. Chem. Chem. Phys. 2000, 2, 4983–4990. [Google Scholar] [CrossRef]
- Pompeo, F.; Nichio, N.N.; González, M.G.; Montes, M. Characterization of Ni/SiO2 and Ni/Li-SiO2 catalysts for methane dry reforming. Catal. Today 2005, 107–108, 856–862. [Google Scholar] [CrossRef]
- Zenboury, L.; Azambre, B.; Weber, J.V. Transient TPSR, DRIFTS-MS and TGA studies of a Pd/ceria-zirconia catalyst in CH4 and NO 2 atmospheres. Catal. Today 2008, 137, 167–173. [Google Scholar] [CrossRef]
- Takahashi, R.; Sato, S.; Tomiyama, S.; Ohashi, T.; Nakamura, N. Pore structure control in Ni/SiO2 catalysts with both macropores and mesopores. Microporous Mesoporous Mater. 2007, 98, 107–114. [Google Scholar] [CrossRef]
- Guevara, J.C.; Wang, J.A.; Chen, L.F.; Valenzuela, M.A.; Salas, P.; García-Ruiz, A.; Toledo, J.A.; Cortes-Jácome, M.A.; Angeles-Chavez, C.; Novaro, O. Ni/Ce-MCM-41 mesostructured catalysts for simultaneous production of hydrogen and nanocarbon methane decomposition. Int. J. Hydrog. Energy 2010, 35, 3509–3521. [Google Scholar] [CrossRef]
- Takenaka, S.; Kobayashi, S.; Ogihara, H.; Otsuka, K. Ni/SiO 2 catalyst effective for methane decomposition into hydrogen and carbon nanofiber. J. Catal. 2003, 217, 79–87. [Google Scholar]
- Zhu, J.; Peng, X.; Yao, L.; Shen, J.; Tong, D.; Hu, C. The promoting effect of La, Mg, Co and Zn on the activity and stability of Ni/SiO2 catalyst for CO2 reforming of methane. Int. J. Hydrog. Energy 2011, 36, 7094–7104. [Google Scholar] [CrossRef]
- Soni, K.; Rana, B.S.; Sinha, A.K.; Bhaumik, A.; Nandi, M.; Kumar, M.; Dhar, G.M. 3-D ordered mesoporous KIT-6 support for effective hydrodesulfurization catalysts. Appl. Catal. B Environ. 2009, 90, 55–63. [Google Scholar] [CrossRef]

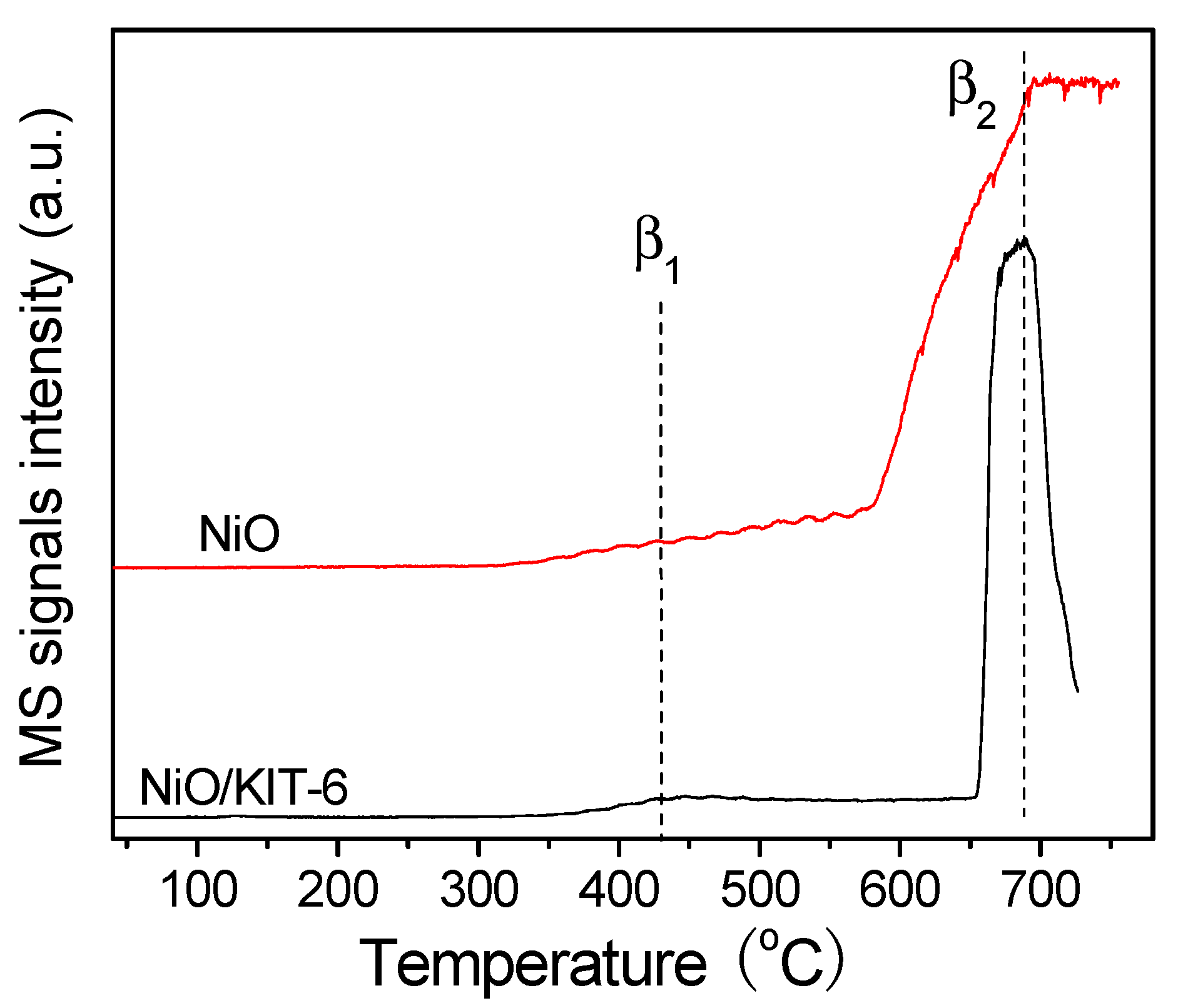
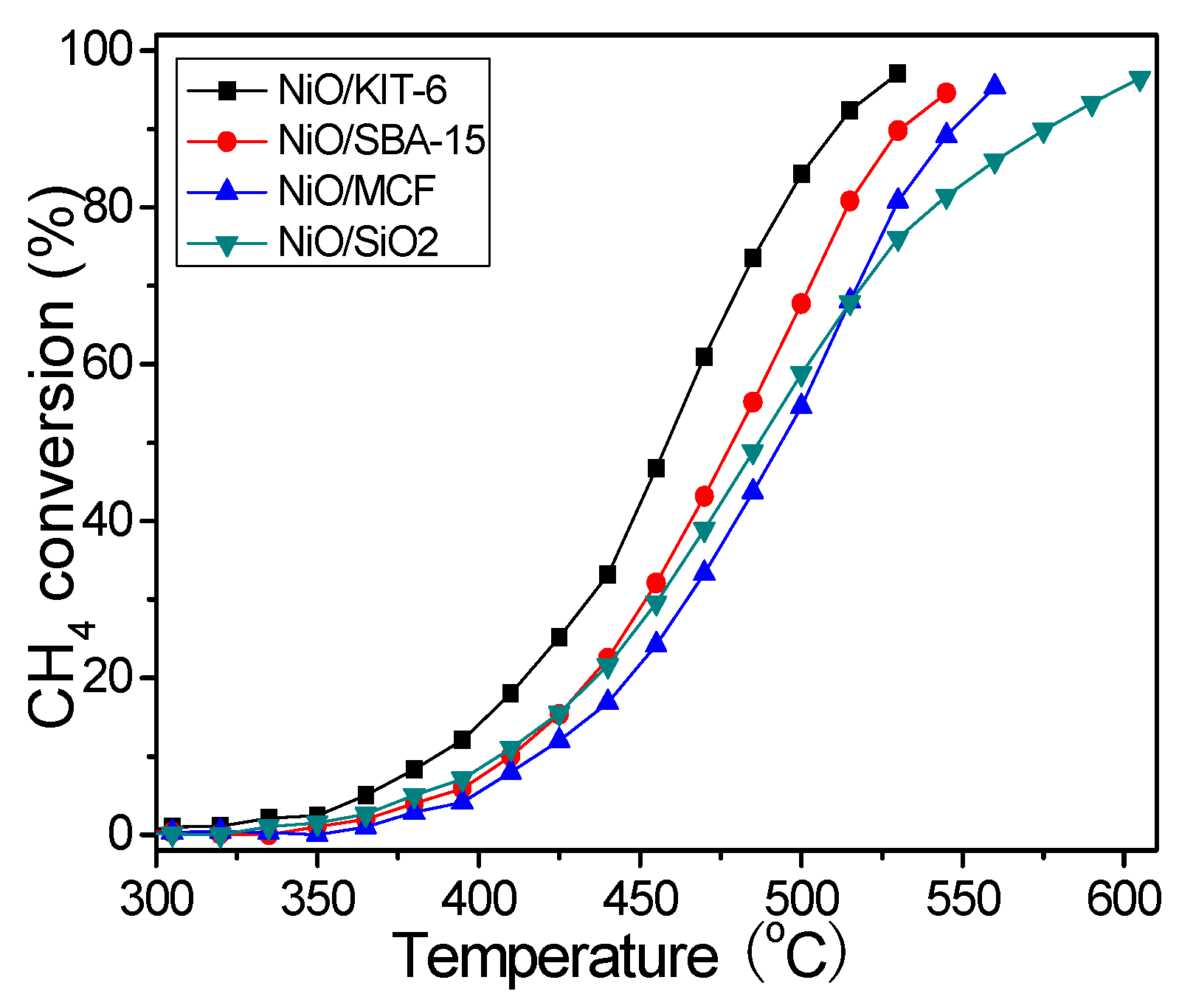
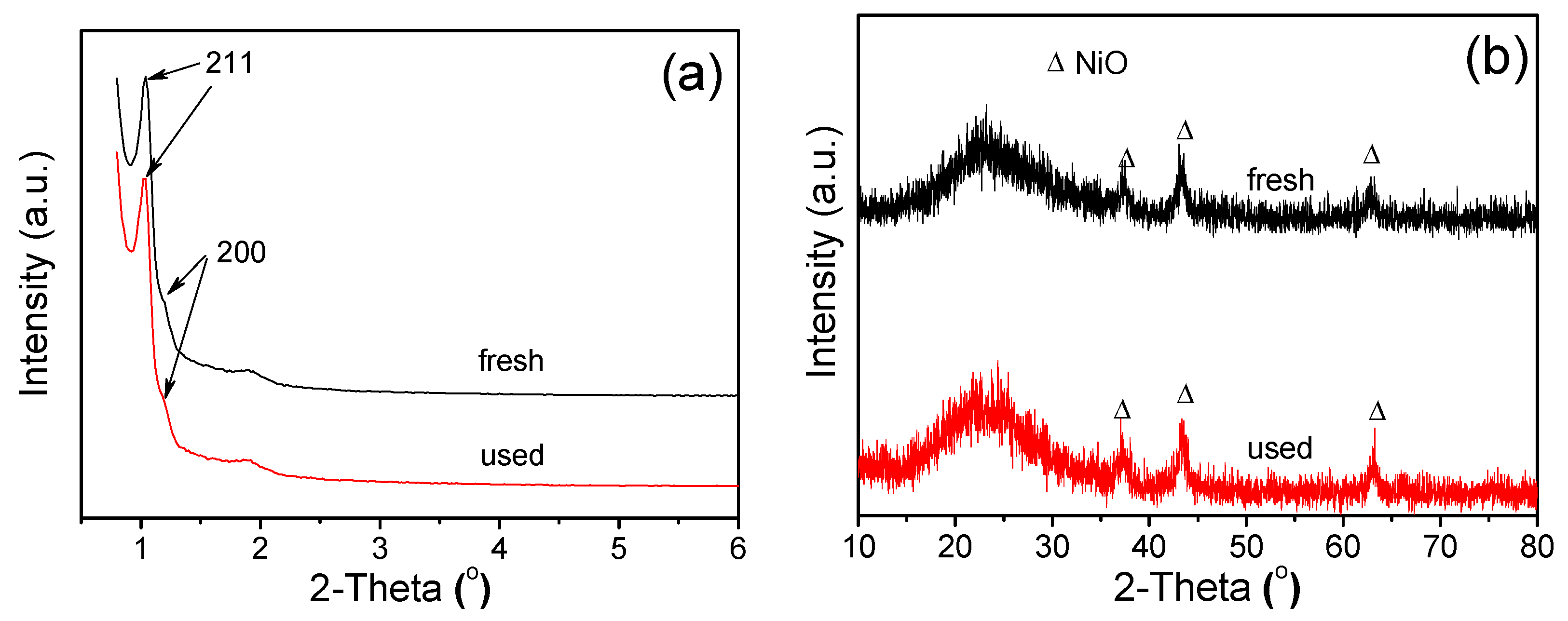

| Samples | NiO Crystallite | BET Surface Area (m2·g−1) | Average Pore Diameter (nm) | Pore Volume (cm3·g−1) | Catalytic Activity in CH4 Combustion | ||
|---|---|---|---|---|---|---|---|
| Size (nm) a | T10 (°C) | T50 (°C) | T90 (°C) | ||||
| KIT-6 | – | 708 | 8.2 | 0.94 | – | – | – |
| 1 wt% NiO/ KIT-6 | 7.63 | 671 | 8.1 | 0.91 | 420 | 523 | 611 |
| 3 wt% NiO/ KIT-6 | 7.39 | 638 | 7.9 | 0.87 | 432 | 515 | 577 |
| 5 wt% NiO/ KIT-6 | 7.60 | 619 | 7.8 | 0.83 | 396 | 496 | 554 |
| 7 wt% NiO/ KIT-6 | 7.41 | 546 | 7.6 | 0.72 | 411 | 485 | 535 |
| 10 wt% NiO/KIT-6 | 7.65 | 468 | 7.5 | 0.60 | 386 | 456 | 507 |
| 15 wt% NiO/KIT-6 | 8.07 | 407 | 7.5 | 0.51 | 386 | 457 | 510 |
| 10 wt% NiO/KIT-6 (used) b | 8.56 | 357 | 7.5 | 0.48 | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Yang, W.; Li, Z.; Lou, Q.; Tian, Y.; Li, J. Nickel Oxide Nanoparticles on KIT-6: An Efficient Catalyst in Methane Combustion. Processes 2023, 11, 1004. https://doi.org/10.3390/pr11041004
Huang X, Yang W, Li Z, Lou Q, Tian Y, Li J. Nickel Oxide Nanoparticles on KIT-6: An Efficient Catalyst in Methane Combustion. Processes. 2023; 11(4):1004. https://doi.org/10.3390/pr11041004
Chicago/Turabian StyleHuang, Xiuhui, Wenkai Yang, Zeqiu Li, Qin Lou, Ying Tian, and Junfeng Li. 2023. "Nickel Oxide Nanoparticles on KIT-6: An Efficient Catalyst in Methane Combustion" Processes 11, no. 4: 1004. https://doi.org/10.3390/pr11041004
APA StyleHuang, X., Yang, W., Li, Z., Lou, Q., Tian, Y., & Li, J. (2023). Nickel Oxide Nanoparticles on KIT-6: An Efficient Catalyst in Methane Combustion. Processes, 11(4), 1004. https://doi.org/10.3390/pr11041004









