Abstract
Thirteen benzothiazolone derivatives (M1–M13) were synthesized and evaluated for their inhibitory activity against cholinesterases (ChEs) and monoamine oxidases (MAOs). All the compounds inhibited ChEs more effectively than MAOs. In addition, most of the compounds showed higher inhibitory activities against butyrylcholinesterase (BChE) than acetylcholinesterase (AChE). Compound M13 most potently inhibited BChE with an IC50 value of 1.21 μM, followed by M2 (IC50 = 1.38 μM). Compound M2 had a higher selectivity index (SI) value for BChE over AChE (28.99) than M13 (4.16). The 6-methoxy indole group of M13 was expected to have a greater effect on BChE inhibitory activity than the other groups. Kinetics and reversibility tests showed that M13 was a reversible noncompetitive BChE inhibitor with a Ki value of 1.14 ± 0.21 μM. In a docking simulation, M13 is predicted to form a hydrogen bond with the backbone carbonyl group of Ser287 of BChE through its methoxy indole moiety and π−π interactions between its benzothiazolone group and the side chain of Trp82 with the five-membered pyrrole ring and with the six-membered benzene ring. From these results, it is suggested that M13 is a BChE inhibitor and a potential candidate agent for the treatment of Alzheimer’s disease.
1. Introduction
As for the general consideration, Alzheimer’s disease (AD) is associated with neuron loss in the brain; however, the pathophysiological events leading to neuron loss are yet to be entirely understood. According to the cholinergic hypothesis, the reduction of acetylcholine, a crucial neurotransmitter, is assumed to be the cause of AD [1,2]. In AD patients, cholinergic neurons in the basal forebrain degenerate, and levels of cholinergic receptors and choline acetyltransferase in the cerebral cortex are found to be significantly decreased [3]. Clinical trials have shown that medications focused on raising acetylcholine levels offer symptomatic alleviation, with the fact that many previous treatment methods were founded on this concept [4,5]. According to recent research on the cholinergic hypothesis, amyloid fibrillation, one of the pathological signs of AD, may be influenced by the use of cholinesterase inhibitors [6,7,8].
Some cholinesterase (ChE) inhibitors, such as rivastigmine, tacrine, galantamine, donepezil, and huperzine A, have been shown to slow the progression of AD. Tacrine (Cognex®), the first drug among these compounds approved by the FDA in the USA for the treatment of AD, is based on the cholinergic hypothesis of AD as an acetylcholinesterase (AChE) inhibitor and was put into clinical use. However, due to its hepatotoxicity, tacrine has been limited in clinical use. By contrast, three other AChE inhibitors, rivastigmine, donepezil, and galantamine, were approved as anti-AD drugs and used clinically. These drugs also show effects other than AChE inhibition. Rivastigmine can block butyrylcholinesterase (BChE), while galantamine can modulate nicotinic acetylcholine receptors [8,9]. Donepezil is a moderate inhibitor of β-amyloid (Aβ) synthesis and the β-secretase (BACE1) responsible for Aβ synthesis, and it can also interact with sigma-1 receptors, which are known for their anti-amnesic activities [10].
Structural features and key binding sites of residues of AChE and BChE active sites have been clarified by crystallographic studies with molecules known to be AChE and BChE inhibitors, such as tacrine, donepezil, and galantamine [11,12,13,14,15,16,17]. Studies have determined that there is a catalytic active site (CAS), peripheral anionic site (PAS), anionic subsite (AS), oxyanion hole, and acyl binding pocket in the active site of the enzyme. It has been reported that the interactions with residues in the CAS, PAS, and AS cause significant changes in the activity of the enzyme. Contrary to tacrine, donepezil and galantamine interact with the cationic active site (CAS), the acyl-binding pocket, and the PAS residues that surround the rim at the entry to the active gorge [12,17,18,19,20]. The acetylcholine bond breaking is catalyzed by the catalytic triad of three amino acids, S203, E334, and H447, which are located at the bottom of this gorge [21]. The ideal scenario would be for an agent to interact with these active regions of the enzyme by inhibiting ChE (Figure 1a). Despite a 65% sequence similarity between BChE and AChE, there are notable differences that are crucial for the enzyme’s selectivity, for example, the larger acyl-binding pocket in BChE [22].
It has been reported that the benzoxazolone ring has many important activities, such as anticancer, antimicrobial, antiviral, antioxidant, analgesic, anti-inflammatory, and ChE inhibitory activities. The benzoxazolone ring was defined as a “privileged skeleton” because of its high chemical reactivity and wide variety of biological activities [23,24,25,26]. In addition, various tertiary amine moieties have been reported as a pharmacophore group in ChE inhibition, including the importance of designing compounds with a benzoxazolone structure containing the tertiary amine moieties with a ChE inhibition effect [27,28].
It has been reported in the literature that various compounds carrying the benzothiazolone skeleton have ChE inhibitory effects. Erdogan et al. investigated the ChE inhibitory activity of some benzothiazol-2-one derivatives and reported the most effective compound (Figure 1b) with an IC50 value of 0.34 µM against AChE [23]. The synthesized compounds (M1-13) were derived from the 3rd position of the benzothiazolone core, and the most active compound (Figure 1b) was derived from the 6th position of the core. When compound M13 and the most active compound are evaluated, both compounds have a benzothiazol-2-one core in the peripheral anionic region. M13 and the most effective compound (Figure 1b) have a 6-methoxyindole group and phenyl ring at the choline-binding site, respectively, as well as an acetohydrazide structure and a piperazine ring at the positive charge center.
In addition, various compounds have been designed and synthesized as ChE inhibitors using Schiff bases as linkers. The compounds were observed to be potentially useful for ChE inhibition and possible treatment for AD (Figure 1c,d) [29,30].
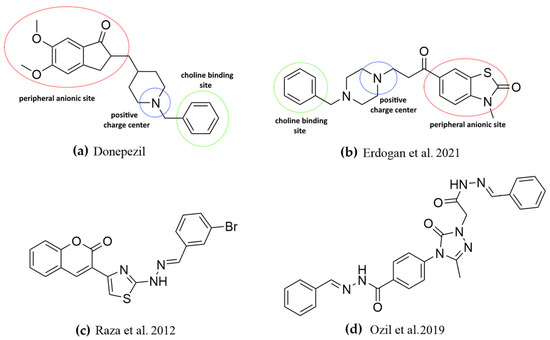
Figure 1.
Structural hypothesis for ChE inhibitors illustrated on donepezil (a) and a bezothiazolone derivative (b) and ChE inhibitor Schiff base derivatives (c,d) [23,29,30].
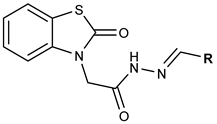
In light of this information, in the present study, we aimed to synthesize and characterize benzothiazolone derivatives, i.e., the M series, and determine their ChE inhibitory activity according to the hypothesis as shown below (Figure 2).
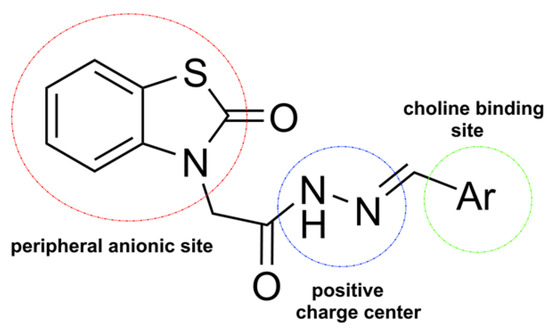
Figure 2.
Structural hypothesis for the M series.
2. Materials and Methods
2.1. Experimental Procedure for Synthesis
General procedure of synthesis:
Benzo[d]thiazol-2(3H)-one (1): A mixture of 2-aminothiophenol [1 eq] and urea [2 eq] was taken into a flask and irradiated in a microwave oven at 140 °C and 500 W for 15 min without a solvent. The reaction was cooled and poured into ice water, and the resulting solid was filtered off. The solid obtained was added to a 10% NaOH solution and filtered, and the filtrate was acidified with concentrated hydrochloric acid. The precipitate was filtered off, dried, and crystallized from water. The product was obtained with an 87% yield. The reaction was monitored with TLC and LC-MS [31].
Ethyl 2-(2-oxobenzo[d]thiazol-3(2H)-yl)acetate (2): Benzo[d]thiazol-2(3H)-one 1 eq], ethyl bromoacetate [1.2 eq], K2CO3 [2 eq], and acetone were mixed at 400 W at 58 °C for 20 min. The reaction was cooled and taken up in ice water, and the precipitated solid was filtered off, washed with water, and dried. The product was obtained in 91% yield. The reaction was monitored with TLC and LC-MS [32].
2-(2-oxobenzo[d]thiazol-3(2H)-yl)acetohydrazide (3): Ethyl 2-(2-oxobenzo[d]thiazol-3(2H)-yl)acetate [1 eq] was dissolved in ethanol and hydrazine hydrate [10 eq] was added dropwise and refluxed at 70 °C for 3 h. The reaction was cooled, and the solid formed was filtered and dried. The product was obtained with an 82% yield. The reaction was monitored with TLC and LC-MS [33].
2-(2-oxobenzo[d]thiazol-3(2H)-yl)-N′-(substitued)acetohydrazide derivatives (M1–M13): 2-(2-Oxobenzo[d]thiazol-3(2H)-yl)acetohydrazide [1 eq] and its aldehyde derivatives [1.2 eq] were mixed in 5 mL of acetic acid at 400 W for 10 min at 85 °C. The reaction was cooled, taken up in ice water, and filtered, and the solid in ethanol:water [8:2] was crystallized, and the crystals were filtered and adjusted. The products were obtained with a 55–75% yield. The reaction was monitored with TLC and LC-MS.
General information about materials and methods for the chemistry is described in the Supplementary information (Method S).
2.2. Inhibition Studies of ChE and MAO
Using 0.5 mM of acetylthiocholine (ATCI) and butyrylthiocholine iodide (BTCI) as substrates, respectively, the inhibitory activity of AChE and BChE were evaluated for 15 min at 412 nm. For the colour development, 0.5 mM of 5,5-dithiobis (2-nitrobenzoic acid) (DTNB) was also added [34]. AChE from Electrophorus electricus and BChE from horse serum were used for the ChEs. Before adding BTCI and DTNB, AChE or BChE was mixed with an inhibitor and preincubated for 10 min. Thiocholine, an AChE or BChE reaction product, and DTNB form the yellowish 5-thio-2-nitrobenzoic acid. According to a prior description, the activities of MAO-A and MAO-B were monitored continuously for 45 min at 316 nm using 0.06 mM of kynuramine and at 250 nm using 0.3 mM of benzylamine, respectively, as described previously [35].
2.3. Enzyme Inhibition and Kinetic Studies
After inhibitory activities of the compounds for ChEs or MAOs were evaluated at a concentration of 10 μM, an IC50 value of each compound showing residual activity of <50% was determined. The IC50 value was calculated by measuring the residual activity at different concentrations of the compound and by using GraphPad Prism software 5 [36,37]. The selectivity index (SI) value of BChE was calculated by (IC50 of AChE)/(IC50 of BChE) [38]. Enzyme kinetic parameters, inhibitor type, and Ki value of compound M13 were determined by measuring enzyme activity at five different substrate concentrations and at three different inhibitor concentrations (0.5–2 times of IC50) [39,40]. The inhibition type and Ki value were determined by the Lineweaver–Burk plots and their secondary plots, respectively.
2.4. Reversibility Analysis of M13
The reversibility of BChE inhibition by M13 was evaluated using dialysis after preincubation of BChE and M13 for 10 min at ~2.0 × IC50 (i.e., 2.42 μM), as previously described [41]. For the reference compound, donepezil (a reference reversible BChE inhibitor) was preincubated at ~2.0 × IC50 (0.36 μM). The reversibility pattern was determined by comparing the activities of dialyzed (AD) and undialyzed (AU) samples.
2.5. Docking Studies
First of all, the three-dimensional X-ray structures of hAChE (PDB ID: 4EY7 [17]) and hBChE (PDB ID: 6RUA [42]) were retrieved from the Protein Data Bank (PDB). The target structures were refined using Protein Preparation Wizard (Schrödinger Suite 2021–4) [43] in order to correct the bond order and to add hydrogen atoms and possible missing amino acidic side chains and loops.
The 3D conformation of the compound M13 was processed by LigPrep (Schrödinger Suite 2021–4) [44] in order to properly generate all the possible tautomers and ionization states at a physiological pH value of 7.4. For the co-crystallized cognate ligands (donepezil and KJT for 4EY7 and 6RUA, respectively), cubic grids for hAChE and hBChE were generated. The edges of the inner and outer boxes were 10 × 10 × 10 Å and 28 × 28 × 28 Å, and 10 × 10 × 10 Å and 24 × 24 × 24 Å, respectively.
Docking simulations were, thus, performed using Grid-based Ligand Docking with Energetics (GLIDE) v.9.1 [45,46], which is a part of the Schrodinger Suite (Schrödinger Suite 2021–4). All the default advanced settings for standard precision (SP) were used, and the Force Field OPLS_2005 was employed. The reliability of an SP simulation protocol was previously challenged by computing the Root Mean Square Deviation (RMSD) values.
3. Results and Discussion
3.1. Chemistry
The title compounds (M1–M13) were synthesized in accordance with the literature, as shown in Scheme 1.
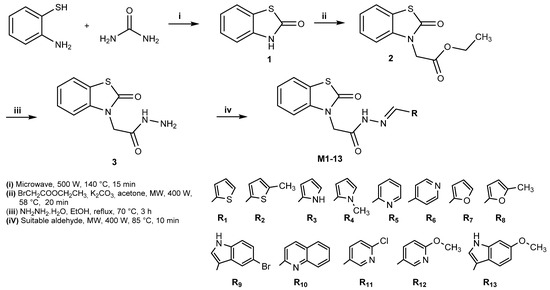
Scheme 1.
Synthesis of the compounds M1-13.
Synthesis-starting material benzo[d]thiazol-2(3H)-one (1) was synthesized using 2-aminothiophenol and urea with an 87% yield according to the microwave irradiation method. As a result of the reaction of the resulting compound (1) with ethyl bromoacetate, ethyl 2-(2-oxobenzo[d]thiazol-3(2H)-yl)acetate (2) was obtained in high yield (91%) by the microwave irradiation method. Compound 2-(2-oxobenzo[d]thiazol-3(2H)-yl)acetohydrazide (3) was synthesized by the reaction of compound 2 with hydrazide hydrate in ethanol, and the reaction was completed in 3 h at 70 °C. In the third step (iii), without microwave irradiation, the reaction time was considerably prolonged, and the yield was reduced. The resulting compounds, the Schiff bases (M1-13), were obtained by the reaction of compound 3 with substituted and unsubstituted benzaldehyde derivatives. The resulting compounds (M1–13) were synthesized in a 55–93% yield (Table 1). The spectral characterization of the compounds (M1–M13) is provided in the supplementary information (Figure S).

Table 1.
Structures, yields, melting points, and molecular formula of the synthesized compounds.
3.2. Inhibition Studies of ChE and MAO
Thirteen benzothiazolone derivatives were synthesized and tested for ChEs’ and MAOs’ inhibitory activities. All derivatives showed more effective inhibitory activity against ChEs than MAOs. Based on the residual activity at 10 μM, three and five compounds showed < 50% for AChE and BChE, respectively (Table 2); however, no compounds showed < 50% for MAO-A and MAO-B, except M10 for MAO-B (Table S1). Compound M13 most potently inhibited BChE with an IC50 value of 1.21 μM, followed by M2 (IC50 = 1.38 μM) (Table 2). In the case of BChE inhibitory activity, the IC50 values of M13 and M2 were lower than those of glycyrol (7.22 μM) from the roots of Glycyrrhiza uralensis [47] and khellactone coumarin 3′,4′-disenecioylkhellactone (7.20 μM) from Peucedanum japonicum Thurnberg [48] and sargachromanol I (13.69 μM) from Sargassum siliquastrum 20 [36]. Structurally, M13 and M9 have a 6-methoxy indole and 5-bromo indole, respectively, whereas M4 and M2 have a 1-methyl pyrrole and 5-methyl thiophene, respectively. It is suggested that the 6-methoxy indole group is expected to have a greater effect on BChE inhibitory activity than the other groups. Compound M2 had a higher selectivity index (SI) value for BChE (28.99) than M13 (4.16). The substrate preference of AChE and BChE is different; AChE prefers ACh, while BChE has a relatively wide substrate range with a low preference for ACh. Therefore, SI values are important in inhibitor screening. On the other hand, all derivatives showed weak MAO inhibitory activities, except M10 for MAO-B (IC50 = 2.75 μM) (Table S1). These results show that M13 is a potent BChE inhibitor.

Table 2.
Inhibitions of ChE by the M series a.
3.3. Kinetic Study
The mode of BChE inhibition by M13 was investigated using Lineweaver–Burk plots. The plots of BChE inhibition by M13 were linear, and the lines seemed to be intersected at a point on the X-axis, showing that M13 was a noncompetitive inhibitor of BChE (Figure 3a). A secondary plot obtained from the slopes of the Lineweaver–Burk plots against inhibitor concentrations showed that the Ki value of M13 was 1.14 ± 0.21 μM (Figure 3b). These results suggest that M13 is a potent noncompetitive inhibitor of BChE. It is similar to the inhibition type of donepezil.
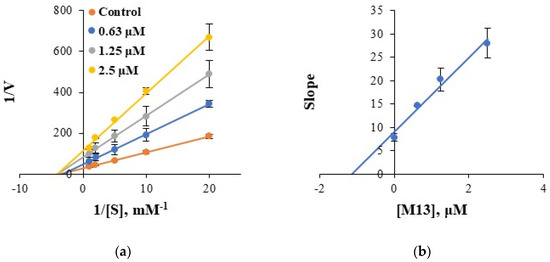
Figure 3.
Lineweaver–Burk plots for BChE inhibition by M13 (a) and its secondary plot (b) of the slopes vs. inhibitor concentrations.
3.4. Reversibility Studies
A reversibility study of BChE inhibition by M13 was analyzed using the dialysis method. In the experiment, the concentrations used were the following: M13 at 2.42 μM and donepezil (a reference reversible inhibitor) at 0.36 μM. After dialysis of the preincubated mixture of BChE and M13, the relative activities for the undialyzed (AU) and dialyzed (AD) samples were compared to determine the reversibility pattern. As a result, the inhibition of BChE by M13 was recovered from 30.6% (AU) to 82.2% (AD) (Figure 4). The recovery value for M13 was similar to that for donepezil (from 29.5% to 88.2%). These results indicate that M13 is a reversible BChE inhibitor.
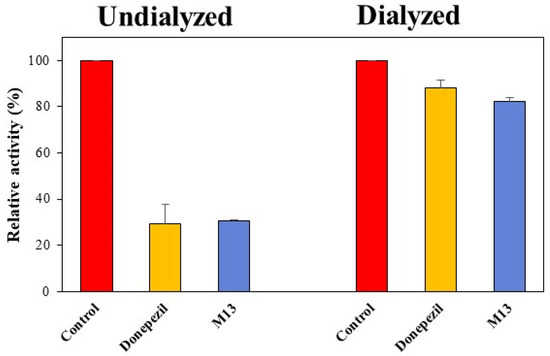
Figure 4.
Recovery of BChE inhibition by M13 using the dialysis method.
3.5. Docking Studies
In order to corroborate the validity of the docking studies, redocking simulations were first performed on the co-crystallized ligands in their binding sites. The cognate ligands moved back to the original positions with RMSD accounting for all the heavy atoms equal to 0.126 Å and 0.686 Å for donepezil and KJT, respectively, whose docking score values were equal to −12.428 and −10.035 kcal/mol, respectively (Figure 5).
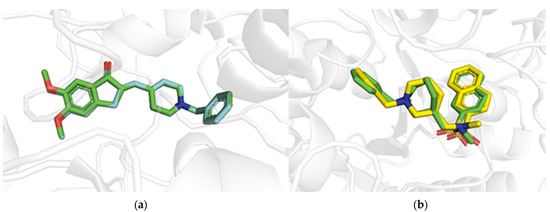
Figure 5.
Overlaps of the X-ray solved (green sticks) and docking poses for donepezil for hAChE (PDB entry: 4EY7) (a) and KJT for hBChE (PDB entry: 6RUA) (b), rendering in cyan and yellow sticks, respectively.
The docking simulations were, thus, exploited to investigate the binding modes of the M13 compound towards the hAChE and hBChE crystal structures. The docking poses of the M13 compound against both the target proteins, as well as the relevant binding residues, are depicted in Figure 6.
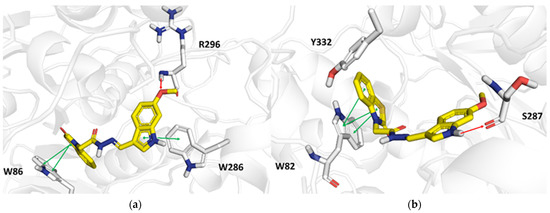
Figure 6.
Docking poses carried out from docking analyses of the M13 compound towards hAChE (PDB entry: 4EY7) (a) and hBChE (PDB entry: 6RUA) (b), respectively. Green and red arrows indicate π−π interactions and hydrogen bonds, respectively. Ligands and the target residues of the binding sites are rendered in yellow and gray sticks, respectively. For the sake of completeness, all the molecular interactions observed in the docking simulations were automatically flagged by GLIDE software.
Overall, the molecular docking analyses of M13 with hAChE and hBChE provide a good rationale for the observed bioactivities. The IC50 values correlated well with the docking scores equal to −9.097 kcal/mol and −8.783 kcal/mol for hAChE and hBChE, respectively, considering that the volume of the catalytic site of BChE is much larger than that of AChE. As a result, the latter is more prone to engage in hydrophobic interactions whose contributions are more effective in enhancing docking scores.
Interactions between M13 and hAChE were observed, and the methoxy group of M13 established a hydrogen bond with the backbone of Arg296 with a distance of 2.2 Å, whereas π−π hydrophobic stacking contacts occurred between the benzothiazolone group of M13 and the side chain of Trp286 (at a distance of 4.1 Å). The side chain of Trp86 (Figure 6a) was, instead, involved in a bidentate interaction involving the engagement of its five- and six-membered rings (at distances of 3.9 and 3.8 Å, respectively) with the benzothiazolone arm of M13.
As far as the interactions between M13 and the hBChE target residues (Figure 6b) are concerned, a hydrogen bond occurred with the backbone carbonyl group of Ser287 (at a distance of 2.1 Å). Interestingly, M13 was predicted to form hydrophobic π−π interactions between the benzothiazolone group and the side chain of Trp82 (at a distance of 3.9 Å) through contacts with its five- and six-membered rings (at distances of 3.5 and 4.1 Å, respectively).
Both AChE and BChE catalyze the hydrolysis of esters and regulate the ACh concentration in glial cells, the hippocampus, and the temporal nerve cortex; therefore, ChE inhibitors can increase ACh levels to treat AD [49]. In this study, M13 was found to be a potent BChE inhibitor (IC50 = 1.21 µM) with an effective AChE inhibitory activity (IC50 = 5.03 µM).
4. Conclusions
In the study, thirteen benzothiazolone derivatives were synthesized, and their ChE and MAO inhibitory activities were investigated. The ChE inhibitory effects of the compounds were considerably higher than the MAO inhibitory effects. M13 (IC50 = 1.21 ± 0.05 µM) and M2 (IC50 = 1.38 ± 0.17 µM) were the most active compounds to BChE. In the kinetics and reversibility studies, the most effective compound, M13, was determined to be a reversible and noncompetitive inhibitor of BChE. These results suggest that M13 is a potential BChE inhibitor to be considered a candidate agent for the treatment of Alzheimer’s disease.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr10091872/s1, Method S: Chemistry; Figure S: 1H-NMR, 13C-NMR, and HRMS spectra of the compounds M1-13 (data 1~39); Table S1: Inhibitions of MAO by M series.
Author Contributions
Conceptualization, M.A.A., G.A. and T.Ö.; synthesis, M.A.A., G.A., T.Ö., A.B.Ö., Z.Ö. and S.S.; Biological evaluation, S.-M.K., J.M.O. and H.K.; Computational studies, D.T. and O.N.; writing—original draft preparation, M.A.A., B.M. and H.K.; Supervision, B.M. and H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Research Promotion Program of SCNU (To H.K.).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brookmeyer, R.; Abdalla, N.; Kawas, C.H.; Corrada, M.M. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement 2018, 14, 121–129. [Google Scholar] [PubMed]
- Ozdemir, Z.; Ozcelik, A.B.; Uysal, M. Approaches based on cholinergic hypothesis and cholinesterase inhibitors in the treatment of alzheimer’s disease. Front. Clin. Drug Res.-Alzheimer Disord. 2019, 8, 154–190. [Google Scholar]
- Shekari, A.; Fahnestock, M. Cholinergic neurodegeneration in Alzheimer disease mouse models. Handb. Clin. Neurol. 2021, 182, 191–209. [Google Scholar]
- Mendiola-Precoma, J.; Berumen, L.C.; Padilla, K.; Garcia-Alcocer, G. Therapies for prevention and treatment of Alzheimer’s disease. Bio. Med. Res. Int. 2016, 2016, 2589276. [Google Scholar]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar]
- Chen, Z.R.; Huang, J.B.; Yang, S.L.; Hong, F.F. Role of cholinergic signaling in Alzheimer’s disease. Molecules 2022, 27, 1816. [Google Scholar]
- Bekdash, R. The cholinergic system, the adrenergic system and the neuropathology of Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 1273. [Google Scholar] [CrossRef] [PubMed]
- Bozbey, I.; Ozdemir, Z.; Uslu, H.; Ozcelik, A.B.; Senol, F.S.; Orhan, I.E.; Uysal, M. A series of new hydrazone derivatives: Synthesis, molecular docking and anticholinesterase activity studies. Mini Rev. Med. Chem. 2020, 20, 1042–1060. [Google Scholar] [PubMed]
- Dos Santos, G.A.A.; Schmidt, C.W.P.; Forlenza, K.P. The use of esterase inhibitors. In Pharmacological Treatment of Alzheimer’s Disease; Santos, G.A.A.D., Ed.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Gabr, M.T.; Abdel-Raziqc, M.S. Structure-based design, synthesis, and evaluation of structurally rigid donepezil analogues as dual AChE and BACE-1 inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 2910–2913. [Google Scholar] [CrossRef]
- Dvir, H.; Silman, I.; Harel, M.; Rosenberry, T.L.; Sussmana, J.L. Acetylcholinesterase: From 3D structure to function. Chem. Biol. Interact. 2010, 187, 10–22. [Google Scholar]
- Costanzo, P.; Cariati, L.; Desiderio, D.; Sgammato, R.; Lamberti, A.; Arcone, R.; Salerno, R.; Nardi, M.; Masullo, M.; Oliverio, M. Design, synthesis, and evaluation of donepezil-like compounds as AChE and BACE-1 inhibitors. Med. Chem. Lett. 2016, 7, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Nachon, F.; Carletti, E.; Ronco, C.; Trovaslet, M.; Nicolet, Y.; Jean, L. Crystal structures of human cholinesterases in complex with huprine W and tacrine: Elements of specificity for anti-Alzheimer’s drugs targeting acetyl- and butyrylcholinesterase. Biochem. J. 2013, 453, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Leon, J.; Marco-Contelles, J. A step further towards multitarget drugs for Alzheimer and neuronal vascular diseases: Targeting the cholinergic system, amyloid-β aggregation and Ca2+ dyshomeostatis. J. Curr. Med. Chem. 2011, 18, 552. [Google Scholar] [CrossRef]
- Simoni, E.; Daniele, S.; Bottegoni, G.; Pizzirani, D.; Trincavelli, M.L.; Goldoni, L.; Tarozzo, G.; Reggiani, A.; Martini, C.; Piomelli, D.; et al. Combining galantamine and memantine in multitargeted, new chemical entities potentially useful in Alzheimer’s disease. J. Med. Chem. 2012, 55, 9708–9721. [Google Scholar] [CrossRef] [PubMed]
- Nepovimova, E.; Uliassi, E.; Korabecny, J.; Peña-Altamira, L.E.; Samez, S.; Pesaresi, A.; Garcia, G.E.; Bartolini, M.; Andrisano, V.; Bergamini, C.; et al. Multitarget drug design strategy: Quinone–tacrine hybrids designedtTo block amyloid-β aggregation and to exert anticholinesterase and antioxidant effects. J. Med. Chem. 2014, 57, 8576–8589. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef]
- Camps, P.; Formosa, X.; Galdeano, C.; Gomez, T.; Munoz-Torrero, D.; Scarpellini, M. Novel donepezil-based inhibitors of acetyl- and butyrylcholinesterase and acetylcholinesterase induced β-amyloid aggregation. J. Med. Chem. 2009, 51, 3588–3598. [Google Scholar] [CrossRef]
- Bourne, Y.; Taylor, P.; Radić, Z.; Marchot, P. Structural insights into ligand interactions at the acetylcholinesterase peripheral anionic site. EMBO J. 2003, 22, 1–12. [Google Scholar] [CrossRef]
- Tougu, V. Acetylcholinesterase: Mechanism of catalysis and inhibition. Curr. Med. Chem. Cent. Nerv. Syst. Agents 2001, 1, 155–170. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Zhang, Y. Catalytic reaction mechanism of acetylcholinesterase determined by born-oppenheimer AB initio QM/MM molecular dynamics simulations. J. Phys. Chem. B 2010, 114, 8817–8825. [Google Scholar] [CrossRef]
- Brus, B.; Kosak, U.; Turk, S.; Pislar, A.; Coquelle, N.; Kos, J.; Stojan, J.; Colletier, J.P.; Gobec, S. Discovery, biological evaluation, and crystal structure of a novel nanomolar selective butyrylcholinesterase inhibitor. J. Med. Chem. 2014, 57, 8167–8179. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, M.; Kilic, B.; Ilıkcı Sagkan, R.; Aksakal, F.; Ercetin, T.; Gulcan, H.O.; Dogruer, D.S. Design, synthesis and biological evaluation of new benzoxazolone/benzothiazolone derivatives as multi-target agents against Alzheimer’s disease. Eur. J. Med. Chem. 2021, 212, 113124. [Google Scholar] [CrossRef] [PubMed]
- Doğruer, D.S.; Ünlü, S.; Yeşilada, E.; Şahin, M.F. N-(2-pyridinyl)-2-[2(3H)-benzazolone-3-yl]acetamides: Synthesis, antinociceptive and anti-inflammatory activity. Farmaco 1997, 52, 745–750. [Google Scholar] [PubMed]
- Abdelazeem, A.H.; Khan, S.I.; White, S.W.; Sufka, K.J.; McCurdy, C.R. Design, synthesis and biological evaluation of bivalent benzoxazolone and benzothiazolone ligands as potential anti-inflammatory/analgesic agents. Bioorg. Med. Chem. 2015, 23, 3248–3259. [Google Scholar] [CrossRef]
- Jacques, P.; Pascal, C.; Evelina, C. 2(3H)-Benzoxazolone and bioisosters as “privileged scaffold” in the design of pharmacological probes. Curr. Med. Chem. 2005, 12, 877–885. [Google Scholar]
- Akrami, H.; Mirjalili, B.H.; Khoobi, M.; Nadri, H.; Moradi, A.; Sakhteman, A.; Emami, S.; Foroumadi, A.; Shafiee, A. Indolinone-based acetylcholinesterase inhibitors: Synthesis, biological activity and molecular modeling. Eur. J. Med. Chem. 2014, 84, 375–381. [Google Scholar] [CrossRef]
- Tripathi, P.N.; Srivastava, P.; Sharma, P.; Kumar Tripathi, M.; Seth, A.; Tripathi, A. Biphenyl-3-oxo-1,2,4-triazine linked piperazine derivatives as potential cholinesterase inhibitors with anti-oxidant property to improve the learning and memory. Bioorg. Chem. 2019, 85, 82–96. [Google Scholar] [CrossRef]
- Raza, R.; Saeed, A.; Arif, M.; Mahmood, S.; Muddassar, M.; Raza, A.; Iqbal, J. Synthesis and Biological Evaluation of 3-Thiazolocoumarinyl Schiff-Base Derivatives as Cholinesterase Inhibitors. Chem. Biol. Drug Des. 2012, 80, 605–615. [Google Scholar] [CrossRef]
- Özil, M.; Balaydın, H.T.; Şentürk, M. Synthesis of 5-Methyl-2,4-Dihydro-3H-1,2,4-Triazole-3-One’s Aryl Schiff Base Derivatives and Investigation of Carbonic Anhydrase and Cholinesterase (AChE, BuChE) Inhibitory Properties. Bioorganic Chem. 2019, 86, 705–713. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Mahmood, K.; Wajid, A.; Maah, M.J.; Yusoff, I. Synthesis, characterization and biological activity of Schiff bases. IPCBEE 2011, 10, 185. [Google Scholar]
- Önkol, T.; Çakir, B.; Ito, S.; Özçlik, B.; Şahin, M.F. Synthesis of some (5-chloro-2 (3H)-benzothiazolinone-3-yl) aceto/propanohydrazides towards antimicrobial and antiviral activity. Turk. J. Pharm. Sci. 2009, 6, 195–206. [Google Scholar]
- Önkol, T.; Yildirim, E.; Erol, K.; Ito, S.; Şahin, M.F. Synthesis and antinociceptive activity of (5-chloro-2 (3H)-benzothiazolon-3-yl) propanamide derivatives. Arch. Pharm. 2004, 337, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Mathew, B.; Oh, J.M.; Khames, A.; Abdelgawad, M.A.; Rangarajan, T.M.; Nath, L.R.; Agoni, C.; Soliman, M.E.S.; Mathew, G.E.; Kim, H. Replacement of chalcone-ethers with chalcone-thioethers as potent and highly selective monoamine oxidase-B inhibitors and their protein-ligand interactions. Pharmaceuticals 2021, 14, 1148. [Google Scholar] [CrossRef]
- Alagöz, M.A.; Oh, J.M.; Zenni, Y.N.; Özdemir, Z.; Abdelgawad, M.A.; Naguib, I.A.; Ghoneim, M.M.; Gambacorta, N.; Nicolotti, O.; Kim, H.; et al. Development of a novel class of pyridazinone derivatives as selective MAO-B inhibitors. Molecules 2022, 27, 3801. [Google Scholar] [CrossRef]
- Oh, J.M.; Jang, H.J.; Kim, W.J.; Kang, M.G.; Baek, S.C.; Lee, J.P.; Park, D.; Oh, S.R.; Kim, H. Calycosin and 8-O-methylretusin isolated from Maackia amurensis as potent and selective reversible inhibitors of human monoamine oxidase-B. Int. J. Biol. Macromol. 2020, 151, 441–448. [Google Scholar] [CrossRef]
- Lee, J.P.; Kang, M.G.; Lee, J.Y.; Oh, J.M.; Baek, S.C.; Leem, H.H.; Park, D.; Cho, M.L.; Kim, H. Potent inhibition of acetylcholinesterase by sargachromanol I from Sargassum siliquastrum and by selected natural compounds. Bioorg. Chem. 2019, 89, 1030. [Google Scholar] [CrossRef]
- Mathew, B.; Oh, J.M.; Abdelgawad, M.A.; Khames, A.; Ghoneim, M.M.; Kumar, S.; Nath, L.R.; Sudevan, S.T.; Parambi, D.G.T.; Agoni, C.; et al. Conjugated dienones from differently substituted cinnamaldehyde as highly potent monoamine oxidase-B inhibitors: Synthesis, biochemistry, and computational chemistry. ACS Omega 2022, 7, 8184–8197. [Google Scholar] [CrossRef]
- Lee, H.W.; Ryu, H.W.; Kang, M.G.; Park, D.; Oh, S.R.; Kim, H. Potent selective monoamine oxidase B inhibition by maackiain, a pterocarpan from the roots of Sophora favescens. Bioorg. Med. Chem. Lett. 2016, 26, 4714–4719. [Google Scholar] [CrossRef]
- Mathew, B.; Oh, J.M.; Baty, R.S.; Batiha, G.E.; Parambi, D.; Gambacorta, N.; Nicolotti, O.; Kim, H. Piperazine-substituted chalcones: A new class of MAO-B, AChE, and BACE-1 inhibitors for the treatment of neurological disorders. Environ. Sci. Pollut. Res. Int. 2021, 28, 38855–38866. [Google Scholar] [CrossRef]
- Pajk, S.; Knez, D.; Košak, U.; Zorović, M.; Brazzolotto, X.; Coquelle, N.; Nachon, F.; Colletier, J.P.; Živin, M.; Stojan, J.; et al. Development of potent reversible selective inhibitors of butyrylcholinesterase as fluorescent probes. J. Enzym. Inhib. Med. Chem. 2020, 35, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2021-4: Protein Preparation Wizard; Epik, Schrödinger, LLC: New York, NY, USA; Impact, Schrödinger, LLC: New York, NY, USA; Prime, Schrödinger, LLC: New York, NY, USA, 2021.
- Schrödinger Release 2021-4: LigPrep; Schrödinger, LLC: New York, NY, USA, 2021.
- Schrödinger Release 2021-4: Glide; Schrödinger, LLC: New York, NY, USA, 2021.
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.S.; Kang, M.G.; Lee, J.Y.; Lee, S.R.; Park, D.; Cho, M.; Kim, H. Inhibition of butyrylcholinesterase and human monoamine oxidase-B by the coumarin glycyrol and liquiritigenin isolated from Glycyrrhiza uralensis. Molecules 2020, 25, 3896. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Eom, B.H.; Ryu, H.W.; Kang, M.G.; Park, J.E.; Kim, D.Y.; Kim, J.H.; Park, D.; Oh, S.R.; Kim, H. Acetylcholinesterase and butyrylcholinesterase inhibitory activities of khellactone coumarin derivatives isolated from Peucedanum japonicum Thurnberg. Sci. Rep. 2020, 10, 21695. [Google Scholar] [CrossRef]
- Oh, J.M.; Kang, Y.; Hwang, J.H.; Park, J.H.; Shin, W.H.; Mun, S.K.; Lee, J.U.; Yee, S.T.; Kim, H. Synthesis of 4-substituted benzyl-2-triazole-linked-tryptamine-paeonol derivatives and evaluation of their selective inhibitions against butyrylcholinesterase and monoamine oxidase-B. Int. J. Biol. Macromol. 2022, 217, 910–921. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).