Abstract
Probiotic strains such as Lactobacillus spp. are already known for their beneficial effect on human health and new research supports their role in colon cancer prevention and treatment. The current study reports the effect of different concentrations of Lacticaseibacillus rhamnosus (LGG, 106–109 CFU/mL), alone or in association with 5-fluorouracil (5-FU, 10 μM), tested against normal HaCaT cells, HT-29 colorectal adenocarcinoma and HCT-116 colorectal carcinoma cell lines. The underlying cytotoxic effect was further investigated. LGG treatment of HT-29 and HCT-116 cells caused a variety of apoptotic-related nuclear morphological changes, as revealed by DAPI staining. ELISA studies showed that LGG treatment increased caspase-3 activity and pro-apoptotic BAX protein levels while decreasing anti-apoptotic Bcl-2 protein levels and the proto-oncogene Cyclin D1. A more detailed examination of the mitochondrial function revealed that high concentrations of LGG can impair mitochondrial function in HT-29 and HCT-116 cancer cells. All of these findings suggest that LGG has a pro-apoptotic, mitochondrial-targeted, cytotoxic effect on both colon cancer cell lines studied.
1. Introduction
The International Scientific Association for Probiotics and Prebiotics (ISAPP) defines probiotics as live bacteria that, when given in sufficient amounts, can benefit human health. Probiotics have been shown to have both local and systemic positive effects upon oral administration [1]. Indeed, the gut microbiota is involved in numerous physiological processes through the uptake of nutrients that are later used by both intestinal and immune cells thus generating bidirectional interactions between the microbial population and various organs [2]. At the intestinal level, the microbiota supports the preservation of gut integrity and peristaltic movements, improves lactose intolerance and is directly engaged in the metabolism of nutrients while also protecting the host against pathogens [3]. Scientific evidence shows that probiotics act effectively against various types of diarrhea, ulcerative colitis and other functional gastrointestinal disorders that are unmistakably related to gut dysbiosis. Additionally, the species, dose, and targeted disease determine the probiotic’s effectiveness and the length of clinical therapy [4]. The effectiveness of probiotics in treating inflammatory bowel disease (IBD), which causes chronic inflammation of the gastrointestinal system and may lead to colorectal cancer, is a significant problem. IBD pathophysiology was clearly linked to gut dysbiosis and impaired intestinal barrier [5].
The connection between gut microbiota and colon cancer has attracted a lot of attention. Several species have been identified as individually linked or found in abundance in colorectal cancer stool or tumor samples [6,7]. Probiotics may act as preventive agents against this pathology but also against the adverse effects of the currently used therapies [8]. The mechanisms of action for probiotics are quite complex and not yet fully understood, being hugely heterogeneous and dependent on the strain. A comprehensive review by Plaza-Diaz et al. has summarized the main mechanisms of action as competitive pathogen exclusion, enzyme modulation resulting in carcinogen inactivation, production of short-chain fatty acids that are able to interact with specific receptors, and modulation of the immune system [9]. However, clear evidence that probiotics are directly effective against cancer has not yet been provided. Also, despite bacterial changes being associated with specific diseases including colon cancer development and progression, a causality relationship could not be proven in humans [10].
Lactic acid bacteria, including Lactobacillus spp., are among the most available probiotics used to restore intestinal microbiota [11,12]. They have also been used as prophylactic agents for colorectal cancer. Lacticaseibacillus rhamnosus is an anaerobic bacterium often associated with the treatment and prophylaxis of gastrointestinal disorders since its significant reduction was found in colitis patients [13]. Apparently, Lacticaseibacillus rhamnosus reduces the risk of colon cancer by modulating gut microbiota and human dendritic cells, blocking altered enzymatic activities, blocking altered enzymatic activity, decreasing inflammatory and angiogenic gene expression, and increasing apoptotic gene expression [14]. Most interestingly, lactobacilli were linked to high immunotherapy response rates which are generally poor in colorectal cancer while also promoting an anti-inflammatory intestinal effect. Although these findings seem contradictory, L. rhamnosus affects the immune system in a proinflammatory manner enhancing immunological and anticancer effects in vivo and thereby limiting tumor burden [15].
The current article aims to elucidate the effect of L. rhamnosus against two types of colon cancer cells, HCT-116 and HT29, as well as against normal HaCaT keratinocytes to clarify its potential antitumor effect and selectivity. Furthermore, the study aims to uncover the underlying mechanism of anticancer activity which has remained unclear despite numerous efforts. In addition, the cytotoxic effect of L. rhamnosus was assessed against 5-fluouracil, a conventional anticancer agent frequently used as therapy in colorectal cancer.
2. Materials and Methods
2.1. Bacteria and Cell Lines
The bacterial strain Lacticaseibacillus rhamnosus GG (LGG, 53103, American Type Culture Collection ATTC, Łomianki, Poland) was cultured under proper conditions in Man—Rogosa—Sharpe (MRS) Agar/Broth (Merck KGaA, Darmstadt, Germany) and grown for 24 h at 37 °C [16]. Following 10 min centrifugation at 3500 rpm, a washing step with PBS and resuspension in PBS, the optical density was adjusted at 600 nm (OD600) to obtain 5 × 109 CFU/mL (colony-forming units per milliliter) (Microbiology Reader LogPhase 600, BioTek Instruments Inc., Winooski, VT, USA).
HaCaT immortalized human keratinocytes, HT-29 human colorectal adenocarcinoma (ATCC® HTB−38™) and HCT-116 human colorectal carcinoma (ATCC® CCL-247™) cell lines were bought from CLS Cell Lines ServiceGmbH (Eppelheim, Germany) and ATTC American Type Culture Collection (Łomianki, Poland), respectively. HT-29 and HCT-116 cells were cultured in McCoy’s 5A Medium while HaCaT cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM). Both mediums were supplemented with 10% FBS and 1% penicillin/streptomycin mixture (10,000 IU/mL). The cells were grown under standard conditions in a humidified incubator with a 5% CO2 atmosphere at 37 °C and used for the experiments after reaching 80–90% confluency.
2.2. Cell Viability
The cellular viability of normal HaCaT and colon cancer HT-29 and HCT-116 cell lines was assessed by using the Alamar Blue assay (Resazurin sodium salt) [17]. Briefly, the cells (10,000 cells/well) were cultured in 96-well plates at 37 °C and 5% CO2 for 24 h until reaching appropriate confluency. HaCaT, HT-29 and HCT-116 cell lines were treated with LGG (106–109 CFU/mL) ± 5-Fluorouracil (5-FU, 10 μM) for 24 and 48 h. After the incubation period with the tested samples was completed, 0.01% Alamar Blue reagent (Merck KGaA, Darmstadt, Germany) was used to counterstain each well. The plates were further incubated for 3 h at 37 °C. Cell viability was determined by measuring the absorbance at 570 and 600 nm using a microplate reader (xMark™ Microplate Spectrophotometer, Biorad, Hercules, CA, USA).
2.3. Immunofluorescence Assay
HaCaT, HT-29 and HCT-116 cells were seeded in 6-well plates, treated with LGG (106 and 109 CFU/mL) ± 10 μM 5-FU and incubated for 48 h. The nuclei were counterstained and morphologically analyzed using the 4,6′-Diamidino-2-Phenylindole (DAPI) staining (Merck KGaA, Darmstadt, Germany). The protocol to which the cells were subjected consisted of the following steps: (i) cell washing 3 times with cold PBS (Phosphate buffered saline, Thermo Fisher Scientific, Boston, MA, USA) after the 48 h stimulation period, (ii) fixation with 4% paraformaldehyde in PBS, (iii) permeabilization with Triton X/PBS 2%, (iv) washing 3 times with cold PBS, (v) blocking with 30% FCS in 0.01% Triton, (vi) washing 3 times with cold PBS, (vii) staining with DAPI (300 nM) and (viii) incubation at 4 °C in the dark [18]. The nuclear alterations were analyzed using the EVOS™ M7000 Imaging System (Thermo Fisher Scientific, Boston, MA, USA).
2.4. Cyclin D1 and Apoptosis Assay
The activities of Cyclin D1, BAX, Bcl-2 and caspase-3 were measured by colorimetric assay kits (ab214571, ab199080, ab119506 and ab39401; Abcam plc, Cambridge, CB2 0AX, UK). HT-29 and HCT-116 cells were cultured in a 6-well plate and treated for 48 h with the test samples (LGG 108 and 109 CFU/mL ± 10 μM 5-FU). The level of cyclin D1, BAX, Bcl-2 and caspase-3 activities were determined following the assay procedure described in the manufacturer’s instructions [19]. The total protein concentration was determined using the Pierce™ Rapid Gold BCA Protein Assay Kit (Thermo Fisher Scientific, Boston, MA, USA) while the absorbance was read using an xMark™ Microplate Spectrophotometer (Bio-Rad, Hercules, CA, USA).
2.5. Polarographic Measurement of Respiration
The cellular O2 consumption was measured by high-resolution respirometry using the Oroboros Oxygraph-2k instrument (Oroboros Instruments, GmbH, Innsbruck, Austria). Data acquisition and analysis were performed using the Datalab software (Oroboros Instruments). HT-29 and HCT-116 cells were cultured in a 6-well plate and treated for 48 h with the test samples (LGG 108 and 109 CFU/mL). The cells (1 × 106/mL) were permeabilized with digitonine (optimal concentration: 35 μg/L × 106 HT-29 cells and 25 μg/L × 106 HCT-116 cells). The measurements were performed at 37 °C in mitochondrial respiration medium (MIRO5: EGTA 0.5 mM, taurine 20 mM, MgCl2 3 mM, K-lactobionate 60 mM, KH2PO4 10 mM, HEPES 20 mM, D-sucrose 110 mM and BSA 1 g/L, pH 7.1) and expressed as pmol∙s−1∙10−6. The cell suspensions were added to each chamber and the O2 signal was allowed to stabilize for 15 min under routine respiration conditions (respiration dependent on endogenous substrates). According to a protocol described by Petruș et al. [20], the following respiratory substrates, uncouplers and inhibitors were added during the measurement of mitochondrial respiration: glutamate (10 mM) + malate (5 mM) → State2CI, ADP (5 mM) + Succinate (10 mM) → OXPHOSCI + II, Cytochrome c (5 mM) → Cyt c, Oligomycin (1 μg/mL) → State4 CI + II, FCCP (1 μM/step) successive titrations → ETSCI + II, and antimycin A (2.5 μM) → ROX.
2.6. Statistical Analysis
The statistical analysis of the cellular viability results and apoptosis/proliferation markers was performed using one-way ANOVA followed by Dunnett’s post-test (GraphPad Prism version 6.0.0, GraphPad Software, San Diego, CA, USA). The IC50 values were calculated using the GraphPad Prism6 software (GraphPad Software, San Diego, CA, USA) based on the correlation between the log[concentration] and cell viability. Mitochondrial respiratory rates were statistically analyzed using two-way ANOVA followed by Bonferroni’s multiple comparisons post-test (GraphPad Prism version 6.0.0, GraphPad Software, San Diego, CA, USA). The differences between groups were considered statistically significant if p < 0.05, as follows: * p < 0.05, ** p < 0.01, and *** p < 0.001.
3. Results
3.1. Cell Viability Assessment
The cytotoxic effect of LGG (106, 107, 108 and 109 CFU/mL), 5-FU (10 μM), and their association, on normal HaCaT, HT-29 colorectal adenocarcinoma and HCT-116 colorectal carcinoma cell lines was evaluated using the Alamar Blue assay. The cytotoxic effect and anti-proliferative activity of LGG, alone or in combination with 5-FU, on HaCaT, HT-29 and HCT-116 cells following a 24 and 48 h stimulation period are presented in Figure 1. The calculated IC50 values of LGG and LGG associated with 5-FU on HaCaT, HT-29 and HCT-116 cell lines are presented in Table 1. In HaCaT cells only the association of LGG 109 CFU/mL + 5-FU decreased cell viability to 88.06% after a 24 h treatment period (Figure 1A). However, by increasing the incubation time to 48 h, LGG 109 CFU/mL alone decreased cell viability to 87.56%, compared with its association with 5-FU (LGG 109 CFU/mL + 5-FU 83.73%), whereas 5-FU alone decreased cell viability to 71.68% (Figure 1B). The vitality of HT-29 cells treated with LGG reduced as the incubation duration increased from 24 to 48 h. Specifically, LGG 108 CFU/mL alone decreased HT-29 cell viability at 24 h but without reaching a statistical significance, whereas LGG 109 CFU/mL alone decreased cell viability to 87.16% (Figure 1C). 5-FU alone and LGG (106–109 CFU/mL) associated with 5-FU decreased cell viability of HT-29 cells after 24 h, as follows: 67.01%, 91.13%, 83.07%, 78.87% and 75.58%. At 48 h, LGG 108 CFU/mL alone decreased cell viability to 83.4% vs. control (100%), reaching now a statistical significance (Figure 1D). Treatment with LGG at 109 CFU/mL alone, as well as 5-FU alone and LGG (106–109 CFU/mL) associated with 5-FU decreased cell viability of HT-29 cells after 48 h, as follows: 72.36%, 45.35%, 76.07%, 66.72%, 59.57% and 51.38% vs. control (Figure 1D). LGG effect was more pronounced in HCT-116 cells; at 108 and 109 CFU/mL LGG alone decreased HCT-116 cell viability to 88.44% and 86.02% after 24 h, and to 66.37% and 57.64% after 48 h (Figure 1E,F). LGG 109 CFU/mL effect at 24 and 48 h was comparable to that of 5-FU (86.02% vs. 83.93% and 57.64%vs. 53.54%) (Figure 1E,F). Association of LGG 106–109 CFU/mL with 5-FU resulted in an increased overall cytotoxic activity, as follows: after 24 h the cell viability decreased to 77.35%, 71.50%, 61.24% and 54.57% and after 48 h to 47.68%, 39.72%, 32.13% and 26.20% (Figure 1E,F).
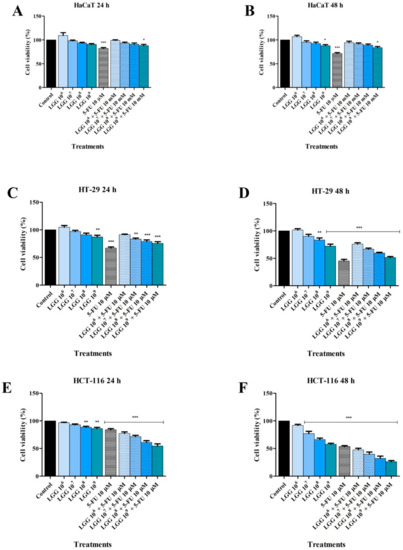
Figure 1.
Cell viability of HaCaT (A,B), HT-29 (C,D) and HCT-116 (E,F) cells following the treatment with LGG 106–109 CFU/mL, 5-FU (10 μΜ), and their association after 24 and 48 h. The results are expressed as viability percentage in comparison with the control group, considered 100% (* p < 0.05, ** p < 0.01 and *** p < 0.001). The data represent the mean values ± SD of three independent experiments performed in triplicate.

Table 1.
The calculated IC50 values.
3.2. Effect of LGG and 5-FU on Nuclear Morphology
The effect of LGG (106 and 109 CFU/mL), alone and in association with 5-FU (10 μM), on the nuclear morphology of HaCaT, HT-29 and HCT-116 cells was investigated using the DAPI staining (Figure 2, Figure 3 and Figure 4). In HaCaT cells the highest concentration of LGG (109 CFU/mL) induced nuclear condensation and shrinkage while its association with 5-FU induced nuclear fragmentation both at 106 and 109 CFU/mL, respectively. 5-FU alone induced nuclear condensation and fragmentation (Figure 2).
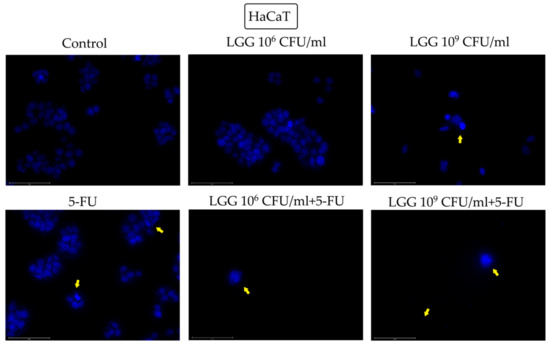
Figure 2.
Nuclear morphological changes in HaCaT cells observed following 48 h treatment with LGG 106 and 109 CFU/mL, alone or in association with 10 μM 5-FU. The yellow arrows represent signs of apoptosis (nuclear shrinkage, fragmentation, condensation, and cellular membrane disruption). The scale bar is 75 μm.
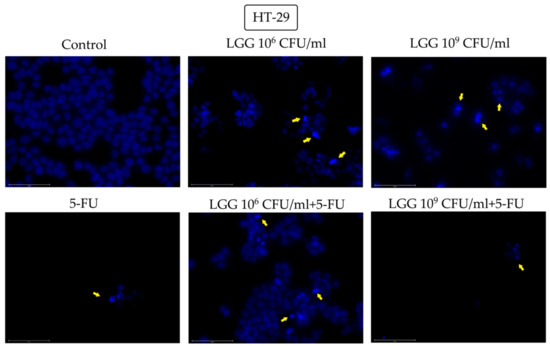
Figure 3.
Nuclear morphological changes in HT-29 cells observed following 48 h treatment with LGG 106 and 109 CFU/mL, alone or in association with 10 μM 5-FU. The yellow arrows represent signs of apoptosis (nuclear shrinkage, fragmentation, condensation, and cellular membrane disruption). The scale bar is 75 μm.
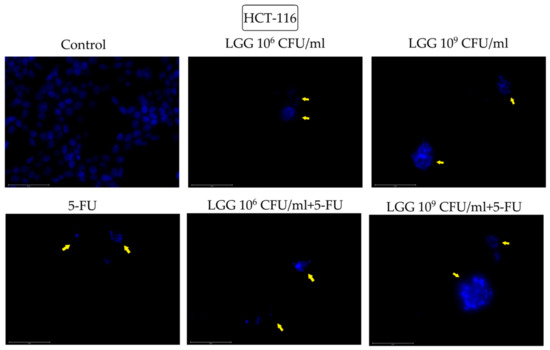
Figure 4.
Nuclear morphological changes in HCT-116 cells observed following 48 h treatment with LGG 106 and 109 CFU/mL, alone or in association with 10 μM 5-FU. The yellow arrows represent signs of apoptosis (nuclear shrinkage, fragmentation, condensation, and cellular membrane disruption). The scale bar is 75 μm.
Signs of nuclei condensation and fragmentation were also observed in HT-29 cells after treatment with 106 and 109 CFU/mL LGG, alone, LGG associated with 5-FU at both tested concentrations and 5-FU alone (Figure 3). When 109 CFU/mL LGG was incubated with 5-FU, the nuclei underwent a severe fragmentation accompanied by cellular membrane disruption followed by the scattering of the genetic material (Figure 3).
In HCT-116 cells treated with 5-FU alone and 106 and 109 FCU/mL LGG, alone and combined with 5-FU, induced similar morphological changes of the nuclei that are consistent with apoptosis (condensation, fragmentation, blebbing and cellular membrane disruption) (Figure 4).
3.3. Evaluation of Cell-Cycle-Related Protein and Pro-/Anti-Apoptotic Markers Levels
LGG was tested alone and in combination with 5-FU (10 μΜ) at the concentrations that produced the strongest reduction in HT-29 and HCT-116 cell viability (108 and 109 CFU/mL). The effect of LGG (108 and 109 CFU/mL) associated with 5-FU, and given alone, on the protein level of cell-cycle-related protein Cyclin D1, pro (BAX) and anti-apoptotic (Bcl-2) markers was determined using the enzyme-linked immunosorbent assay (ELISA). The caspase-3 activity was determined using a colorimetric caspase assay.
The cyclin D1 expression was measured in HT-29 and HCT-116 cell lines treated with LGG (108 and 109 CFU/mL) alone or in combination with 5-FU (10 μΜ). The results revealed a significant reduction in protein levels after treatment with the investigated substances at any concentration tested. (Figure 5A). Specifically, compared to 5-FU alone (HT-29: 4.27 ± 0.28 and HCT-116: 0.94 ± 0.04) and control (HT-29: 5.52 ± 0.23 and HCT-116: 3.27 ± 0.28), LGG alone was able to decrease Cyclin D1 protein level in HT-29 and HCT-116 cells to 4.55 ± 0.29 and 2.68 ± 0.26 at 108 CFU/mL and to 4.29 ± 0.15 and 1.27 ± 0.15 at 109 CFU/mL (Figure 1A). Moreover, the association of LGG with 5-FU produced an even sharper decrease in the Cyclin D1 level, as follows: 3.57 ± 0.021 and 0.75 ± 0.12 (LGG 108 CFU/mL) 2.86 ± 0.022 and 0.22 ± 0.02 (LGG 109 CFU/mL) (Figure 5A).
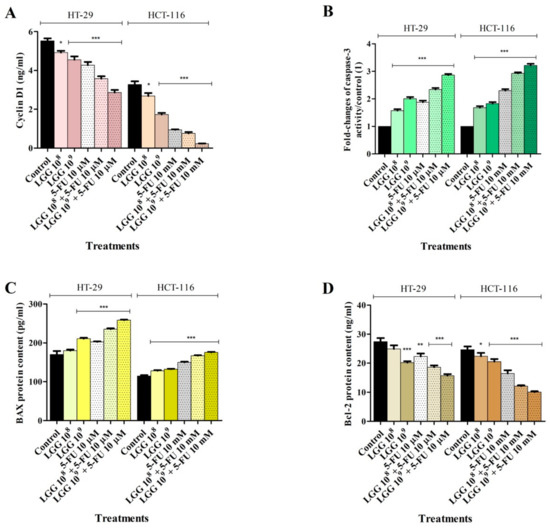
Figure 5.
Effect of LGG (108 and 109 CFU/mL) ± 5-FU (10 μΜ) on Cyclin D1 protein expression (A), caspase-3 activity (B) and BAX (C) and Bcl-2 (D) protein levels in HT-29 and HCT-116 colorectal cancer cell lines after 48 h treatment. The results were reported as mean values ± SD with p < 0.05 (*), p < 0.01 (**), p < 0.081 (***), when compared to control. All experiments were performed in triplicate.
Given that numerous cytotoxic agents act by inducing apoptosis as their main mechanism and considering the effect of LGG on cancer cell viability and nuclear morphology of HT-29 and HCT-116 cell lines, the protein levels of the well-known pro and anti-apoptotic markers, BAX and Bcl-2, along with caspase-3 activity, were determined following 48 h treatment with LGG (108 and 109 CFU/mL) ± 5-FU (10 μΜ). In HT-29 cell lines, LGG alone increased both BAX protein level and caspase-3 activity at both 108 (179.8 ± 3.48 and 1.57 ± 0.09) and 109 CFU/mL (210.9 ± 3.72 and 2.08 ± 0.01), respectively vs. control (170.28 ± 8.89 and 1) and 5-FU (202.3 ± 2.78 and 1.87 ± 0.11) (Figure 5B,C). The association of LGG with 5-FU increased BAX protein levels to 235 ± 3.83 (LGG 108 + 5-FU) and to 258 ± 2.64 (LGG 109 + 5-FU), while the same concentrations increased caspase-3 activity to 2.33 ± 0.09 and 2.86 ± 0.07 (Figure 5B,C). In the same cell line, the level of the anti-apoptotic protein Bcl-2 decreased as a result of LGG treatment, as follows: 24.93 ± 2.09 (LGG 108), 20.18 ± 0.85 (LGG 109), 18.61 ± 1.05 (LGG 108 + 5-FU) and 15.72 ± 0.89 (LGG 109 + 5-FU) vs. control (27.42 ± 2.20) and 5-FU (22.32) (Figure 5D).
In HCT-116 cell lines a similar increase of pro-apoptotic BAX protein level and caspase-3 activity was recorded upon LGG treatment. Compared to the control (114.6 ± 5.86) and to 5-FU (149 ± 3.67), LGG at 108 and 109 alone and in combination with 5-FU increased BAX level to 128.0 ± 3.27, 132.18 ± 1.86, 166.87 ± 2.25 and 175.68 ± 1.72. Caspase-3 activity levels were 1.68 ± 0.09 (LGG 108), 1.82 ± 10.08 (LGG 109), 2.92 ± 0.07 (LGG 108 + 5-FU) and 3.22 ± 0.13 (LGG 109 + 5-FU) vs. control (1) and 5-FU (2.32 ± 0.17) (Figure 5B,C). Bcl-2 protein levels obtained in HCT-116 cells were as follows: 22.43 ± 1.91 (LGG 108), 20.55 ± 1.57 (LGG 109), 16.46 ± 1.19 (5-FU), 12.08 ± 0.51 (LGG 108 + 5-FU) and 10.07 ± 0.49 (LGG 109 + 5-FU) vs. 24.66 ± 1.94 (control) (Figure 5D).
3.4. Evaluation of Mitochondrial Respiration
The mitochondrial function of permeabilized HT-29 and HCT-116 cells was evaluated by high-resolution respirometry at 37 °C after 48 h treatment with LGG (108 and 109 CFU/mL).
LGG tested at 108 and 109 CFU/mL inhibit the mitochondrial respiration of both HT-29 and HCT-116 cells (Figure 6) by firstly reducing routine respiration in a dose-dependent manner vs. control. Both State2 dependent on CI and State4 dependent on CI + II (LEAK respiration) were significantly reduced, regardless of the concentration tested, and thus, LGG treatment can decrease basal cell respiration (Figure 6). The probiotic suppressed OXPHOSCI+II and ETSCI+II. These results suggest that LGG treatment decreases the oxidation of reduced fuel substrates, a process that is coupled to the phosphorylation of ADP to ATP, while also decreasing the electron transfer-pathway capacity, and hence, LGG decreases the respiratory capacity of the mitochondria and mitochondrial function. The mean values ± SD of the mitochondrial respiratory rates (pmol∙s−1∙10−6 cells) obtained after the treatment of HT-29 and HCT-116 cells with LGG 108 and 109 CFU/mL are presented in Table 2.
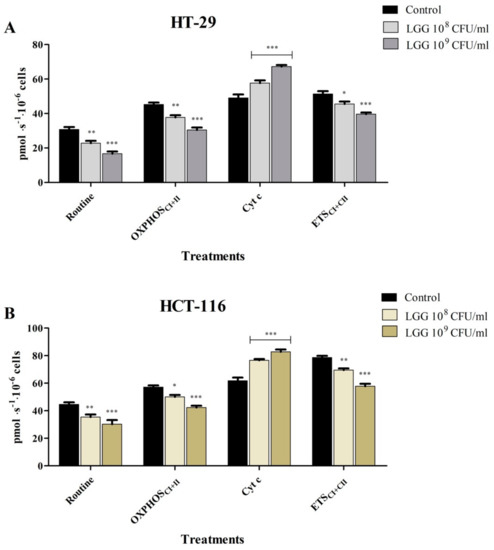
Figure 6.
Mitochondrial respiratory rates (O2 consumption) of HT-29 (A) and HCT-116 (B) cells after 48 h treatment with LGG 108 and 109 CFU/mL. The experiments were performed in triplicate and the results are presented as mean values ± SD. Values with p < 0.05 were considered to be statistically different (* p < 0.05, ** p < 0.01 and *** p < 0.001). The respiratory parameters displayed represent the following: Routine —respiration in a substrate-free media, based on endogenous substrates; OXPHOSCI+II —the maximal active respiration driven by both CI and CII; Cyt c—evaluation of mitochondrial membrane integrity and ETSCI+II—the maximal respiratory capacity of the electron transport system in the fully noncoupled state.

Table 2.
Mitochondrial respiratory rates of HT-29 and HCT-116 cells treated with LGG (108 and 109 CFU/mL).
4. Discussion
Colorectal cancer is associated with high mortality and exhibits high incidence worldwide regardless of gender. In addition to cases in the older population, which present special age-related issues such as the risk of complications from conventional therapy, there is a rising number of cases (younger than 50 years) with an advanced stage and a bad prognosis. Although its underlying mechanisms are not fully known, dietary factors are definitely involved [21]. The administration of probiotics is a useful strategy in both the prevention and treatment of severe gastrointestinal diseases including colorectal cancer due to various mechanisms that include competitive colonization and anti-inflammatory effects [22]. Moreover, probiotics are able to reduce the incidence and severity of adverse effects associated with conventional chemo- and radiotherapy [23,24]. The most widely used probiotics belong to the group of lactic acid-producing bacteria that include Lactobacillus spp; Lacticaseibacillus rhamnosus GG (LGG) is an anaerobe Gram-positive strain isolated from stool samples of a healthy human adult and designated as probiotic due to its resistance to acid and bile, easy growth and strong adhesion to the gut epithelial layer [25]. The LGG strain is known to exhibit anti-inflammatory effects through the stimulation and production of several cytokines; tests in animal models have revealed its ability to prevent the development of colorectal cancer by modulating gut microbiota and downregulating pro-inflammatory factors [26].
In the current study, the cytotoxic effect of various concentrations of LGG was assessed against normal keratinocytes as well as two types of colon cancer cells, HCT-116 (colorectal carcinoma) and HT-29 (colorectal adenocarcinoma) by means of Alamar Blue assay. 5-fluorouracil was used as a reference; its combination with the bacterial strain was also tested. We chose the HaCat cell line as the healthy cell group. While this cell line may not be a good representation of healthy colon cells, it is a good benchmark for assessing compound cytotoxicity against healthy cells as it is widely used in anticancer in vitro studies despite the fact that the cancers cell lines tested are of different types [27,28]. In normal HaCaT cells, only the highest LGG concentration (109 CFU/mL) induced a slight reduction, that reached statistical significance, of cell viability after 48 h stimulation. Despite this finding, there is little cause for concern because this concentration is not typically up-scaled in vivo studies, which means it reaches lower concentrations after oral administration. A previous study found no cytotoxic effects in the control animal group that received 109 CFU/mL LGG, indicating the safety of this dose [29]. Association of 5-FU with LGG 109 CFU/mL reduced cell viability after both 24 h and 48 h, respectively, an effect that was observed for LGG 109 CFU/mL, only after 48 h, but not after the 24 h treatment period. In HT-29 cancer cells, reduced cell viability following 24 h treatment with LGG 109 CFU/mL alone, 5-FU and LGG 106–109 CFU/mL + 5-FU was statistically significant; however, after 48 h of stimulation, the percentage of cell viability dropped significantly for those concentrations and additionally for LGG 108 CFU/mL alone. The association of 5-FU with LGG-induced cell death; however, the effects are comparable to 5-FU alone. In HCT-116 cells, the cytotoxic effects were more dramatic: after 48 h stimulation, the reduction of cell viability following LGG application (109 CFU/mL) was similar to 5-FU alone (~ 50%) while the combination of both agents induced a sharp reduction of cell viability to approximately 25%; the cytotoxic effect was time and dose-dependent in all cases. The experimental results are consistent with literature reports that indicate a significant cytotoxic effect of L. rhamnosus against HT-29 cells; the study indicated a 99% inhibition of HT-29 cells after 72 h stimulation with 30 mg/mL LGG [30]. HT-29 cells are known to exhibit an intermediate ability to differentiate while HCT-116 cells have little or no ability in this regard which makes it a highly aggressive cell line [31]. Therefore, HCT-116 and HT-29 cells are used as models for the more and less aggressive types of colorectal cancer, respectively. As such, HCT-116 cells were assimilated to the TNM 3 stage cancer, with higher percentages of cancer stem cells presumably responsible for cancer recurrence while HT-29 cells correspond to the TNM 2 stage being less invasive [32]. In our previous study, the combination of Lactobacillus sporogenes and Clostridium butyricum induced a stronger inhibition of HT-29 cells compared to HCT-116 cells [33]. The presence of C. butyricum, which has the ability to target the mucus glycoprotein whose production varied dramatically across differentiated and undifferentiated cell lines, was attributed to the effect. However, in the current study, the cytotoxic activity of L. rhamnosus was more pronounced against HCT-116 cells. Similarly, Escamilla et al. [34] reported that the cell-free supernatant from LGG strongly reduced HCT-116 cell invasion. The authors established that the probiotic downregulated the level and activity of matrix metalloproteinase-9 (MMP-9) and upregulated the level of zona occludens-1 (ZO-1) in HCT-116 cells; highly relevant is the fact that other bacterial species were unable to produce inhibitory activities which led the authors to conclude that specific secretory macromolecule metabolites of L. rhamnosus ranging between 50–100 Da or > 100 Da were responsible for the reported cytotoxic effect [34]. Another study assessed the cytotoxicity of several Lactobacillus spp. against HT-29 and HCT-116 colon cancer cells using bleomycin as a positive control. All probiotic supernatants exhibited robust and specific cytotoxicity against both cell lines, comparable to or even superior to bleomycin, but with no significant differences between the two cell lines [35]. Considering these rather controversial results, we may assume that specific metabolites of L. rhamnosus are responsible for the higher cytotoxicity against the more aggressive HCT-116 cells. In addition, the association of LGG with 5-FU induced a stronger cytotoxic effect compared to both agents alone. In fact, these results can be corroborated by the study of El Hadad et al. [36] who reported that the in vivo association of LGG and 5-FU led to a reduction of inflammatory processes and optimized innate and adaptive immune responses. Overall, the experimental results confirm the selective cytotoxic activity of LGG against colon cancer cells with the strongest effect exerted on the most aggressive type of cancer cells.
The morphological cell features before and after stimulation with LGG, 5-FU or their combination were assessed by means of the DAPI technique that uses blue 4′,6-diamidino-2-phenylindole (DAPI) fluorescent dye that binds stoichiometrically to nuclear DNA in order to determine nuclear morphology changes [37]. The tested compounds induced various morphological changes that are consistent with apoptotic processes. In contrast to necrosis, where the nuclei stay relatively intact, the nuclei degenerate in apoptosis, resulting in morphological alterations that can be considered indicators of programmed cell death [38]. In healthy HaCaT cells, only the highest concentration of LGG (109 CFU/mL) was able to induce nuclear condensation and shrinkage. However, its association with 5-FU triggered nuclear fragmentation both at 106 and 109 CFU/mL, while 5-FU alone induced nuclear condensation and fragmentation, thus reflecting the less selective cytotoxic effect of 5-FU. In HT-29 cells, nuclear condensation and fragmentation occurred after treatment with 106 and 109 CFU/mL LGG, alone and associated with 5-FU (Figure 3). Similarly to normal keratinocytes, the addition of 5-FU caused more severe nuclear fragmentation, which, in conjunction with the cellular membrane breakdown, resulted in the scattering of genetic material, an effect that was not detected when 5-FU was tested alone on HT-29 cells. Apoptotic signs such as nuclear condensation, fragmentation, blebbing as well as cell membrane disruption were also recorded in HCT-116 cells after stimulation with 106 and 109 CFU/mL LGG. The apoptotic activity of LGG in HT-29 cancer cells in a time- and dose-dependent manner has been previously reported by Dehghani et al. [30] who indicated the use of LGG as a prophylactic and therapeutic agent against colorectal cancer due to its low cost and accessibility. Similar results with shrinking and condensed nuclei also appeared in HeLa cells (cervix cancer) after stimulation with L. rhamnosus isolated from human breast milk [39] thus indicating apoptotic changes. On the other hand, LGG is able to exert antioxidative and anti-apoptotic effects through its exopolysaccharides which can effectively oppose the damaging effects of hydrogen peroxide (H2O2) in intestinal porcine epithelial cells thus providing a potentially effective therapeutic strategy against oxidation-induced gastrointestinal disorders [40]. Lactobacilli also have the ability to generate H2O2 in nanomolar concentrations thus exerting a beneficial role in inflammatory gut diseases [41]. Taking into consideration that reactive oxygen species (ROS) play such a complex role in both cancer cell proliferation and cell death, ROS-modulating agents like LGG may indeed exhibit therapeutic potential in cancer prophylaxis but also as adjuvants to other systemic approaches [42].
Considering the robust results obtained after the evaluation of the cytotoxic potential and the nuclear morphological assessment that indicated apoptosis, a more in-depth analysis regarding the mechanism of action of LGG, alone and associated with 5-FU (LGG ± 5-FU), was necessary; therefore, the levels of cyclin D1, caspase-3, Bax and Bcl-2 proteins were quantified. Cyclin D1 is a proto-oncogene that regulates the G1–S phase of the cell cycle in mammalian cells; its overexpression has been linked with cancer cell progression, differentiation and reduced overall survival in patients with colon cancer [43,44]. Caspase-3, Bax and Bcl-2 are part of the Bcl-2 and caspase families of proteins that initiate and promote the mitochondria-mediated intrinsic apoptosis; effector caspases such as caspase-3 are responsible for the occurrence of the degradation phase of apoptosis described above, including nuclear DNA fragmentation, cell shrinkage and membrane blebbing [45]. LGG produced the decrease of Cyclin D1 level with a potency similar to 5-FU in both cell lines while their combination achieved an additive effect; LGG alone was able to increase the expression of the pro-apoptotic Bax and caspase-3 and decrease the anti-apoptotic Bcl-2 level thus confirming the overall apoptotic effect of lactobacilli. The addition of 5-FU induced a stronger apoptotic effect on both cancer cell lines. These experimental results confirm previous literature reports. Gamallat et al. [29] revealed the epithelial cell apoptosis by L. rhamnosus in an animal model where LGG acted successfully against colon carcinogenesis by modulating the levels of inflammatory as well as pro-and anti-apoptotic proteins thus qualifying as a bio-therapeutic dietary agent. Another study reported that prior administration of LGG and celecoxib reduced tumor initiation and progression in a chemically-induced colon cancer animal model due to increased apoptosis. The authors concluded that probiotics have the ability to increase Bax expression which later neutralizes Bcl-2 activity as a caspase activator [46]. In addition to confirming the apoptotic activity of L. rhamnosus on HT-29 colon cancer cells, Tukenmez et al. [47] highlighted the importance of exopolysaccharide composition in mannose and glucose for apoptotic potency. L. rhamnosus induced the highest Bax increase after 24 h cell stimulation combined with survival suppression which increases effector caspases like caspase-3 [47]. Interestingly enough, the combination of LGG with 5-FU induced stronger effects on all quantified markers thus suggesting an additive apoptotic activity; on the other hand, L. rhamnosus was shown to alleviate 5-FU gastrointestinal injuries by upregulating Bcl-2 expression in intestinal cells and diminishing the production of inflammatory markers thus significantly prolonging animal survival [48]. Therefore, we may assume that probiotics act differently on cancer versus healthy cells thus displaying the necessary degree of selectivity in anticancer therapy; their mechanism of action is based on mitochondrial apoptosis which is specifically regulated in normal and cancer cells, respectively.
Given the importance of the mitochondria and the Bcl-2 protein family in the intrinsic apoptotic pathway, as well as the findings regarding the influence of LGG on pro-apoptotic BAX and anti-apoptotic Bcl-2 protein levels, the effect of LGG alone on mitochondrial function was examined further. The mitochondrial function of permeabilized HT-29 and HCT-116 cells was evaluated by high-resolution respirometry at 37 °C after 48 h treatment with LGG (108 and 109 CFU/mL).
Mitochondria dysfunction occurs as a pathophysiological factor in numerous diseases including cancer [49,50]. As such, it might induce a swift energy metabolism in cancer cells from OXPHOS to glycolysis thus contributing to cancer progression [51]. However, colorectal cancer cells cannot be considered hypoxic cells since they have been reported to exhibit higher OXPHOS rates than healthy colon cells. Moreover, they exert an in vivo effect on surrounding cells which acquire similar bioenergetic parameters with tumor cells and, as a result, display OXPHOS upregulation and increased values of basal respiration [52].
LGG significantly inhibited mitochondrial respiration in both cancer cell lines starting with routine respiration which diminished in a dose-dependent manner. Membrane permeabilization with digitonine allowed cellular entry of exogenous oxidizable substrates able to generate electrons to the respiratory system complexes. Membrane permeabilization enables the assessment of individual capacities of respiratory complexes and the extended OXPHOS parameters by disrupting the barrier between external media and cytosol and allowing molecular transit to reach equilibrium [53]. Experimental results showed that both State2 dependent on CI and State4 dependent on CI + II (LEAK respiration) were significantly reduced in the presence of LGG, suggesting that LGG may reduce basal respiration, regardless of concentration. Moreover, the probiotic suppressed OXPHOSCI + II and ETSCI + II, thus suggesting that it can inhibit the active respiration and electron transport pathway capacity while impairing ATP production. Currently, there are many anticancer agents that act as inhibitors of the electron transport chain and alter the function of respiratory complexes thus inducing high levels of reactive oxygen species (ROS) which in turn have the ability to kill cancer cells [54]. As previously mentioned, studies have found that colorectal cancer cells have higher OXPHOS respiratory rates compared to normal colon cells and are dependent on OXPHOS rather than glycolysis [52,55]. Considering these findings, the disruption of mitochondrial function and OXPHOS inhibition can be viewed as an effective method for colon cancer treatment. Therefore, the inhibition of mitochondrial respiration in cancer cells by LGG triggers mitochondria-mediated intrinsic apoptosis which can be identified as one pivotal mechanism in LGG’s anticancer activity.
5. Conclusions
The current study describes Lacticaseibacillus rhamnosus’s selective antitumor effect against two types of colon cancer cells, HCT-116 and HT29, as well as the underlying mechanism. The probiotic demonstrated mitochondrial targeted pro-apoptotic inclined cytotoxicity against both colon cancer cell lines tested, with HCT-116 being more sensitive to LGG’s anticancer activity. Naturally, some issues remain debatable and may provide useful perspectives for future research. It is unknown why higher concentrations of LGG have a minor effect on the HaCaT cell lines, and what specific bacterial metabolites are responsible for the induced pro-apoptotic effect. Nonetheless, the evidence presented suggests that treatment with probiotics, specifically LGG, alone or in combination with 5FU can induce apoptosis in colon cancer cells. These findings could pave the way for the development of alternative chemopreventive/chemotherapeutic agents for various types of colon cancer.
Author Contributions
Conceptualization, O.B. and C.D.B.; methodology, O.B., C.D.B., D.F.L. and C.S.; validation, C.D.B. and C.S.; investigation, O.B., C.D.B., M.M., A.M. (Alexandra Mioc), G.M., A.P. and A.M. (Andreea Milan); writing—original draft preparation, O.B.; writing—review and editing C.D.B., L.S. and D.F.L.; visualization, A.M. (Alexandra Mioc), M.M.; formal analysis, A.P., A.M. (Andreea Milan) and G.M.; supervision C.S. and L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shang, F.; Jiang, X.; Wang, H.; Chen, S.; Wang, X.; Liu, Y.; Guo, S.; Li, D.; Yu, W.; Zhao, Z.; et al. The inhibitory effects of probiotics on colon cancer cells: In vitro and in vivo studies. J. Gastrointest. Oncol. 2020, 11, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Varela-Trinidad, G.U.; Domínguez-Díaz, C.; Solórzano-Castanedo, K.; Íñiguez-Gutiérrez, L.; Hernández-Flores, T.D.J.; Fafutis-Morris, M. Probiotics: Protecting Our Health from the Gut. Microorganisms 2022, 10, 1428. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Totino, V.; Cacciotti, F.; Iebba, V.; Neroni, B.; Bonfiglio, G.; Trancassini, M.; Passariello, C.; Pantanella, F.; Schippa, S. Rebuilding the Gut Microbiota Ecosystem. Int. J. Environ. Res. Public Health 2018, 15, 1679. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, T.; Sequoia, J. Probiotics for Gastrointestinal Conditions: A Summary of the Evidence. Am. Fam. Physician 2017, 96, 170–178. [Google Scholar] [PubMed]
- Zhou, J.; Li, M.; Chen, Q.; Li, X.; Chen, L.; Dong, Z.; Zhu, W.; Yang, Y.; Liu, Z.; Chen, Q. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat. Commun. 2022, 13, 3432. [Google Scholar] [CrossRef]
- Rebersek, M. Gut microbiome and its role in colorectal cancer. BMC Cancer 2021, 21, 1325. [Google Scholar] [CrossRef]
- Hong, J.; Fang, J.-Y. Gut Microbiota Impacts on the Efficacy of Anticancer Treatment of Colorectal Cancer. In Microbiome in Gastrointestinal Cancer; Yu, J., Ed.; Springer Nature Singapore: Singapore, 2023; pp. 237–249. ISBN 978-981-19-4492-5. [Google Scholar]
- Drago, L. Probiotics and Colon Cancer. Microorganisms 2019, 7, 66. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Chen, G. The Role of the Gut Microbiome in Colorectal Cancer. Clin. Colon Rectal Surg. 2018, 31, 192–198. [Google Scholar] [CrossRef]
- Ali, U.; Saeed, M.; Ahmad, Z.; Shah, F.-H.; Rehman, M.A.; Mehmood, T.; Waseem, M.; Hafeez, H.; Azam, M.; Rahman, A. Stability and Survivability of Alginate Gum-Coated Lactobacillus rhamnosus GG in Simulated Gastrointestinal Conditions and Probiotic Juice Development. J. Food Qual. 2023, 2023, 3660968. [Google Scholar] [CrossRef]
- Demarinis, C.; Verni, M.; Pinto, L.; Rizzello, C.G.; Baruzzi, F. Use of Selected Lactic Acid Bacteria for the Fermentation of Legume-Based Water Extracts. Foods 2022, 11, 3346. [Google Scholar] [CrossRef]
- Kim, J.; Balasubramanian, I.; Bandyopadhyay, S.; Nadler, I.; Singh, R.; Harlan, D.; Bumber, A.; He, Y.; Kerkhof, L.J.; Gao, N.; et al. Lactobacillus rhamnosus GG modifies the metabolome of pathobionts in gnotobiotic mice. BMC Microbiol. 2021, 21, 165. [Google Scholar] [CrossRef]
- Huang, J.; Wang, D.; Zhang, A.; Zhong, Q.; Huang, Q. Lactobacillus rhamnosus confers protection against colorectal cancer in rats. Trop. J. Pharm. Res. 2021, 18, 1449–1454. [Google Scholar] [CrossRef]
- Owens, J.A.; Saeedi, B.J.; Naudin, C.R.; Hunter-Chang, S.; Barbian, M.E.; Eboka, R.U.; Askew, L.; Darby, T.M.; Robinson, B.S.; Jones, R.M. Lactobacillus rhamnosus GG Orchestrates an Antitumor Immune Response. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1311–1327. [Google Scholar] [CrossRef]
- Śliżewska, K.; Chlebicz-Wójcik, A. Growth Kinetics of Probiotic Lactobacillus Strains in the Alternative, Cost-Efficient Semi-Solid Fermentation Medium. Biology 2020, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Bonnier, F.; Keating, M.; Wrobel, T.; Majzner, K.; Baranska, M.; Garcia, A.; Blanco, A.; Byrne, H. Cell viability assessment using the Alamar blue assay: A comparison of 2D and 3D cell culture models. Toxicol. In Vitro 2014, 29, 124–131. [Google Scholar] [CrossRef]
- Mioc, M.; Mioc, A.; Prodea, A.; Milan, A.; Balan-Porcarasu, M.; Racoviceanu, R.; Ghiulai, R.; Iovanescu, G.; Macasoi, I.; Draghici, G.; et al. Novel Triterpenic Acid—Benzotriazole Esters Act as Pro-Apoptotic Antimelanoma Agents. Int. J. Mol. Sci. 2022, 23, 9992. [Google Scholar] [CrossRef] [PubMed]
- Assay Procedure Can Be Found. Available online: https://www.abcam.com/?gclsrc=aw.ds&gclid=Cj0KCQiAutyfBhCMARIsAMgcRJTqXuV8ejoRZWlnUJ1b5th64NWvCAUQZCJ3vXNt-0T9tgWXRutKeaMaAvwCEALw_wcB&gclsrc=aw.ds (accessed on 7 October 2022).
- Petrus, A.; Ratiu, C.; Noveanu, L.; Lighezan, R.; Rosca, M.; Muntean, D.; Duicu, O. Assessment of Mitochondrial Respiration in Human Platelets. Rev. Chim. 2017, 68, 768–771. [Google Scholar] [CrossRef]
- Guren, M.G. The global challenge of colorectal cancer. Lancet Gastroenterol. Hepatol. 2019, 4, 894–895. [Google Scholar] [CrossRef]
- Karbalaei, M.; Keikha, M. Probiotic as anti-colorectal cancer agents: Challenges and further perspective. Ann. Med. Surg. 2022, 80, 104189. [Google Scholar] [CrossRef]
- Feng, J.; Gao, M.; Zhao, C.; Yang, J.; Gao, H.; Lu, X.; Ju, R.; Zhang, X.; Zhang, Y. Oral Administration of Probiotics Reduces Chemotherapy-Induced Diarrhea and Oral Mucositis: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 823288. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Heus, P.; Van de Wetering, F.T.; Van Tienhoven, G.; Verleye, L.; Scholten, R.J. Probiotics for the prevention or treatment of chemotherapy- or radiotherapy-related diarrhoea in people with cancer. Cochrane Database Syst. Rev. 2018, 8, CD008831. [Google Scholar] [CrossRef] [PubMed]
- Segers, M.E.; Lebeer, S. Towards a better understanding of Lactobacillus rhamnosus GG—Host interactions. Microb. Cell Fact. 2014, 13, S7. [Google Scholar] [CrossRef] [PubMed]
- Banna, G.L.; Torino, F.; Marletta, F.; Santagati, M.; Salemi, R.; Cannarozzo, E.; Falzone, L.; Ferraù, F.; Libra, M. Lactobacillus rhamnosus GG: An Overview to Explore the Rationale of Its Use in Cancer. Front. Pharmacol. 2017, 8, 603. [Google Scholar] [CrossRef]
- Mielczarek-Puta, M.; Otto-Ślusarczyk, D.; Chrzanowska, A.; Filipek, A.; Graboń, W. Telmisartan Influences the Antiproliferative Activity of Linoleic Acid in Human Colon Cancer Cells. Nutr. Cancer 2020, 72, 98–109. [Google Scholar] [CrossRef]
- Otto-Ślusarczyk, D.; Mielczarek-Puta, M.; Graboń, W. The Real Cytotoxic Effect of Artemisinins on Colon Cancer Cells in a Physiological Cell Culture Setting. How Composition of the Culture Medium Biases Experimental Findings. Pharmaceuticals 2021, 14, 976. [Google Scholar] [CrossRef]
- Gamallat, Y.; Meyiah, A.; Kuugbee, E.D.; Hago, A.M.; Chiwala, G.; Awadasseid, A.; Bamba, D.; Zhang, X.; Shang, X.; Luo, F.; et al. Lactobacillus rhamnosus induced epithelial cell apoptosis, ameliorates inflammation and prevents colon cancer development in an animal model. Biomed. Pharmacother. 2016, 83, 536–541. [Google Scholar] [CrossRef]
- Dehghani, N.; Tafvizi, F.; Jafari, P. Cell cycle arrest and anti-cancer potential of probiotic Lactobacillus rhamnosus against HT-29 cancer cells. BioImpacts 2020, 11, 245–252. [Google Scholar] [CrossRef]
- Yeung, T.M.; Gandhi, S.C.; Wilding, J.L.; Muschel, R.; Bodmer, W.F. Cancer stem cells from colorectal cancer-derived cell lines. Proc. Natl. Acad. Sci. USA 2010, 107, 3722–3727. [Google Scholar] [CrossRef]
- Olejniczak, A.; Szaryńska, M.; Kmieć, Z. In vitro characterization of spheres derived from colorectal cancer cell lines. Int. J. Oncol. 2017, 52, 599–612. [Google Scholar] [CrossRef]
- Budu, O.; Banciu, C.; Pinzaru, I.; Sarău, C.; Lighezan, D.; Șoica, C.; Dehelean, C.; Drăghici, G.; Dolghi, A.; Prodea, A.; et al. A Combination of Two Probiotics, Lactobacillus sporogenes and Clostridium butyricum, Inhibits Colon Cancer Development: An In Vitro Study. Microorganisms 2022, 10, 1692. [Google Scholar] [CrossRef] [PubMed]
- Escamilla, J.; Lane, M.A.; Maitin, V. Cell-Free Supernatants from Probiotic Lactobacillus casei and Lactobacillus rhamnosus GG Decrease Colon Cancer Cell Invasion In Vitro. Nutr. Cancer 2012, 64, 871–878. [Google Scholar] [CrossRef]
- Shyu, P.T.; Oyong, G.G.; Cabrera, E.C. Cytotoxicity of Probiotics from Philippine Commercial Dairy Products on Cancer Cells and the Effect on Expression of cfos and cjun Early Apoptotic-Promoting Genes and Interleukin-1 β and Tumor Necrosis Factor- α Proinflammatory Cytokine Genes. BioMed Res. Int. 2014, 2014, 491740. [Google Scholar] [CrossRef]
- Hadad, S.E.; Al Hazmi, B.; Alhebshi, A.; Aldahlawi, A.M.; Bassam, R. Al Lactobacillus rhamnosus Enhances the Immunological Antitumor Effect of 5-Fluorouracil against Colon Cancer. Pakistan J. Biol. Sci. 2019, 22, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Ferro, A.; Mestre, T.; Carneiro, P.; Sahumbaiev, I.; Seruca, R.; Sanches, J.M. Blue intensity matters for cell cycle profiling in fluorescence DAPI-stained images. Lab. Investig. 2017, 97, 615–625. [Google Scholar] [CrossRef]
- Eidet, J.R.; Pasovic, L.; Maria, R.; Jackson, C.J.; Utheim, T.P. Objective assessment of changes in nuclear morphology and cell distribution following induction of apoptosis. Diagn. Pathol. 2014, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Riaz Rajoka, M.S.; Zhao, H.; Mehwish, H.M.; Li, N.; Lu, Y.; Lian, Z.; Shao, D.; Jin, M.; Li, Q.; Zhao, L.; et al. Anti-tumor potential of cell free culture supernatant of Lactobacillus rhamnosus strains isolated from human breast milk. Food Res. Int. 2019, 123, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Q.; Gao, N.; Wang, Z.; Li, F.; Li, J.; Shan, A. Exopolysaccharides produced by Lactobacillus rhamnosus GG alleviate hydrogen peroxide-induced intestinal oxidative damage and apoptosis through the Keap1/Nrf2 and Bax/Bcl-2 pathways in vitro. Food Funct. 2021, 12, 9632–9641. [Google Scholar] [CrossRef]
- Singh, A.K.; Hertzberger, R.Y.; Knaus, U.G. Hydrogen peroxide production by lactobacilli promotes epithelial restitution during colitis. Redox Biol. 2018, 16, 11–20. [Google Scholar] [CrossRef]
- Sorolla, M.A.; Hidalgo, I.; Sorolla, A.; Montal, R.; Pallisé, O.; Salud, A.; Parisi, E. Microenvironmental Reactive Oxygen Species in Colorectal Cancer: Involved Processes and Therapeutic Opportunities. Cancers 2021, 13, 5037. [Google Scholar] [CrossRef]
- Bahnassy, A.A.; Zekri, A.-R.N.; El-Houssini, S.; El-Shehaby, A.M.; Mahmoud, M.R.; Abdallah, S.; El-Serafi, M. Cyclin A and cyclin D1 as significant prognostic markers in colorectal cancer patients. BMC Gastroenterol. 2004, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, L. Degradation strategy of cyclin D1 in cancer cells and the potential clinical application. Front. Oncol. 2022, 12, 4343. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, L.K.; Sharma, M.; Chandel, D.; Shukla, G. Prophylactic intervention of probiotics (L.acidophilus, L.rhamnosus GG) and celecoxib modulate Bax-mediated apoptosis in 1,2-dimethylhydrazine-induced experimental colon carcinogenesis. BMC Cancer 2018, 18, 1111. [Google Scholar] [CrossRef]
- Tukenmez, U.; Aktas, B.; Aslim, B.; Yavuz, S. The relationship between the structural characteristics of lactobacilli-EPS and its ability to induce apoptosis in colon cancer cells in vitro. Sci. Rep. 2019, 9, 8268. [Google Scholar] [CrossRef]
- Hu, M.; Wu, X.; Luo, M.; Wei, H.; Xu, D.; Xu, F. Lactobacillus rhamnosus FLRH93 protects against intestinal damage in mice induced by 5-fluorouracil. J. Dairy Sci. 2020, 103, 5003–5018. [Google Scholar] [CrossRef]
- Evinova, A.; Cizmarova, B.; Hatokova, Z.; Racay, P. Correction to: High-Resolution Respirometry in Assessment of Mitochondrial Function in Neuroblastoma SH-SY5Y Intact Cells. J. Membr. Biol. 2020, 253, 137. [Google Scholar] [CrossRef]
- Bedreag, O.; Papurica, M.; Rogobete, A.; Sandesc, D.; Dumache, R.; Cradigati, C.; Sarandan, M.; Bratu, L.; Popovici, S.; Sima, L. Using Circulating miRNAs as Biomarkers for the Evaluation and Monitoring of the Mitochondrial Damage in the Critically Ill Polytrauma Patients. Clin. Lab. 2016, 62, 1397–1403. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Tseng, L.-M.; Lee, H.-C. Role of mitochondrial dysfunction in cancer progression. Exp. Biol. Med. 2016, 241, 1281–1295. [Google Scholar] [CrossRef]
- Chekulayev, V.; Mado, K.; Shevchuk, I.; Koit, A.; Kaldma, A.; Klepinin, A.; Timohhina, N.; Tepp, K.; Kandashvili, M.; Ounpuu, L.; et al. Metabolic remodeling in human colorectal cancer and surrounding tissues: Alterations in regulation of mitochondrial respiration and metabolic fluxes. Biochem. Biophys. Rep. 2015, 4, 111–125. [Google Scholar] [CrossRef]
- Djafarzadeh, S.; Jakob, S.M. High-resolution Respirometry to Assess Mitochondrial Function in Permeabilized and Intact Cells. J. Vis. Exp. 2017, 120, e54985. [Google Scholar] [CrossRef]
- Dong, L.; Gopalan, V.; Holland, O.; Neuzil, J. Mitocans Revisited: Mitochondrial Targeting as Efficient Anti-Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 7941. [Google Scholar] [CrossRef] [PubMed]
- Kaldma, A.; Klepinin, A.; Chekulayev, V.; Mado, K.; Shevchuk, I.; Timohhina, N.; Tepp, K.; Kandashvili, M.; Varikmaa, M.; Koit, A.; et al. An in situ study of bioenergetic properties of human colorectal cancer: The regulation of mitochondrial respiration and distribution of flux control among the components of ATP synthasome. Int. J. Biochem. Cell Biol. 2014, 55, 171–186. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).