Abstract
Livestock manure has a high ammonium content that can limit its direct application on soil as a fertiliser in nitrate-vulnerable zones. Treatment technologies that are able to extract ammonium from livestock manure allow it to be concentrated in small volumes, making it cheaper and easier to transport and use as fertiliser in crop areas where there is a deficit of nitrogen. This study proposed using low-temperature vacuum evaporation to treat pig slurry in order to obtain marketable products that can be used as fertilisers and help close the nitrogen cycle. Two different configurations and scales were used. The first was a seven-litre laboratory-scale evaporator complemented with a condenser, a condensate trapper, an acid trap and a vacuum pump operated at −90 kPa vacuum pressure and at three different temperatures: 50.1 ± 0.2 °C, 46.0 ± 0.1 °C and 45.3 ± 1.3 °C. The second, Ammoneva, is an on-farm pilot-scale evaporator (6.4 m3), capable of working in four-hour batches of 1 t of liquid fraction of pig slurry with an operating temperature of 40–45 °C and −80 kPa vacuum pressure. The laboratory-scale evaporator, which features several novel improvements focused on increasing ammonia recovery, showed a higher nitrogen removal efficiency from the liquid fraction of pig slurry than the on-farm pilot plant, achieving 84% at 50.1 °C operation, and recovering most of it in ammonia solution (up to 77% of the initial nitrogen), with 7% of the ammonia not recovered. The Ammoneva pilot plant achieved a treated liquid fraction with 41% of initial nitrogen on average, recovering 15% in the ammonia solution in the acid trap; so, the NH3 gas absorption step needs to be further optimised. However, due to the simplicity of the Ammoneva pilot plant, which is easily placed inside a 20-foot container, and the complete automation of the process, it is suitable as an on-farm treatment for decentralised pig slurry management. The implementation of the novel design developed at laboratory-scale could help further increase recovery efficiencies at the pilot-plant scale.
1. Introduction
Livestock manure requires correct management due to its high organic matter content and concentration of nutrients, especially phosphorus and nitrogen. In some cases, owing to the high concentration of livestock, a treatment to recover nutrients is advisable. Ammonia recovery from livestock manure can produce marketable products, such as ammonium salt fertilisers (e.g., ammonium sulphate, ammonium nitrate and ammonium lactate), and allows the nitrogen loop to be closed. Treatment technologies able to extract ammonium from livestock manure enable it to be concentrated in reduced volumes, simplifying its transport and use as a fertiliser in crop areas where there is a nitrogen deficit. Furthermore, ammonium recovery from livestock manure can help reduce ammonia gas emissions into the atmosphere and its environmental impact. Ammonia acts as an aerosol precursor in the troposphere by reacting with sulfuric and nitric acids to form particulate matter (PM2.5 and PM10) and is one of the compounds responsible for the generation of secondary N2O emissions, a greenhouse gas [1].
Several technologies have been tested to recover ammonium from livestock manure, such as nutrient chemical precipitation in the form of struvite [2,3], water evaporation [4,5], the use of hydrophobic membranes [6,7,8] and other mostly emergent technologies, e.g., bioelectrochemical systems [9,10]. One of the most extended technologies for ammonia recovery is air stripping and absorption [11,12,13]. This process is based on the ammonium–ammonia equilibrium (Reaction (1)). Livestock manure has a high concentration of nitrogen in the form of ammonium (), and it is possible to displace this equilibrium to ammonia (NH3), which is a gas and easier to recover by absorption in an acid. Ammonium can be converted into ammonia mainly by increasing pH and/or temperature (Equation (2)) [1].
where [NH3] is the concentration of ammonia in the solution, TAN is the total ammonia concentration and T is the temperature (K).
Although it will depend on the substrate and reactor configuration, typical values of the parameters favouring air stripping are a pH of between 10.0 and 11.0 and a temperature of up to 80 °C [14]. However, increasing the temperature to favour ammonium conversion to ammonia is an energy-intensive step, and more efficient processes have been developed, such as low-temperature vacuum evaporation [15,16,17]. When a vacuum is applied to an enclosed reactor, boiling point temperature decreases to below normal, thus reducing energy costs due to a 56% decrease in energy demand when operating at 65 °C compared to 102 °C [17]. In addition, gas-phase ammonia mass transfer is boosted by the suction effect of the applied vacuum [17]. Energy consumption for vacuum application can be further reduced by taking advantage of the temperature gradient between the evaporator and a cold condenser, a novel distillation technology called Cool Steam, and successfully tested for obtaining fresh water. The Cool Steam method takes profit advantage of a net heat flux between a warm and a cold source or surface. Water vapour acts as a heat carrier, travels to the cooler surface and condensates in contact with it. This novel method avoids continuous vacuum application by using switches and solenoid valves to maintain the whole system’s tightness under strict sealing conditions [18]. The recovered ammonia can be in the form of, among others, an ammonium sulphate or nitrate solution, depending on the absorption acid. Low-temperature vacuum evaporation of ammonia has been applied to dairy manure digestate and achieved an efficiency above 93% in three hours when operated at 50 °C and 16.6 kPa [17].
Most low-temperature vacuum evaporator set-ups consist of a boiling reactor, and limitations on its feeding depth have been reported since boiling intensity is less vigorous in a deeper feed due to a greater hydrostatic pressure [16]. Improvements in the design of this technology are needed to increase its efficiency. Furthermore, up-to-date, mostly laboratory-scale set-ups (up to 40 L) have been tested using anaerobic digester effluent, livestock manure and urine [16,17]. Therefore, field plants must be developed and optimised to increase the liquid–gas contact surface area and boost ammonia evaporation. Automation of the process is also needed to simplify operations in order for it to be a technology that can easily be placed on farms and operated by farmers.
The objective of this study was to evaluate the ammonia recovery efficiency of low-temperature vacuum evaporation from raw pig slurry in two different systems. First, a novel laboratory-scale set-up was developed, which was focused on increasing the liquid–gas contact surface in combination with Cool Steam technology to reduce the consumption of electrical energy due to the continuous vacuum pump operation. Second, an automated vacuum evaporation field plant to obtain a salt solution from pig slurry that can be used as a fertiliser was monitored, including its gaseous emissions, and a mass balance was performed.
2. Materials and Methods
Two different configurations were tested. The first was a 7 L laboratory-scale evaporator, and the second was a 6.4 m3 on-farm pilot-scale evaporator.
2.1. Laboratory-Scale Low-Temperature Evaporator Set-up
The laboratory-scale low-temperature evaporation system was constructed in methacrylate and comprised of an evaporator, a condenser, a condensate trapper, an acid trap and a vacuum pump (VACUUBRANDTM MZ2C NT, Vacuubrand GmbH and Co., Wertheim, Germany) (Figure 1 and Figure 2), following a design used in previous studies [19]. The evaporator consisted of two concentric cylinders (15 cm internal diameter of the inner cylinder, 21 cm internal diameter of the external cylinder, 1 cm thickness, 40 cm height, SUMMEPLAST, S.L., Barcelona, Spain) and upper and lower lids. The space delimited by both cylinders was used as a heating jacket to circulate hot water from a thermostatic bath. A stainless-steel spiral was placed in this heating jacket as a pre-heating step. The inner cylinder was equipped with a plastic ramp to facilitate the slow drop of the liquid fraction of the pig slurry. Although methacrylate has a low transmission of heat, this material was chosen in order to check visually that the pig slurry was going down the ramp as slowly as possible. The evaporator was equipped with a temperature probe (405-113, TC Medida y Control de Temperatura, S.A., Madrid, Spain) and a pressure probe (PSE 61-01, SMC España, S.A., Álava, Spain). The evaporator had a gas exit in the centre of the upper lid connected to the condenser with polyurethane tubing. The condenser was a methacrylate cylinder (15 cm diameter, 30 cm height) with upper and lower lids and a stainless-steel spiral for the circulation of the gas and vapour inside. The condenser was filled with cold water that was recirculated with a pump in order to condense water and ammonia. The exit of the refrigeration spiral was connected to the condensate trapper and acid trap so as to accumulate the evaporated water and ammonia. Each of the traps consisted of a methacrylate cylinder (9 cm diameter, 30 cm length) with upper and lower lids and an external cool bath for the condensate trapper. The system was equipped with automatic vacuum solenoid valves (VX234KGAXB, SMC España, S.A., Álava, Spain) to control the input and output flux of the different components and maintain the whole system tightness under strict sealing conditions.
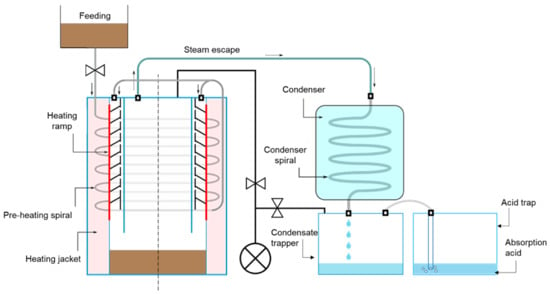
Figure 1.
Diagram of the laboratory-scale low-temperature evaporation set-up.

Figure 2.
Laboratory-scale low-temperature evaporation set-up: ① evaporator equipped with a heating jacket and heating spiral, ② condenser with a condenser spiral, ③ condensate trapper, ④ acid trap, ⑤ vacuum pump and ⑥ cooling tank.
2.2. Operation of the Laboratory-Scale Low-Temperature Evaporation Set-up
Pig slurry was collected from a farm in Puigllong (Catalonia), sieved (500 µm) and stored at 4 °C until use. The main characteristics of the liquid fraction of pig slurry were a pH value of 8.1 ± 0.2, ammonia nitrogen (-N) of 2197 ± 24 mg L−1, total Kjeldahl nitrogen (TKN) of 3364 ± 133 mg L−1 and chemical oxygen demand (COD) of 30814 ± 3304 mg O2 L−1. To boost ammonia evaporation, the pH of the liquid fraction was increased up to a value of 11 by the addition of 40% NaOH prior to each assay. This pH value was chosen to use the most similar parameters to the pilot-scale plant, which was operated at this same pH.
The whole system was operated at near vacuum conditions with a vacuum pump (absolute pressure between 5 and 11 kPa). The vacuum was performed at the start of the experiment, switching off the vacuum pump when the vacuum setpoint was achieved, with no need for it to be activated during the process, thus reducing energy consumption. Once the vacuum was set, feeding of the evaporator with the basified liquid fraction started. A volume of 2.5 L of the substrate was introduced to the vacuum in a 5 L Cubitainer® (Fisher Scientific, S.L., Madrid, Spain) that was connected to the evaporator. The feeding valve was opened intermittently to allow the substrate to go into the pre-heating spiral and slowly down the ramp for 30 min (flowrate of 5 L h−1). Once all the volume of the substrate had entered the evaporator, it remained at the bottom for two hours. Concomitant evaporation of water and ammonia occurred. The vapour generated exited the evaporator through the upper lid, was directed to the condensation spiral and recovered in the condensate trapper and acid trap—the latter containing 10% H2SO4 to convert ammonia into ammonium and avoid losses. Ammonium was recovered preferably in the condensate to reduce the use of reagents (acid) and the volume of the recovered product. The evaporator was operated at three different temperatures, 50.1 ± 0.2 °C, 46.0 ± 0.1 °C and 45.3 ± 1.3 °C, and each assay was performed in duplicate. The pressure and temperature inside the evaporator were recorded during the assay.
A nitrogen mass balance was performed for each assay, measuring the volumes and analysing the concentration of -N in the feeding liquid fraction, the treated liquid fraction, the condensed ammonia solution and the acid at the start and end of each batch. Ammonia removal efficiency was calculated as the quotient between the difference in mass of the feeding and treated liquid fraction, and the mass in the feeding liquid fraction.
2.3. On-Farm Pilot Plant: Set-up and Operation
The pilot plant develops a process called Ammoneva [20] and has been in place since 2020 in Viver i Serrateix (Catalonia, Spain) on a farm belonging to UPB GENETIC WORLD, S.L. It was manufactured and launched between 2017 and 2018. The pilot system is comprised of an influent pit, a solid–liquid separator, a closed pit for a basification step, the Ammoneva container (Figure 3) and the effluent pit. The main components of the system are placed inside a 20-foot container with the system controllers: an evaporator (6.4 m3) (Figure 4a), an acid trap (0.7 m3) (Figure 4b) that contains lactic acid to absorb ammonia, a vacuum pump and a basic trap, which is placed outside the container. Lactic acid was used instead of conventional sulfuric acid in order to market the recovered product as a root stimulant. Previous studies have shown that lactic acid stimulates soil microbial activity and the release of soluble phosphates [21].

Figure 3.
Picture of the 20-foot Ammoneva container.
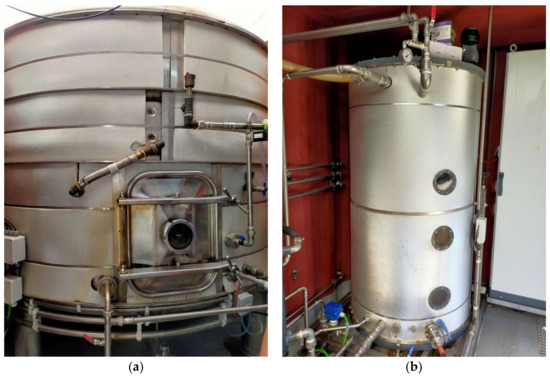
Figure 4.
(a) Evaporator and (b) acid trap placed inside the Ammoneva container.
Figure 5 shows the process diagram. First, pig slurry is directed towards the solid–liquid separator. The pig slurry liquid fraction exiting the separator is directed to a covered pit, where pH value is modified to 11 with a Ca(OH)2 solution (8% w/w) and homogenised. At this point, due to the pH increase, there is a precipitation of phosphates, mainly apatite, that can also be recovered and used as fertiliser. Once the liquid fraction is basified, it is diverted to the evaporator. The evaporator works in batches of 1 t of liquid fraction of pig slurry and lasts for four hours, with an operating temperature of 40–45 °C and −80 kPa vacuum pressure. Thanks to the low pressure and the basic pH value, ammonium–ammonia equilibrium is displaced to the second species and evaporated at this relatively low temperature. The special internal mixing design of the evaporator favours liquid–gas contact. Three concentric cylinders inside the evaporator increase the evaporation surface of the liquid fraction, continuously recirculated internally from the bottom to the top of the reactor. Ammonia is absorbed by the acid since the low pH value displaces equilibrium towards the ammonium species. Finally, gases exiting the acid trap are directed to a basic trap to minimise atmospheric emissions (H2S, hydrogen sulphide). At the end of the cycle, processed livestock manure is obtained that has a lower nitrogen content. Furthermore, an ammonium lactate solution is produced, which can also be used as a fertiliser.
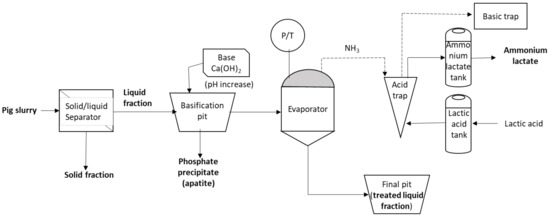
Figure 5.
Flux diagram of the Ammoneva process.
The operation of the pilot plant was monitored in 12 sampling campaigns. Table 1 shows the main parameters of the pig slurry used in these campaigns.

Table 1.
Characterisation of the main parameters of the pig slurry used in the pilot-plant assays (n = 12).
2.4. Analytical Methods and Calculations
The COD, NH4+-N, TKN, total and volatile solids (TS, VS), pH, and conductivity (EC) of liquid samples were determined according to Standard Methods [22]. The bulk solution pH in each sample was measured using a CRISON 2000 pH electrode (HACH LANGE SPAIN, S.L.U., Barcelona, Spain). N-NH4+ and TKN were analysed by a Büchi KjelFlex K-360 distiller (BUCHI Ibérica, S.L.U., Barcelona, Spain) and a Metrohm 702 SM autotitrator (Metrohm, Herisau, Switzerland). Phosphate (PO43−), sulphate (SO42−) and potassium (K+) concentrations were measured by ionic chromatography (IC) with an 861 Advanced Compact IC (Metrohm, Herisau, Switzerland). A Metrosep A Supp 4 (Metrohm, Herisau, Switzerland) column with a Metrospt A Supp 4/5 Guard pre-column and a CO2 suppressor were used for anion determination. A Metrohm C4 150/4.0 column (Metrohm, Herisau, Switzerland) and a Metrosep C4 Guard pre-column were used for cation determination. Prior to IC analysis, samples were diluted and filtrated with nylon (0.45 mm) and BonElut JR C18 microfilters (Varian, Palo Alto, CA, USA). Ammonia emissions to the atmosphere in the on-farm pilot plant were monitored using a GX-6000 gas sensor (RKI Analytical Instruments GmbH, Bad Homburg vor der Höhe, Germany) at the gas exit of the basic trap at four specific moments throughout the process (filling of the evaporator, t = 0 h, t = 2 h and t = 4 h). A Lindvall hood was used to monitor emissions from the influent and effluent pits in the Ammoneva plant. Air samples (30 mL) for N2O emission measurements were collected with a syringe from the pipe connected to the hood outlet or the basic trap gas exit and injected into a vacutainer tube with a butyl rubber stopper. N2O concentration was determined with an Agilent 7820A (Agilent, Santa Clara, CA, USA) gas chromatography system using an electron capture detector (ECD).
3. Results and Discussion
3.1. Laboratory-Scale Low-Temperature Evaporation Plant
As mentioned above, prior to feeding the pig slurry into the evaporator, the pH was increased from pH 8 to pH 11, requiring a dosage of 9.5 ± 0.8 g NaOH L−1.
The three different average operation temperatures inside the evaporator of 50.1 ± 0.2 °C, 46.0 ± 0.1 °C and 45.3 ± 1.3 °C, were achieved by operating the thermostatic bath at three different temperatures, 75 °C, 65 °C and 55 °C, due to the low heat transmission of methacrylate. The vacuum pressure was maintained at −90.0 ± 0.2 kPa, −91.5 ± 0.1 kPa and −91.5 ± 0.9 kPa when operating at 50.1 ± 0.2 °C, 46.0 ± 0.1 °C and 45.3 ± 1.3 °C, respectively.
The ammonium removal efficiency was similar for operation at 50.1 °C and 46.0 °C, achieving 84% (Table 2), while it was 63% when operating at 45.3 °C. Although the temperature reached inside the evaporator was enough for ammonia evaporation at vacuum conditions in the three assays, the assay performed at the lowest temperature produced a return of the evaporated water and ammonia to the evaporator that partly condensed along the pipe before reaching the condenser. Ammonia was recovered mainly in the condensate trapper, while little or no ammonia was found in the acid trap. NH4+-N concentration was lower in the assays performed at 50.1 °C and 46.0 °C, with a higher condensate volume than in the assay performed at 45.3 °C, indicating a larger amount of evaporated water. NH4+-N concentration of the treated slurry was reduced to 446 ± 54, 459 ± 57 and 1123 ± 281 NH4+-N mg L−1 at 50.1 °C, 46.0 °C and 45.3 °C, respectively.

Table 2.
Summary of the results of the laboratory-scale low-temperature ammonia evaporation set-up.
Previous studies have reported similar or slightly higher removal efficiencies, albeit operating at higher temperatures and for longer periods. Ukwuani and Tao (2016) tested seven combinations of temperature and pressure, their lowest temperature of 50 °C being equivalent to the highest one in this assay (evaporator at 50.1 °C), in combination with an absolute pressure of 16.6 kPa. They found that after 1.5 h, 21.3% of the ammonia remained in the dairy manure digestate [17], while in the present study, 16% of the ammonia remained in the pig slurry after two hours. High ammonium removal has been achieved using high-strength synthetic wastewater in a rotary evaporator, such as 97.84% at a temperature of 65.5 °C, 73.3 kPa vacuum pressure and 59.6 min of operation [15]. Later, the same authors found that when using digested liquid dairy manure at a higher temperature of 69.6 °C with a vacuum pressure of 43.5 kPa and treatment time of 87.65 min, the removal efficiency fell to 93.58% [23]. Han et al. (2022), using a 40 L stainless-steel vacuum vessel fed with digestate heated at 65 °C and 25–27 kPa, removed 97.4–99.4% of the ammonia in eight hours [24]. Tao et al. (2018) also removed up to 95.7% of ammonia from digested dairy manure at a temperature and vacuum combination of 65 °C and 27 kPa in five hours [16].
With a focus on scaling up the system, previous studies have shown feeding depth to be an important limitation that cannot be addressed by mechanical mixing. Tao et al. (2018) found a substantial decrease in stripping performance when increasing the feeding depth in the boiler since feeding depths of 8.5 cm and 17.0 cm removed 92.2% and 56.5% ammonia, respectively, in five hours, with a temperature and vacuum combination of 65 °C and 27 kPa [16]. In the present study, the new design allowed ammonia evaporation while pig slurry was circulating in the pre-heating spiral and on the ramp, finishing with a feeding depth of 14 cm in the evaporator. Monitoring of the accumulation of condensate during each assay showed that the fastest evaporation occurred while the pig slurry was being fed into the evaporator, circulating in the pre-heating spiral and on the ramp (Figure 6).
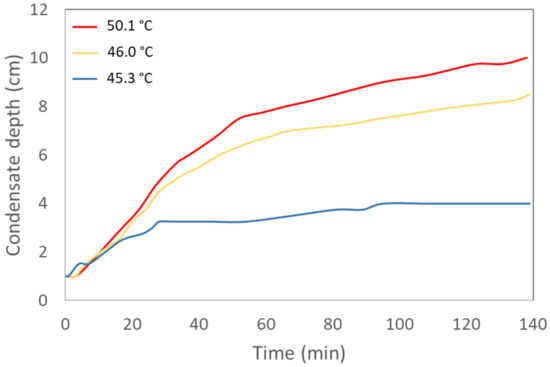
Figure 6.
Condensate accumulation (depth monitoring) in the condensate trapper in the experiment performed at 50.1 °C, 46.0 °C and 45.3 °C.
Energy consumption for vacuum production at the start of the process was 3 Wh (1.2 Wh L−1 of pig slurry), which is much lower than the values previously estimated since evaporation occurs with the vacuum pump off thanks to vacuum solenoid valves and the temperature gradient between the hot and cold points. Vacuum evaporation from hydrolysed urine has shown ammonia removal efficiencies of 72% in 12 h with a combination of 65 °C and 27 kPa, estimating a vacuum energy consumption of 3.7 kWh for the treatment of 1 m3 of hydrolysed human urine (4 Wh L−1 of hydrolysed urine) [25]. Han et al. (2022) estimated the energy consumption of the vacuum pump working 24 h per day in a scaled-up vacuum evaporator (65 °C, 25–27 kPa) to be less than 18 kWh (2 Wh L−1 of digestate) [24]. Thus, the novel set-up of the ammonia vacuum evaporator has demonstrated important improvements regarding reducing energy consumption and avoiding feeding depth limitations.
3.2. On-Farm Low-Temperature Evaporation Pilot Plant
Table 3 shows the average characterisation of the different fractions obtained in the process: solid fraction, ammonium lactate and treated liquid fraction after four hours. Ammonium accumulated as ammonium lactate while different cycles were performed, reaching a maximum of 12 g N L−1 in one of the characterisations. The acid in the acid trap was replaced on average every 5 evaporator batches, although it could vary in a range between 1 and 10 in the different sampling campaigns that were carried out, explaining the high variability and standard deviations. Both the ammonium lactate and the solid fraction, rich in phosphorus, can be exported and reused as fertilisers. The treated liquid fraction, which has a lower ammonium content, can be applied to nearby crops.

Table 3.
Average characterisation of the different fractions obtained in the pilot plant (n = 12).
The mass balance (Figure 7) showed that, on average, 41% of the initial nitrogen remained in the treated liquid fraction, while 15% of it was recovered as an ammonia solution in the acid trap, and 4% was found in the solid fraction obtained in the separator. It was found that 12% of the nitrogen was emitted into the atmospherein the basification pit or basic trap, N2O representing between 5 and 37% of the total emitted nitrogen. On average, 10% of the nitrogen accumulated in the intermediate tank. This represents a difference in the nitrogen balance of 26%; thus, ammonia absorption needs further optimisation. Another much smaller pilot plant (40 L) removed 97.4–99.4% of ammonia from digestate at 25–27 kPa, although at a higher temperature (65 °C) and over a longer operation time (8 h) [25].
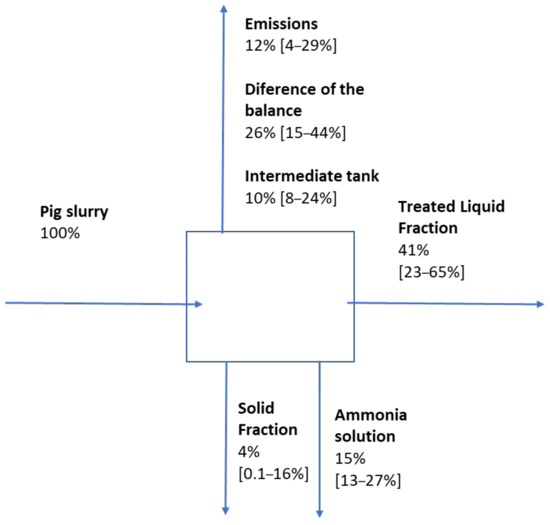
Figure 7.
Scheme of the mass balance for nitrogen, with the mean value of the 12 samples and their range in brackets.
The difference in the mass balance can be due to several facts related to the difficulties of working at the field level. First, other points of diffused emissions were detected (such as the solid–liquid separator), which were not monitored. Second, the monitoring of the gaseous emissions of the basic trap was carried out through four specific samples throughout the process, assuming that they were representative of each period, although the concentration oscillated over time. Discontinuous sampling could cause a loss of information. Furthermore, the basification pit accumulated different batches of liquid–solid separation, which made it possible for the evaporator input to be slightly different from the raw pig slurry sampled at the beginning of the process.
The electricity consumption, including for the vacuum, recirculation, mixing, feeding and drain pumps, solid–liquid separator and refrigerator, was 5.4 kWh t−1 of treated pig slurry. Compared with a case study of the thermal stripping of ammonia from manure digestate [26], 4895 kWh were required to heat 66.6 m3 of digestate from 37 °C to 102 °C, which is 73.5 kWh m−3, i.e., more than 13 times the energy required to operate the Ammoneva plant at 45 °C. In the absence of actual data from other vacuum ammonia evaporation pilot plants, previous estimations about scaled-up vacuum systems have estimated the energy consumption of the vacuum pump to be less than 18 kWh d−1 [24], which is in the range of this study’s on-farm pilot plant taking all the equipment into account.
This technology can be applied directly to raw livestock manure to avoid ammonia gas emissions to the atmosphere or as a subsequent step in an anaerobic digestion process. Due to the simplicity of the plant, it is suitable for use as an on-farm treatment for decentralised pig slurry management.
4. Conclusions
The assays performed in the laboratory-scale plant showed a greater nitrogen removal efficiency from the liquid fraction of pig slurry than the on-farm pilot plant, achieving 84% at 50.1 °C operation and recovering most of it in the ammonia solution (up to 77% of the initial nitrogen), with 7% of ammonia not recovered. The use of heat-conductive materials for the evaporator’s construction rather than methacrylate could allow the thermostatic bath’s temperature to be reduced. The novel design, based on the evaporator’s pre-heating spiral and internal ramp, has addressed the limitation of feeding depth found in previous studies. Moreover, the use of vacuum solenoid valves and the combination with Cool Steam technology helped reduce the consumption of electrical energy due to continuous vacuum pump operation while achieving high recovery efficiencies with only half of the energy consumption reported to date for vacuum evaporation.
The Ammoneva pilot plant achieved a treated liquid fraction with, on average, 41% of the initial nitrogen, recovering 15% of it in the ammonia solution of the acid trap. Thus, ammonia absorption needs to be further optimised. The treatment allows the solid fraction and ammonium lactate to be exported to crop areas deficient in nitrogen, while facilitating the application of the treated liquid fraction in nitrate-vulnerable zones. Due to the simplicity of the Ammoneva plant, since it is easy to place in a 20-foot container, and the complete automation of the process, it is suitable to become an on-farm treatment for decentralised pig slurry management.
Author Contributions
Conceptualisation, A.B. and R.E.; methodology, A.B.; validation, M.C.; formal analysis, M.C; investigation, M.C., M.M. and L.B.; resources, A.B., D.C. and R.E.; data curation, M.C.; writing—original draft preparation, M.C.; writing—review and editing, M.C., L.B., J.S., N.N., A.B., P.A.A. and R.E.; visualisation, M.C.; supervision, A.B; project administration, A.B.; funding acquisition, D.C., R.E. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Nutri2Cycle project funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement No 773682. The support of the CERCA Programme and of the Consolidated Research Group of Sustainability in Biosystems (ref. 2021 SGR 01568), both from the Generalitat de Catalunya, is also acknowledged.
Data Availability Statement
Data will be supplied upon request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Behera, S.N.; Sharma, M.; Aneja, V.P.; Balasubramanian, R. Ammonia in the atmosphere: A review on emission sources, atmospheric chemistry and deposition on terrestrial bodies. Environ. Sci. Pollut. Res. 2013, 20, 8092–8131. [Google Scholar] [CrossRef]
- Nagarajan, A.; Goyette, B.; Raghavan, V.; Bhaskar, A.; Rajagopal, R. Nutrient recovery via struvite production from livestock manure-digestate streams: Towards closed loop bio-economy. Process Saf. Environ. Prot. 2023, 171, 273–288. [Google Scholar] [CrossRef]
- Siciliano, A.; Limonti, C.; Curcio, G.M.; Molinari, R. Advances in struvite precipitation technologies for nutrients removal and recovery from aqueous waste and wastewater. Sustainability 2020, 12, 7538. [Google Scholar] [CrossRef]
- Vondra, M.; Máša, V.; Bobák, P. The energy performance of vacuum evaporators for liquid digestate treatment in biogas plants. Energy 2018, 146, 141–155. [Google Scholar] [CrossRef]
- Bonmatí, A.; Campos, E.; Flotats, X. Concentration of pig slurry by evaporation: Anaerobic digestion as the key process. Water Sci. Technol. 2003, 48, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Vecino, X.; Reig, M.; Bhushan, B.; Gibert, O.; Valderrama, C.; Cortina, J.L. Liquid fertilizer production by ammonia recovery from treated ammonia-rich regenerated streams using liquid-liquid membrane contactors. Chem. Eng. J. 2019, 360, 890–899. [Google Scholar] [CrossRef]
- Brennan, B.; Briciu-Burghina, C.; Hickey, S.; Abadie, T.; Al Ma Awali, S.M.; Delaure, Y.; Durkan, J.; Holland, L.; Quilty, B.; Tajparast, M.; et al. Pilot scale study: First demonstration of hydrophobic membranes for the removal of ammonia molecules from rendering condensate wastewater. Int. J. Mol. Sci. 2020, 21, 3914. [Google Scholar] [CrossRef]
- Cerrillo, M.; Burgos, L.; Serrano-Finetti, E.; Riau, V.; Noguerol, J.; Bonmatí, A. Hydrophobic membranes for ammonia recovery from digestates in microbial electrolysis cells: Assessment of different configurations. J. Environ. Chem. Eng. 2021, 9, 105289. [Google Scholar] [CrossRef]
- Burns, M.; Qin, M. Ammonia recovery from organic nitrogen in synthetic dairy manure with a microbial fuel cell. Chemosphere 2023, 325, 138388. [Google Scholar] [CrossRef]
- Cerrillo, M.; Riau, V.; Bonmatí, A. Recent Advances in Bioelectrochemical Systems for Nitrogen and Phosphorus Recovery Using Membranes. Membranes 2023, 13, 186. [Google Scholar] [CrossRef]
- Heidarzadeh Vazifehkhoran, A.; Finzi, A.; Perazzolo, F.; Riva, E.; Ferrari, O.; Provolo, G. Nitrogen Recovery from Different Livestock Slurries with an Innovative Stripping Process. Sustainability 2022, 14, 7709. [Google Scholar] [CrossRef]
- Kinidi, L.; Tan, I.A.W.; Abdul Wahab, N.B.; Tamrin, K.F.B.; Hipolito, C.N.; Salleh, S.F. Recent development in ammonia stripping process for industrial wastewater treatment. Int. J. Chem. Eng. 2018, 2018, 3181087. [Google Scholar] [CrossRef]
- Abbà, A.; Domini, M.; Baldi, M.; Pedrazzani, R.; Bertanza, G. Ammonia Recovery from Livestock Manure Digestate through an Air-Bubble Stripping Reactor: Evaluation of Performance and Energy Balance. Energies 2023, 16, 1643. [Google Scholar] [CrossRef]
- Folino, A.; Zema, D.A.; Calabrò, P.S. Environmental and economic sustainability of swine wastewater treatments using ammonia stripping and anaerobic digestion: A short review. Sustainability 2020, 12, 4971. [Google Scholar] [CrossRef]
- Reza, A.; Chen, L. Optimization and Modeling of Ammonia Nitrogen Removal from High Strength Synthetic Wastewater Using Vacuum Thermal Stripping. Processes 2021, 9, 2059. [Google Scholar] [CrossRef]
- Tao, W.; Ukwuani, A.T.; Agyeman, F. Recovery of ammonia in anaerobic digestate using vacuum thermal stripping—Acid absorption process: Scale-up considerations. Water Sci. Technol. 2018, 78, 878–885. [Google Scholar] [CrossRef]
- Ukwuani, A.T.; Tao, W. Developing a vacuum thermal stripping—Acid absorption process for ammonia recovery from anaerobic digester effluent. Water Res. 2016, 106, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Arnau, P.; Navarro, N.; Soraluce, J.; Martínez-Iglesias, J.; Illas, J.; Oñate, E. Cool Steam Method for Desalinating Seawater. Water 2019, 11, 2385. [Google Scholar] [CrossRef]
- Estéfano, R.; Flotats, X.; Cuadras, A. AMMONEVA system. In Proceedings of the 2019 AIChE Annual Meeting, Orlando, FL, USA, 10–15 November 2019. [Google Scholar]
- Estéfano Lagarrigue, R. Procedure and Equipment for the Obtaining/Recovery of Nitrogen in the form of Ammonia (Bio Ammonia) from Animal and Vegetable Biomasses Spanish Patent ES2676622A1, 23 July 2018.
- Rodríguez-Morgado, B.; Jiménez, P.C.; Moral, M.T.; Parrado, J. Effect of L-lactic acid from whey wastes on enzyme activities and bacterial diversity of soil. Biol Fertil Soils 2017, 53, 389–396. [Google Scholar] [CrossRef]
- APHA/AWWA/WEF. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Whasington, DC, USA, 2012. [Google Scholar]
- Reza, A.; Chen, L. Optimization and modeling of ammonia nitrogen removal from anaerobically digested liquid dairy manure using vacuum thermal stripping process. Sci. Total Environ. 2022, 851, 158321. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Agyeman, F.; Green, H.; Tao, W. Stable, high-rate anaerobic digestion through vacuum stripping of digestate. Bioresour. Technol. 2022, 343, 126133. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Bayrakdar, A.; Wang, Y.; Agyeman, F. Three-stage treatment for nitrogen and phosphorus recovery from human urine: Hydrolysis, precipitation and vacuum stripping. J. Environ. Manage. 2019, 249, 109435. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.W.; Tao, W. Cost benefit assessment of a novel thermal stripping—Acid absorption process for ammonia recovery from anaerobically digested dairy manure. Water Pract. Technol. 2016, 11, 355–364. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).