Ericaria amentacea Algae Extracts: A Sustainable Approach for the Green Synthesis of Silver Oxide Nanoparticles and Their Effectiveness against Leishmaniasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.1.1. Chemical Reagents
2.1.2. Preparation of Seaweed Extracts
2.1.3. Phenolic Chemical Profiles of the Extracts
2.1.4. Synthesis of Nanoparticles Using Seaweed Extracts
2.2. Analytical Instruments for Characterization
- (i)
- UV-VIS spectrophotometer: a Shidmadzu UV-1601, manufactured by Shimadzu Corporation in Tokyo, Japan, was employed to perform the spectrophotometric measurements.
- (ii)
- Transmission electron microscopy: a Philips JOEL TEM (New York, NY, USA) was utilized for this purpose.
- (iii)
- X-ray diffraction: a Bruker D8 Advance diffractometer with Cu Kα radiation (wavelength: 1.54 Å), manufactured by Bruker Corporation in Billerica, MA, USA, was utilized to conduct the XRD analysis.
- (iv)
- Thermal gravimetry analysis: a thermal analyzer, specifically the DTG-60H system manufactured by Shimadzu in Kyoto, Japan, was used to perform the thermal gravimetry analysis. The analysis involved heating the samples in a temperature range of 0 to 700 °C at a rate of 10 °C/min.
- (v)
- Fourier transform infrared spectra: The FTIR spectra were obtained using a spectrophotometer known as the BRUKER VERTEX 70, manufactured by Bruker Corporation in Billerica, MA, USA. The spectra were collected within the range of 4000–400 cm−1 with a resolution of 4 cm−1.
- (vi)
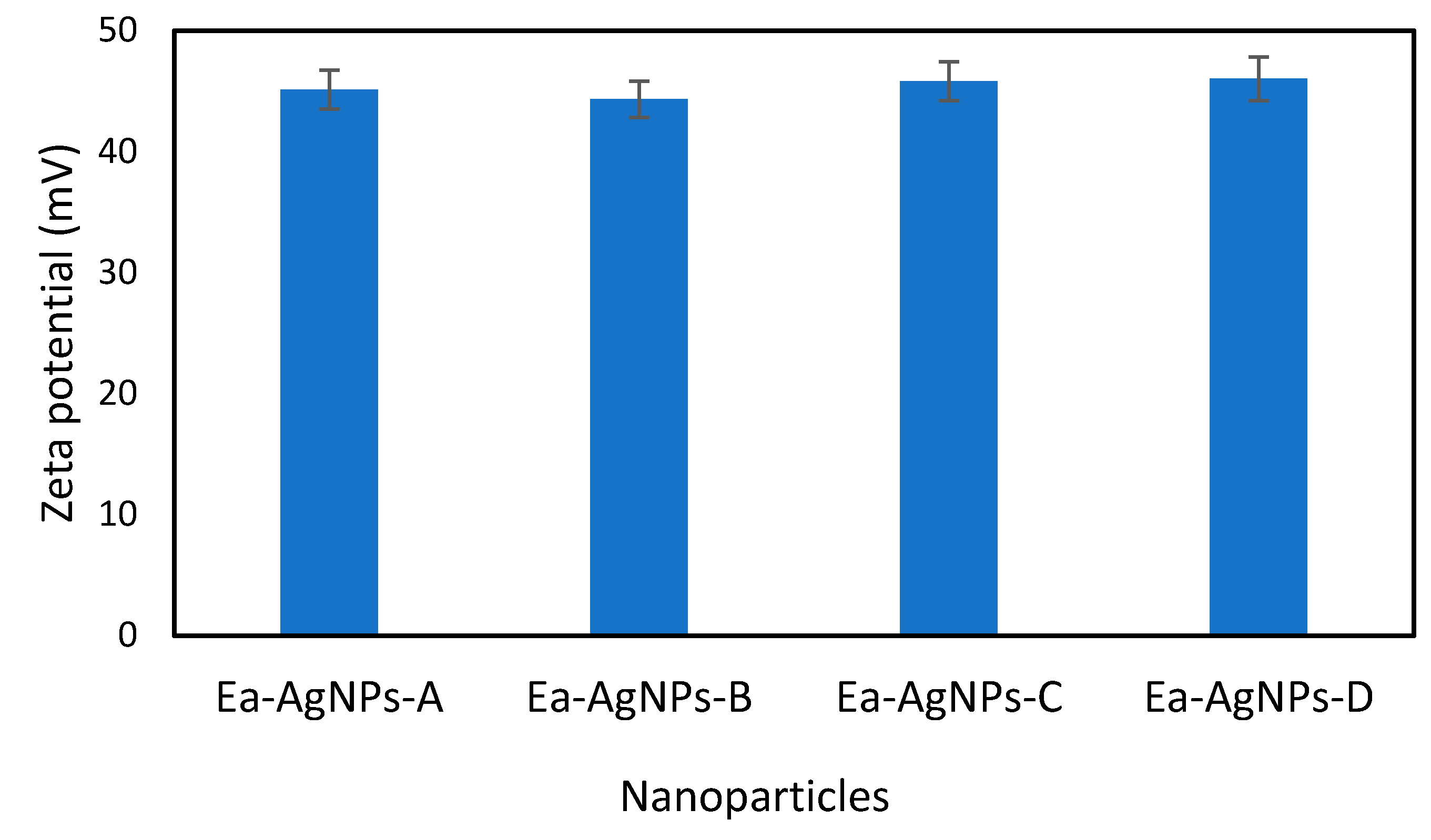
- Zeta potential analysis: the zeta potentials of each nanoparticle were measured using a Zetasizer model 3000HS, Malvern Instrument Ltd., Malvern, UK.
2.3. Biological Activities
2.3.1. Antileishmanial Tests
2.3.2. Cytotoxicity Tests
2.4. Statistical Analysis
2.5. Ethics Approval
3. Results
- The 1st weight loss, accounting for less than 1% of the total weight, occurs between 50 and 100 °C. This weight loss is attributed to the decomposition of organic compounds present on the surface of the nanoparticles.
- The 2nd weight loss, accounting for approximately 3.5%, takes place between 120 and 245 °C. It corresponds to the decomposition of AgO, leading to the formation of Ag2O and the release of O2 gas [33]. This reaction can be represented as:
- The 3rd weight loss, accounting for approximately 8%, occurs between 350 and 500 °C. It is associated with the decomposition of Ag2O into Ag [34]. The reaction can be described as:
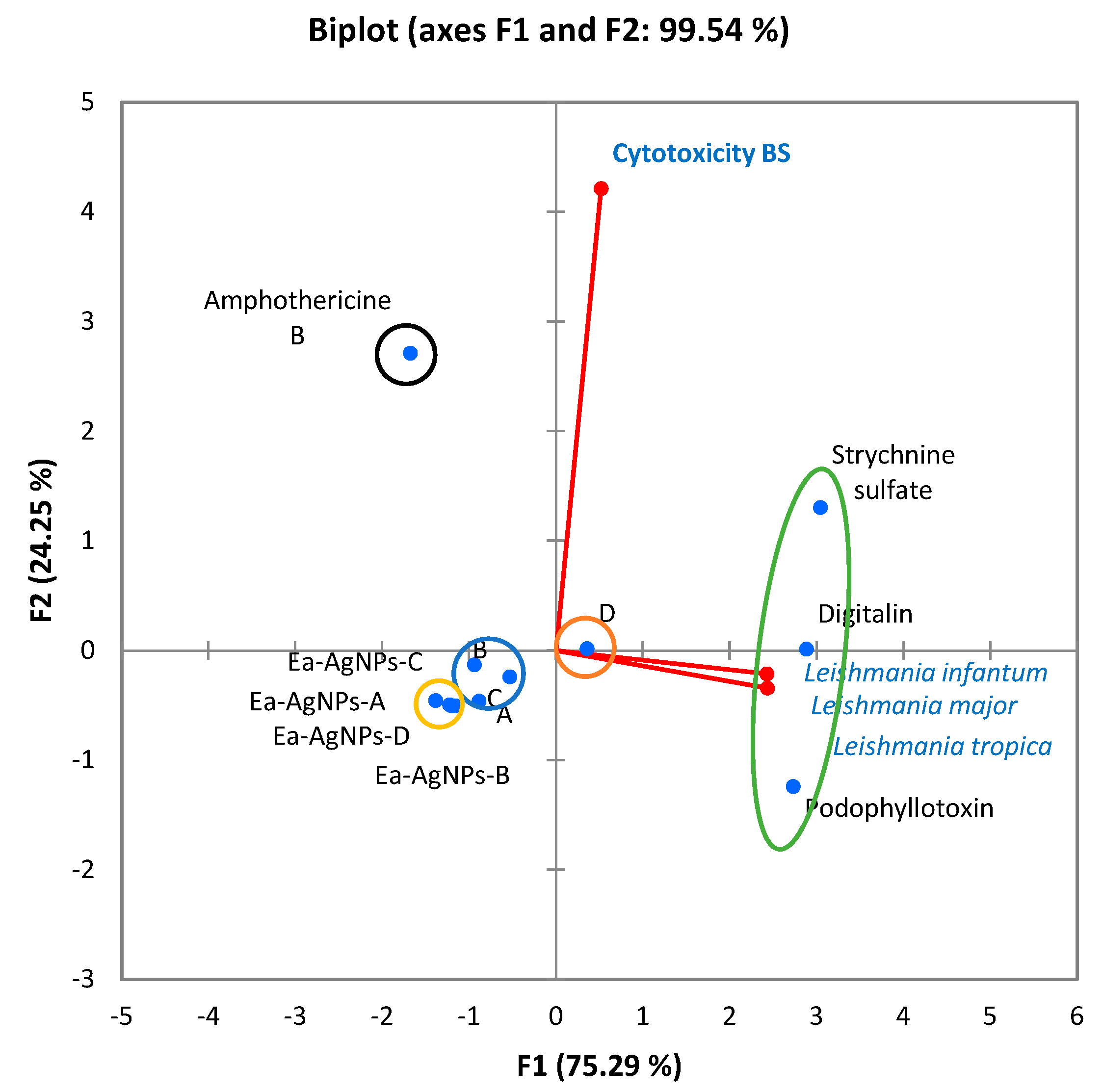
- The first class comprises only amphotericin, which shows promise as a therapeutic alternative in leishmaniasis treatment.
- The second class consists of cytotoxic activity control substances, namely podophyllotoxin, digitalin, and strychnine sulphate.
- The third class includes the four nanoparticles Ea-AgNPs-A, Ea-AgNPs-B, Ea-AgNPs-C, and Ea-AgNPs-D.
- The fourth class comprises extracts A, B, and C.
- The fifth class includes extract D.
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mengarda, A.C.; Iles, B.; Longo, J.P.F.; de Moraes, J. Recent approaches in nanocarrier-based therapies for neglected tropical diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1852. [Google Scholar] [CrossRef] [PubMed]
- Ornellas-Garcia, U.; Cuervo, P.; Ribeiro-Gomes, F.L. Malaria and leishmaniasis: Updates on co-infection. Front. Immunol. 2023, 14, 1122411. [Google Scholar] [CrossRef]
- Sampaio, R.N.R. Pharmacotherapy in leishmaniasis: Old, new treatments, their impacts and expert opinion. Expert Opin. Pharmacother. 2023, 24, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Teli, G.; Matada, G.S.P. The role of natural anti-parasitic guided development of synthetic drugs for leishmaniasis. Eur. J. Med. Chem. 2023, 258, 115609. [Google Scholar] [CrossRef] [PubMed]
- Chastonay, A.H.; Chastonay, O.J. Housing risk factors of four tropical neglected diseases: A brief review of the recent literature. Trop. Med. Infect. Dis. 2022, 7, 143. [Google Scholar] [CrossRef]
- Majumder, N.; Banerjee, A.; Saha, S. A review on new natural and synthetic anti-leishmanial chemotherapeutic agents and current perspective of treatment approaches. Acta Trop. 2023, 240, 106846. [Google Scholar] [CrossRef]
- García-Estrada, C.; Pérez-Pertejo, Y.; Domínguez-Asenjo, B.; Holanda, V.N.; Murugesan, S.; Martínez-Valladares, M.; Reguera, R.M. Further Investigations of Nitroheterocyclic Compounds as Potential Antikinetoplastid Drug Candidates. Biomolecules 2023, 13, 637. [Google Scholar] [CrossRef]
- Registre, C.; Soares, R.D.; Rubio, K.T.; Santos, O.D.; Carneiro, S.P. A Systematic Review of Drug-Carrying Nanosystems Used in the Treatment of Leishmaniasis. ACS Infect. Dis. 2023, 9, 423–449. [Google Scholar] [CrossRef]
- Imran, M.; Khan, S.A.; Abida; Alshrari, A.S.; EltahirMudawi, M.M.; Alshammari, M.K.; Alshammari, N.A. Small molecules as kinetoplastid specific proteasome inhibitors for Leishmaniasis: A patent review from 1998 to 2021. Expert Opin. Ther. Pat. 2022, 32, 591–604. [Google Scholar] [CrossRef]
- Daher, E.D.F.; da Silva Junior, G.B.; Trivedi, M.; Fayad, T.; Srisawat, N.; Nair, S.; Jha, V. Kidney complications of parasitic diseases. Nat. Rev. Nephrol. 2022, 18, 396–406. [Google Scholar] [CrossRef]
- Abdoul-Latif, F.M.; Elmi, A.; Merito, A.; Nour, M.; Risler, A.; Ainane, A.; Ainane, T. Essential oils of Tagetesminuta and Lavandulacoronopifolia from Djibouti: Chemical composition, antibacterial activity and cytotoxic activity against various human cancer cell lines. Int. J. Plant Biol. 2022, 13, 315–329. [Google Scholar] [CrossRef]
- Abdoul-Latif, F.M.; Ainane, A.; Merito, A.; Ainane, T. Chemical composition and biological activities of essential oils from Djibouti. J. Anal. Sci. Appl. Biotechnol. 2022, 4, 1–9. [Google Scholar]
- Ainane, A.; Cherroud, S.; El Kouali, M.; Abba, E.H.; Ainane, T. Chemical compositions, insecticidal and antimicrobial activities of two moroccan essential oils of Citrus limonum and Syzygiumaromaticum. Pharmacologyonline 2020, 30, 190–199. [Google Scholar]
- de Castro Levatti, E.V.; Costa-Silva, T.A.; Morais, T.R.; Fernandes, J.P.S.; Lago, J.H.G.; Tempone, A.G. Lethal action of Licarin A derivatives in Leishmania (L.) infantum: Imbalance of calcium and bioenergetic metabolism. Biochimie 2023, 208, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Ainane, T.; Abourriche, A.; Kabbaj, M.; Elkouali, M.; Bennamara, A.; Charrouf, M.; Lemrani, M. Biological activities of extracts from seaweed Cystoseiratamariscifolia: Antibacterial activity, antileishmanial activity and cytotoxicity. J. Chem. Pharm. Res 2014, 6, 607–611. [Google Scholar]
- Ainane, T.; Abourriche, A.; Bennamara, A.; Talbi, M.; Lemrani, M. Activité anti-leishmanienne des extraits d'une algue brune Bifurcariabifurcata de la côte atlantique du Maroc. Phytothérapie 2018, 16, 68–73. [Google Scholar] [CrossRef]
- Assolini, J.P.; Carloto, A.C.M.; da Silva Bortoleti, B.T.; Gonçalves, M.D.; Pellissier, F.T.; Feuser, P.E.; Pavanelli, W.R. Nanomedicine in leishmaniasis: A promising tool for diagnosis, treatment and prevention of disease-An update overview. Eur. J. Pharmacol. 2022, 923, 174934. [Google Scholar] [CrossRef]
- Tuon, F.F.; Dantas, L.R.; de Souza, R.M.; Ribeiro, V.S.T.; Amato, V.S. Liposomal drug delivery systems for the treatment of leishmaniasis. Parasitol. Res. 2022, 121, 3073–3082. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, Y.; He, Y.; Zhang, W.; Zou, J.; Magar, K.T.; Boucetta, H.; Teng, C.; He, W. Approved Nanomedicine against Diseases. Pharmaceutics 2023, 15, 774. [Google Scholar] [CrossRef]
- Abpeikar, Z.; Safaei, M.; Alizadeh, A.A.; Goodarzi, A.; Hatam, G. The novel treatments based on tissue engineering, cell therapy and nanotechnology for cutaneous leishmaniasis. Int. J. Pharm. 2023, 633, 122615. [Google Scholar] [CrossRef]
- Gopu, B.; Kour, P.; Pandian, R.; Singh, K. Insights into the drug screening approaches in leishmaniasis. Int. Immunopharmacol. 2023, 114, 109591. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, C.; Kaliannagounder, V.K.; Rajan, R.; Ramesh, T.; Kim, C.S.; Park, C.H.; Yun, S.I. Silver nanoparticles decorated reduced graphene oxide: Eco-friendly synthesis, characterization, biological activities and embryo toxicity studies. Environ. Res. 2022, 210, 112864. [Google Scholar] [CrossRef] [PubMed]
- de Santana, N.S.; de Oliveira de Siqueira, L.B.; do Nascimento, T.; Santos-Oliveira, R.; dos Santos Matos, A.P.; Ricci-Júnior, E. Nanoparticles for the treatment of visceral leishmaniasis. J. Nanopart. Res. 2023, 25, 24. [Google Scholar] [CrossRef]
- González, M.A.C.; Gonçalves, A.A.M.; Ottino, J.; Leite, J.C.; Resende, L.A.; Melo-Júnior, O.A.; Giunchetti, R.C. Vaccination with formulation of nanoparticles loaded with Leishmania amazonensis antigens confers protection against experimental visceral Leishmaniasis in hamster. Vaccines 2023, 11, 111. [Google Scholar] [CrossRef]
- Khan, M.M.; Zaidi, S.S.; Siyal, F.J.; Khan, S.U.; Ishrat, G.; Batool, S.; Din, F. Statistical optimization of co-loaded rifampicin and pentamidine polymeric nanoparticles for the treatment of cutaneous leishmaniasis. J. Drug Deliv. Sci. Technol. 2023, 79, 104005. [Google Scholar] [CrossRef]
- Elbouny, H.; Ouahzizi, B.; Bakali, A.H.; Sellam, K.; Chakib, A.L.E.M. Phytochemical study and antioxidant activity of two Moroccan Lamiaceae species: Nepeta nepetella subsp. amethystina and Sideritis arborescens Salzm. ex Benth. J. Anal. Sci. Appl. Biotechnol. 2022, 4, 15–21. [Google Scholar]
- Borah, A.; Selvaraj, S.; Holla, S.R.; De, S. Extraction and characterization of total phenolic and flavonoid contents from bark of Swietenia macrophylla and their antimicrobial and antioxidant properties. Arab. J. Chem. 2022, 15, 104370. [Google Scholar] [CrossRef]
- Al-Dalahmeh, Y.; Al-Bataineh, N.; Al-Balawi, S.S.; Lahham, J.N.; Al-Momani, I.F.; Al-Sheraideh, M.S.; Mayyas, A.S.; Abu Orabi, S.T.; Al-Qudah, M.A. LC-MS/MS screening, total phenolic, flavonoid and antioxidant contents of crude extracts from three asclepiadaceae species growing in Jordan. Molecules 2022, 27, 859. [Google Scholar] [CrossRef]
- Bennamara, A.; Abourriche, A. Alkaloids 8-Hydroxyquinoline derivatives: Synthesis and biological activities. J. Anal. Sci. Appl. Biotechnol. 2020, 2, 57–62. [Google Scholar]
- Maduraimuthu, V.; Ranishree, J.K.; Gopalakrishnan, R.M.; Ayyadurai, B.; Raja, R.; Heese, K. Antioxidant Activities of Photoinduced Phycogenic Silver Nanoparticles and Their Potential Applications. Antioxidants 2023, 12, 1298. [Google Scholar] [CrossRef]
- Gecer, E.N.; Erenler, R. Biogenic synthesis of silver nanoparticles using Echium vulgare: Characterisation, quantitative analysis of bioactive compounds, antioxidant activity and catalytic degradation. J. Indian Chem. Soc. 2023, 100, 101003. [Google Scholar] [CrossRef]
- Sharma, A.; Sagar, A.; Rana, J.; Rani, R. Green synthesis of silver nanoparticles and its antibacterial activity using fungus Talaromycespurpureogenus isolated from Taxus baccata Linn. Micro Nano Syst. Lett. 2022, 10, 2. [Google Scholar] [CrossRef]
- Kipke, A.; Hofmeister, H. Formation of silver nanoparticles in low-alkali borosilicate glass via silver oxide intermediates. Mater. Chem. Phys. 2008, 111, 254–259. [Google Scholar] [CrossRef]
- Kayed, K. The optical properties of individual silver nanoparticles in Ag/Ag2O composites synthesized by oxygen plasma treatment of silver thin films. Plasmonics 2020, 15, 1439–1449. [Google Scholar] [CrossRef]
- Khammour, F.; Abdoul-Latif, F.M.; Ainane, A.; Mohamed, J.; Ainane, T. Eco-friendly adsorbent from waste of mint: Application for the removal of hexavalent chromium. J. Chem. 2021, 2021, 8848964. [Google Scholar] [CrossRef]
- Espina, A.; Sanchez-Cortes, S.; Jurašeková, Z. Vibrational study (Raman, SERS, and IR) of plant gallnut polyphenols related to the fabrication of iron gall inks. Molecules 2022, 27, 279. [Google Scholar] [CrossRef]
- Petrisor, G.; Motelica, L.; Ficai, D.; Trusca, R.D.; Surdu, V.A.; Voicu, G.; Oprea, C.; Ficai, A.; Andronescu, E. New mesoporous silica materials loaded with polyphenols: Caffeic acid, ferulic acid and p-coumaric acid as dietary supplements for oral administration. Materials 2022, 15, 7982. [Google Scholar] [CrossRef]
- Saroha, V.; Khan, H.; Raghuvanshi, S.; Dutt, D. Development of polyvinyl alcohol-based antioxidant nanocomposite films with nanokaolin impregnated with polyphenols from pomegranate peel extract. Food Packag. Shelf Life 2022, 32, 100848. [Google Scholar] [CrossRef]
- Allaka, G.; King, M.F.L.; Yepuri, V.; Narayana, R.L. Synthesis of silver oxide nanoparticles using gomutra mediation and their investigations on anti-oxidant property. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Rab, S.; Suman, R. Applications of Nanotechnology in Medical field. Glob. Health J. 2023, 3, 5–11. [Google Scholar]
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.V.K.S.; Nair, M.P.; Verma, H.K. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. J. Drug Deliv. Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- Modi, S.; Prajapati, R.; Inwati, G.K.; Deepa, N.; Tirth, V.; Yadav, V.K.; Yadav, K.K.; Islam, S.; Gupta, P.; Kim, D.-H.; et al. Recent trends in fascinating applications of nanotechnology in allied health sciences. Crystals 2021, 12, 39. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, M.; Cho, M.H.; Khan, M.M. Selected nanotechnologies and nanostructures for drug delivery, nanomedicine and cure. Bioprocess Biosyst. Eng. 2020, 43, 1339–1357. [Google Scholar] [CrossRef] [PubMed]
- Grodzinski, P.; Kircher, M.; Goldberg, M.; Gabizon, A. Integrating nanotechnology into cancer care. ACS Nano. 2019, 13, 7370–7376. [Google Scholar] [CrossRef]
- Konvičková, Z.; Schröfel, A.; Kolenčík, M.; Dědková, K.; Peikertová, P.; Žídek, M.; Kratošová, G. Antimicrobial bionanocomposite–from precursors to the functional material in one simple step. J. Nanopart. 2016, 18, 368. [Google Scholar] [CrossRef]
- Almeida, J.R.; Gomes, A.; Mendes, B.; Aguiar, L.; Ferreira, M.; Brioschi, M.B.C.; Duarte, D.; Nogueira, F.; Cortes, S.; Salazar-Valenzuela, D.; et al. Unlocking the potential of snake venom-based molecules against the malaria, Chagas disease, and leishmaniasis triad. Int. J. Biol. Macromol. 2023, 242, 124745. [Google Scholar] [CrossRef]
- Kumi, R.O.; Oti, B.; Abo-Dya, N.E.; Alahmdi, M.I.; Soliman, M.E. Bridging the gap in Malaria Parasite Resistance, current interventions, and the Way Forward from in Silico Perspective: A review. Molecules 2022, 27, 7915. [Google Scholar] [CrossRef]
- Martínez-Peinado, N.; Cortes-Serra, N.; Losada-Galvan, I.; Alonso-Vega, C.; Urbina, J.A.; Rodríguez, A.; VandeBerg, J.L.; Cortes-Serra, N.; Gascon, J.; Alonso-Padilla, J. Emerging agents for the treatment of Chagas disease: What is in the preclinical and clinical development pipeline? Expert Opin. Investig. Drugs 2020, 29, 947–959. [Google Scholar] [CrossRef]
- Khetmalis, Y.M.; Shivani, M.; Murugesan, S.; Sekhar, K.V.G.C. Oxindole and its derivatives: A review on recent progress in biological activities. Biomed. Pharmacother. 2021, 141, 111842. [Google Scholar] [CrossRef]
- Kuzminac, I.Z.; Savić, M.P.; Ajduković, J.; Nikolić, A.R. Steroid and triterpenoid compounds with antiparasitic properties. Curr. Top. Med. Chem. 2023, 23, 791–815. [Google Scholar]
- Zhang, M.; Zhang, Q.; Zhang, Q.; Cui, X.; Zhu, L. Promising Antiparasitic Natural and Synthetic Products from Marine Invertebrates and Microorganisms. Mar. Drugs 2023, 21, 84. [Google Scholar] [CrossRef] [PubMed]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial activity of metal and metal-oxide based nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, H.; Wang, Y.; Zhang, N.; Trépout, S.; Tang, B.Z.; Gasser, G.; Li, M.H. Polymersomes with Red/Near-Infrared Emission and Reactive Oxygen Species Generation. Macromol. Rapid Commun. 2023, 44, 2200716. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.R.; Pal, S.; Sarkar, A.K.; Jana, N.R. Hemin-based cell therapy via nanoparticle-assisted uptake, intracellular reactive oxygen species generation and autophagy induction. New J. Chem. 2022, 46, 21746–21755. [Google Scholar] [CrossRef]
- Wang, Y.; Pu, M.; Yan, J.; Zhang, J.; Wei, H.; Yu, L.; Yan, X.; He, Z. 1,2-Bis(2-aminophenoxy)ethane-N,N,N′, N′-tetraacetic Acid Acetoxymethyl Ester Loaded Reactive Oxygen Species Responsive Hyaluronic Acid–Bilirubin Nanoparticles for Acute Kidney Injury Therapy via Alleviating Calcium Overload Mediated Endoplasmic Reticulum Stress. ACS Nano 2022, 17, 472–491. [Google Scholar]
- Khan, T.A.; Mnasri, A.; Al Nasr, I.S.; Özdemir, I.; Gürbüzd, N.; Hamdi, N.; Koko, W.S. Activity of benzimidazole derivatives and their N-heterocyclic carbene silver complexes against leishmania major promastigotes and amastigotes. Biointerface Res. Appl. Chem. 2022, 13, 132–133. [Google Scholar]
- Martínez-Esquivias, F.; Guzmán-Flores, J.M.; Pérez-Larios, A.; González Silva, N.; Becerra-Ruiz, J.S. A review of the antimicrobial activity of selenium nanoparticles. J. Nanosci. Nanotechnol. 2021, 21, 5383–5398. [Google Scholar] [CrossRef]
- Hoseinzadeh, E.; Makhdoumi, P.; Taha, P.; Hossini, H.; Stelling, J.; Amjad Kamal, M.; Md Ashraf, G. A review on nano-antimicrobials: Metal nanoparticles, methods and mechanisms. Currentdrugmetabolism 2017, 18, 120–128. [Google Scholar] [CrossRef]
- Yan, L.; Gu, Z.; Zhao, Y. Chemical mechanisms of the toxicological properties of nanomaterials: Generation of intracellular reactive oxygen species. Chem.–Asian J. 2013, 8, 2342–2353. [Google Scholar] [CrossRef]
- Konvičková, Z.; Holišová, V.; Kolenčík, M.; Niide, T.; Kratošová, G.; Umetsu, M.; Seidlerová, J. Phytosynthesis of colloidal Ag-AgCl nanoparticles mediated by Tilia sp. leachate, evaluation of their behaviour in liquid phase and catalytic properties. Colloid Polym. Sci. 2018, 296, 677–687. [Google Scholar] [CrossRef]
- Unciti-Broceta, J.D.; Arias, J.L.; Maceira, J.; Soriano, M.; Ortiz-González, M.; Hernández-Quero, J.; Garcia-Salcedo, J.A. Specific cell targeting therapy bypasses drug resistance mechanisms in African trypanosomiasis. PLoSpathogens 2015, 11, e1004942. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Langbang, L.; Haque, M.; Belwal, V.K.; Aguan, K.; Roy, A.S. Biocompatible silver nanoparticles: An investigation into their protein binding efficacies, anti-bacterial effects and cell cytotoxicity studies. J. Pharm. Anal. 2021, 11, 422–434. [Google Scholar] [CrossRef]
- Holsing, J.J.; Gaikwad, J. Electrically modified lyophilic silver hydrosol inhibits bacterial growth in a dose-dependent manner in vitro and in vivo. Bios 2023, 94, 36–41. [Google Scholar] [CrossRef]
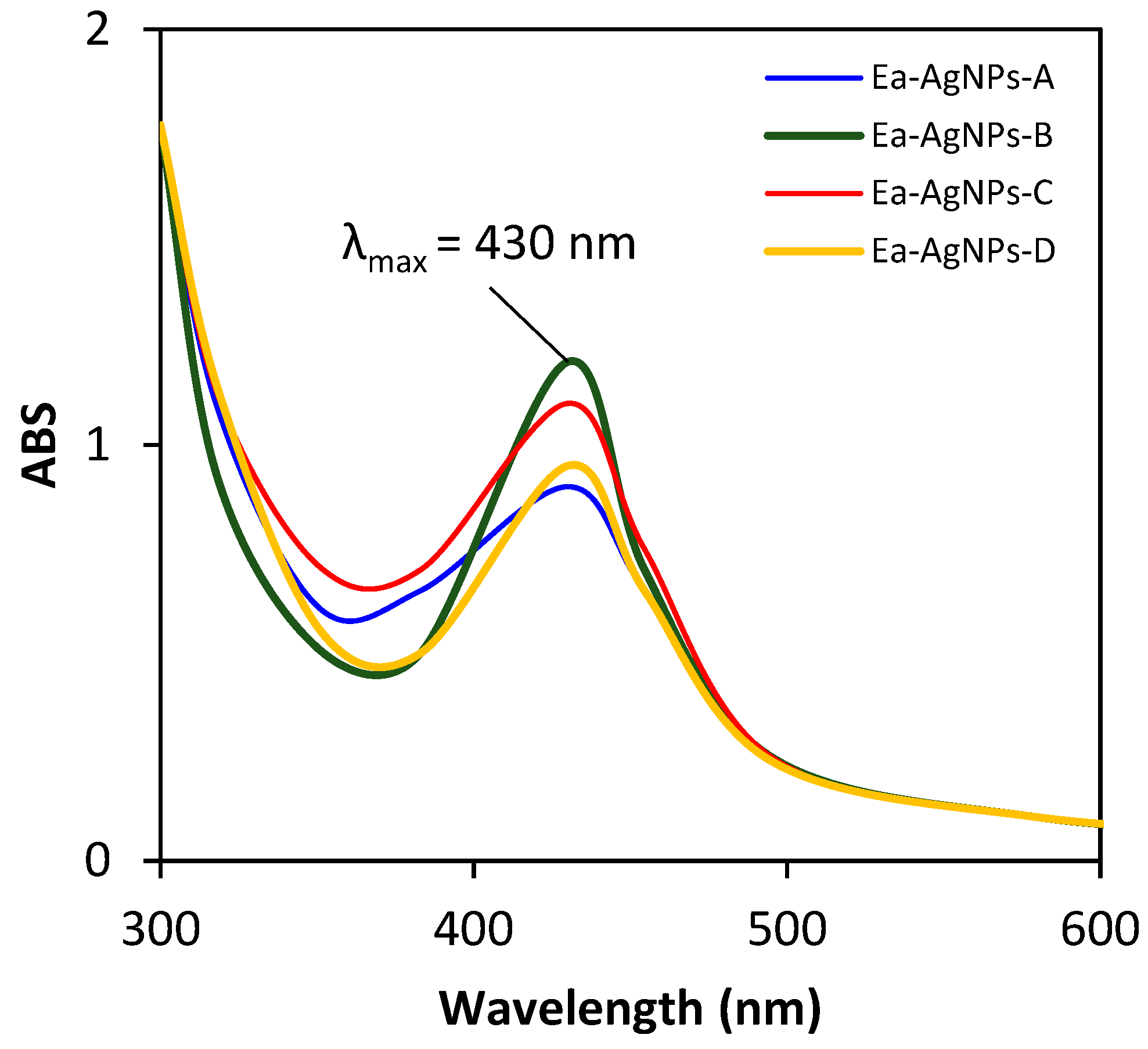
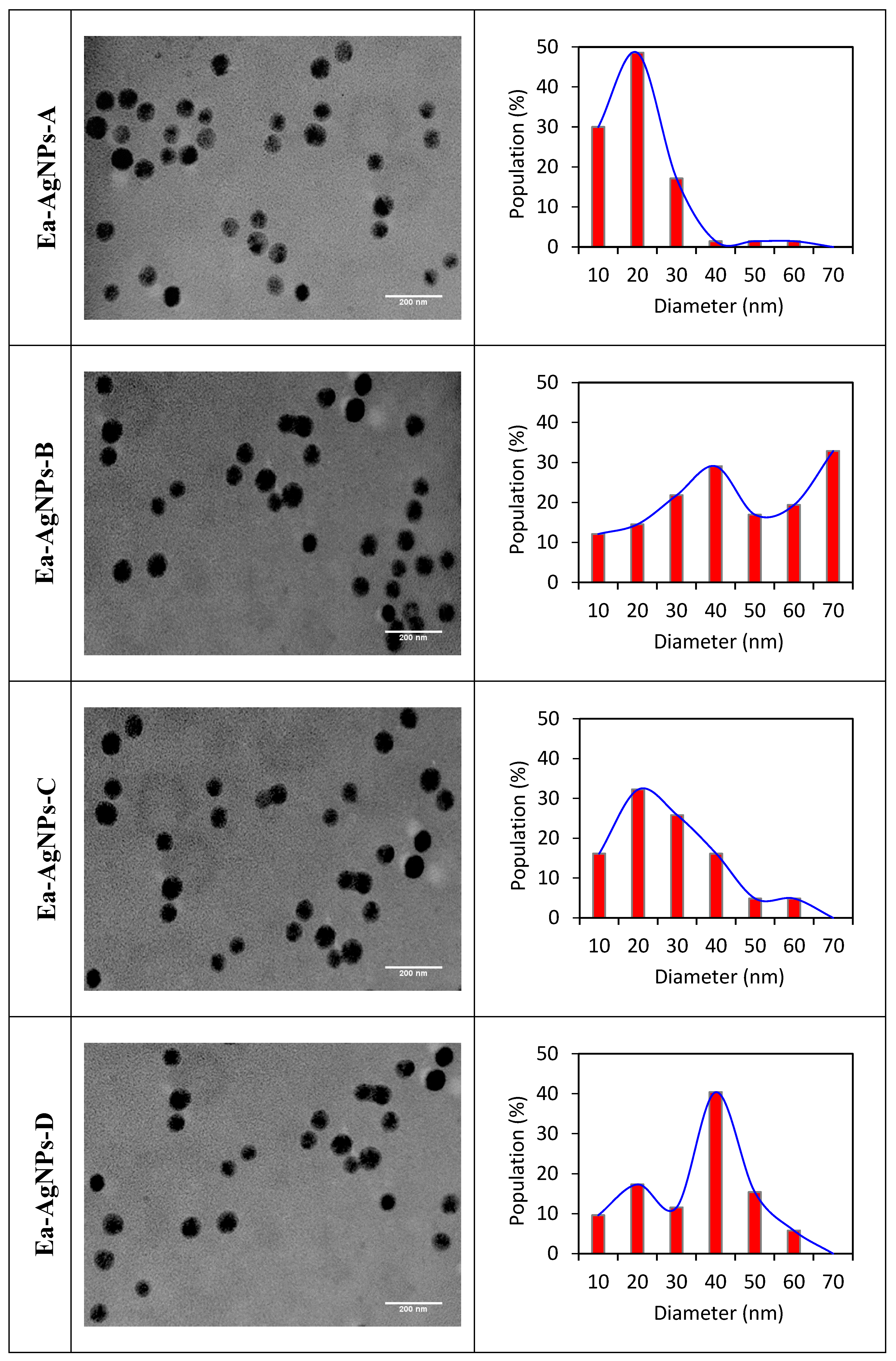

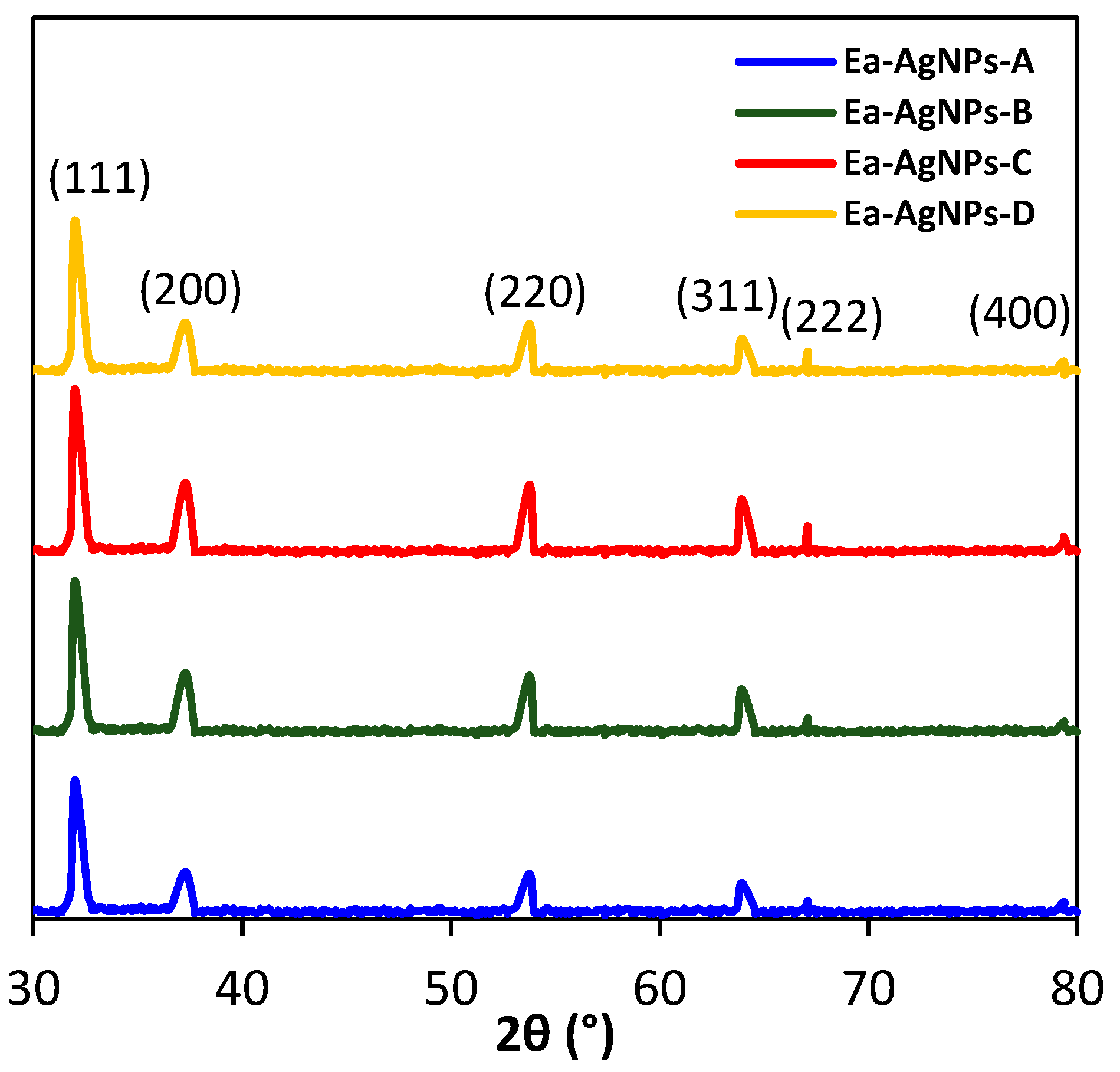
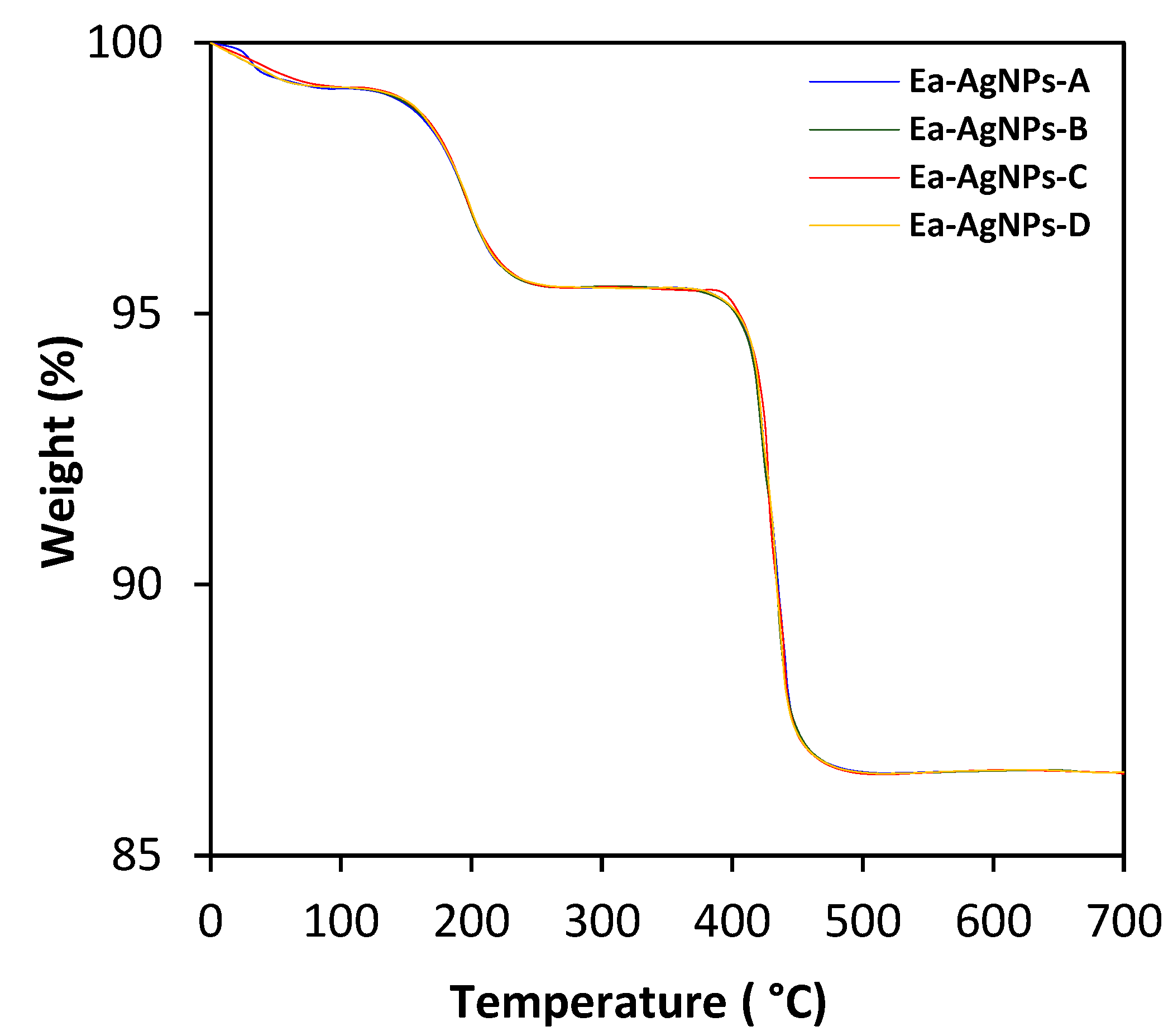

| Extract | Color | Yield (%) |
|---|---|---|
| A | Yellow–green | 3.84 |
| B | Green | 0.95 |
| C | Dark green | 1.33 |
| D | Green–black | 9.27 |
| Extract | A | B | C | D |
|---|---|---|---|---|
| Total phenols (mg GAE g−1) | 5.974 ± 0.194 a | 6.078 ± 0.212 a | 5.231 ± 0.190 b | 4.664 ± 0.183 c |
| Total flavonoids (mg QE g−1) | 0.611 ± 0.132 a | 0.885 ± 0.141 b | 0.602 ± 0.133 a | 0.458 ± 0.125 c |
| Total tannins (mg TAE g−1) | 0.050 ± 0.015 a | 0.057 ± 0.013 a | 0.048 ± 0.008 b | 0.037 ± 0.007 b |
| Product | L. infantum | L. tropica | L. major | ANOVA | Cytotoxicity BS | |
|---|---|---|---|---|---|---|
| IC 50 (μg/mL) | F-Ratio | p-Value | LD 50 (μg/mL) | |||
| A | 48.77 ± 2.14 | 50.45 ± 3.08 | 46.39 ± 2.05 | 0.63 | 0.52 | 21.80 ± 3.05 |
| B | 42.14 ± 1.87 a | 42.08 ± 1.82 a | 49.86 ± 3.77 b | 214.22 | <0.05 * | 40.46 ± 4.18 |
| C | 66.11 ± 2.52 a | 56.41 ± 3.14 b | 62.74 ± 1.90 c | 174.92 | <0.05 * | 37.05 ± 3.33 |
| D | 90.52 ± 3.01 a | 94.78 ± 3.14 b | 105.21 ± 5.24 c | 154.07 | <0.05 * | 58.24 ± 4.46 |
| Ea-AgNPs-A | 33.12 ± 2.54 | 35.74 ± 2.88 | 35.75 ± 2.94 | 0.52 | 0.61 | 17.41 ± 2.82 |
| Ea-AgNPs-B | 27.41 ± 2.32 | 27.16 ± 2.34 | 29.53 ± 2.78 | 0.71 | 0.48 | 18.42 ± 2.87 |
| Ea-AgNPs-C | 35.87 ± 2.12 | 36.25 ± 2.55 | 35.74 ± 2.26 | 0.48 | 0.75 | 17.08 ± 2.01 |
| Ea-AgNPs-D | 37.7 ± 2.87 | 38.18 ± 2.63 | 37.49 ± 2.71 | 0.68 | 0.57 | 17.25 ± 2.50 |
| Amphothericine B | 0.24 ± 0.05 | 0.26 ± 0.05 | 0.23 ± 0.05 | 0.33 | 0.81 | - |
| Podophyllotoxin | - | - | - | - | - | 2.45 ± 0.41 |
| Digitalin | - | - | - | - | - | 76.23 ± 5.17 |
| Strychnine sulfate | - | - | - | - | - | 152.14 ± 6.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed Abdoul-Latif, F.; Ainane, A.; Aboubaker, I.H.; Houssein Kidar, B.; Mohamed, J.; Lemrani, M.; Abourriche, A.; Ainane, T. Ericaria amentacea Algae Extracts: A Sustainable Approach for the Green Synthesis of Silver Oxide Nanoparticles and Their Effectiveness against Leishmaniasis. Processes 2023, 11, 3227. https://doi.org/10.3390/pr11113227
Mohamed Abdoul-Latif F, Ainane A, Aboubaker IH, Houssein Kidar B, Mohamed J, Lemrani M, Abourriche A, Ainane T. Ericaria amentacea Algae Extracts: A Sustainable Approach for the Green Synthesis of Silver Oxide Nanoparticles and Their Effectiveness against Leishmaniasis. Processes. 2023; 11(11):3227. https://doi.org/10.3390/pr11113227
Chicago/Turabian StyleMohamed Abdoul-Latif, Fatouma, Ayoub Ainane, Ibrahim Houmed Aboubaker, Barwako Houssein Kidar, Jalludin Mohamed, Meryem Lemrani, Abdelmjid Abourriche, and Tarik Ainane. 2023. "Ericaria amentacea Algae Extracts: A Sustainable Approach for the Green Synthesis of Silver Oxide Nanoparticles and Their Effectiveness against Leishmaniasis" Processes 11, no. 11: 3227. https://doi.org/10.3390/pr11113227
APA StyleMohamed Abdoul-Latif, F., Ainane, A., Aboubaker, I. H., Houssein Kidar, B., Mohamed, J., Lemrani, M., Abourriche, A., & Ainane, T. (2023). Ericaria amentacea Algae Extracts: A Sustainable Approach for the Green Synthesis of Silver Oxide Nanoparticles and Their Effectiveness against Leishmaniasis. Processes, 11(11), 3227. https://doi.org/10.3390/pr11113227








