1. Introduction
The disposal of industrial waste and the creation of low-waste industries is one of the main fields for improving environmental safety in the chemical industry and in energy, metallurgy, and other industries. At the same time, the priority task is the utilization and neutralization of generated industrial waste at the source of their formation [
1]. For enterprises producing phosphoric acid and phosphate fertilizers, the problem of environmental safety is largely related to preventing the contact of solid wastes such as phosphogypsum and lime mud with moving components of the natural environment.
Currently, the global cumulative release of phosphogypsum is about 6 billion tons, which is increasing at a rate of 200 million tons per year. It is predicted that between 2025 and 2045, the total amount of phosphogypsum will double [
2,
3,
4,
5,
6], and the problem of disposal of this waste has not yet been resolved. It is taken outside the factories and stored in specially designated storage areas [
7,
8]. Within the territory of phosphogypsum dumps, various geological processes are widely developed under the influence of exogenous agents. The surface of dumps is intensively eroded as a result of drip-rain spraying of soil particles and erosion by temporary stream flows, and the process of deflation of loose material occurs. The disintegration of dump material under the influence of physical and chemical weathering leads to the migration of mobile chemical elements [
9].
The material composition of phosphogypsum dumps is dominated by gypsum (about 97%, mainly in the form of calcium sulfate dihydrate CaSO
4·2H
2O) [
10], and about 3% is phosphates of iron and aluminum, orthophosphoric acid, potassium and sodium fluorosilicates, calcium fluorides, heavy metals, and radioactive elements [
11,
12,
13,
14]. The main impurities in phosphogypsum fall into three categories: phosphorus, fluorine, and organic impurities. Phosphorus impurities in phosphogypsum mainly consist of soluble phosphorus (H
3PO
4, H
2PO
4− and HPO
42−), insoluble phosphorus (Ca
3(PO
4)
2), and eutectic phosphorus (CaHPO
4·2H
2O). Fluorine-containing impurities in phosphogypsum consist mainly of soluble fluorine (NaF and KF) and insoluble fluorine (Na
3AlF
6, CaSiF
6, CaF
2). Organic impurities in phosphogypsum mainly consist of organic matter inherent in phosphate ore and organic additives added during the production process [
15,
16]. Underground infiltration and surface runoff from phosphogypsum dumps are the main permanent sources of contamination of soils and surface and groundwater, mainly with sulfates and phosphates. Some of the pollutants from phosphogypsum dumps are transported by air with a dust cloud.
Further handling of the resulting waste depends on many factors: the type and quality, the presence of useful components, and the possibility of processing for re-extracting useful components or disposal due to unsuitability for further use. Storage and disposal of waste are one of the main ways to solve the problem of preventing environmental pollution, but recently, the direction providing for the reuse or extraction of useful components has become widespread, which is the most rational solution, both in terms of saving resources and environmental protection.
There are various studies on the application of phosphogypsum in the construction industry. Garbaya et al. [
17] studied wet natural phosphogypsum, which was obtained directly from the dump at the Tunisian Chemical Group M’dhilla plant (Gafsa, Tunisia). It was found that phosphogypsum is formed mainly by gypsum with varying degrees of hydration (CaSO
4, CaSO
4∙1/2H
2O, and CaSO
4∙2H
2O) with a small amount of mineral and organic impurities of phosphorite rock (with a low percentage of Si, Ti, Na, Mg, and Fe, such as in the initial phosphate ore). Based on the obtained results, the authors propose considering phosphogypsum as a source of gypsum-based material. The physicochemical characteristics of phosphogypsum showed that the different degree of hydration allows it to exchange water with the external environment, creating a water pump, when the degree of hydration change from 2 to 0, and vice versa, depending on temperature. It is noted that the mechanical properties of phosphogypsum allow it to be used as building materials for non-bearing structures, finishing materials, or for insulation in the construction of buildings.
Ajam and Kammoun [
18] proved that the use of Tunisian phosphogypsum in the production of non-structural bricks requires low energy and consumes a lot of waste, which greatly reduces environmental pollution in addition to high socio-economic benefits. It was established that the radioactive emission of the components of this brick is below the limits recommended by standards, which means that its use is safe. Hamdi et al. [
19] confirmed the possibility of using Tunisian phosphogypsum in the construction industry as paving slabs.
Coarse-grained phosphogypsum produced by a phosphorus chemical enterprise in the Deyang Region (Deyang, China), containing CaSO
4·2H
2O at 80.65% and water at less than 5%, was studied by Xiao et al. [
20]. It was established that the main mineral of phosphogypsum is CaSO
4·2H
2O with impurities of brushite, quartz, muscovite, and zoisite. Harmful elements such as silicon, phosphorus, and fluorine are mainly concentrated in the +0.15 mm and −0.025 mm fractions; they can be pre-selected and removed with sorting to increase the CaSO
4·2H
2O content and reduce processing costs. For the extraction of gypsum from the −0.15 to +0.025 mm fraction, a direct flotation process was used, consisting of one pre-treatment operation, one cleaning, and two post-treatment operations. The test results showed that the gypsum concentrate containing 98.94% of CaSO
4·2H
2O and a CaSO
4·2H
2O extraction rate of 80.02% can be used as a high-quality raw material for obtaining α-semihydrate of high-strength gypsum or β-semihydrate of building plaster.
Lin et al. [
21] suggested a simple and effective method for obtaining high-purity CaSO
4·2H
2O (DH) and α-CaSO
4·0.5H
2O (α-HH) whiskers from phosphogypsum (Chinese waste from the production of phosphoric acid using the limestone–gypsum wet method), which are new types of inorganic fiber material with high-added value, excellent workability, high strength, and favorable biocompatibility [
22,
23].
Pyagai et al. [
24] studied the waste phosphogypsum from the Phosagro industrial site (Volkhov), which is a gray-white powder with a mass fraction of 60% of the main substance in terms of calcium sulfate. The study found that acidic impurities (phosphoric and sulfuric acids) in the phosphogypsum are an obstacle to its use in the production of building materials. The effect of an acidic environment on phosphogypsum was studied using Na
2CO
3, K
2CO
3, and (NH
4)
2CO
3 salts. Special regimes for washing the initial phosphogypsum were developed, in which the reactivity increased by 1.2 times due to the removal of acidic impurities. During the reaction with sodium carbonate, the yield of CaCO
3 was 70.6%, with potassium carbonate at 65.0%. It was established that the obtained laboratory sample of calcium carbonate can be used in the construction or paper industry as a bleaching agent.
Hou et al. [
25] studied phosphogypsum from the surface layer of a tailing dump in Hubei Province (China), with an average moisture content of 56.7%. Environmentally friendly three-component cement based on powders of phosphogypsum, fly ash, and Portland cement were obtained. The thermodynamic characteristics of the solid phase, solid solution phase, and aqueous solution during the hydration of the triple-binder system of phosphogypsum–dust–cement were studied.
The binding material with ultra-low alkalinity based on industrial wastes of phosphogypsum, granulated blast-furnace slag, and sulfoaluminate cement, which was obtained in [
26], has a broad application in the construction of islands, reefs, and river restoration. The new type of binder allows the use of a large amount of industrial waste and has the advantages of short setting time, low heat of hydration, high strength of the cement slurry, and the ability to fix harmful substances in phosphogypsum, such as phosphates, fluorides, and Cr and Ba elements.
Gong et al. [
27] used phosphogypsum from Kailin Chemical Fertilizer Co., Ltd. (Guiyang, China) (white-gray, density 2.37 g/cm
3, containing small amounts of P
2O
5, F
-, Pb, Cr, and Ni impurities), as an additive for the production of a composite binder with good characteristics. It was established that the addition of no more than 30% phosphogypsum in the manufacture of the composite cement material leads to an improvement in mechanical characteristics and pore size distribution, a decrease in porosity, and compaction of the microstructure.
Alkali-activated binders were developed using the phosphogypsum from Kailin Chemical Fertilizer Co., Ltd., Guiyang, China (white-gray, with a density of 2.212 g/cm
3, and a specific surface area of 115 m
2/kg). Phosphogypsum was preliminarily subjected to ultrasonic washing with water and dried at 105 °C to reduce the content of impurities (P
2O
5, F
−, Pb, Cr, Ni). The pretreated phosphogypsum obtained using this method (without high-temperature autoclaving) had a low activity. Therefore, it was compounded with granulated blast-furnace slag and activated with a NaOH solution [
28].
Phosphogypsum obtained from phosphate fertilizer production in Guiyang (China) (dark gray, main component is CaSO
4∙2H
2O, pH of about 4.8, and humidity over 13.1%) was used in [
29] together with processed fine powder and slag to obtain a stable geopolymer.
Wu et al. [
30] studied the effect of the hardening of various modification compositions on hazardous components of phosphogypsum (Yunnan Yuntianhua Environmental Protection Technology Co., Ltd., Wuhan, China) using calcium carbide slag from industrial solid waste (CCS) as an alkaline regulator, Portland cement (PC), polyaluminum chloride (PAC), and calcium chloride as the main raw materials of the solidification, and the water content stabilization formula in phosphogypsum as the reaction medium. The results showed that CCS (0.5%), PC (0.4%), and PAC (0.3%) have a more significant hardening effect on phosphorus (P) and fluoride (F
−), which makes it possible to obtain materials and fillers based on phosphogypsum with low economic costs.
Tian et al. [
31] studied phosphogypsum waste (Kunming, China) with a high initial water content and easy caking. In their study, the method of cyclic spraying of a solution of bacteria (urease-positive
Sporosarcina pasteurii microorganisms) of various concentrations, having high urease activity and ubiquitous distribution in the soil, was used to modify phosphogypsum material.
Sporosarcina pasteurii can produce a large amount of precipitation in a short period of time due to the formation of adenosine triphosphate through the secretion of urease. It was found that phosphogypsum slope treated with microbial treatment can significantly increase water erosion resistance and reduce slope erosion. Additionally, in the process of microbial modification, the ionic states of heavy metals in phosphogypsum were extracted and fixed by combining them with carbonate through microbial complexation, which changes the chemical form of heavy metal elements and brings them into line with emission standards. It was shown that phosphogypsum can be used as a hardened material to simulate artificial slopes.
Phosphogypsum is one of the best wastes in the chemical industry, which can be used as an ameliorant, soil improver, and multi-component fertilizer. Phosphogypsum contains calcium and sulfur, as well as phosphorus, silicon, and various trace elements, including zinc, which is necessary for plant nutrition. According to [
32,
33], most of the components of organic phosphogypsum are gypsum (CaSO
4∙2H
2O). This organic phosphogypsum does not contain significant amounts of radionuclides other than naturally occurring radionuclides and is not a radioactive material. Therefore, applying organic phosphogypsum to the soil as a fertilizer has no effect on soil, plants, or water [
33]. Organic phosphogypsum is a natural compound with a pH of ~7 and macro- (P, Ca) and microelements (Zn, Cu, B, Co, Mn, and Mo), which are important for plant growth and development. Other metal ions, which can contribute to soil and water pollution and be toxic to plants and humans, are found at very low levels. However, for the growth and development of crops, the amount of macronutrients in organic phosphogypsum is small and not enough to support the plant’s nutrient needs. Therefore, when using organic phosphogypsum as a fertilizer, additional fertilizer is needed either alone or in combination with several elements [
33].
Komissarov et al. [
34] established the possibility of using phosphogypsum (mineral fertilizer plant in Meleuzovsky district, Republic of Bashkortostan, Russia) and poultry waste (turkey litter) as an ameliorant/fertilizer for erosion-prone soils. The effect of treatments on soil moisture reserves, soil structure, microaggregate and granulometric composition, aggregative stability (water resistance), the content of organic carbon, ammonium, nitrate and alkaline hydrolysable nitrogen, available phosphorus, and exchangeable potassium was studied. The results obtained suggest the use of phosphogypsum to improve the fertility of black soil and minimize its erodibility without soil and plant pollution.
Millions of tons of phosphogypsum, which is formed during the production of mineral fertilizers, can become a source of rare earth elements (REEs) for use in high-tech industries. From phosphogypsum, it is possible to obtain REEs of the medium–heavy group, which are in dire need in the world industry and for energy. Phosphogypsum obtained from Yunnan Phosphate Chemical Group (Kunming, China) was studied in [
35], the main component of which was gypsum (CaSO
4·2H
2O), as well as a small amount of brushite (CaHPO
4·2H
2O) and quartz (SiO
2). In addition to the main elements S, O, and Ca, the phosphogypsum also contained impurities such as Si, P, Al, F, and rare earth elements (REEs), of which Y, La, Ce, and Nd were the most abundant. It is shown that according to the distribution of REEs, the largest share falls on the oxide form of metals, then the residual, organic, and ion-exchange fractions, and the smallest is in the form of carbonates. It was found that HCl is potentially the best leaching agent for leaching REEs from phosphogypsum, and suitable operating conditions are an acid concentration of 1.65 mol/L, a S/L ratio of 1/10, and a reaction temperature of 60 °C. Under optimal conditions, the maximum extraction during leaching of ∑REE was 65.6%, of which the yttrium leaching coefficient was the highest and reached 73.8%.
However, so far, these methods have not found wide use, and with their help, only a small amount of phosphogypsum is utilized. The organization of its processing remains an important technical, economic, and environmental task. To solve all these issues, it is necessary to conduct comprehensive studies, the task of which is to determine the chemical and mineralogical composition of phosphate raw material processing wastes and the possibility of the presence of natural radionuclides in them. These studies also obtain data on the degree of toxicity and hazard class of the studied phosphate raw materials processing wastes for humans and the environment, allowing for the determination of the method and conditions of their possible reuse.
On the territory of Kazakhstan, the largest deposit of phosphorites is concentrated in the Karatau mountains with a total reserve of 3000 million tons, including the forecast, with a content of . In addition to fluorapatite, , phosphorites contain fluorocarbonate apatite francolite kurskite , etc. As impurities, they contain calcite , dolomite limonite quartz , glauconite , where clay, etc.
A chemical complex for the processing of phosphates planned for construction will be designed for the processing of finely ground phosphate rock to obtain dicalcium phosphate (calcium hydrogen phosphate dihydride). Based on the results of a preliminary analysis of various methods for processing the studied ore, it was established that the use of traditional chemical processing methods (decomposition with sulfuric acid and nitric acid) is impossible due to critical technical limitations caused by the high magnesium content in the processed raw materials; the use of flotation is ineffective due to the low level of extraction. The use of non-traditional processing methods (demagnesiation and Novaphos) is associated with risks due to the low degree of technology maturity. With a P2O5 content of 24%, the theoretical yield of phosphogypsum will be more than 4 tons per ton of P2O5, which is an additional limitation. The optimal method of ore processing, considering technical limitations and economic efficiency, is the hydrochloric acid decomposition method.
This method will make it possible to process almost any ore from the Karatau Basin into various products, including high-quality products, for example, feed phosphates. The degree of extraction of useful substances depends directly on the quality of the final product. In the case of the production of fertilizer-quality phosphates using this method, without removing heavy metals, the extraction is about 90%, including 51% of P2O5 and 0% of MgO, and heavy metals are not regulated in the finished product. In the case of feed phosphate production, the recovery is about 78%, where P2O5 is 41%, F is less than 0.2%, MgO is 0%, heavy metals (As, Cd, Pb) are below 10 ppm each, and Hg is less than 0.2 ppm, in accordance with European standards. Hydrochloric acid decomposition technology has been confirmed by a number of pilot industrial tests and demonstrates the greatest economic efficiency.
The production of dicalcium phosphate (DCP) consists of the following main technological stages:
(1) Processing of phosphorite raw materials with a solution of hydrochloric acid with the transfer of a valuable component into a soluble state in the form of a production solution and the removal of a solid residue.
Hydrofluoric acid reacts with silicic acid to form
. Other compounds are also formed in the solution, among which iron and aluminum chlorides are of the greatest importance:
The insoluble residue consists of undecomposed minerals of phosphate raw materials, as well as insoluble products of their decomposition (mainly). The solution obtained as a result of the decomposition of phosphate with hydrochloric acid contains significantly less than that obtained with sulfuric acid decomposition due to the reduced solubility of natural iron compounds in hydrochloric acid. Therefore, in this case, lower-quality phosphate raw materials can be used for processing.
(2) Treatment of the production solution with ground limestone with precipitation of DCP, drying of the resulting product, and obtaining a solution of calcium chloride.
(3) Treatment of the calcium chloride solution with slaked lime with the precipitation of magnesium, aluminum, and iron impurities in the hydrate cake and obtaining a purified calcium chloride solution, which is removed from the process for further processing.
(4) Treatment of part of the calcium chloride solution with sulfuric acid to obtain a regenerated hydrochloric acid solution and a gypsum precipitate.
The study object of this work is phosphogypsum (according to the terminology of the enterprise—synthetic gypsum), a sample of waste from the production of simple superphosphate (SSP), obtained using hydrochloric acid decomposition of phosphorites at a semi-industrial Technophos plant (Technophos by Prayon Technophos EAD, Devnya, Bulgaria) for the projected plant for the production of mineral fertilizers EuroChem-Karatau (Kazakhstan). A study of waste samples was carried out to determine the degree of their safety for subsequent use or safe disposal.
2. Materials and Methods
In the present study, the hazard class of waste from the processing of phosphate raw materials was established using the calculation method. The calculation method is used if the qualitative and quantitative composition of the waste is known, and the literature contains the necessary information to determine the hazard indicators of the waste components. The composition of the waste was previously determined using the methods of physical, physicochemical, and chemical analysis, considering the composition of the primary raw material from which the waste was formed and the technological regimes to which this raw material was subjected.
Testing of the analyzed sample was carried out using a JSM-6390LV (JEOL, Tokyo, Japan) scanning electron microscope. The sample was preliminarily dried to constant weight at a temperature of 105 °C, abraded to a particle size of 0.071 mm, placed on a conductive carbon film, and examined using a scanning electron microscope with an energy-dispersive elemental microanalysis attachment in a low vacuum mode (at a pressure of 50 Pa).
Mass spectrometric analysis of the test sample was carried out using an inductively coupled plasma mass spectrometer ICP-MS Agilent 7500cx (Agilent technologies, Santa Clara, CA, USA). The analyzed sample was previously dried to constant weight at a temperature of 105 °C, ground to a particle size of 0.071 mm, and decomposed in a mixture of hydrofluoric, nitric, and hydrochloric acids. The following conditions were used for testing on an inductively coupled plasma mass spectrometer: temperature of 20 °C, humidity of 51%, and atmospheric pressure of 100.2 kPa.
When performing atomic emission (spectral) analysis, the sample was transferred to the atomic state (plasma). The basis of qualitative emission spectral analysis is the characteristic of line spectra, and the functional dependence between the concentration of an element in a sample and the intensity of its spectral lines is the basis of quantitative determinations. The plasma radiation was converted into a spectrum in a spectral device and then the spectral lines were identified (qualitative analysis) and their intensity was measured (quantitative analysis).
The silicate analysis included the determination of the content of macrocomponents: silicon, aluminum, total iron, titanium, calcium, magnesium, and manganese in the form of their oxides. The studied sample of phosphogypsum does not completely dissolve in acids. To determine the content of macrocomponents, fusion with alkaline fluxes was used. When determining the content of individual elements, mixtures of acids were used to decompose the sample—a mixture of hydrofluoric and sulfuric or nitric acids. When carrying out the silicate analysis, a classical analysis scheme was used based on the fusion of silicate (0.5 g sample) with alkaline fluxes, leaching with hydrochloric acid, and subsequent gravimetric determination of silicon and complexometric determination of aluminum, total iron, calcium, and magnesium. The content of titanium and manganese was determined using the photometric method. Iron (II) was determined from a separate sample after decomposition in an inert medium using the titrimetric method. Alkali metals—sodium and potassium—were determined after the acid decomposition of silicates using flame photometry.
The determination of the total sulfur content was carried out using the gravimetric method, which is based on the decomposition of a sample of phosphogypsum with a mixture of nitric and hydrochloric acids, followed by the precipitation of sulfur in the form of barium sulfate and the determination of the mass of the latter.
The general steps of the calculation methodology are described in [
36,
37]. The quantitative composition, i.e., the relative concentration of each component in the total mass of waste
, is expressed in mg/kg. The relative content of each component (
, in %) in the total mass of waste is the upper limit of the concentration of a given component in the total mass of waste, that is, it corresponds to the term “no more”. The sum of values for all components
of which the waste is composed is close to 100%, but not less than 95%.
The assignment of waste to the hazard class using the calculation method is carried out based on the value of the total hazard index (), calculated as the sum of the hazard indicators of the substances that make up the waste (). The probability of harmful effects of individual waste components is determined using toxicological, physicochemical, as well as sanitary, and epidemiological indicators for each individual waste component. The search for the specified parameters of toxic–hygienic safety is carried out using officially published reference books.
The list of waste components and their quantitative content was established based on the results of qualitative and quantitative chemical (mineralogical) analyses, taking into account the composition of the raw material and the technology of its processing.
The list of indicators used for the calculation also includes the indicator of information support to consider the lack of information on the primary indicators for the degree of danger of the waste components. The information provision indicator is calculated by dividing the number of established indicators (i.e., indicators for which information is available in the relevant regulations and official reference books) by the number of indicators for the complete system.
To assess each parameter of the toxic and hygienic safety of waste, four characteristics are indicated, corresponding to four levels of toxic and hygienic safety, where each level of toxic and hygienic safety corresponds to a certain score. The corresponding score is set for the information support of the system of parameters. The value of the relative parameter of toxic–hygienic safety (
) is determined by dividing the sum of points for all parameters for which information is available by the number of these parameters. The total number of parameters in the system, considering the indicator of information support, is equal to
, and for the complete system, according to the priority list, will be equal to 13, according to
Table 1 and the Waste Hazard Classes [
36].
The relative parameter of toxic–hygienic safety for the
i-th waste component
is related to the unified relative parameter of toxic–hygienic safety
by the ratio:
The relationship between the standardized norm of toxic ecological safety of the
-th component of a substance
and the standardized relative parameter of the toxic–hygienic safety of the
-th component
is established by one of the following formulas:
The toxicity index of the
-th component of the product is calculated using the formula:
where
is the content of the component in the product, mg/kg, and
is the standard for toxic and environmental safety of the
-th component of the substance. The product toxicity index is calculated using the formula:
where
is product toxicity index;
is toxicity index of the
-th component; and
is the number of components in the product. When calculating
, the condition of full accounting of all components included in the waste is observed, that is:
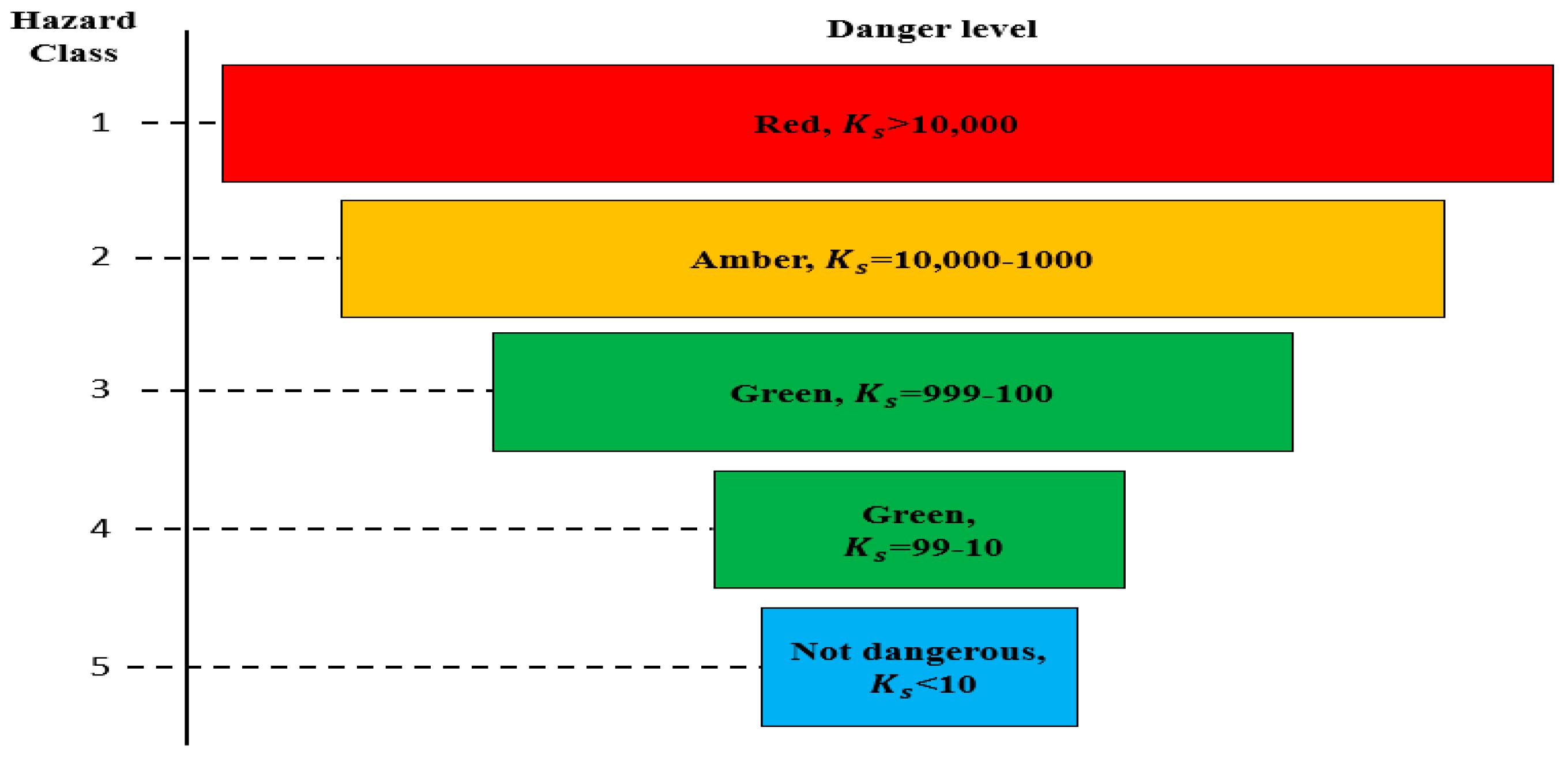
The value of the waste hazard class was determined using the values of its total hazard index (
), guided by the data given in
Figure 1.
The value of the hazard class of the waste is determined using the values of its toxicity index (
), guided by the data in
Table 2 for the intervals of the total value of the toxicity index of the product components for different hazard classes.
4. Discussion
According to the results of the X-ray analysis of the chemical composition, it was established that the main elements that make up the phosphogypsum sample are calcium (35.33–19.73%) and phosphorus (20.64–15.99%). The content of highly toxic elements such as barium, copper, zinc, cadmium, lead, cobalt, molybdenum, arsenic, and antimony and a group of rare earth metals (strontium, rubidium, zirconium, rhodium, yttrium, cerium) was not found in the studied waste. It follows from the results (
Table 3) that phosphogypsum is practically a fluorine-free product since the fluorine content in the studied sample was not established. The raster electronic analysis program used has automated software for peak identification, considering possible factors influencing errors. The number of counts in an X-ray peak is proportional to the amount of an element in the sample, so large peaks are major constituents and small peaks are secondary. However, there are many factors that affect peak size (corrections for absorbance, fluorescence, and detector sensitivity). The results of quantitative X-ray analysis can be achieved with an accuracy of 1%. However, when peaks overlap by more than 40%, the measurement accuracy drops sharply, which does not always lead to a reliable quantitative result. It should also be taken into account that in the present study, using scanning electron spectroscopy, the determination of the elemental composition of the samples under study was carried out in local areas, which provides reliable information only on qualitative elemental analysis.
According to the results of the mass spectrometric analysis (
Table 4), it was found that the main elements that make up the test sample of phosphogypsum are calcium (22.97%), phosphorus (17.42%), and magnesium (1.69%). The content of highly toxic elements such as copper, zinc, cadmium, lead, cobalt, molybdenum, chromium, and arsenic and a group of rare earth metals (strontium, rubidium, zirconium, rhodium, yttrium, cerium) ranges from tenths to thousandths of a percent.
The results of the atomic emission spectral analysis (
Table 5) show that the main element in it is calcium (≥10%), the masking background of which interferes with the determination of Ba, Zn, Yb, Y, and Sr. The presence of phosphorus (>1%) was also established. The content of highly toxic elements such as copper, arsenic, cadmium, lead, chromium, and molybdenum and a group of rare earth metals (zirconium, radium) ranges from tenths to thousandths of a percent. The elements Au, B, Hf, Hg, In, Nb, Pt, Ta, Te, Th, U, and W were not detected.
As can be seen from
Table 7, the specific effective activity of the studied sample does not exceed the level of the natural background or the norms established in Kazakhstan by sanitary rules [
39].
According to the silicate analysis (
Table 6), the studied sample, phosphogypsum, is predominantly represented by calcium (CaO—38.48%) and phosphorus (P
2O
5—18.32%). The content of silicon, potassium, magnesium, sodium, aluminum, and iron is negligible. The results of the determination of total sulfur by type for the studied sample of waste from the processing of phosphate raw materials are satisfactory (
Table 7).
Thus, taking into account the prevailing content of chemical elements in the sample that are characterized by moderate toxicity and an insignificant content of highly toxic substances, the results suggest that the hazard of the considered waste from the processing of phosphorus-containing raw materials will be determined by the quantitative content of elements with moderate toxicity in it. The results obtained for the total toxicity index allow for the establishment of the four hazard classes for the test sample and waste from the processing of phosphate raw materials. However, it should be taken into account that if the amount of toxic and highly toxic substances in the composition of the waste exceeds 1%, the waste will become hazardous [
40]. In the test sample, this contribution can be made by heavy metals, radionuclides, and fluorine contained in the waste and initial components. In this study, products from the processing of phosphate raw materials formed more than one year ago were provided for study, and the process of their storage and transportation was not monitored. Based on the results of scanning microscopy, the presence of traces of fluorine was established, but its content in the initially formed products cannot be reliably determined. Considering the migration ability of fluorine as an element (in its water-soluble forms), there is a risk of losing this dangerous component over storage time. This issue is beyond the scope of this research and requires additional study.