Separation of Ternary System 1,2-Ethanediol + 1,3-Propanediol + 1,4-Butanediol by Liquid-Only Transfer Dividing Wall Column
Abstract
:1. Introduction
2. Experimental and Process Modeling
2.1. Materials
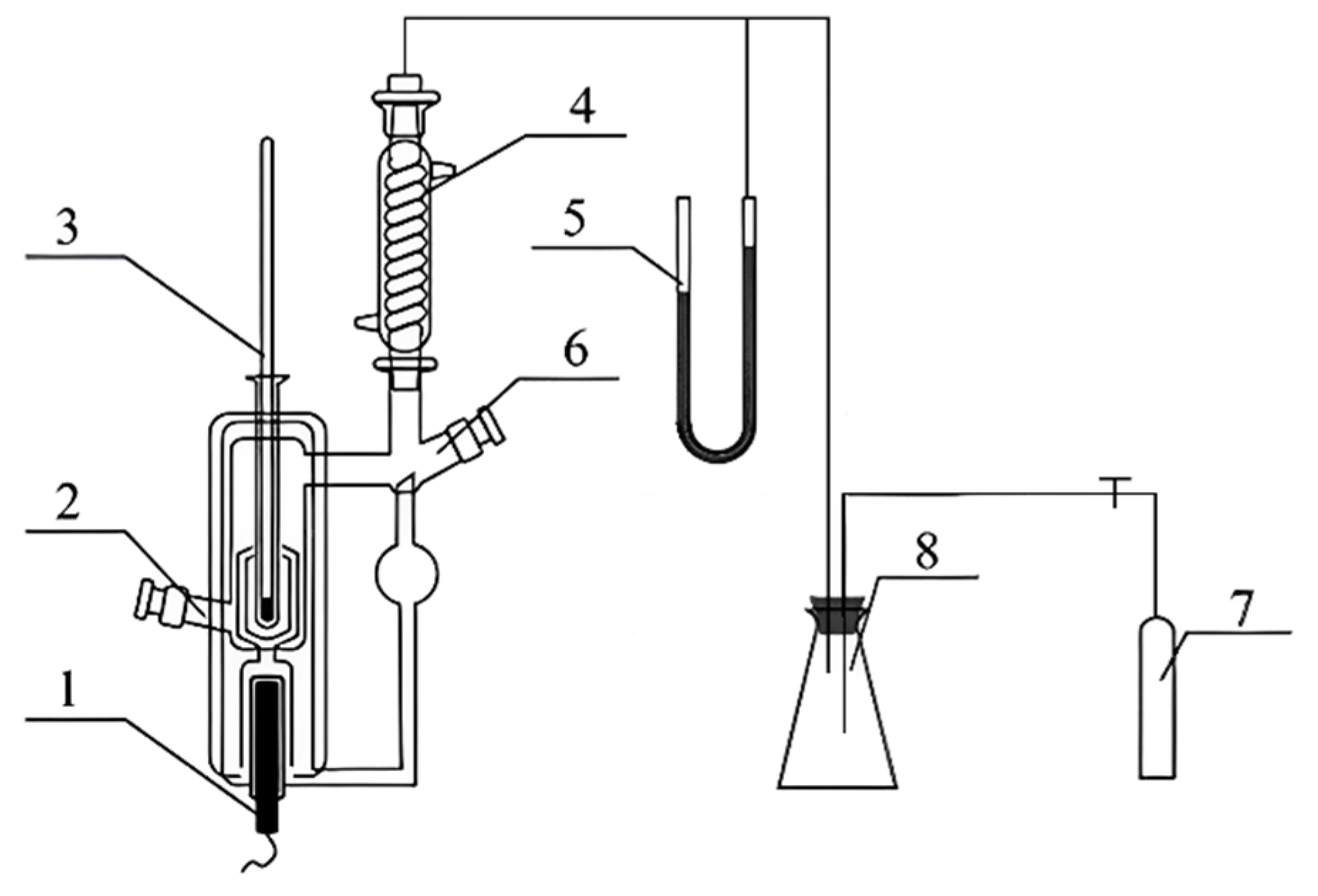
2.2. Methods
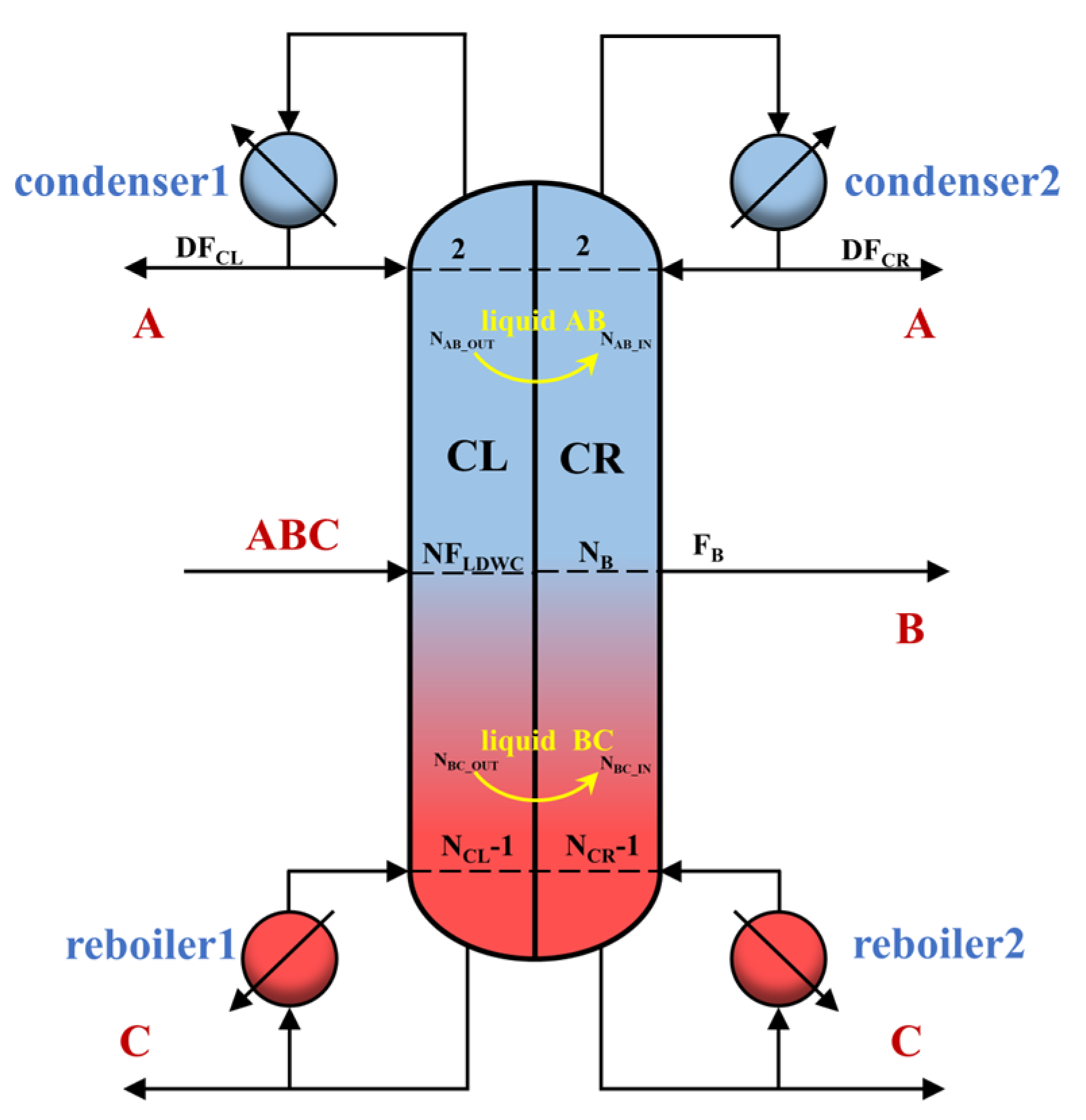
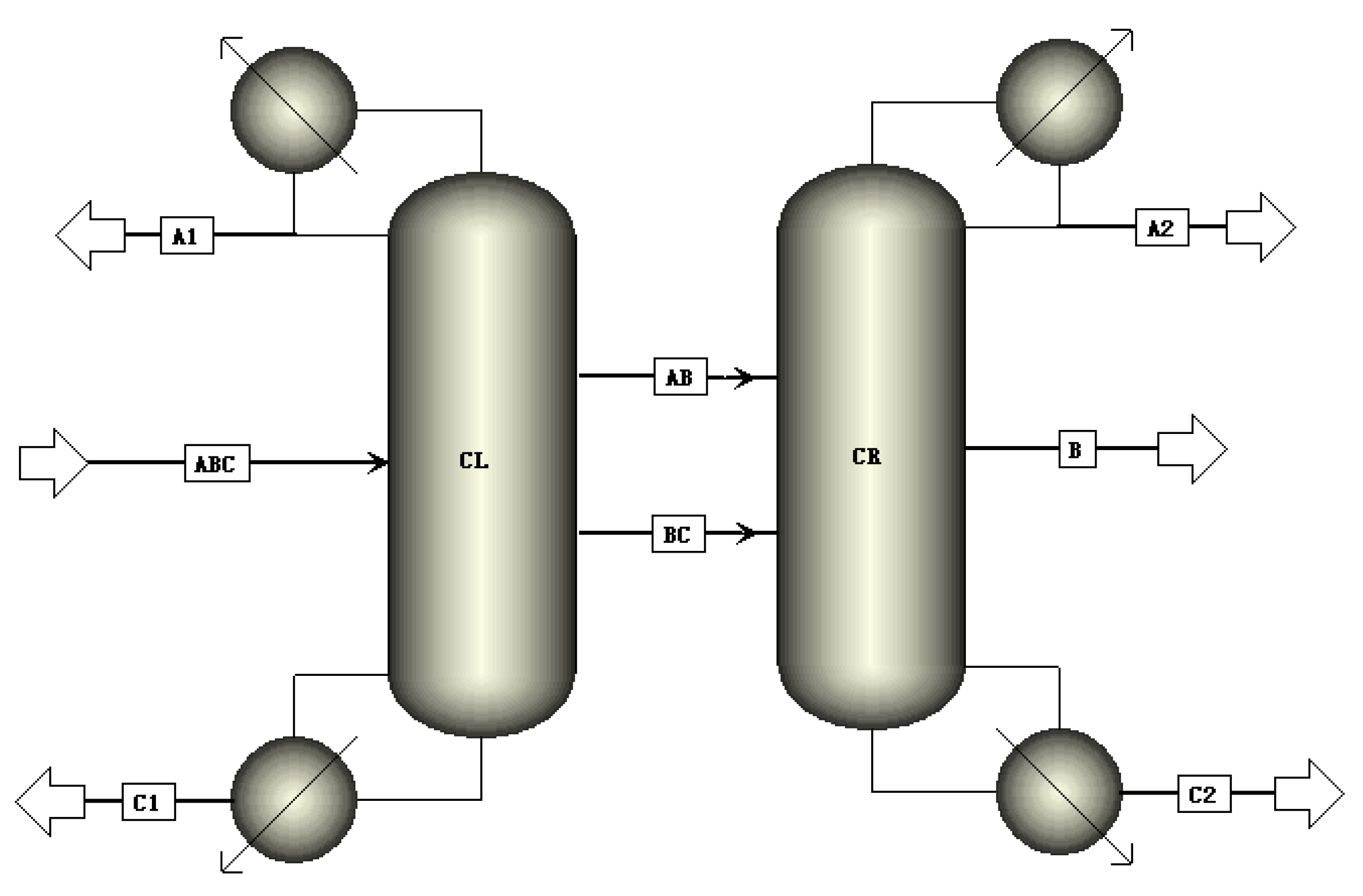
2.3. Simulation
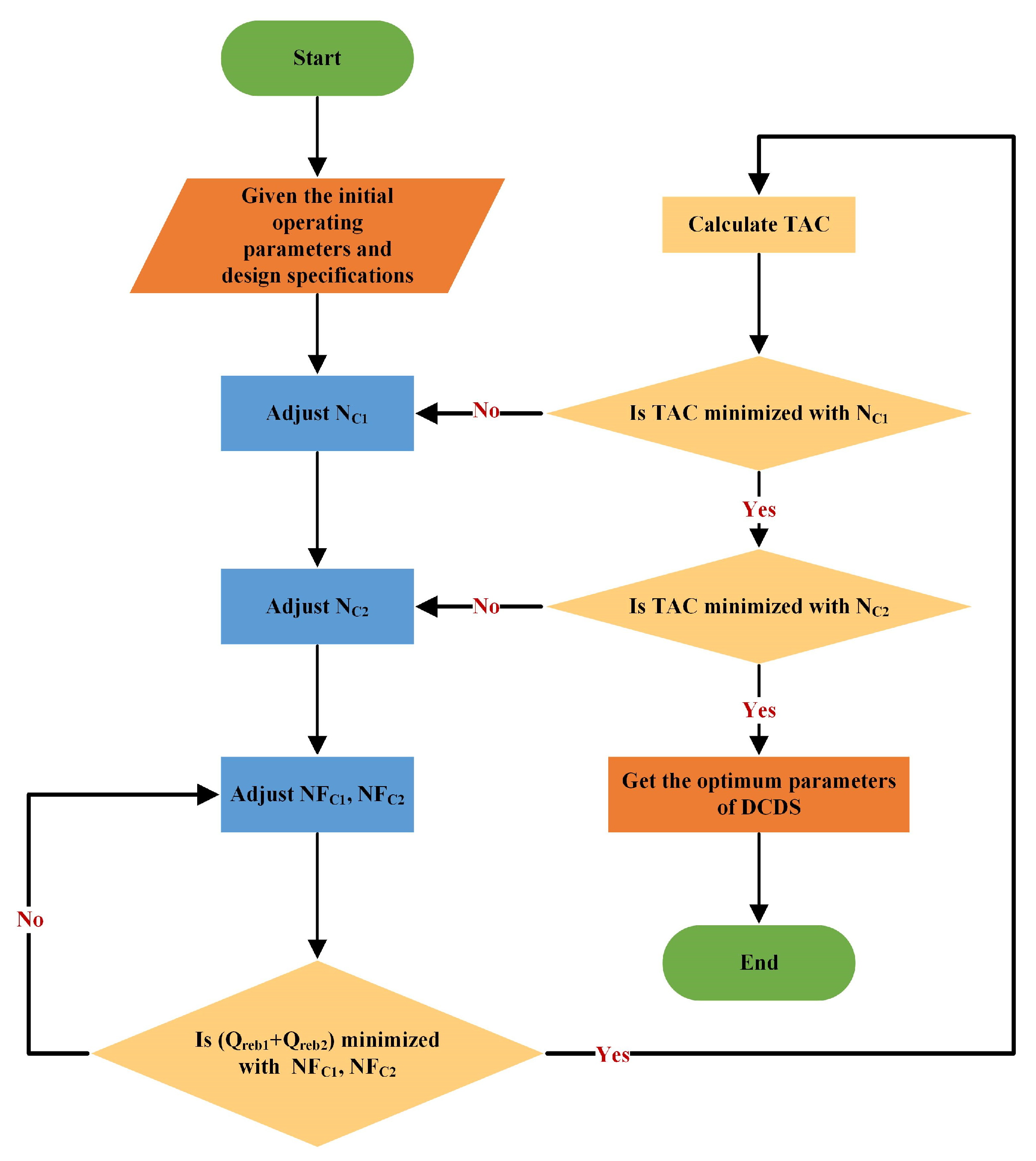
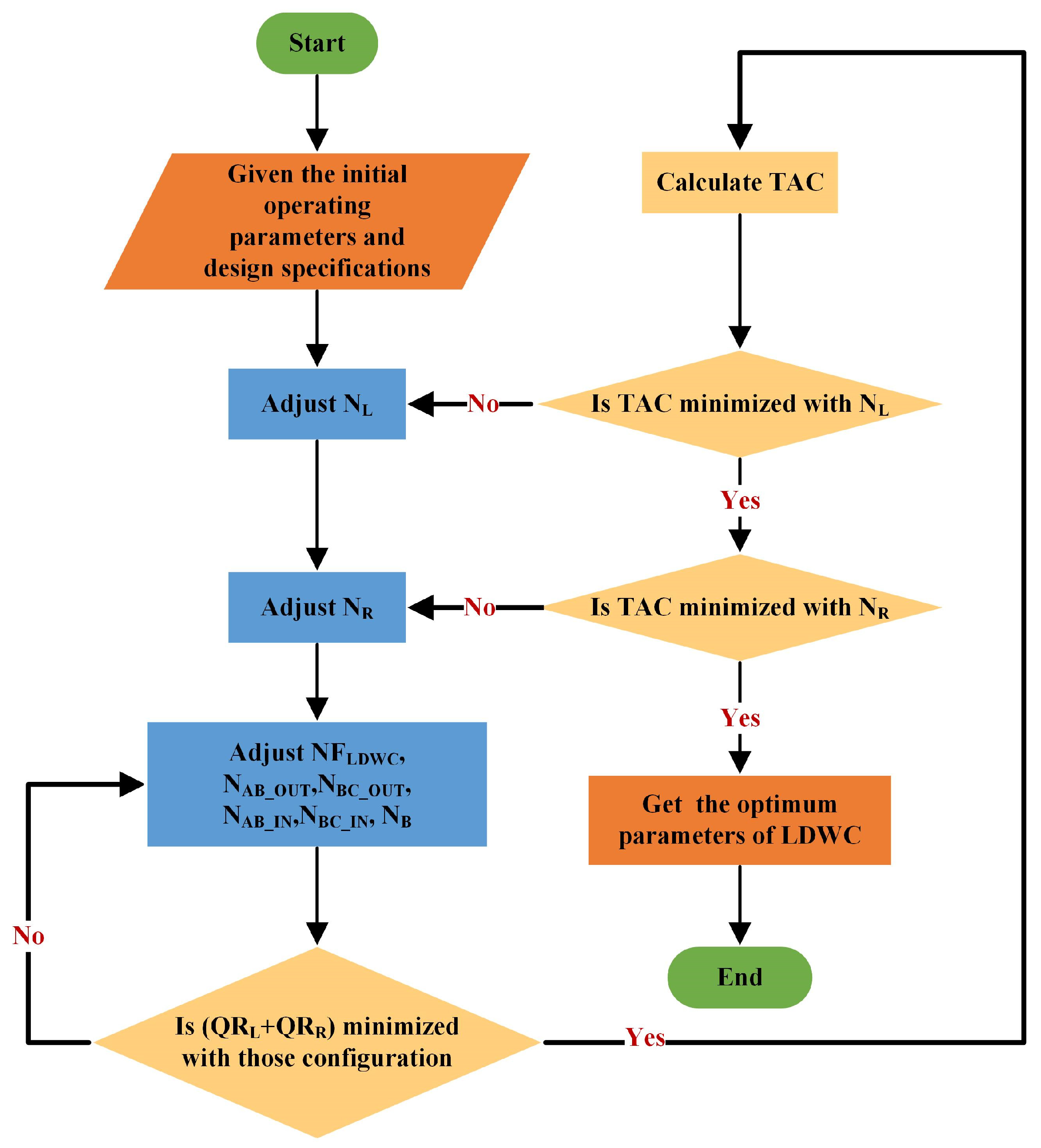
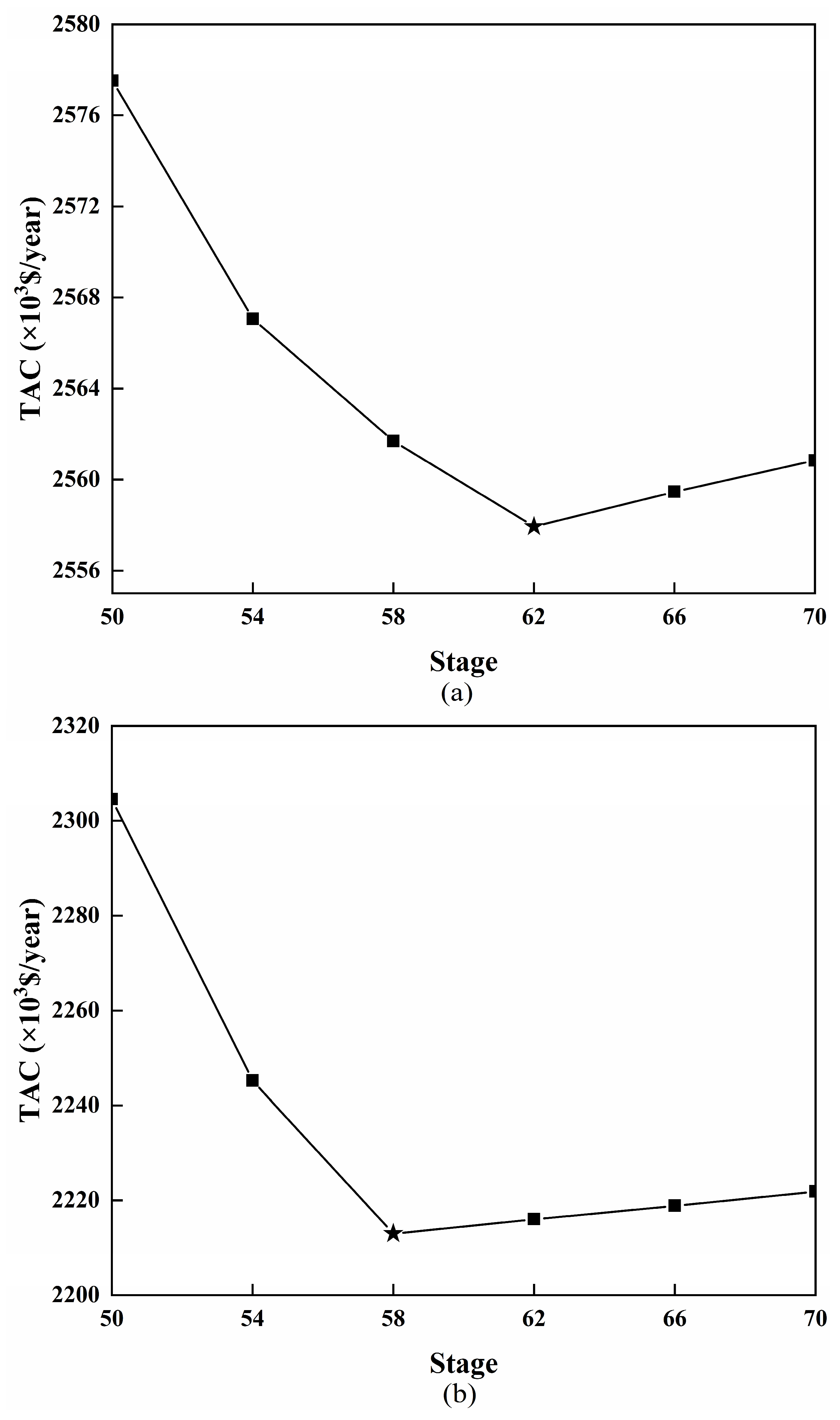
2.4. Optimization
- (1)
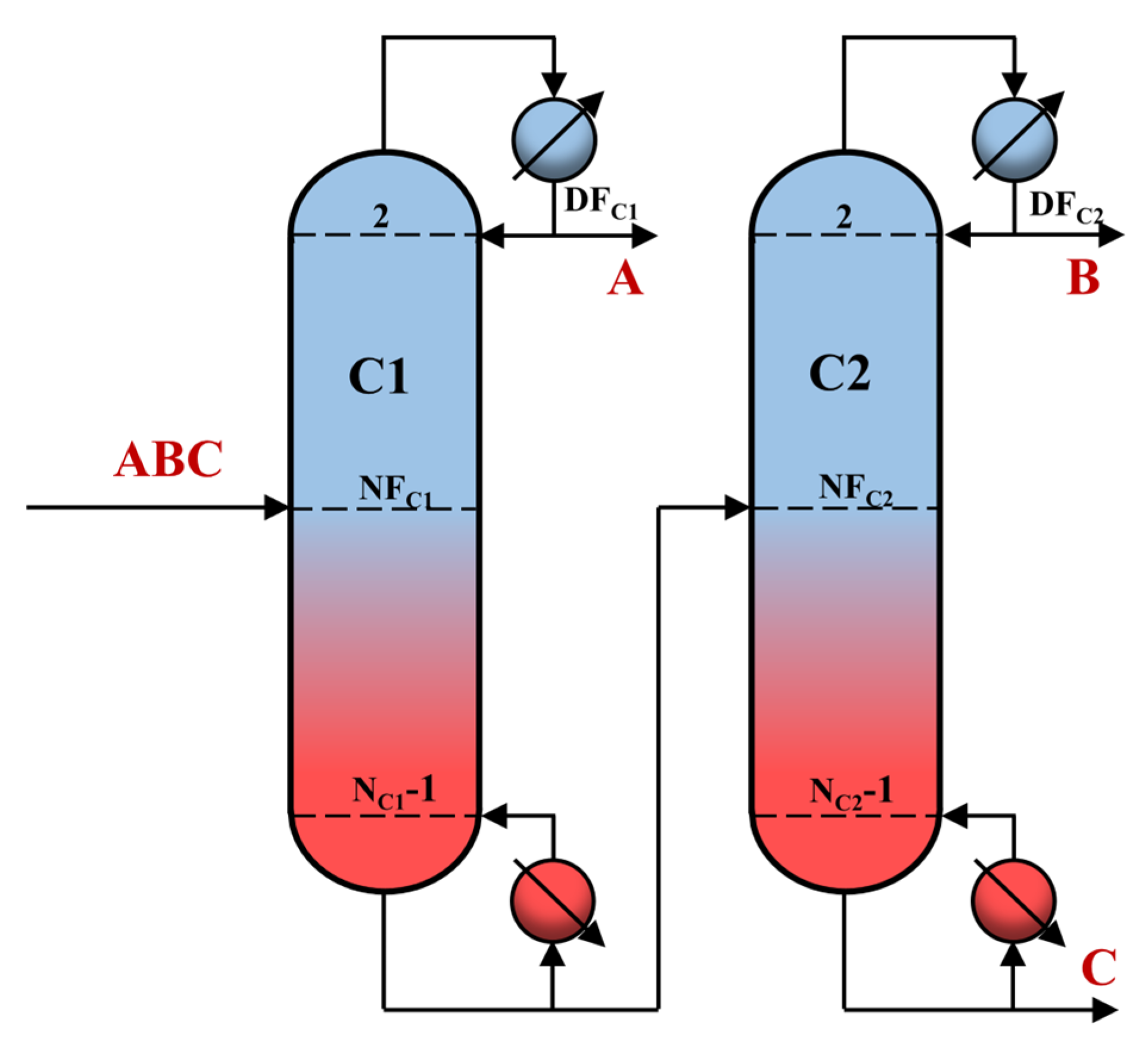
- Specify the initial operation parameters and use Design specifications and vary modules in simulation to maintain target purity. Herein, target purity is achieved by manipulating DFC1, DFC2, RRC1, and RRC2 using these two modules.
- (2)
- Adjust NFC1 and NFC2 to minimize the total heat duty (Qreb1 + Qreb2).
- (3)
- If the TAC is minimized with NC1, obtain the optimum NC1; if not, adjust NC1 and go back to step 2.
- (4)
- If the TAC is minimized with NC2, obtain the optimum NC2; if not, adjust NC2 and go back to step 2.
- (5)
- Obtain the optimum parameters of DCDS.
- (1)
- Specify the initial operation parameters and use design specifications in Aspen Plus to maintain target purity. Herein, target purity is obtained by manipulating DFCL, DFCR, RRCL, RRCR, and FB using these two modules.
- (2)
- Adjust NFLDWC, NAB_OUT, NBC_OUT, NAB_IN, NBC_IN, and NB to minimize the total heat duty (QRL + QRR).
- (3)
- If the TAC is minimized with NL, get the optimum NL; if not, adjust NL and go back to step 2.
- (4)
- If the TAC is minimized with NR, get the optimum NR; if not, adjust NR and go back to step 2.
- (5)
- Get the optimum parameters of DCDS.
3. Results and Discussion
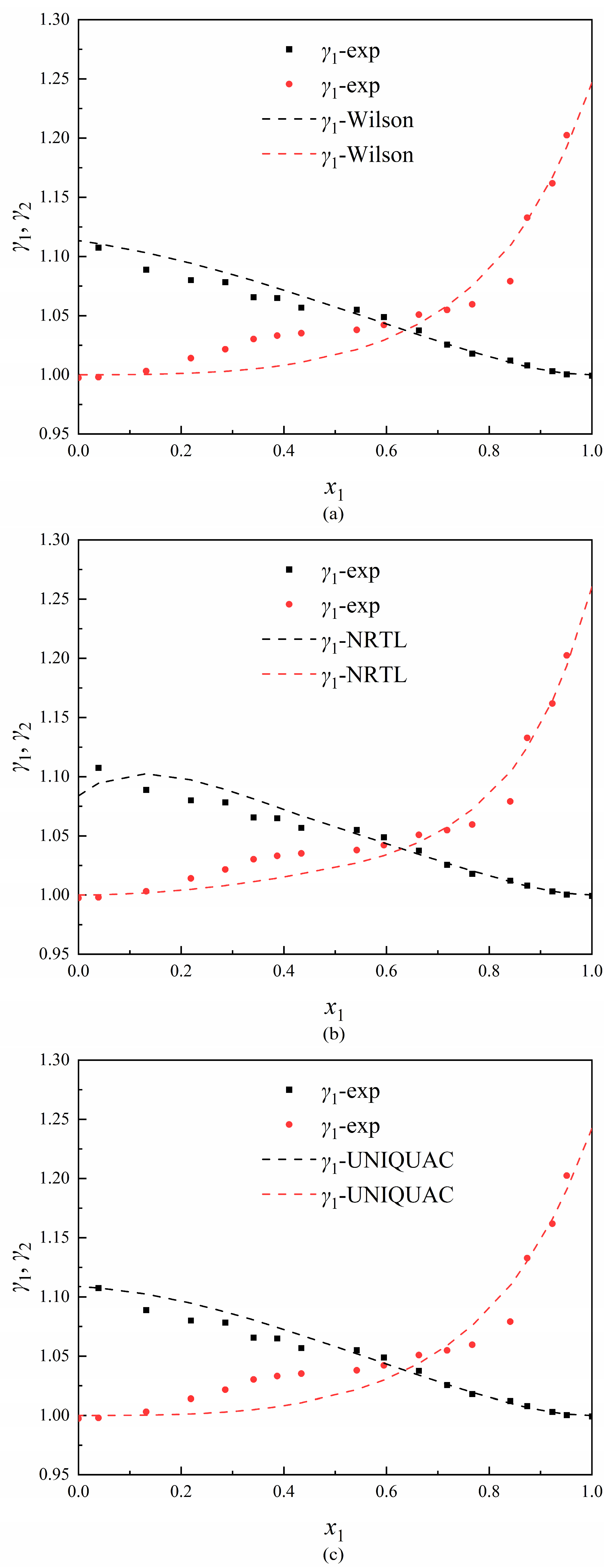
3.1. Phase Equilibrium Data
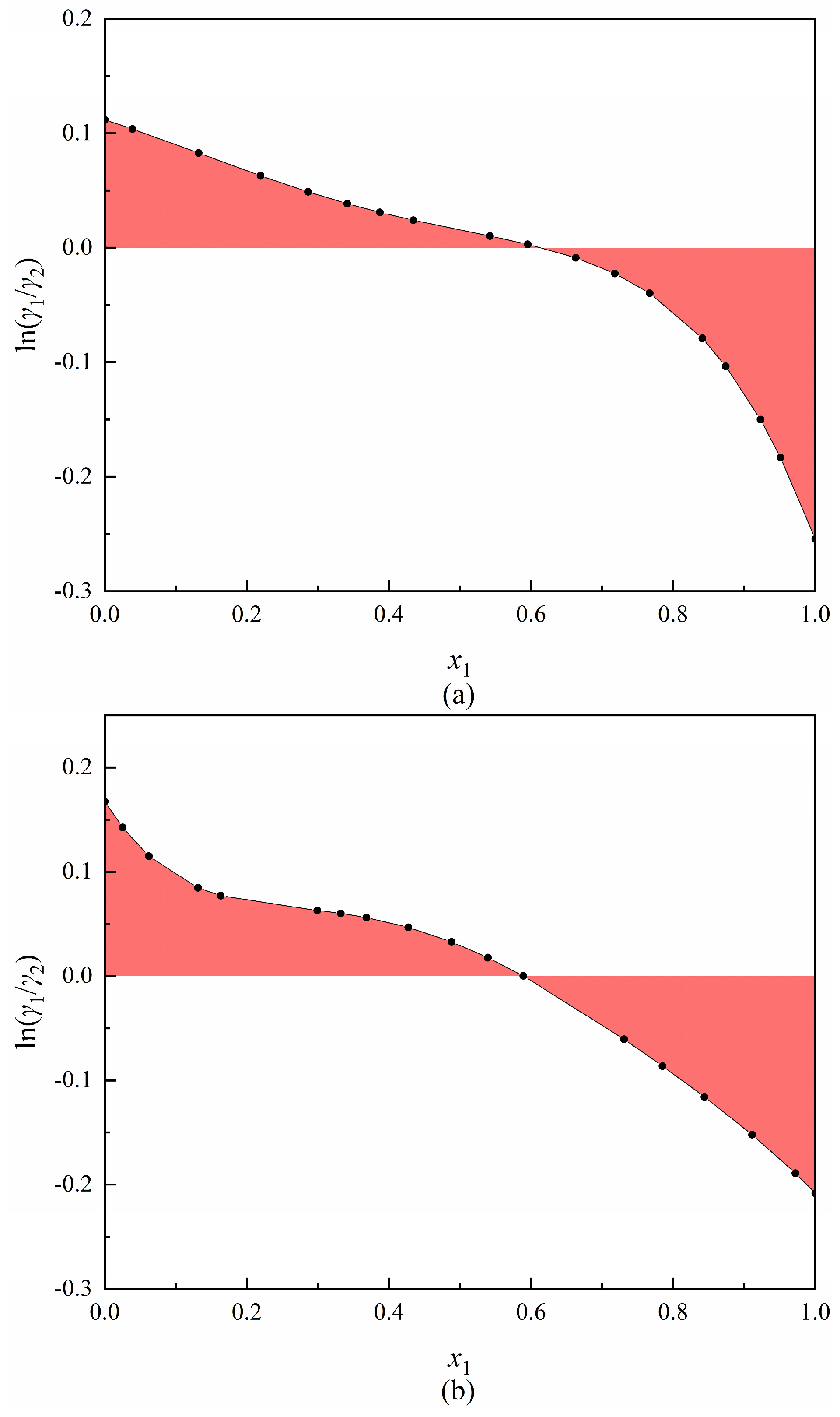
3.2. Thermodynamic Consistency Test
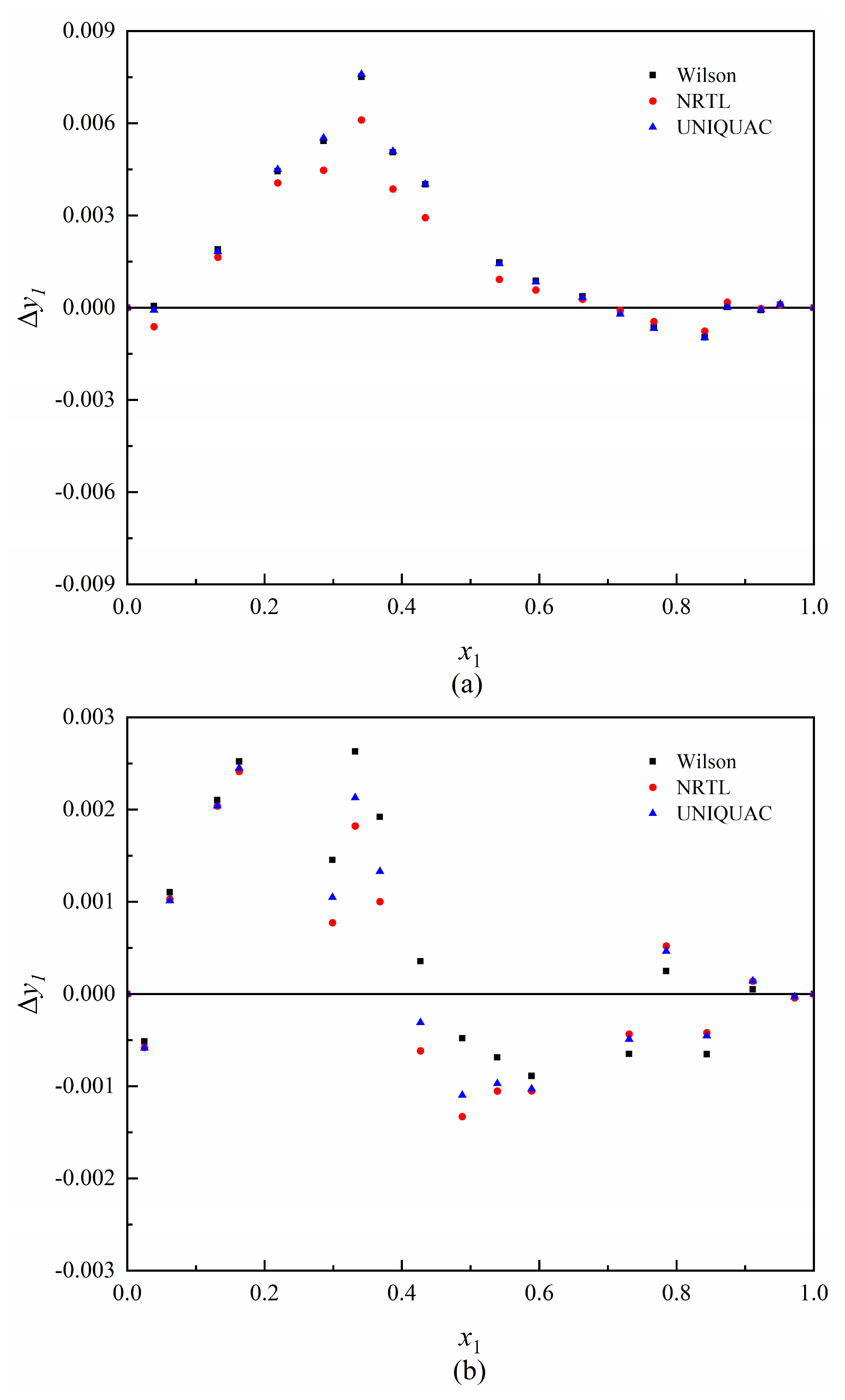
3.3. Data Regression
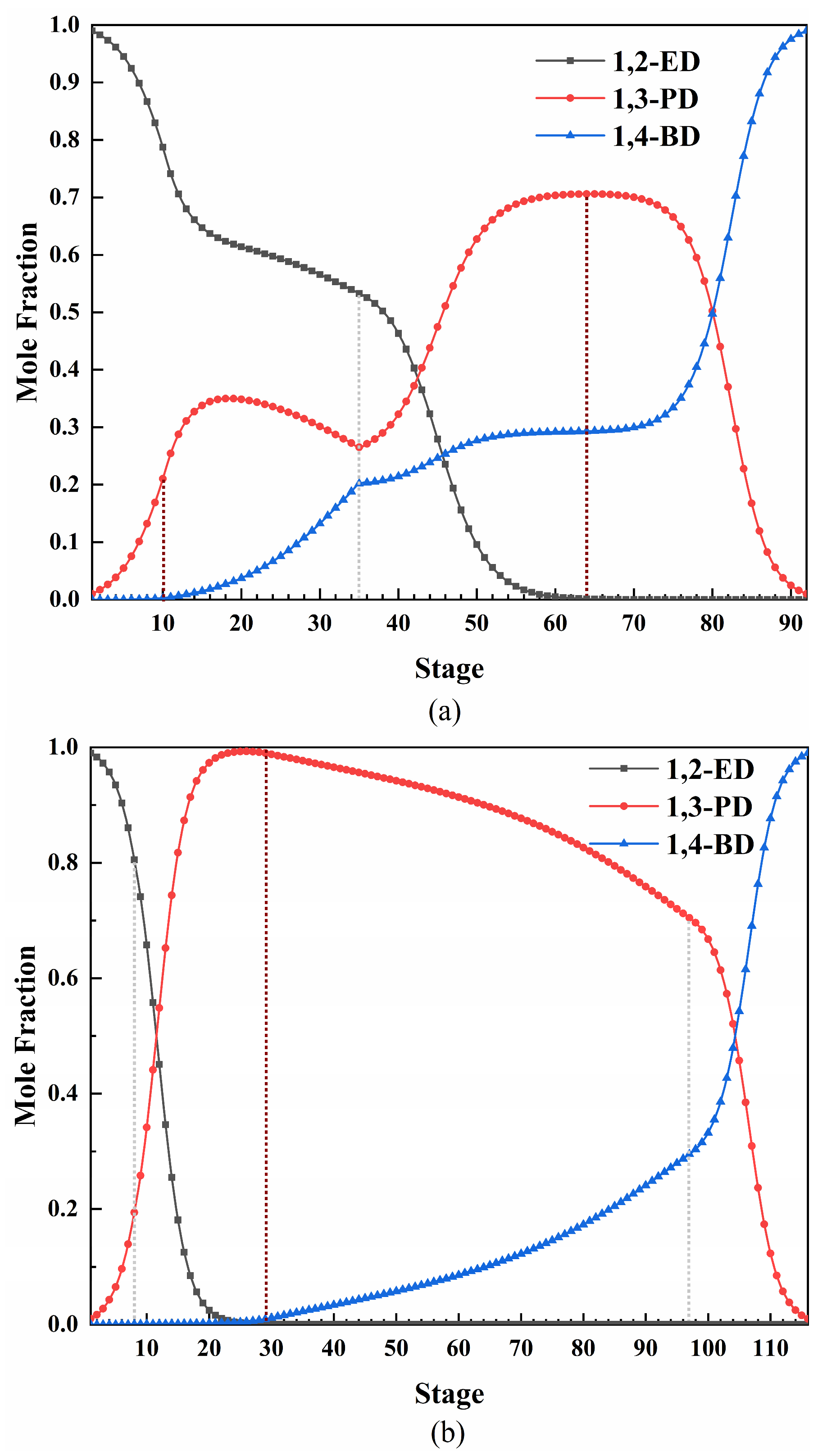
3.4. Optimization Results and Comparison
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. TAC Estimation
| Steam Pressure (BarG) | Latent Heat of Vaporization (kJ/kg) | Price ($/GJ) | Temperature (K) |
|---|---|---|---|
| 40 | 1705.71 | 9.18 | 525 |
| 46 | 1661.15 | 9.42 | 533 |
Appendix B. Experimental VLE Data
| T/K | x1 | y1 | γ1exp | γ2exp |
|---|---|---|---|---|
| 470.3 | 1.000 | 1.000 | 0.999 | |
| 471.2 | 0.951 | 0.978 | 1.000 | 1.202 |
| 471.7 | 0.923 | 0.966 | 1.003 | 1.162 |
| 472.6 | 0.874 | 0.944 | 1.008 | 1.133 |
| 473.3 | 0.841 | 0.931 | 1.012 | 1.079 |
| 474.9 | 0.767 | 0.895 | 1.018 | 1.060 |
| 475.9 | 0.718 | 0.869 | 1.026 | 1.055 |
| 477.0 | 0.663 | 0.838 | 1.038 | 1.051 |
| 478.6 | 0.595 | 0.796 | 1.049 | 1.042 |
| 480.0 | 0.542 | 0.759 | 1.055 | 1.038 |
| 483.3 | 0.434 | 0.668 | 1.057 | 1.035 |
| 484.7 | 0.387 | 0.624 | 1.065 | 1.033 |
| 486.3 | 0.341 | 0.575 | 1.066 | 1.030 |
| 488.2 | 0.286 | 0.514 | 1.078 | 1.022 |
| 490.9 | 0.219 | 0.424 | 1.080 | 1.014 |
| 494.7 | 0.132 | 0.285 | 1.089 | 1.003 |
| 499.1 | 0.039 | 0.096 | 1.107 | 0.998 |
| 501.1 | 0.000 | 0.000 | 0.997 |
| T/K | x1 | y1 | γ1exp | γ2exp |
|---|---|---|---|---|
| 487.4 | 1.000 | 1.000 | 0.997 | |
| 487.5 | 0.972 | 0.978 | 1.000 | 1.207 |
| 488.0 | 0.911 | 0.931 | 1.002 | 1.171 |
| 488.5 | 0.844 | 0.882 | 1.010 | 1.124 |
| 489.1 | 0.785 | 0.836 | 1.011 | 1.112 |
| 489.6 | 0.731 | 0.797 | 1.021 | 1.082 |
| 491.1 | 0.589 | 0.687 | 1.047 | 1.040 |
| 491.7 | 0.539 | 0.644 | 1.054 | 1.035 |
| 492.4 | 0.488 | 0.598 | 1.060 | 1.029 |
| 493.1 | 0.427 | 0.541 | 1.075 | 1.026 |
| 494.0 | 0.368 | 0.48 | 1.079 | 1.025 |
| 494.6 | 0.332 | 0.441 | 1.080 | 1.022 |
| 495.1 | 0.299 | 0.407 | 1.092 | 1.017 |
| 497.6 | 0.163 | 0.239 | 1.098 | 1.011 |
| 498.3 | 0.131 | 0.196 | 1.099 | 1.006 |
| 499.7 | 0.062 | 0.097 | 1.106 | 1.003 |
| 500.5 | 0.025 | 0.042 | 1.162 | 0.998 |
| 501.1 | 0.000 | 0.000 | 0.997 |
Appendix C. Activity Coefficient Models
References
- Liu, Y.; Wang, W.; Zeng, A.-P. Biosynthesizing structurally diverse diols via a general route combining oxidative and reductive formations of OH-groups. Nat. Commun. 2022, 13, 1595. [Google Scholar] [CrossRef] [PubMed]
- Tronconi, E.; Ferlazzo, N.; Forzatti, P.; Pasquon, I.; Casale, B. A mathematical model for the catalytic hydrogenolysis of carbohydrates. Chem. Eng. Sci. 1992, 47, 2451–2456. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Liu, R.; Huang, K.; Chen, H.; Yuan, Y.; Zhang, L.; Wang, S. Comparison of Temperature Control and Temperature Difference Control for a Kaibel Dividing Wall Column. Processes 2019, 7, 773. [Google Scholar] [CrossRef]
- Qian, X.; Liu, R.; Huang, K.; Chen, H.; Yuan, Y.; Zhang, L.; Wang, S. Controllability Comparison of the Four-Product Petlyuk Dividing Wall Distillation Column Using Temperature Control Schemes. Processes 2020, 8, 116. [Google Scholar] [CrossRef]
- Rangaiah, G.P.; Feng, Z.; Hoadley, A.F. Multi-Objective Optimization Applications in Chemical Process Engineering: Tutorial and Review. Processes 2020, 8, 508. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, Y.; Pan, H.; Zhou, W.; Yuan, P. Design and Control of Extractive Dividing Wall Column for Separating Dipropyl Ether/1-Propyl Alcohol Mixture. Processes 2022, 10, 665. [Google Scholar] [CrossRef]
- Kaibel, G. Distillation columns with vertical partitions. Chem. Eng. Technol. 1987, 10, 92–98. [Google Scholar] [CrossRef]
- Dejanović, I.; Matijašević, L.; Halvorsen, I.J.; Skogestad, S.; Jansen, H.; Kaibel, B.; Olujić, Ž. Designing four-product dividing wall columns for separation of a multicomponent aromatics mixture. Chem. Eng. Res. Des. 2011, 89, 1155–1167. [Google Scholar] [CrossRef]
- Kolbe, B.; Wenzel, S. Novel distillation concepts using one-shell columns. Chem. Eng. Process. Process Intensif. 2004, 43, 339–346. [Google Scholar] [CrossRef]
- Ling, H.; Luyben, W.L. Temperature Control of the BTX Divided-Wall Column. Ind. Eng. Chem. Res. 2010, 49, 189–203. [Google Scholar] [CrossRef]
- Si, Z.; Chen, H.; Cong, H.; Li, X. Energy, exergy, economic and environmental analysis of a novel steam-driven vapor recompression and organic Rankine cycle intensified dividing wall column. Sep. Purif. Technol. 2022, 295, 121285. [Google Scholar] [CrossRef]
- Ramapriya, G.M.; Tawarmalani, M.; Agrawal, R. Thermal coupling links to liquid-only transfer streams: A path for new dividing wall columns. AIChE J. 2014, 60, 2949–2961. [Google Scholar] [CrossRef]
- Cui, C.; Zhang, X.; Sun, J. Design and optimization of energy-efficient liquid-only side-stream distillation configurations using a stochastic algorithm. Chem. Eng. Res. Des. 2019, 145, 48–52. [Google Scholar] [CrossRef]
- Cui, C.; Zhang, Q.; Zhang, X.; Sun, J. Eliminating the vapor split in dividing wall columns through controllable double liquid-only side-stream distillation configuration. Sep. Purif. Technol. 2020, 242, 116837. [Google Scholar] [CrossRef]
- Zhang, T.; Li, M.; Pan, H.; Ling, H. Dynamic control of liquid-only transfer Kaibel dividing-wall column. Chem. Eng. Sci. 2023, 272, 118589. [Google Scholar] [CrossRef]
- Qu, X.C.; Wu, Y.Y.; Zhu, J.W.; Chen, K.; Wu, B.; Ji, L.J. Separation of ternary system (1,2-ethanediol+1,3-butanediol+1,3-propanediol) by distillation. Sep. Sci. Technol. 2018, 53, 2435–2443. [Google Scholar] [CrossRef]
- Yang, C.; Sun, Y.; Qin, Z.; Feng, Y.; Zhang, P.; Feng, X. Isobaric Vapor–Liquid Equilibrium for Four Binary Systems of Ethane-1,2-diol, Butane-1,4-diol, 2-(2-Hydroxyethoxy)ethan-1-ol, and 2-[2-(2-Hydroxyethoxy)ethoxy]ethanol at 10.0 kPa, 20.0 kPa, and 40.0 kPa. J. Chem. Eng. Data 2014, 59, 1273–1280. [Google Scholar] [CrossRef]
- Wilson, G.M. Vapor-liquid equilibrium. XI. A new expression for the excess free energy of mixing. J. Am. Chem. Soc. 1964, 86, 127–130. [Google Scholar] [CrossRef]
- Renon, H.; Prausnitz, J.M. Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 1968, 14, 135–144. [Google Scholar] [CrossRef]
- Abrams, D.S.; Prausnitz, J.M. Statistical thermodynamics of liquid mixtures: A new expression for the excess Gibbs energy of partly or completely miscible systems. AIChE J. 1975, 21, 116–128. [Google Scholar] [CrossRef]
- Rodrigues, M.; Francesconi, A.Z. Experimental Study of the Excess Molar Volumes of Binary and Ternary Mixtures Containing Water +(1,2-Ethanediol, or 1,2-Propanediol, or 1,3-Propanediol, or 1,2-Butanediol) + (1-n-Butyl-3-methylimidazolium Bromide) at 298.15 K and Atmospheric Pressure. J. Solut. Chem. 2011, 40, 1863–1873. [Google Scholar] [CrossRef]
- Zhang, K.; Meng, X.Y.; Wu, J.T. Flammability Limits of Binary Mixtures of 1,2-Ethanediol plus Steam and 1,2-Propanediol plus Steam. J. Chem. Eng. Data 2013, 58, 2681–2686. [Google Scholar] [CrossRef]
- Lin, Y.F.; Tu, C.H. Isobaric vapor-liquid equilibria for the binary and ternary mixtures of 2-propanol, water, and 1,3-propanediol at P = 101.3 kPa: Effect of the 1,3-propanediol addition. Fluid Phase Equilib. 2014, 368, 104–111. [Google Scholar] [CrossRef]
- Zhong, Y.; Wu, Y.Y.; Zhu, J.W.; Chen, K.; Wu, B.; Ji, L.J.; Shen, Y.L. The Distillation Process Design for the Ternary System 1,2-Butanediol + 1,4-Butanediol + 2,3-Butanediol. Sep. Sci. Technol. 2015, 50, 2545–2552. [Google Scholar] [CrossRef]
- Zhai, X.; Wu, Y.; Tian, B.; Yao, Y.; Wu, B.; Chen, K.; Ji, L. Isobaric Vapor-Liquid Equilibrium for the Binary System of Cumene plus Acetophenone at 101.3, 30.0, and 10.0 kPa. J. Chem. Eng. Data 2022, 67, 669–675. [Google Scholar] [CrossRef]
- Yi, Q.; Chen, W.P.; Zhang, X.Y.; Wu, Y.Y.; Wu, B.; Chen, K.; Ji, L.J. Isobaric Vapor–Liquid Equilibrium for the Binary Systems of 2-Phenylethanol + 2-Ethylphenol, 1-Phenyl-2-propanol + 2-Ethylphenol, 2-Phenylethanol + 1-Phenyl-2-propanol and 2-Phenyl-1-propanol + 2-Ethylphenol under 101.3 kPa. J. Chem. Eng. Data 2021, 66, 4351–4360. [Google Scholar] [CrossRef]
- Chen, M.; Yu, N.; Cong, L.; Wang, J.; Zhu, M.; Sun, L. Design and Control of a Heat Pump-Assisted Azeotropic Dividing Wall Column for EDA/Water Separation. Ind. Eng. Chem. Res. 2017, 56, 9770–9777. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Yeh, M.-H.; Chen, Y.-Y.; Chen, C.-L. Design and control of a comprehensive Ethylenediamine (EDA) process with external/internal heat integration. Sep. Purif. Technol. 2022, 293, 121137. [Google Scholar] [CrossRef]
- Liu, Y.; Zhai, J.; Li, L.; Sun, L.; Zhai, C. Heat pump assisted reactive and azeotropic distillations in dividing wall columns. Chem. Eng. Process. Process Intensif. 2015, 95, 289–301. [Google Scholar] [CrossRef]
- Douglas, J.M. Conceptual Design of Chemical Processes; McGraw-Hill: New York, NY, USA, 1988. [Google Scholar]
- Zheng, H.; Luo, X.; Yin, G.; Chen, J.; Zhao, S. Vapor Pressure and Isobaric Vapor–Liquid Equilibrium for Binary Systems of Furfural, 2-Acetylfuran, and 5-Methylfurfural at 3.60 and 5.18 kPa. J. Chem. Eng. Data 2018, 63, 49–56. [Google Scholar] [CrossRef]
- Redlich, O.; Kister, A.T. Thermodynamics of Nonelectrolyte Solutions-x-y-t relations in a Binary System. Ind. Eng. Chem. Res. 1948, 40, 341–345. [Google Scholar] [CrossRef]
- Fredenslund, A.; Gmehling, J.; Rasmussen, P. Vapor–Liquid Equilibria Using UNIFAC—A Group Contribution Model; Elsevier: Amsterdam, The Netehrlands, 1977. [Google Scholar]
- Mathias, P.M. Effect of VLE uncertainties on the design of separation sequences by distillation—Study of the benzene–chloroform–acetone system. Fluid Phase Equilib. 2016, 408, 265–272. [Google Scholar] [CrossRef]
- Sosa, A.; Ortega, J.; Fernández, L.; Pacheco, J.M.; Wisniak, J.; Romero, A. Further Advance to a Practical Methodology to Assess Vapor–Liquid Equilibrium Data: Influence on Binaries Rectification. J. Chem. Eng. Data 2019, 64, 3933–3944. [Google Scholar] [CrossRef]
- Kiss, A.A. Advanced Distillation Technologies: Design, Control, and Applications; Wiley: Chichester, UK, 2013. [Google Scholar]
- Cui, C.; Li, X.; Guo, D.; Sun, J. Towards energy efficient styrene distillation scheme: From grassroots design to retrofit. Energy 2017, 134, 193–205. [Google Scholar] [CrossRef]
- Luyben, W.L. Distillation Design and Control Using Aspen™ Simulation; John Wiley & Sons, Inc.: Hoboken, NY, USA, 2013. [Google Scholar]
- Song, Z.-W.; Cui, W.; Wu, Y.-Y.; Wu, B.; Chen, K.; Ji, L.-J. Energy, exergy, economic, and environmental analysis of a novel liquid-only transfer dividing wall column with vapor recompression. Sep. Purif. Technol. 2024, 329, 125122. [Google Scholar] [CrossRef]
- Li, Q.; Feng, Z.; Rangaiah, G.P.; Dong, L. Process Optimization of Heat-Integrated Extractive Dividing-Wall Columns for Energy-Saving Separation of CO2 and Hydrocarbons. Ind. Eng. Chem. Res. 2020, 59, 11000–11011. [Google Scholar] [CrossRef]
- Sun, L.-Y.; Chang, X.-W.; Qi, C.-X.; Li, Q.-S. Implementation of Ethanol Dehydration Using Dividing-Wall Heterogeneous Azeotropic Distillation Column. Sep. Sci. Technol. 2011, 46, 1365–1375. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Q.; Li, L.; Zhai, J.; Liu, Y. Design and Control of Extractive Dividing Wall Column for Separating Benzene/Cyclohexane Mixtures. Ind. Eng. Chem. Res. 2014, 53, 8120–8131. [Google Scholar] [CrossRef]






| Component | CAS | Supplier | Purity (wt %) | Density (g·cm−3) | Boiling Point (K) | Analysis Method | ||
|---|---|---|---|---|---|---|---|---|
| Exp c | Lit d | Exp e | Lit f | |||||
| 1,2-ED | 107-21-1 | Sinopharm, Beijing, China | ≥99.0 | 1112 | 1110 [22] | 470.25 | 470.45 [23] | GC b |
| 1,3-PD | 504-63-2 | Accela, Shanghai, China | ≥98.0 | 1053 | 1050 [24] | 487.35 | 487.55 [24] | |
| 1,4-BD | 110-63-4 | Sinopharm, Beijing, China | ≥99.0 | 1012 | 1016.9 [25] | 501.05 | 501.15 [25] | |
| Component | C1i | C2i | C3i | C4i | C5i | C6i | C7i | C8i | C9i |
|---|---|---|---|---|---|---|---|---|---|
| 1,2-ED b | 84.09 | 10411 | 0 | 0 | 8.1976 | 1.65 × 10−18 | 6 | 260.15 | 720 |
| 1,3-PD b | 115.58 | −11732 | 0 | 0 | −13.174 | 6.55 × 10−6 | 2 | 246.45 | 724 |
| 1,4-BD b | 105.76 | −12811 | 0 | 0 | −11.069 | 9.44 × 10−18 | 6 | 293.05 | 667 |
| System | D | Δy1 a |
|---|---|---|
| 1,2-ED (1)—1,4-BD (2) | 1.77 | 0.0014 |
| 1,3-PD (1)—1,4-BD (2) | 0.22 | 0.0008 |
| Model | a12 | a21 | b12 | b21 | α |
|---|---|---|---|---|---|
| 1,2-ED (1) + 1,4-BD (2) | |||||
| Wilson | 1.54 | −5.40 | −479.79 | 2111.72 | - |
| NRTL | −19.57 | 12.58 | 9926.34 | 6373.18 | 0.3 |
| UNIQUAC | −3.57 | 2.00 | 1497.94 | −823.53 | - |
| 1,3-PD (1) + 1,4-BD (2) | |||||
| Wilson | 4.50 | −8.26 | −2057.22 | 3767.51 | - |
| NRTL | −30.09 | 22.47 | 15,144.8 | −11,261.3 | 0.3 |
| UNIQUAC | 11.57 | −9.60 | −5820.44 | 4809.73 | - |
| 1,2-ED (1) + 1,3-PD (2) α | |||||
| Wilson | −141.12 | 91.50 | 0 | 0 | - |
| NRTL | −14.78 | 50.33 | 0 | 0 | 0.3 |
| UNIQUAC | 116.52 | −154.33 | 0 | 0 | - |
| Model | AAD (T) a | AAD (y1) a | RMSD (T) b | RMSD (y1) b |
|---|---|---|---|---|
| 1,2-ED (1) + 1,4-BD (2) | ||||
| Wilson | 0.10 | 0.0018 | 0.12 | 0.0029 |
| NRTL | 0.07 | 0.0014 | 0.08 | 0.0024 |
| UNIQUAC | 0.09 | 0.0018 | 0.11 | 0.0030 |
| 1,3-PD (1) + 1,4-BD (2) | ||||
| Wilson | 0.06 | 0.0009 | 0.08 | 0.0012 |
| NRTL | 0.05 | 0.0008 | 0.06 | 0.0011 |
| UNIQUAC | 0.05 | 0.0008 | 0.06 | 0.0011 |
| DCDS | LDWC | |
|---|---|---|
| Capital cost ($) | 2,028,300 | 2,178,246 |
| Shell | 1,196,753 | 1,409,168 |
| Tray | 89,091 | 128,568 |
| Heat exchanger | 742,456 | 640,510 |
| CC Saving (%) | 0 | −7.39 |
| Operating cost ($/year) | 2,010,187 | 1,621,942 |
| Heating utilities | 1,936,378 | 1,586,162 |
| Cooling utilities | 73,809 | 35,779 |
| OC Saving (%) | 0 | 19.31 |
| TAC * ($/year) | 2,213,017 | 1,839,766 |
| TAC Saving (%) | 0 | 16.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-Y.; Song, Z.-W.; Rao, J.-B.; Yao, Y.-X.; Wu, B.; Chen, K.; Ji, L.-J. Separation of Ternary System 1,2-Ethanediol + 1,3-Propanediol + 1,4-Butanediol by Liquid-Only Transfer Dividing Wall Column. Processes 2023, 11, 3150. https://doi.org/10.3390/pr11113150
Wu Y-Y, Song Z-W, Rao J-B, Yao Y-X, Wu B, Chen K, Ji L-J. Separation of Ternary System 1,2-Ethanediol + 1,3-Propanediol + 1,4-Butanediol by Liquid-Only Transfer Dividing Wall Column. Processes. 2023; 11(11):3150. https://doi.org/10.3390/pr11113150
Chicago/Turabian StyleWu, Yan-Yang, Zhong-Wen Song, Jia-Bo Rao, Yu-Xian Yao, Bin Wu, Kui Chen, and Li-Jun Ji. 2023. "Separation of Ternary System 1,2-Ethanediol + 1,3-Propanediol + 1,4-Butanediol by Liquid-Only Transfer Dividing Wall Column" Processes 11, no. 11: 3150. https://doi.org/10.3390/pr11113150
APA StyleWu, Y.-Y., Song, Z.-W., Rao, J.-B., Yao, Y.-X., Wu, B., Chen, K., & Ji, L.-J. (2023). Separation of Ternary System 1,2-Ethanediol + 1,3-Propanediol + 1,4-Butanediol by Liquid-Only Transfer Dividing Wall Column. Processes, 11(11), 3150. https://doi.org/10.3390/pr11113150






