Effects of Drinking Electrolyzed Alkaline-Reduced Water on Functional Dyspepsia: A Randomized, Double-Blind, Controlled Prospective Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design
2.3. Study Participants
2.4. Research Equipment
2.5. Interventions
2.6. Outcome Measurements
2.6.1. Primary Outcome Measures
GSRS Score
FD-QoL
NDI-K
2.6.2. Secondary Outcome Measures
Measurement of Inflammatory Cytokines Activities
Blood Sample Analysis for Complete Blood Count (CBC)
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants
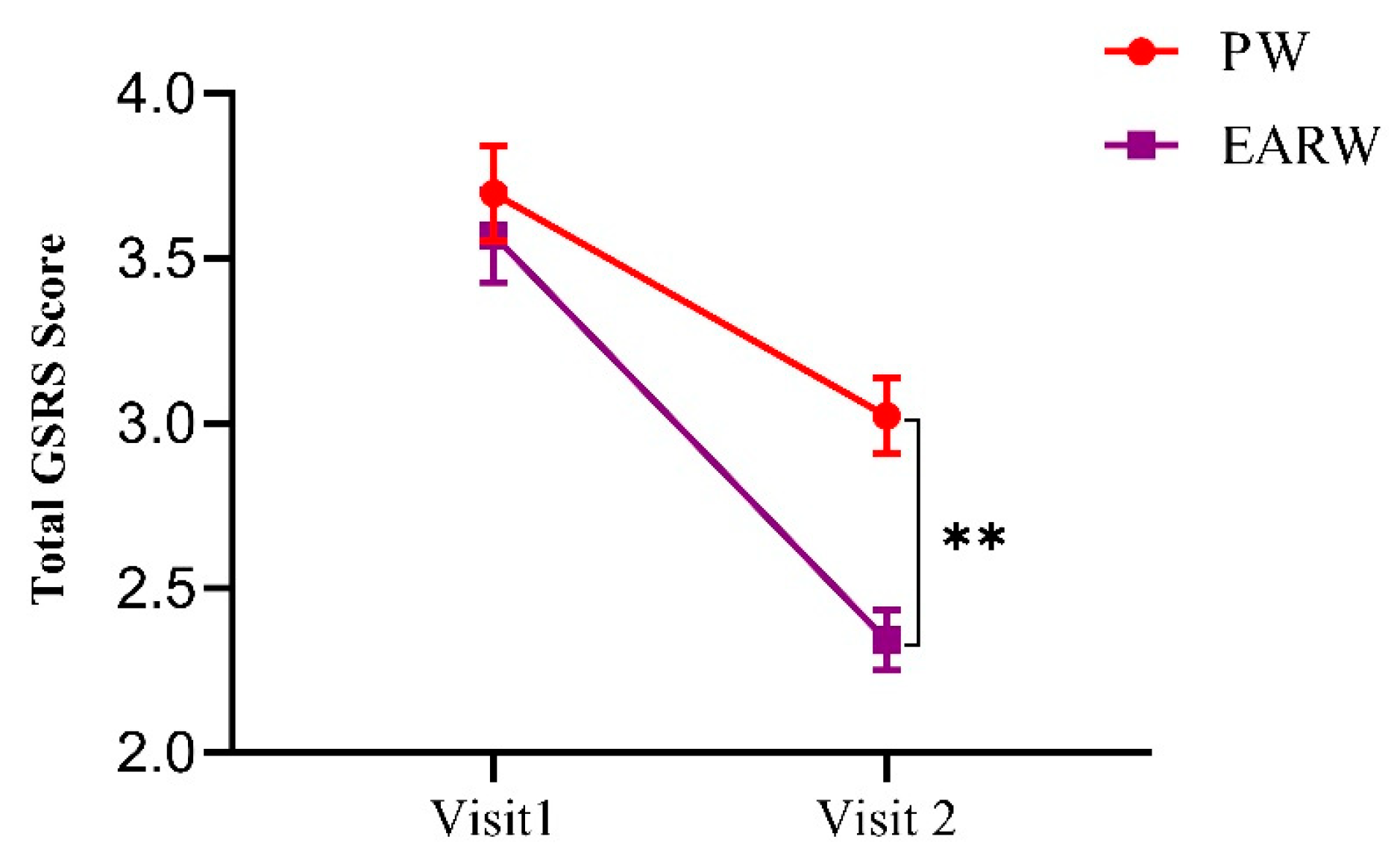
3.2. Effects of Drinking EARW Evaluated by GSRS Score in FD Patients
3.3. Effects of Drinking EARW Assessed by FD-QoL in FD Patients
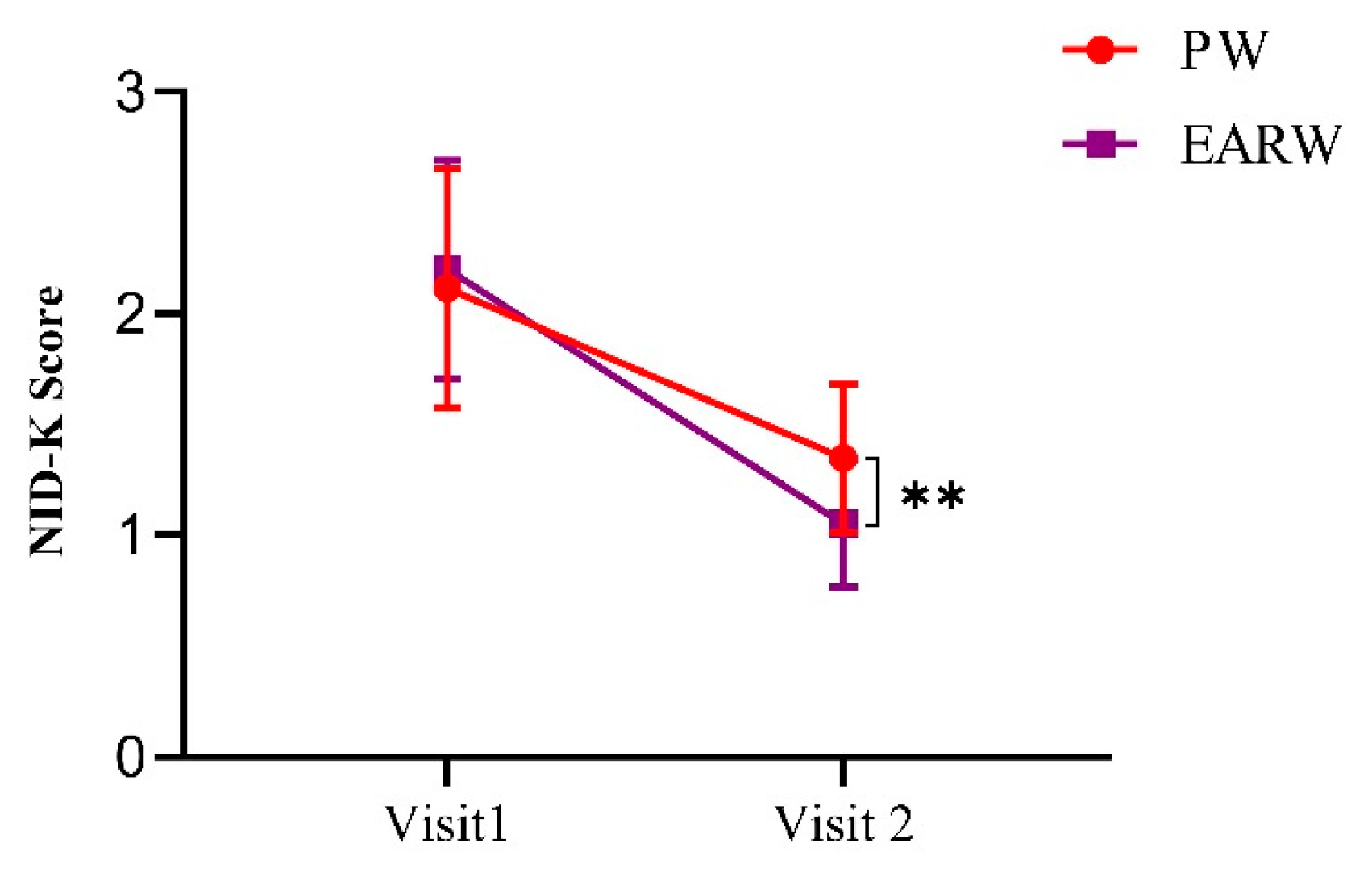
3.4. Drinking Effects of EARW Assessed by NDI-K in FD Patients
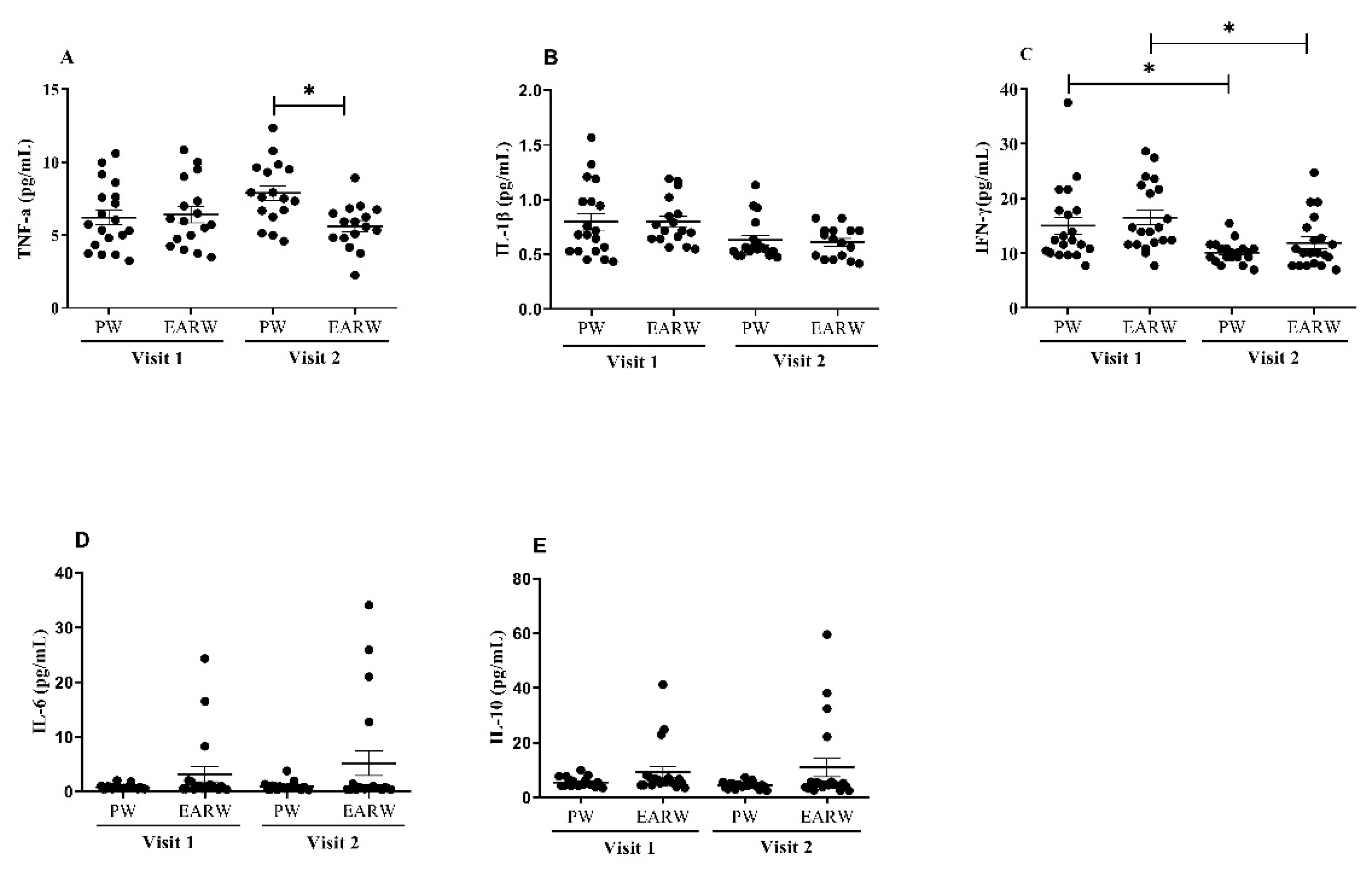
3.5. Effects of Drinking EARW on Levels of Inflammatory Cytokines in FD Patients
3.6. Total CBC Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zacharakis, G.; Al-Ghamdi, S.; Alzahrani, J.; Almasoud, A.; Arahmane, O.; Alshehri, A.; Alharbi, M.H.; Alsalmi, M.M.; Alotibi, S.B.; Algaradi, Y.A.; et al. Effects of the Rome IV criteria to functional dyspepsia symptoms in Saudi Arabia: Epidemiology and clinical practice. Korean J. Gastroenterol. 2020, 76, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.M. Overlap between postprandial distress and epigastric pain syndromes in functional dyspepsia: Its implications for research and clinical practice. Am. J. Gastroenterol. 2013, 108, 74. [Google Scholar] [CrossRef]
- Kim, S.E.; Kim, N.; Lee, J.Y.; Park, K.S.; Shin, J.E.; Nam, K.; Kim, H.J.; Song, H.J.; Joo, Y.E.; Myung, D.S.; et al. Prevalence and risk factors of functional dyspepsia in health check-up population: A nationwide multicenter prospective study. J. Neurogastroenterol. Motil. 2018, 24, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Singh, R.; Chang, F.Y.; Hou, X.; Wong, B.C.; Kachintorn, U.; Functional Dyspepsia Consensus Team of the Asian Neurogastroenterology and Motility Association and the Asian Pacific Association of Gastroenterology. Epidemiology of uninvestigated and functional dyspepsia in Asia: Facts and fiction. J. Neurogastroenterol. Motil. 2011, 17, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Locke, G.R.; Zinsmeister, A.R.; Fett, S.L.; Melton, L.J.; Talley, N.J. Overlap of gastrointestinal symptom complexes in a US community. Neurogastroenterol. Motil. 2005, 17, 29–34. [Google Scholar] [CrossRef]
- Kim, S.E.; Park, H.K.; Kim, N.; Joo, Y.E.; Baik, G.H.; Shin, J.E.; Seo, G.S.; Kim, G.H.; Kim, H.U.; Kim, H.Y.; et al. Prevalence and risk factors of functional dyspepsia: A nationwide multicenter prospective study in Korea. J. Clin. Gastroenterol. 2014, 48, e12–e18. [Google Scholar] [CrossRef]
- Lacy, B.E.; Weiser, K.T.; Kennedy, A.T.; Crowell, M.D.; Talley, N.J. Functional dyspepsia: The economic impact to patients. Aliment. Pharmacol. Ther. 2013, 38, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Hantoro, I.F.; Syam, A.F.; Mudjaddid, E.; Setiati, S.; Abdullah, M. Factors associated with health-related quality of life in patients with functional dyspepsia. Health Qual. Life Outcomes. 2018, 16, 83. [Google Scholar] [CrossRef]
- Wang, W.H.; Huang, J.Q.; Zheng, G.F.; Xia, H.H.; Wong, W.M.; Liu, X.G.; Karlberg, J.; Wong, B.C. Effects of proton-pump inhibitors on functional dyspepsia: A meta-analysis of randomized placebo-controlled trials. Clin. Gastroenterol. Hepatol. 2007, 5, 178–185. [Google Scholar] [CrossRef]
- Talley, N.J.; Locke, G.R.; Saito, Y.A.; Almazar, A.E.; Bouras, E.P.; Howden, C.W.; Lacy, B.E.; Dibaise, J.K.; Prather, C.M.; Abraham, B.P.; et al. Effect of amitriptyline and escitalopram on functional dyspepsia: A multicenter, randomized controlled study. Gastroenterology 2015, 149, 340–349. [Google Scholar] [CrossRef]
- Harper, A.; Naghibi, M.M.; Garcha, D. The role of bacteria, probiotics and diet in irritable bowel syndrome. Foods 2018, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Koufman, J.A.; Johnston, N. Potential benefits of pH 8.8 alkaline drinking water as an adjunct in the treatment of reflux disease. Ann. Otol. Rhinol. Laryngol. 2012, 121, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, M.; Olivieri, F.; Manghetti, M.; Boccolini, E.; Bellomini, M.G.; Blandizzi, C.; Bonino, F.; Del Tacca, M. Effects of a bicarbonate-alkaline mineral water on gastric functions and functional dyspepsia: A preclinical and clinical study. Pharmacol. Res. 2002, 46, 525–531. [Google Scholar] [CrossRef]
- Shirahata, S.; Hamasaki, T.; Teruya, K. Advanced research on the health benefit of reduced water. Trends Food Sci. Technol. 2012, 23, 124–131. [Google Scholar] [CrossRef]
- Bajgai, J.; Kim, C.-S.; Rahman, M.H.; Jeong, E.-S.; Jang, H.-Y.; Kim, K.-E.; Choi, J.; Cho, I.-Y.; Lee, K.-J.; Lee, M. Effects of alkaline-reduced water on gastrointestinal diseases. Processes 2022, 10, 87. [Google Scholar] [CrossRef]
- Ignacio, R.M.C.; Joo, K.-B.; Lee, K.-J. Clinical effect and mechanism of alkaline reduced water. J. Food Drug Anal. 2012, 20, 394–397. [Google Scholar] [CrossRef]
- Sharma, S.; Lee, K.-J.; Bajgai, J.; Trinh, T.T.; Antonio, J.M.; Rahman, M.H.; Vira, K.; Sofian, A.-N.; Cho, S.H.; Kim, C.-S.; et al. Anti-oxidative and anti-diabetic effects of electrolyzed weakly alkaline reduced water on renal proximal tubular epithelial cells. Processes 2022, 10, 2025. [Google Scholar] [CrossRef]
- Jin, D.; Kim, D.H.; Teng, Y.C.; Xufeng, Q.; Lee, K.J. The effect of mineral-induced alkaline reduced water on the DSS-induced acute inflammatory bowel disease mouse model. Appl. Microsc. 2008, 38, 81–87. [Google Scholar]
- Nassini, R.; Andrè, E.; Gazzieri, D.; De Siena, G.; Zanasi, A.; Geppetti, P.; Materazzi, S. A bicarbonate-alkaline mineral water protects from ethanol-induced hemorrhagic gastric lesions in mice. Biol. Pharm. Bull. 2010, 33, 1319–1323. [Google Scholar] [CrossRef]
- Tanaka, Y.; Saihara, Y.; Izumotani, K.; Nakamura, H. Daily ingestion of alkaline electrolyzed water containing hydrogen influences human health, including gastrointestinal symptoms. Med. Gas Res. 2018, 8, 160–166. [Google Scholar] [CrossRef]
- Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects; The World Medical Association, Inc.: Ferney-Voltaire, France, 2008; Available online: https://www.wma.net/wp-content/uploads/2018/07/DoH-Oct2008.pdf (accessed on 2 January 2023).
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A.G. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Revicki, D.A.; Wood, M.; Wiklund, I.; Crawley, J. Reliability and validity of the gastrointestinal Symptom Rating Scale in patients with gastroesophageal reflux disease. Qual. Life Res. 1998, 7, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Matsumoto, T.; Miwa, H. Analysis of the gastrointestinal symptoms of uninvestigated dyspepsia and irritable bowel syndrome. Gut Liver 2009, 3, 192–196. [Google Scholar] [CrossRef]
- Lee, E.H.; Hahm, K.B.; Lee, J.H.; Park, J.J.; Lee, D.H.; Kim, S.K.; Choi, S.R.; Lee, S.T. Development and validation of a Functional Dyspepsia-Related Quality of Life (FD-QOL) scale in South Korea. J. Gastroenterol. Hepatol. 2006, 21, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J.; Haque, M.; Wyeth, J.W.; Stace, N.H.; Tytgat, G.N.; Stanghellini, V.; Holtmann, G.; Verlinden, M.; Jones, M. Development of a new dyspepsia impact scale: The Nepean Dyspepsia Index. Aliment. Pharmacol. Ther. 1999, 13, 225–235. [Google Scholar] [CrossRef]
- Cho, Y.K.; Choi, M.G.; Kim, S.; Lee, I.; Kim, S.; Jung, I.; Lee, S.; Choi, S.; Seol, S. Effects of Mosaprid on the quality of life of functional dyspepsia. Korean J. Gastroenterol. 2004, 43, 160–167. [Google Scholar] [PubMed]
- Lee, M.; Fadriquela, A.; Antonio, J.M.; Kim, C.-S.; Cho, I.-Y.; Kim, K.-E.; An, W.-S.; Jang, H.-Y.; Bajgai, J.; Lee, K.-J. Effects of alkaline-reduced water on exercise-induced oxidative stress and fatigue in young male healthy adults. Processes 2022, 10, 1543. [Google Scholar] [CrossRef]
- Lacy, B.E.; Talley, N.J.; Locke III, G.R.; Bouras, E.P.; Dibaise, J.K.; El-Serag, H.B.; Abraham, B.P.; Howden, C.W.; Moayyedi, P.; Prather, C. Review article: Current treatment options and management of functional dyspepsia. Aliment. Pharmacol. Ther. 2012, 36, 3–15. [Google Scholar] [CrossRef]
- Brook, R.A.; Kleinman, N.L.; Choung, R.S.; Melkonian, A.K.; Smeeding, J.E.; Talley, N.J. Functional dyspepsia impacts absenteeism and direct and indirect costs. Clin. Gastroenterol. Hepatol. 2010, 8, 498–503. [Google Scholar] [CrossRef]
- Ha, N.Y.; Kim, S.; Ko, S.J.; Park, J.W.; Kim, J. A clinical study on safety and efficacy of Naesohwajung-tang on functional dyspepsia. Medicine 2020, 99, e19910. [Google Scholar] [CrossRef]
- Manabe, N.; Wong, B.S.; Camilleri, M. New-generation 5-HT4 receptor agonists: Potential for treatment of gastrointestinal motility disorders. Expert Opin. Investig. Drugs 2010, 19, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Chaves, J.R.; de Souza, C.R.T.; Modesto, A.A.C.; Moreira, F.C.; Teixeira, E.B.; Sarraf, J.S.; Allen, T.S.R.; Ara, T.M.T.; Khayat, A.S. Effects of alkaline water intake on gastritis and miRNA expression (mir-7, mir-155, mir-135b and mir-29c). Am. J. Transl. Res. 2020, 12, 4043–4050. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Shang, G.; Tanaka, Y.; Saihara, Y.; Hou, L.; Velasquez, N.; Liu, W.; Lu, Y. Dose-dependent inhibition of gastric injury by hydrogen in alkaline electrolyzed drinking water. BMC Complement. Altern. Med. 2014, 14, 81. [Google Scholar] [CrossRef]
- Tashiro, H.; Kitahora, T.; Fujiyama, Y.; Banba, T. Clinical evaluation of alkali-ionized water for chronic diarrhea placebo controlled double-blind study. Dig. Absorpt. 2000, 23, 52–56. [Google Scholar]
- Shin, D.W.; Yoon, H.; Kim, H.S.; Choi, Y.J.; Shin, C.M.; Park, Y.S.; Kim, N.; Lee, D.H. Effects of alkaline-reduced drinking water on irritable bowel syndrome with diarrhea: A randomized double-blind, placebo-controlled pilot study. Evid. Based Complement. Alternat. Med. 2018, 2018, 9147914. [Google Scholar] [CrossRef]
- Vorobjeva, N.V. Selective stimulation of the growth of anaerobic microflora in the human intestinal tract by electrolyzed reducing water. Med. Hypotheses 2005, 64, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kiuchi, M.; Higashimura, Y.; Naito, Y.; Koyama, K. The effects of ingestion of hydrogen-dissolved alkaline electrolyzed water on stool consistency and gut microbiota: A double-blind randomized trial. Med. Gas. Res. 2021, 11, 138–144. [Google Scholar] [CrossRef]
- Atreya, I.; Atreya, R.; Neurath, M.F. Nf-kappab in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef]
- Fiorucci, S.; Santucci, L.; Federici, B.; Antonelli, E.; Distrutti, E.; Morelli, O.; Renzo, G.D.; Coata, G.; Cirino, G.; Soldato, P.D. Nitric oxide-releasing NSAIDs inhibit interleukin-1beta converting enzyme-like cysteine proteases and protect endothelial cells from apoptosis induced by TNFalpha. Aliment. Pharmacol. Ther. 1999, 13, 421–435. [Google Scholar] [CrossRef]


| Inclusion Criteria |
|
| Exclusion Criteria |
|
| Variable | PW Group | EARW Group | p-Value |
|---|---|---|---|
| Sex | |||
| Total | 20 | 20 | - |
| Male | 8 | 8 | - |
| Female | 12 | 12 | - |
| Age (years) | |||
| Total | 48.25 ± 10.74 | 50.50 ± 11.92 | 0.987 |
| Male | 49.00 ± 8.49 | 42.38 ± 10.41 | 0.831 |
| Female | 47.75 ± 12.36 | 55.92 ± 9.82 | 0.457 |
| Height (cm) | |||
| Total | 159.89 ± 9.30 | 159.63 ± 10.00 | >0.999 |
| Male | 169.33 ± 6.16 | 164.99 ± 9.83 | 0.915 |
| Female | 153.60 ± 4.17 | 156.05 ± 8.74 | 0.982 |
| Weight (kg) | |||
| Total | 63.93 ± 14.04 | 65.25 ± 11.20 | >0.999 |
| Male | 74.14 ± 14.52 | 69.15 ± 7.99 | 0.961 |
| Female | 57.12 ± 8.95 | 62.54 ±12.55 | 0.868 |
| Blood pressure (mmHg) | |||
| Systolic | 126.00 ± 9.67 | 134.05 ± 10.57 | 0.217 |
| Male | 129.12 ± 10.53 | 130.88 ± 8.22 | >0.999 |
| Female | 123.50 ± 8.65 | 136.17 ± 11.74 | 0.062 |
| Diastolic | 86.78 ± 11.10 | 85.85 ± 9.21 | 0.843 |
| Male | 92.50 ± 10.11 | 87.25 ± 11.46 | 0.996 |
| Female | 82.02 ± 10.04 | 84.92 ± 7.78 | 0.385 |
| PW Group (n = 20) Visit 1 vs. Visit 2 | EARW Group (n = 20) Visit 1 vs. Visit 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | PW (V1) | PW (V2) | P.D. (%) | p-value | EARW (V1) | EARW (V2) | P.D. (%) | p-value |
| Total GSRS score | 3.69 ± 0.54 | 3.02 ± 0.42 | 18.16 | ** p < 0.01 | 3.56 ± 0.53 | 2.34 ± 0.34 | 34.27 | *** p < 0.001 |
| Abdominal pain score | 3.75 ± 1.41 | 2.95 ± 1.31 | 21.33 | *** p < 0.001 | 3.90 ± 1.86 | 2.20 ± 1.28 | 43.59 | *** p < 0.001 |
| Reflux syndrome score | 3.70 ± 1.75 | 3.00 ± 1.41 | 18.92 | *** p < 0.001 | 2.95 ± 1.43 | 1.80 ± 0.89 | 38.98 | *** p < 0.001 |
| Diarrhea score | 3.35 ± 1.76 | 2.65 ± 1.53 | 20.90 | ** p < 0.01 | 2.95 ± 1.54 | 2.20 ± 1.19 | 25.42 | *** p < 0.001 |
| Indigestion score | 4.33 ± 1.3 | 3.40 ± 1.72 | 21.48 | *** p < 0.001 | 4.60 ± 1.5 | 2.95 ± 1.43 | 35.87 | *** p < 0.001 |
| Constipation score | 2.80 ± 2.01 | 2.50 ± 1.95 | 10.71 | n.s. | 3.20 ± 1.96 | 2.15 ± 1.18 | 32.81 | *** p < 0.001 |
| Parameter | PW Group | EARW Group | Normal Range | ||
|---|---|---|---|---|---|
| (Visit 1) | (Visit 2) | (Visit 1) | (Visit 2) | ||
| Hemoglobin (g/dL) | 14.09 ± 0.34 | 13.75 ± 0.35 | 14.29 ± 0.31 | 13.81 ± 0.28 | 12–17.5 |
| Hematocrit (%) | 43.52 ± 0.88 | 42.79 ± 0.922 | 44.11 ± 0.88 | 43.39 ± 0.78 | 36–54 |
| RBC (106/µL) | 4.63 ± 0.09 | 4.56 ± 0.09 | 4.62 ± 0.10 | 4.51 ± 0.09 | 4–6.5 |
| WBC (103/µL) | 6.55 ± 0.33 | 5.55 ± 0.48 | 6.17 ± 0.26 | 4.69 ± 0.35 | 4–10 |
| Platelet (106/µL) | 269 ± 21.89 | 238.7 ± 19.25 | 255.1 ± 10.55 | 223.5 ± 10.57 | 150–450 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajgai, J.; Lee, M.; Jang, Y.-G.; Lee, K.; Sharma, S.; Jeong, Y.J.; Park, H.J.; Goh, S.H.; Kim, C.-S.; Kim, H.I.; et al. Effects of Drinking Electrolyzed Alkaline-Reduced Water on Functional Dyspepsia: A Randomized, Double-Blind, Controlled Prospective Trial. Processes 2023, 11, 968. https://doi.org/10.3390/pr11030968
Bajgai J, Lee M, Jang Y-G, Lee K, Sharma S, Jeong YJ, Park HJ, Goh SH, Kim C-S, Kim HI, et al. Effects of Drinking Electrolyzed Alkaline-Reduced Water on Functional Dyspepsia: A Randomized, Double-Blind, Controlled Prospective Trial. Processes. 2023; 11(3):968. https://doi.org/10.3390/pr11030968
Chicago/Turabian StyleBajgai, Johny, Mihyun Lee, Yeon-Gyu Jang, Kiwon Lee, Subham Sharma, Yun Ju Jeong, Hong Jun Park, Seong Hoon Goh, Cheol-Su Kim, Hyun Il Kim, and et al. 2023. "Effects of Drinking Electrolyzed Alkaline-Reduced Water on Functional Dyspepsia: A Randomized, Double-Blind, Controlled Prospective Trial" Processes 11, no. 3: 968. https://doi.org/10.3390/pr11030968
APA StyleBajgai, J., Lee, M., Jang, Y.-G., Lee, K., Sharma, S., Jeong, Y. J., Park, H. J., Goh, S. H., Kim, C.-S., Kim, H. I., & Lee, K.-J. (2023). Effects of Drinking Electrolyzed Alkaline-Reduced Water on Functional Dyspepsia: A Randomized, Double-Blind, Controlled Prospective Trial. Processes, 11(3), 968. https://doi.org/10.3390/pr11030968









