Synthesis of Urea-Formaldehyde Fertilizers and Analysis of Factors Affecting These Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Preparation of Urea Formaldehyde
2.2.1. Preparation Principle
2.2.2. Experimental Design and Methods
2.3. Determination of Urea Formaldehyde
Measurement of Cold Water-Insoluble Nitrogen and Hot Water-Insoluble Nitrogen:
2.4. Micro Chemical Characterization of Urea Formaldehyde
3. Result
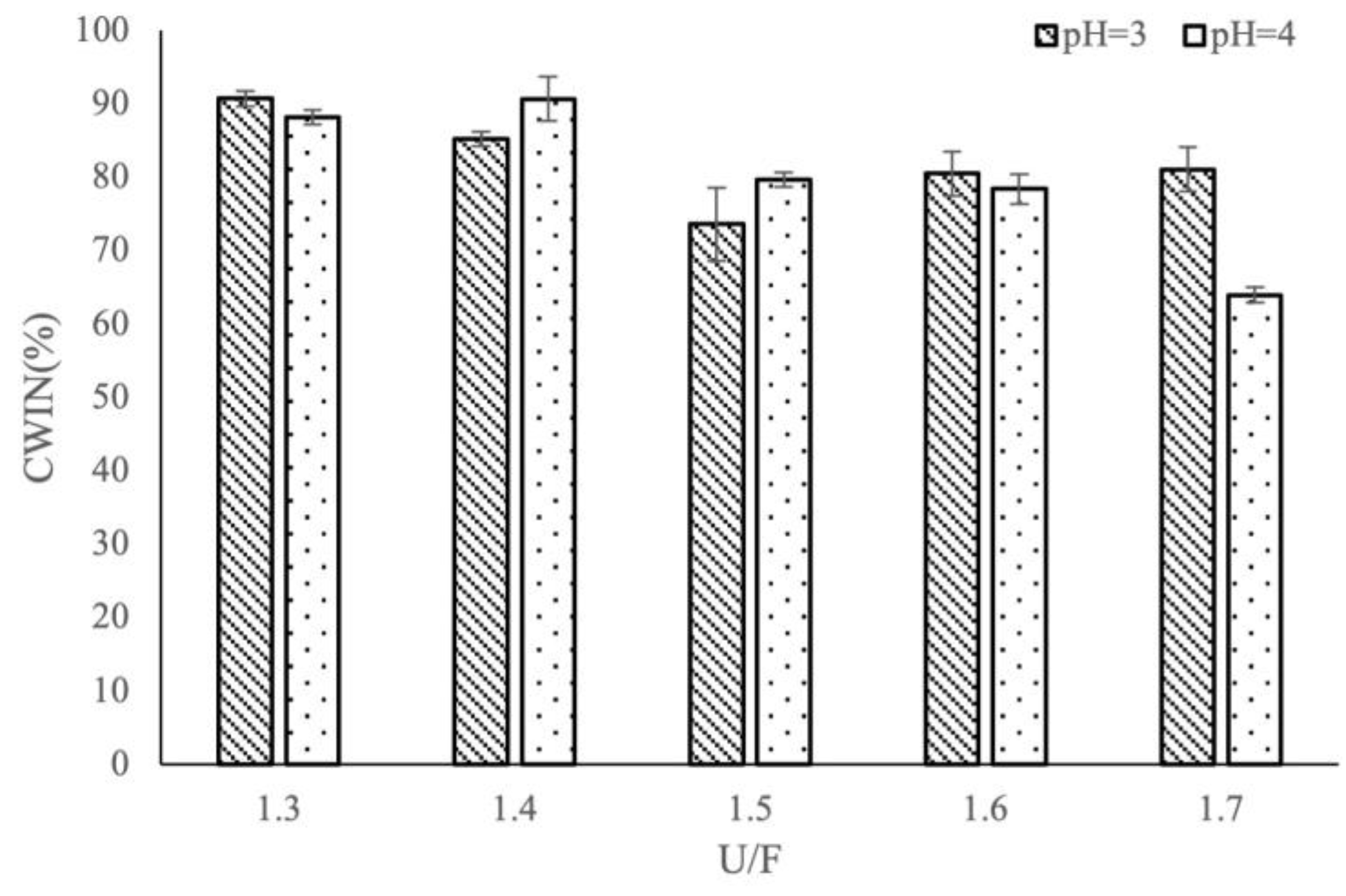
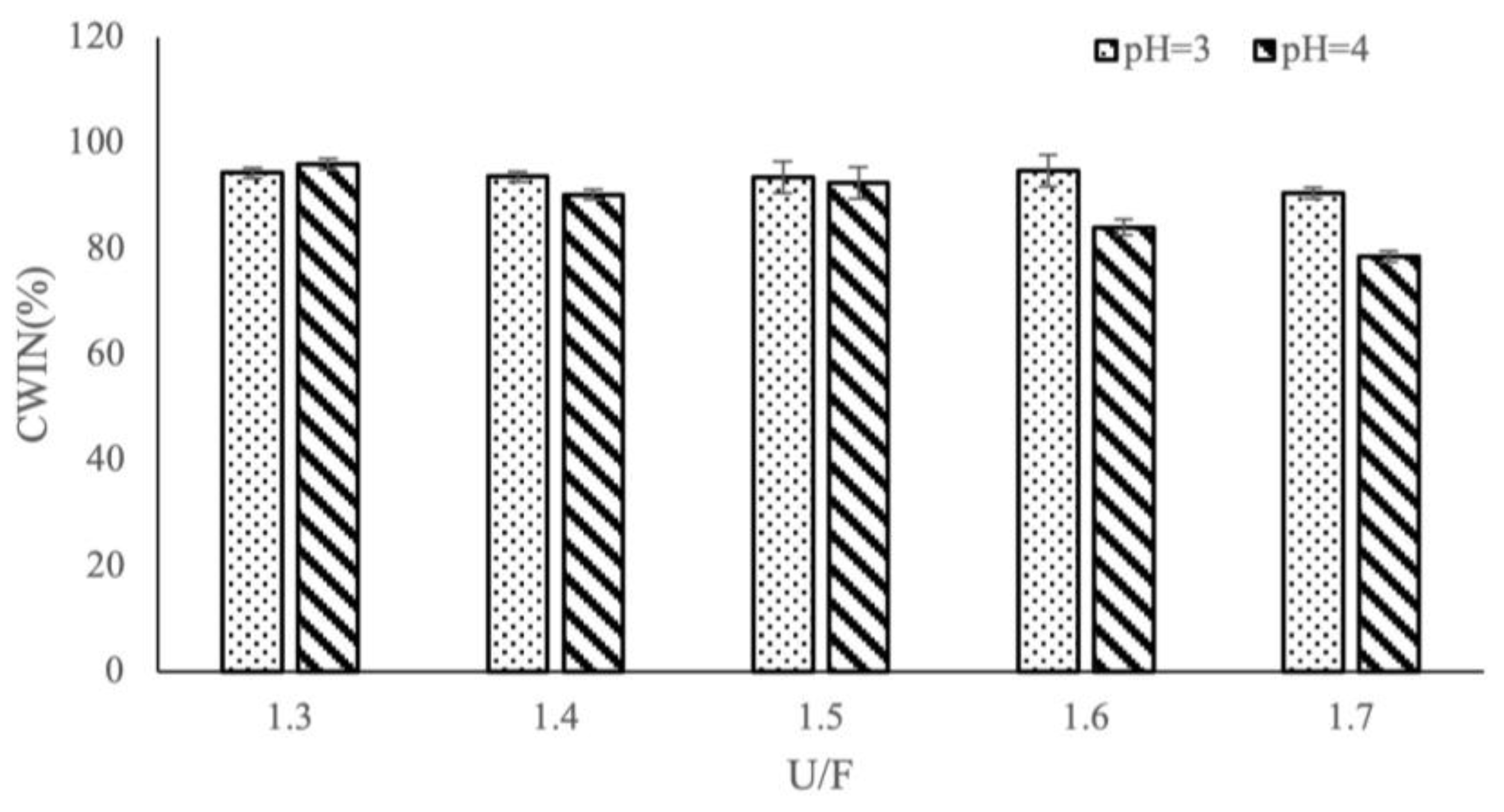
3.1. Effects of Different Reaction Factors on CWIN
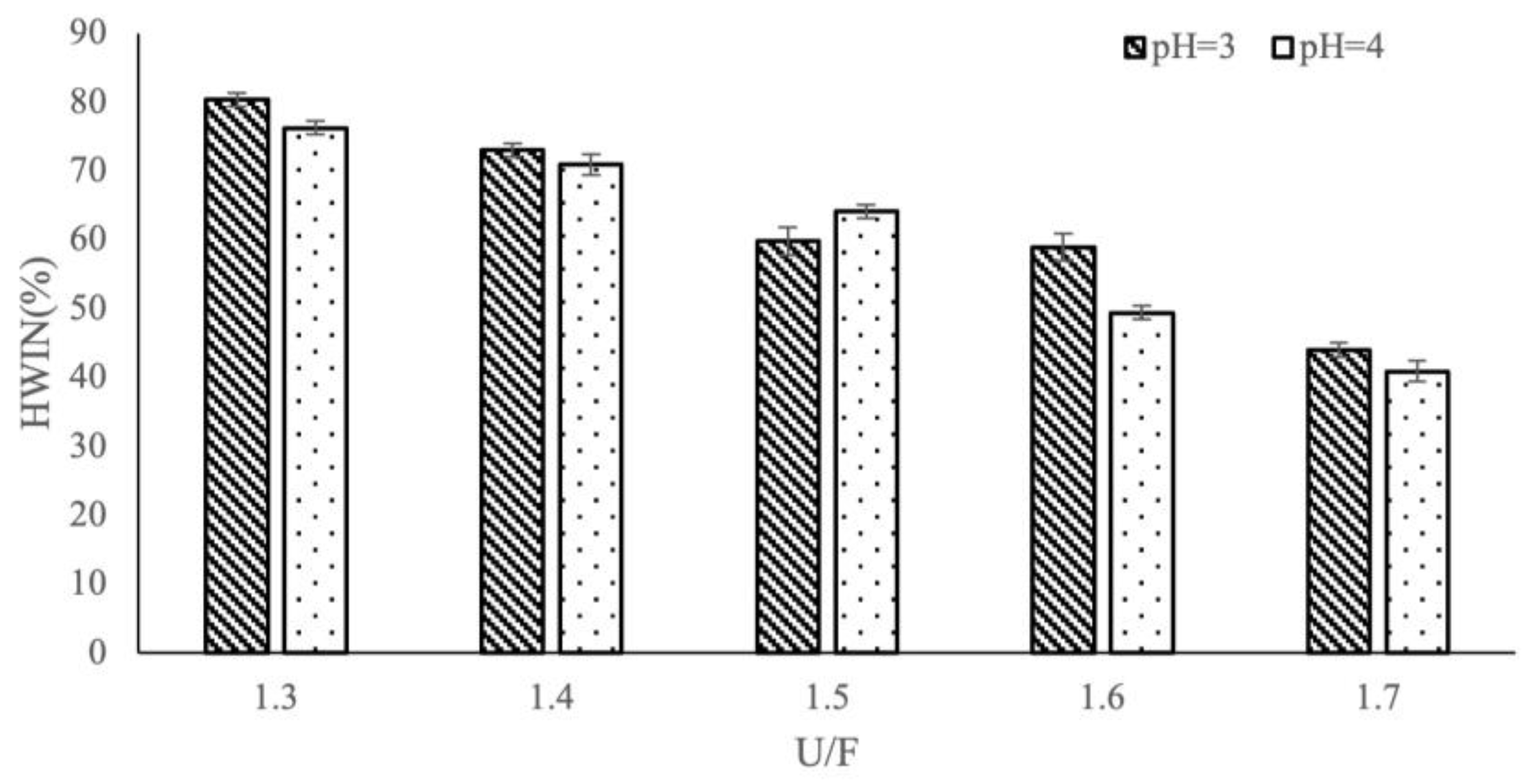
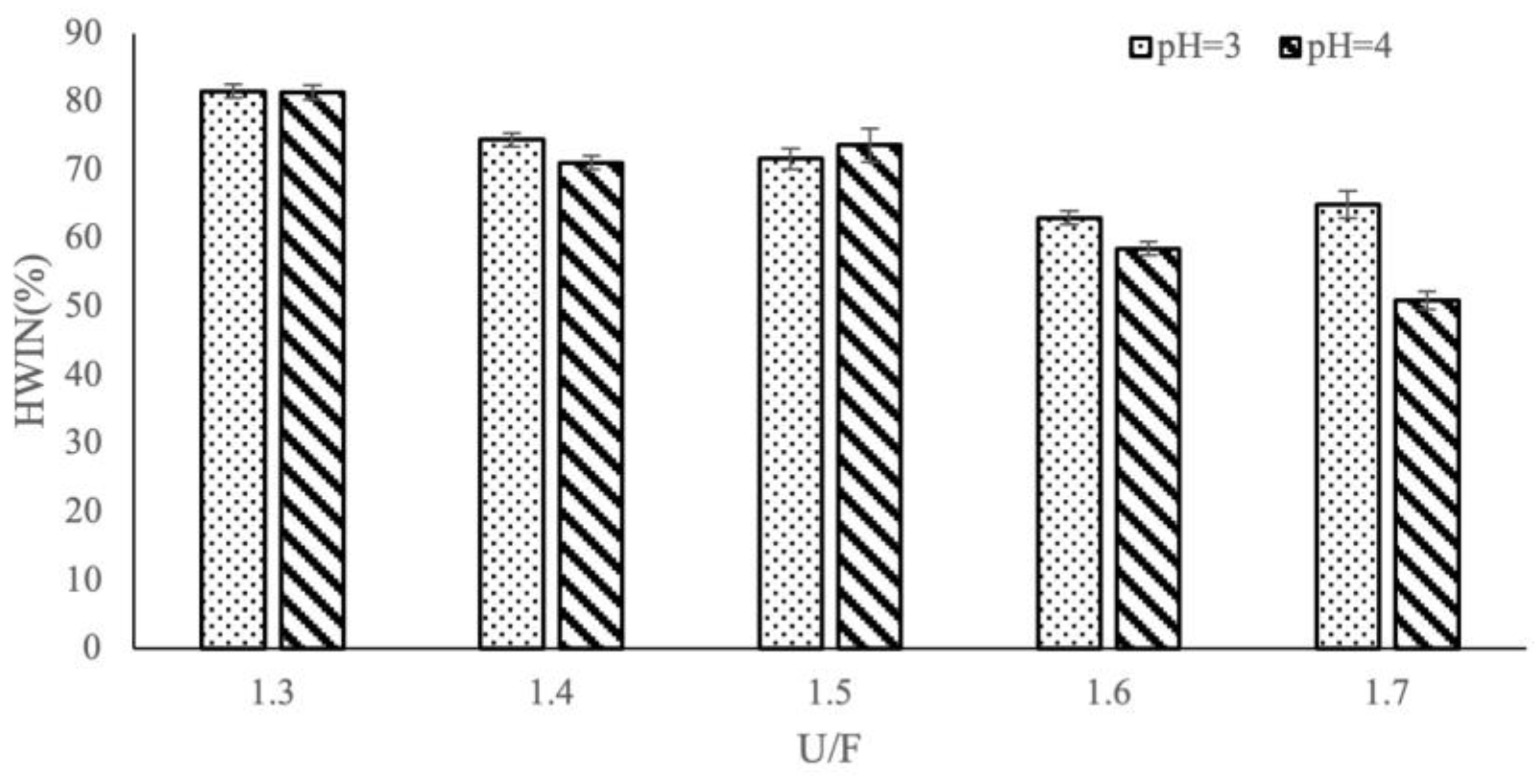
3.2. Effect of Different Reaction Factors on HWIN
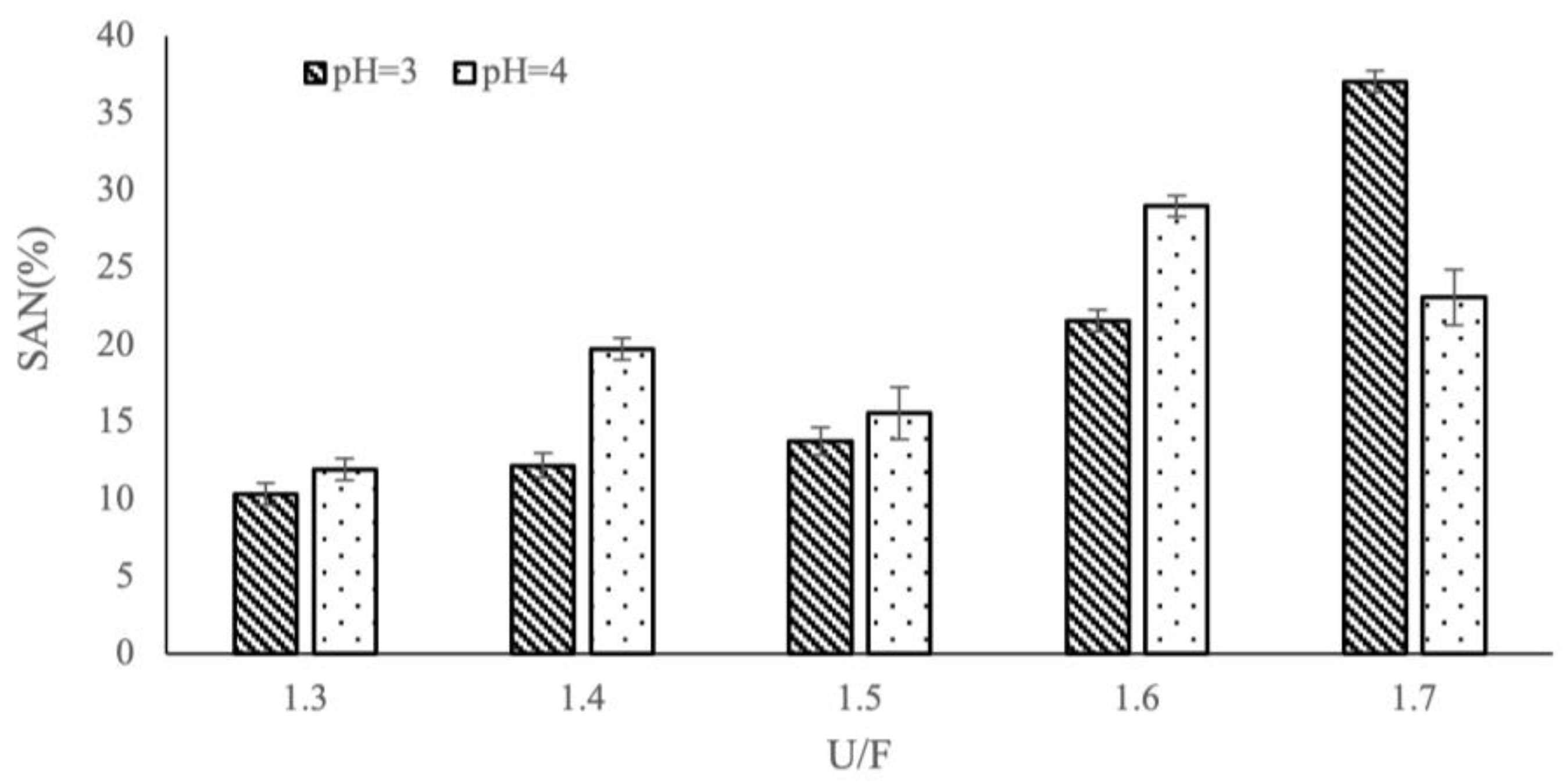
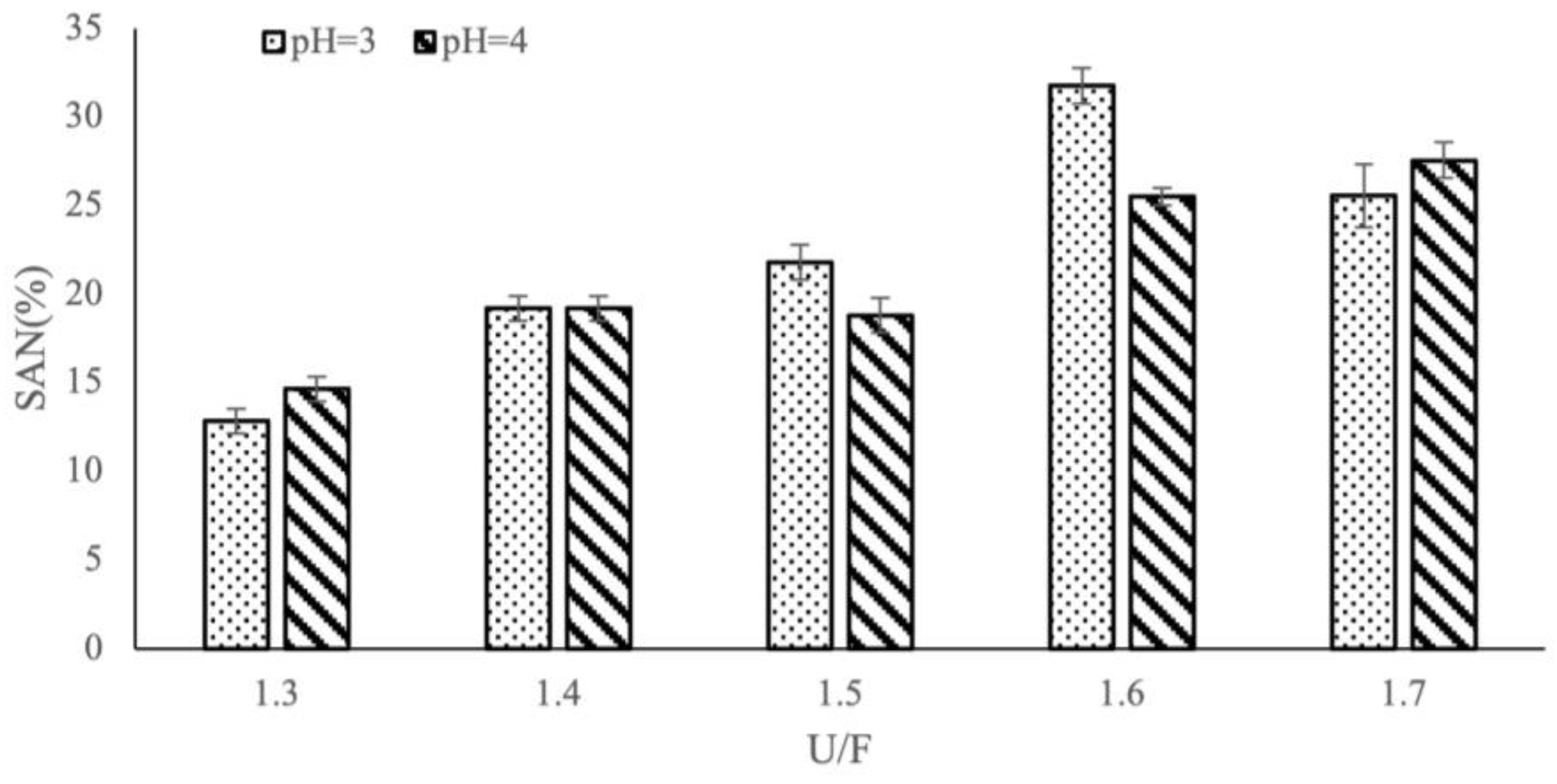
3.3. Effect of Different Reaction Factors on SAN
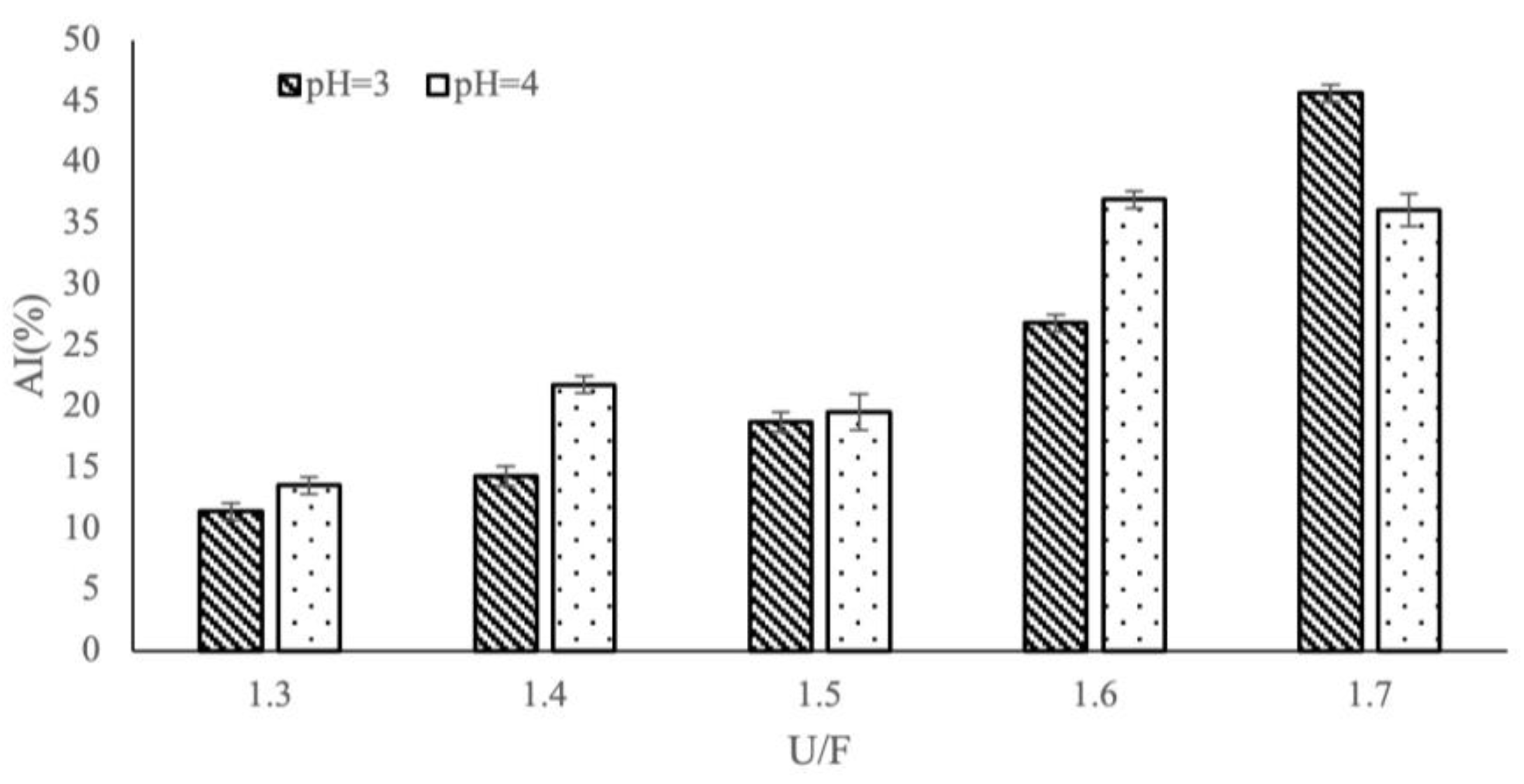
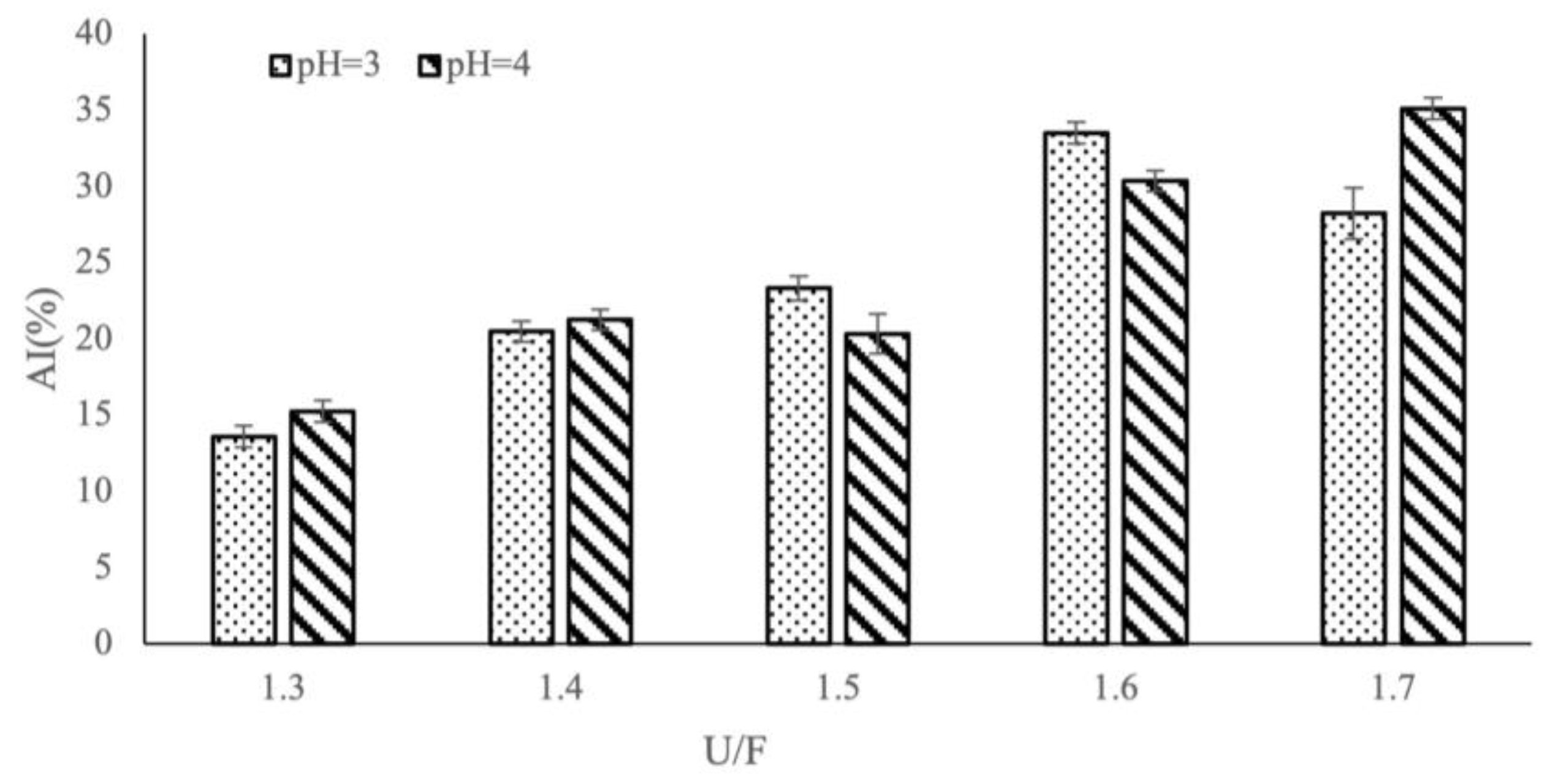
3.4. Influence of Different Reaction Factors on AI
3.5. Analysis of Characteristic Urea Formaldehyde Samples
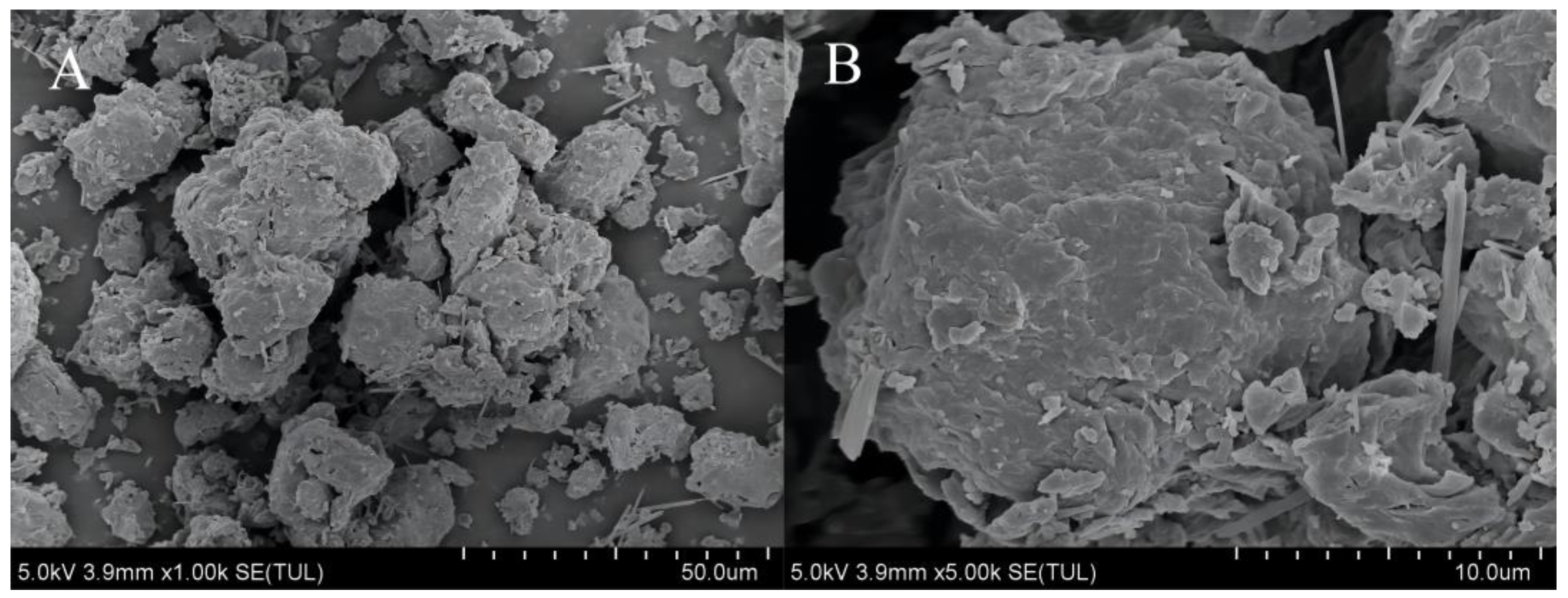
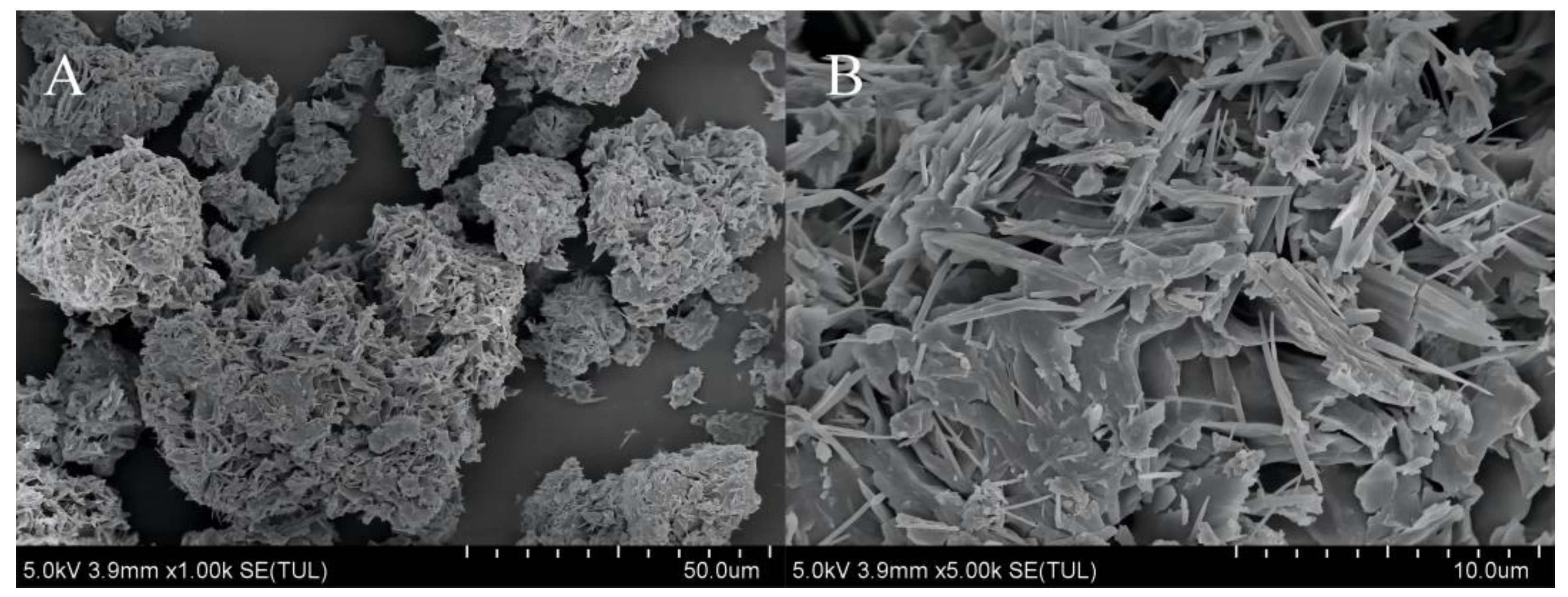
3.5.1. Microscopic Morphology of Urea Formaldehyde Samples
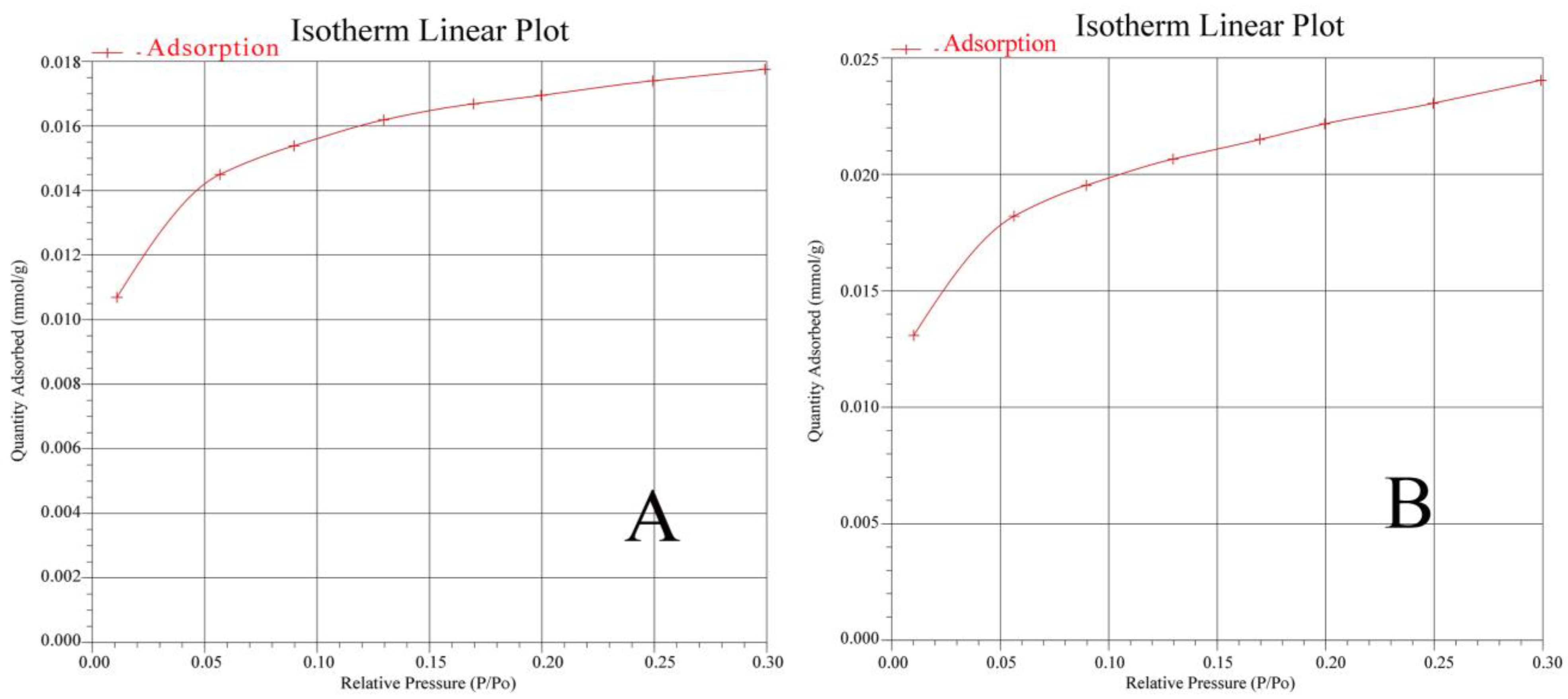
3.5.2. BET Analysis of Characteristic Samples
3.5.3. FT-IR Analysis of Characteristic Samples
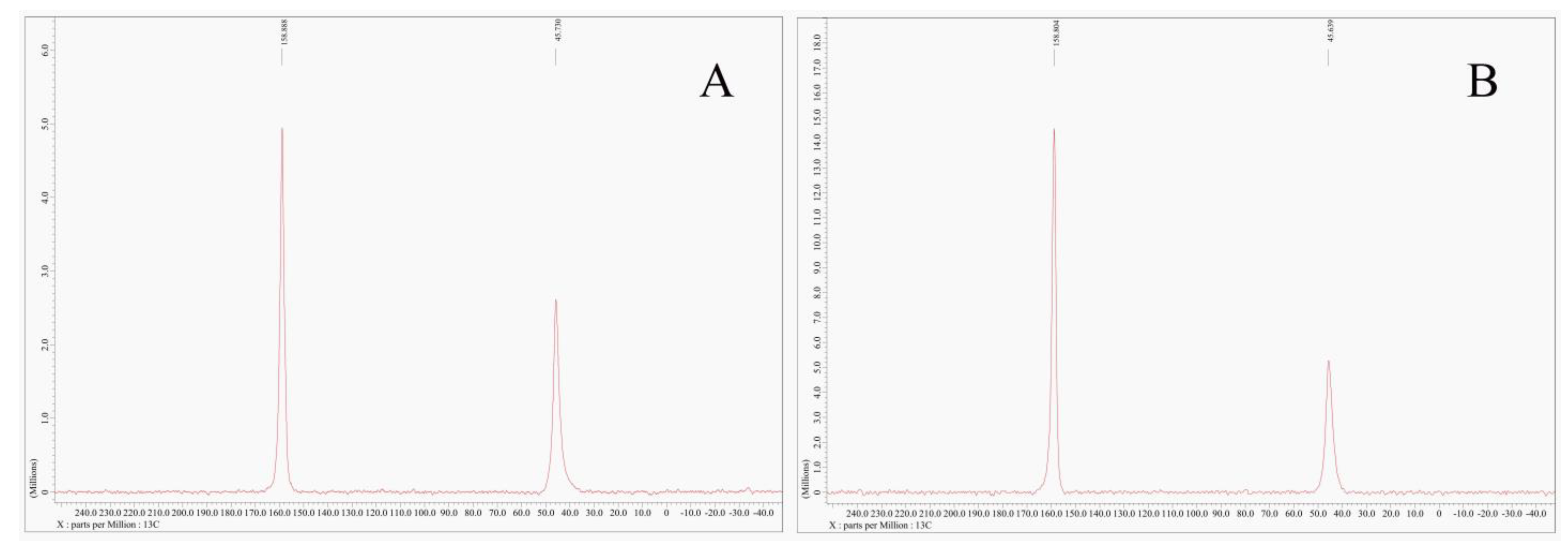
3.5.4. NMR Analysis of Characteristic Samples
3.5.5. Mass Spectrometry Analysis of Characteristic Samples
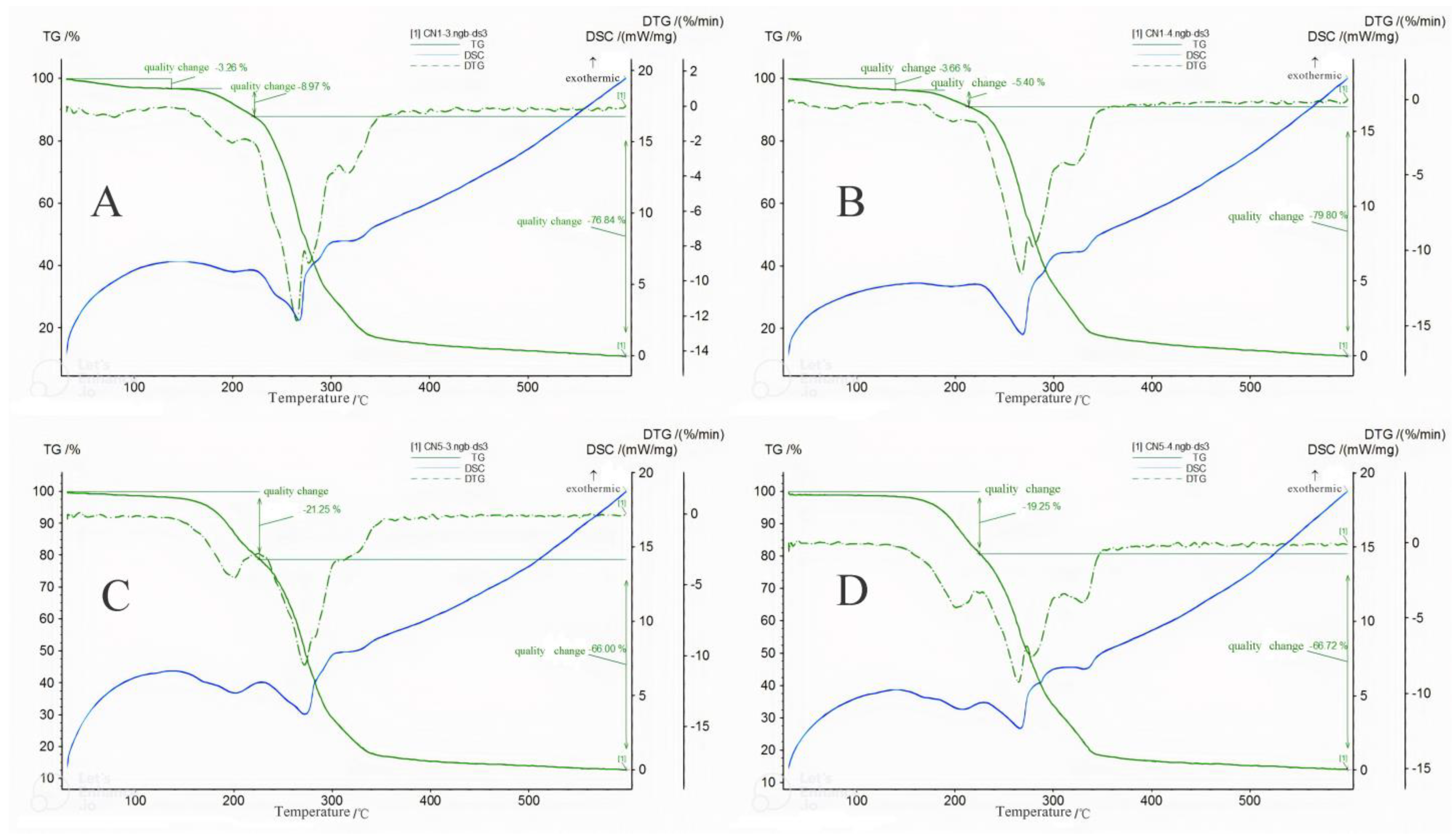
3.5.6. Thermal Stability Analysis of Characteristic Samples
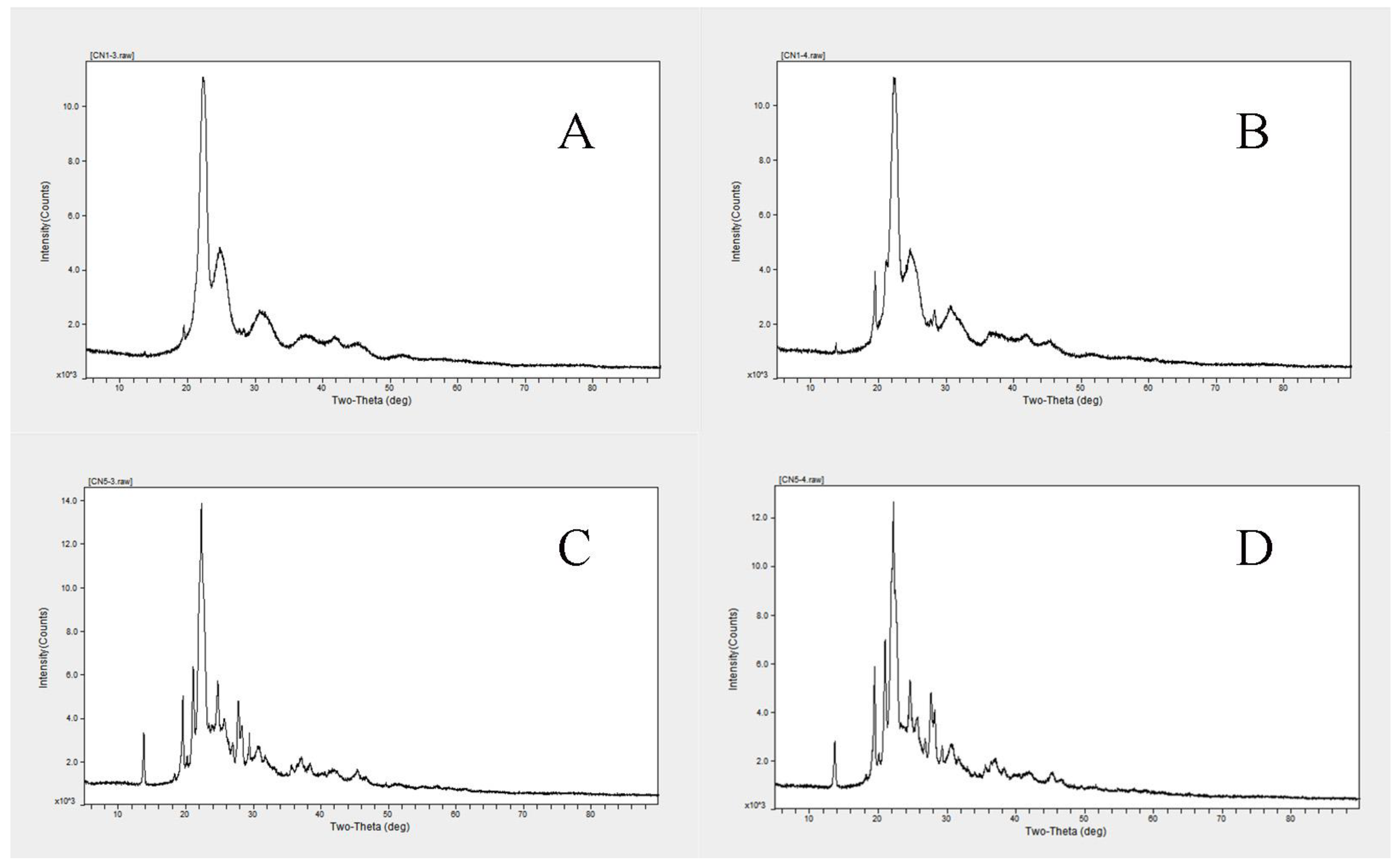
3.5.7. XRD Analysis of Characteristic Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunter, M.C.; Smith, R.G.; Schipanski, M.E.; Atwood, L.W.; Mortensen, D.A. Agriculture in 2050: Recalibrating targets for sustainable intensification. Bioscience 2017, 67, 386–391. [Google Scholar] [CrossRef]
- Beig, B.; Niazi, M.B.K.; Jahan, Z.; Pervaiz, E.; Shah, G.A.; Haq, M.U.; Zafar, M.I.; Zia, M. Slow-release urea prills developed using organic and inorganic blends in fluidized bed coater and their effect on spinach productivity. Sustainability 2020, 12, 5944. [Google Scholar] [CrossRef]
- Calicioglu, O.; Flammini, A.; Bracco, S.; Bellù, L.; Sims, R. The future challenges of food and agriculture: An integrated analysis of trends and solutions. Sustainability 2019, 11, 222. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, H.; Li, W.; Liu, Z.; Li, J. Synthesis and characterization of double-network hydrogels based on sodium alginate and halloysite for slow release fertilizers. Int. J. Biol. Macromol. 2020, 164, 557–565. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Snyder, C.S.; Bruulsema, T.W.; Jensen, T.L.; Fixen, P.E. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric. Ecosyst. Environ. 2009, 133, 247–266. [Google Scholar] [CrossRef]
- Serrano-Silva, N.; Luna-Guido, M.; Fernández-Luqueno, F.; Marsch, R.; Dendooven, L. Emission of greenhouse gases from an agricultural soil amended with urea: A laboratory study. Appl. Soil Ecol. 2011, 47, 92–97. [Google Scholar] [CrossRef]
- Tomasz, S.; Ewa, S.; Magdalena, S.; Wojciech, S. N2O emission and nitrogen and carbon leaching from the soil in relation to long-term and current mineral and organic fertilization—A laboratory study. Plant Soil Environ. 2017, 63, 97–104. [Google Scholar]
- Sim, D.; Tan, I.; Lim, L.; Hameed, B. Encapsulated biochar-based sustained release fertilizer for precision agriculture: A review. J. Clean. Prod. 2021, 303, 127018. [Google Scholar] [CrossRef]
- Swami, K.; Sahu, B.K.; Nagargade, M.; Kaur, K.; Pathak, A.D.; Shukla, S.K.; Stobdan, T.; Shanmugam, V. Starch wall of urea: Facile starch modification to residue-free stable urea coating for sustained release and crop productivity. Carbohydr. Polym. 2023, 317, 121042. [Google Scholar] [CrossRef]
- Salimi, M.; Channab, B.-E.; El Idrissi, A.; Zahouily, M.; Motamedi, E. A comprehensive review on starch: Structure, modification, and applications in slow/controlled-release fertilizers in agriculture. Carbohydr. Polym. 2023, 322, 121326. [Google Scholar] [CrossRef]
- Ni, B.; Lu, S.; Liu, M. Novel multinutrient fertilizer and its effect on slow release, water holding, and soil amending. Ind. Eng. Chem. Res. 2012, 51, 12993–13000. [Google Scholar] [CrossRef]
- Kottegoda, N.; Sandaruwan, C.; Priyadarshana, G.; Siriwardhana, A.; Rathnayake, U.A.; Berugoda Arachchige, D.M.; Kumarasinghe, A.R.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A. Urea-hydroxyapatite nanohybrids for slow release of nitrogen. ACS Nano 2017, 11, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Souri, M.K.; Naiji, M.; Kianmehr, M.H. Nitrogen release dynamics of a slow release urea pellet and its effect on growth, yield, and nutrient uptake of sweet basil (Ocimum basilicum L.). J. Plant Nutr. 2019, 42, 604–614. [Google Scholar] [CrossRef]
- Naz, M.Y.; Sulaiman, S.A. Slow release coating remedy for nitrogen loss from conventional urea: A review. J. Control. Release 2016, 225, 109–120. [Google Scholar] [CrossRef]
- Sahu, B.K.; Nagargade, M.; Chandel, M.; Kaur, K.; Swami, K.; Kumar, P.; Palanisami, M.; Pathak, A.D.; Shukla, S.K.; Shanmugam, V. Eco-friendly urea nanosack: Jute grafted silica nanoring woven fertilizer to control urea release and enhance crop productivity. ACS Sustain. Chem. Eng. 2022, 10, 13357–13366. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Ma, R.; Dong, Y.; Huang, S.; Sao, J.; Jiang, Y.; Ma, L.; Cheng, D. Determination of residual formaldehyde in urea-formaldehyde fertilizer and formaldehyde release from urea-formaldehyde fertilizer during decomposition. J. Polym. Environ. 2020, 28, 2191–2198. [Google Scholar] [CrossRef]
- Nardi, P.; Neri, U.; Matteo, G.D. Nitrogen release from slow-release fertilizers in soils with different microbial activities. Pedosphere 2018, 28, 332–340. [Google Scholar] [CrossRef]
- Pereira, E.I.; Minussi, F.B.; da Cruz, C.C.; Bernardi, A.C.; Ribeiro, C. Urea-montmorillonite-extruded nanocomposites: A novel slow-release material. J. Agric. Food Chem. 2012, 60, 5267–5272. [Google Scholar] [CrossRef]
- Adnan, N.; Nordin, S.M.; Bahruddin, M.A.; Tareq, A.H. A state-of-the-art review on facilitating sustainable agriculture through green fertilizer technology adoption: Assessing farmers behavior. Trends Food Sci. Technol. 2019, 86, 439–452. [Google Scholar] [CrossRef]
- Fan, X.; Li, Y. Nitrogen release from slow-release fertilizers as affected by soil type and temperature. Soil Sci. Soc. Am. J. 2010, 74, 1635–1641. [Google Scholar] [CrossRef]
- Jahns, T.; Ewen, H.; Kaltwasser, H. Biodegradability of urea-aldehyde condensation products. J. Polym. Environ. 2003, 11, 155–159. [Google Scholar] [CrossRef]
- Cheng, D.; Zhao, G.; Bai, T.; Liu, Y. Preparation and nutrient release mechanism of a polymer as slow-release compound fertilizer. J. Chem. Soc. Pak. 2014, 36, 647–653. [Google Scholar]
- Zhao, G.; Liu, Y.; Tian, Y.; Sun, Y.; Cao, Y. Preparation and properties of macromelecular slow-release fertilizer containing nitrogen, phosphorus and potassium. J. Polym. Res. 2010, 17, 119–125. [Google Scholar] [CrossRef]
- Yamamoto, C.F.; Pereira, E.I.; Mattoso, L.H.; Matsunaka, T.; Ribeiro, C. Slow release fertilizers based on urea/urea-formaldehyde polymer nanocomposites. Chem. Eng. J. 2016, 287, 390–397. [Google Scholar] [CrossRef]
- Li, T.; Guo, X.; Liang, J.; Wang, H.; Xie, X.; Du, G. Competitive formation of the methylene and methylene ether bridges in the urea-formaldehyde reaction in alkaline solution: A combined experimental and theoretical study. Wood Sci. Technol. 2015, 49, 475–493. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, M.; Liu, Z.; Tian, X.; Zhang, S.; Zhao, C.; Lu, H. Modeling and optimizing the synthesis of urea-formaldehyde fertilizers and analyses of factors affecting these processes. Sci. Rep. 2018, 8, 4504. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Peng, Z.; Feng, Q.; Lou, Y. Study on the properties of urea-formaldehyde resin in repairing microcracks of cement stone in oil well under CO2 acid environment. React. Funct. Polym. 2023, 191, 105688. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, Y.; Hou, S.; Cheng, D.; Yao, Y.; Zhang, S.; Xie, J.; Wang, X.; Ma, X.; Yu, Z. Multifunctional iron–humic acid fertilizer from ball milling double-shelled Fe–N-doped hollow mesoporous carbon microspheres with Lignite. ACS Sustain. Chem. Eng. 2021, 9, 717–731. [Google Scholar] [CrossRef]
- Kovács, K.; Czech, V.r.; Fodor, F.; Solti, A.; Lucena, J.J.; Santos-Rosell, S.; Hernández-Apaolaza, L. Characterization of Fe-leonardite complexes as novel natural iron fertilizers. J. Agric. Food Chem. 2013, 61, 12200–12210. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, Z.; Zhang, M.; Tian, X.; Chen, J.; Sun, L. Synthesis and application of urea-formaldehyde for manufacturing a controlled-release potassium fertilizer. Ind. Eng. Chem. Res. 2018, 57, 1593–1606. [Google Scholar] [CrossRef]
- Khoeini, M.; Najafi, A.; Rastegar, H.; Amani, M. Improvement of hollow mesoporous silica nanoparticles synthesis by hard-templating method via CTAB surfactant. Ceram. Int. 2019, 45, 12700–12707. [Google Scholar] [CrossRef]
- Pereira, E.I.; A. Nogueira, A.R.; Cruz, C.C.; Guimaraes, G.G.; Foschini, M.M.; Bernardi, A.C.; Ribeiro, C. Controlled urea release employing nanocomposites increases the efficiency of nitrogen use by forage. ACS Sustain. Chem. Eng. 2017, 5, 9993–10001. [Google Scholar] [CrossRef]
- Huang, J.; Bai, J.; Demir, M.; Hu, X.; Jiang, Z.; Wang, L. Efficient N-doped porous carbonaceous CO2 adsorbents derived from commercial urea-formaldehyde resin. Energy Fuels 2022, 36, 5825–5832. [Google Scholar] [CrossRef]
- Zorba, T.; Papadopoulou, E.; Hatjiissaak, A.; Paraskevopoulos, K.; Chrissafis, K. Urea-formaldehyde resins characterized by thermal analysis and FTIR method. J. Therm. Anal. Calorim. 2008, 92, 29–33. [Google Scholar] [CrossRef]
- Zhang, W.; Xiang, Y.; Fan, H.; Wang, L.; Xie, Y.; Zhao, G.; Liu, Y. Biodegradable urea-formaldehyde/PBS and its ternary nanocomposite prepared by a novel and scalable reactive extrusion process for slow-release applications in agriculture. J. Agric. Food Chem. 2020, 68, 4595–4606. [Google Scholar] [CrossRef]
- Li, D.; Yu, L.; Li, L.; Liang, J.; Wu, Z.; Xu, X.; Zhong, X.; Gong, F. Melamine-urea-formaldehyde resin adhesive modified with recycling lignin: Preparation, structures and properties. Forests 2023, 14, 1625. [Google Scholar] [CrossRef]
- Lü, S.; Gao, C.; Wang, X.; Xu, X.; Bai, X.; Gao, N.; Feng, C.; Wei, Y.; Wu, L.; Liu, M. Synthesis of a starch derivative and its application in fertilizer for slow nutrient release and water-holding. RSC Adv. 2014, 4, 51208–51214. [Google Scholar] [CrossRef]
- Roumeli, E.; Papadopoulou, E.; Pavlidou, E.; Vourlias, G.; Bikiaris, D.; Paraskevopoulos, K.; Chrissafis, K. Synthesis, characterization and thermal analysis of urea-formaldehyde/nano SiO2 resins. Thermochim. Acta 2012, 527, 33–39. [Google Scholar] [CrossRef]
- Lin, C.f.; Karlsson, O.; Martinka, J.; Rantuch, P.; Garskaite, E.; Mantanis, G.I.; Jones, D.; Sandberg, D. Approaching highly leaching-resistant fire-retardant wood by in situ polymerization with melamine formaldehyde resin. ACS Omega 2021, 6, 12733–12745. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Park, B.D. Crystalline lamellar structure of thermosetting urea-formaldehyde resins at a low molar ratio. Macromolecules 2021, 54, 2366–2375. [Google Scholar] [CrossRef]
- Kibrik, É.J.; Steinhof, O.; Scherr, G.n.; Thiel, W.R.; Hasse, H. On-line NMR spectroscopic reaction kinetic study of urea-formaldehyde resin synthesis. Ind. Eng. Chem. Res. 2014, 53, 12602–12613. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, M.; Du, G.; Duan, Z.; Zhou, X.; Li, T. Highly branched polyurea-enhanced urea-formaldehyde resin. ACS Appl. Polym. Mater. 2021, 3, 1157–1170. [Google Scholar] [CrossRef]
- Ding, Z.; Ding, Z.; Ma, T.; Zhang, H. Condensation reaction and crystallization of urea-formaldehyde resin during the curing process. BioResources 2020, 15, 2924–2936. [Google Scholar] [CrossRef]
- Park, B.D.; Jeong, H.W. Hydrolytic stability and crystallinity of cured urea-formaldehyde resin adhesives with different formaldehyde/urea mole ratios. Int. J. Adhes. Adhes. 2011, 31, 524–529. [Google Scholar] [CrossRef]
- Xiang, Y.; Ru, X.; Shi, J.; Song, J.; Zhao, H.; Liu, Y.; Zhao, G. Granular, slow-release fertilizer from urea-formaldehyde, ammonium polyphosphate, and amorphous silica gel: A new strategy using cold extrusion. J. Agric. Food chem. 2018, 66, 7606–7615. [Google Scholar] [CrossRef]
- Li, K.; Li, H.; Cui, Y.; Li, Z.; Ji, J.; Feng, Y.; Chen, S.; Zhang, M.; Wang, H. Dual-functional coatings with self-lubricating and self-healing properties by combining poly (urea-formaldehyde)/SiO2 hybrid microcapsules containing linseed oil. Ind. Eng. Chem. Res. 2019, 58, 22032–22039. [Google Scholar] [CrossRef]
- Nuryawan, A.; Park, B.D.; Singh, A.P. Comparison of thermal curing behavior of liquid and solid urea-formaldehyde resins with different formaldehyde/urea mole ratios. J. Therm. Anal. Calorim 2014, 118, 397–404. [Google Scholar] [CrossRef]
- Nuryawan, A.; Singh, A.P.; Zanetti, M.; Park, B.D.; Causin, V. Insights into the development of crystallinity in liquid urea-formaldehyde resins. Int. J. Adhes. Adhes. 2017, 72, 62–69. [Google Scholar] [CrossRef]
- Singh, A.P.; Causin, V.; Nuryawan, A.; Park, B.D. Morphological, chemical and crystalline features of urea-formaldehyde resin cured in contact with wood. Eur. Polym. J. 2014, 56, 185–193. [Google Scholar] [CrossRef]
- Park, B.D.; Causin, V. Crystallinity and domain size of cured urea-formaldehyde resin adhesives with different formaldehyde/urea mole ratios. Eur. Polym. J. 2013, 49, 532–537. [Google Scholar] [CrossRef]
- Chen, X.Y.; Chen, C.; Zhang, Z.J.; Xie, D.H.; Deng, X. Nitrogen-doped porous carbon prepared from urea formaldehyde resins by template carbonization method for supercapacitors. Ind. Eng. Chem. Res. 2013, 52, 10181–10188. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, M.; Liu, Z.; Zhao, C.; Lu, H.; Zheng, L.; Li, Y.C. Applying and optimizing water-soluble, slow-release nitrogen fertilizers for water-saving agriculture. ACS Omega 2020, 5, 11342–11351. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Tian, J. Influence of pH condition on the hydrolysis stability and crystallinity of cured urea-formaldehyde resin during curing process. Sci. Silvae Sin. 2017, 53, 120–125. [Google Scholar]
- Wibowo, E.S.; Park, B.D. Cure kinetics of low-molar-ratio urea-formaldehyde resins reinforced with modified nanoclay using different kinetic analysis methods. Thermochim. Acta 2020, 686, 178552. [Google Scholar] [CrossRef]
- Shi, R.; Liu, K.; Liu, B.; Chen, H.; Xu, X.; Ren, Y.; Qiu, J.; Zeng, Z.; Li, L. New insight into toluene adsorption mechanism of melamine urea-formaldehyde resin based porous carbon: Experiment and theory calculation. Colloids Surf. A 2022, 632, 127600. [Google Scholar] [CrossRef]
- Abbasi, M.K.; Khizar, A. Microbial biomass carbon and nitrogen transformations in a loam soil amended with organic-inorganic N sources and their effect on growth and N-uptake in maize. Ecol. Eng. 2012, 39, 123–132. [Google Scholar] [CrossRef]
- Lubis, M.A.R.; Park, B.D.; Lee, S.M. Microencapsulation of polymeric isocyanate for the modification of urea-formaldehyde resins. Int. J. Adhes. Adhes. 2020, 100, 102599. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Lubis, M.A.R.; Park, B.D.; Kim, J.S.; Causin, V. Converting crystalline thermosetting urea-formaldehyde resins to amorphous polymer using modified nanoclay. J. Ind. Eng. Chem. 2020, 87, 78–89. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Liu, J.; Cui, X.; Hou, T.; Cheng, D. A novel synthetic slow release fertilizer with low energy production for efficient nutrient management. Sci. Total Environ. 2022, 831, 154844. [Google Scholar] [CrossRef]
- Wang, C.; Luo, D.; Zhang, X.; Huang, R.; Cao, Y.; Liu, G.; Zhang, Y.; Wang, H. Biochar-based slow-release of fertilizers for sustainable agriculture: A mini review. Environ. Sci. Ecotechnol. 2022, 10, 100167. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Cheng, M.; Zhao, C.; Li, B.; Peng, H.; Zhang, Y.; Shao, Q.; Hassan, M. Application of lignin in preparation of slow-release fertilizer: Current status and future perspectives. Ind. Crops Prod. 2022, 176, 114267. [Google Scholar] [CrossRef]


| Number | Processing Number | U/F | Urea m1, g | Formol m2, g | Catalyst (Ammonium Chloride) | pH(2) |
|---|---|---|---|---|---|---|
| 1 | N1-3 | 1.3 | 105.82 | 110 | not added | 3 |
| 2 | N1-4 | 1.3 | 105.82 | 110 | not added | 4 |
| 3 | N2-3 | 1.4 | 113.96 | 110 | not added | 3 |
| 4 | N2-4 | 1.4 | 113.96 | 110 | not added | 4 |
| 5 | N3-3 | 1.5 | 122.10 | 110 | not added | 3 |
| 6 | N3-4 | 1.5 | 122.10 | 110 | not added | 4 |
| 7 | N4-3 | 1.6 | 130.24 | 110 | not added | 3 |
| 8 | N4-4 | 1.6 | 130.24 | 110 | not added | 4 |
| 9 | N5-3 | 1.7 | 138.38 | 110 | not added | 3 |
| 10 | N5-4 | 1.7 | 138.38 | 110 | not added | 4 |
| 11 | CN1-3 | 1.3 | 105.82 | 110 | added | 3 |
| 12 | CN1-4 | 1.3 | 105.82 | 110 | added | 4 |
| 13 | CN2-3 | 1.4 | 113.96 | 110 | added | 3 |
| 14 | CN2-4 | 1.4 | 113.96 | 110 | added | 4 |
| 15 | CN3-3 | 1.5 | 122.10 | 110 | added | 3 |
| 16 | CN3-4 | 1.5 | 122.10 | 110 | added | 4 |
| 17 | CN4-3 | 1.6 | 130.24 | 110 | added | 3 |
| 18 | CN4-4 | 1.6 | 130.24 | 110 | added | 4 |
| 19 | CN5-3 | 1.7 | 138.38 | 110 | added | 3 |
| 20 | CN5-4 | 1.7 | 138.38 | 110 | added | 4 |
| Sample | Specific Surface Area/(m2·g−1) |
|---|---|
| CN1-3 | 1.2135 |
| CN5-3 | 1.6520 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Shi, Y.; Cui, Q.; Zai, X.; Zhang, S.; Lu, H.; Feng, G. Synthesis of Urea-Formaldehyde Fertilizers and Analysis of Factors Affecting These Processes. Processes 2023, 11, 3251. https://doi.org/10.3390/pr11113251
Guo Y, Shi Y, Cui Q, Zai X, Zhang S, Lu H, Feng G. Synthesis of Urea-Formaldehyde Fertilizers and Analysis of Factors Affecting These Processes. Processes. 2023; 11(11):3251. https://doi.org/10.3390/pr11113251
Chicago/Turabian StyleGuo, Yanle, Yiyun Shi, Qunxiang Cui, Xueming Zai, Shugang Zhang, Hao Lu, and Gucheng Feng. 2023. "Synthesis of Urea-Formaldehyde Fertilizers and Analysis of Factors Affecting These Processes" Processes 11, no. 11: 3251. https://doi.org/10.3390/pr11113251
APA StyleGuo, Y., Shi, Y., Cui, Q., Zai, X., Zhang, S., Lu, H., & Feng, G. (2023). Synthesis of Urea-Formaldehyde Fertilizers and Analysis of Factors Affecting These Processes. Processes, 11(11), 3251. https://doi.org/10.3390/pr11113251







