Durability of Repair Metakaolin Geopolymeric Cement under Different Factors
Abstract
:1. Introduction
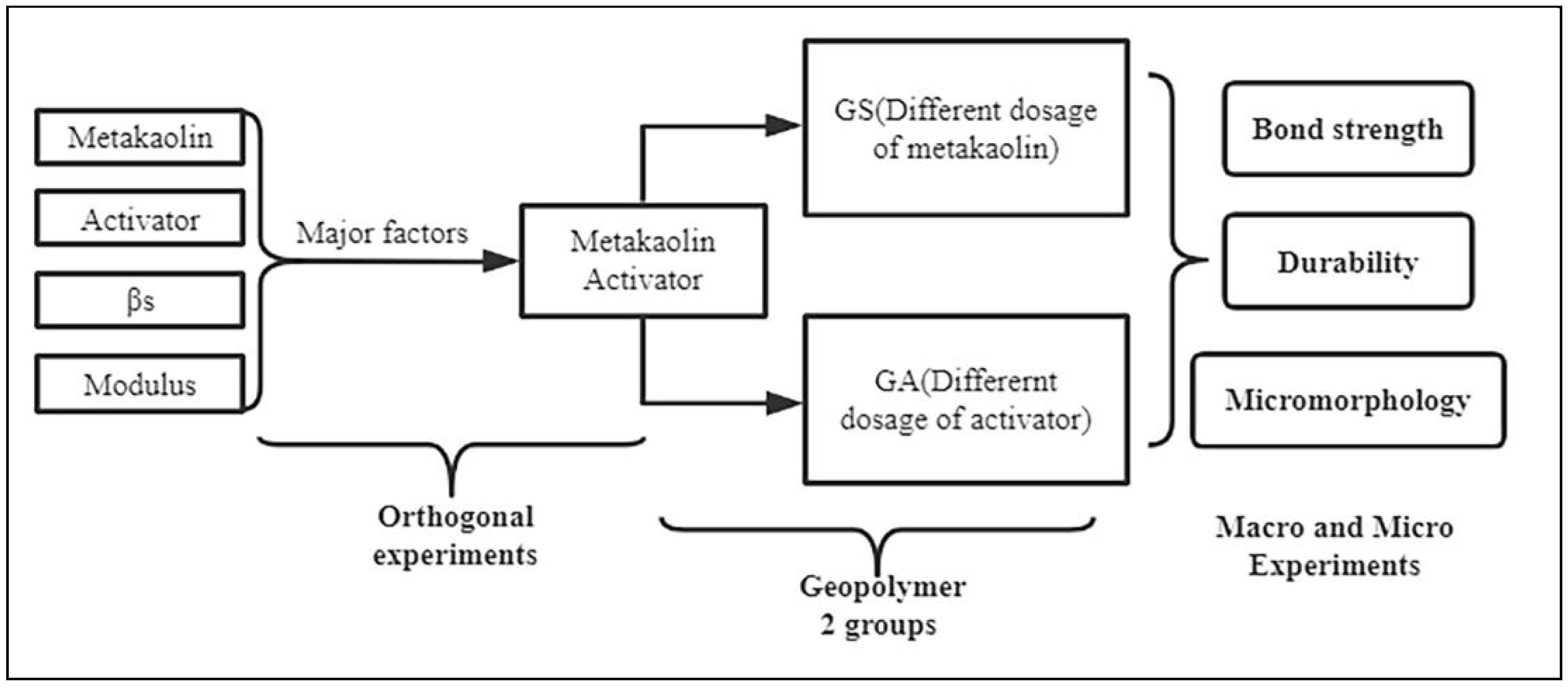
2. Experimental Methods
2.1. Materials
2.1.1. Metakaolin and OPC
2.1.2. Alkali Activator
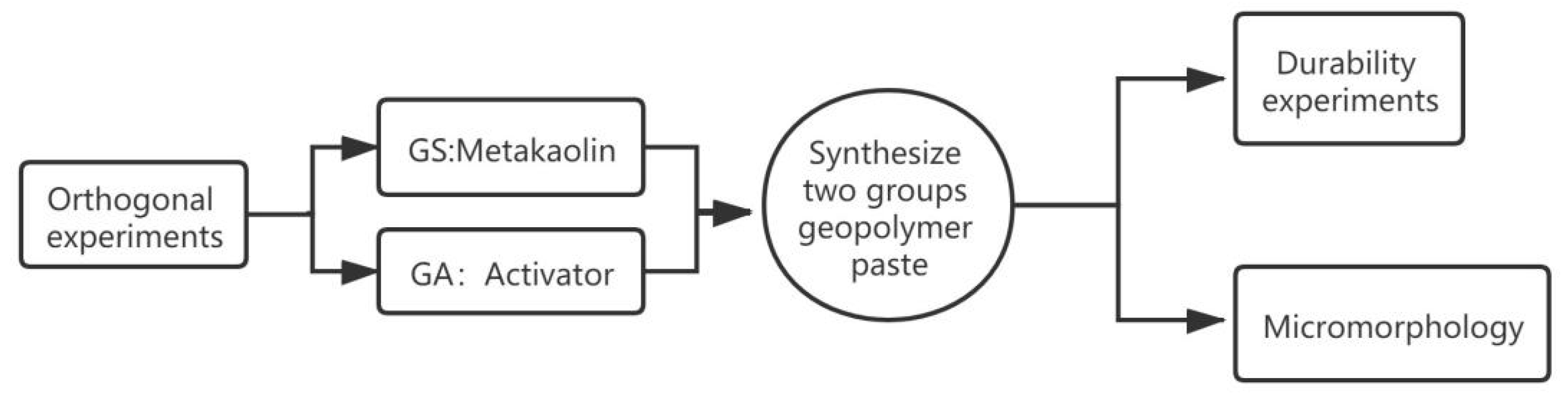
2.2. Preparation
2.3. Characterisation
2.3.1. Permeability Resistance
2.3.2. Sulphate Corrosion Resistance
2.3.3. Freeze-Thaw Resistance
2.3.4. Bond Strength
2.3.5. Scanning Electron Microscopy
2.3.6. Fourier Transform-Infrared Spectroscopy
3. Results and Discussion
3.1. Permeability Resistance
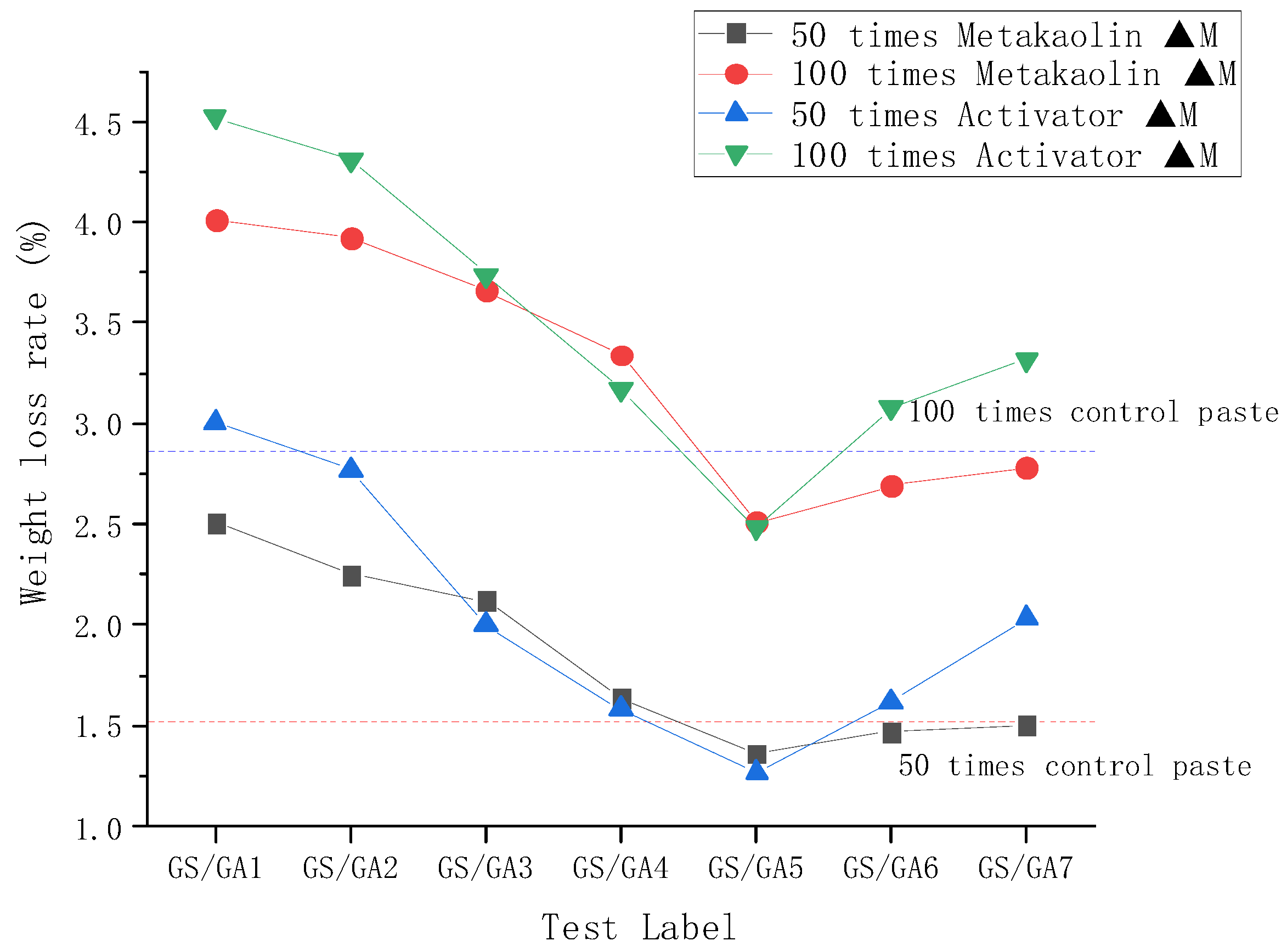
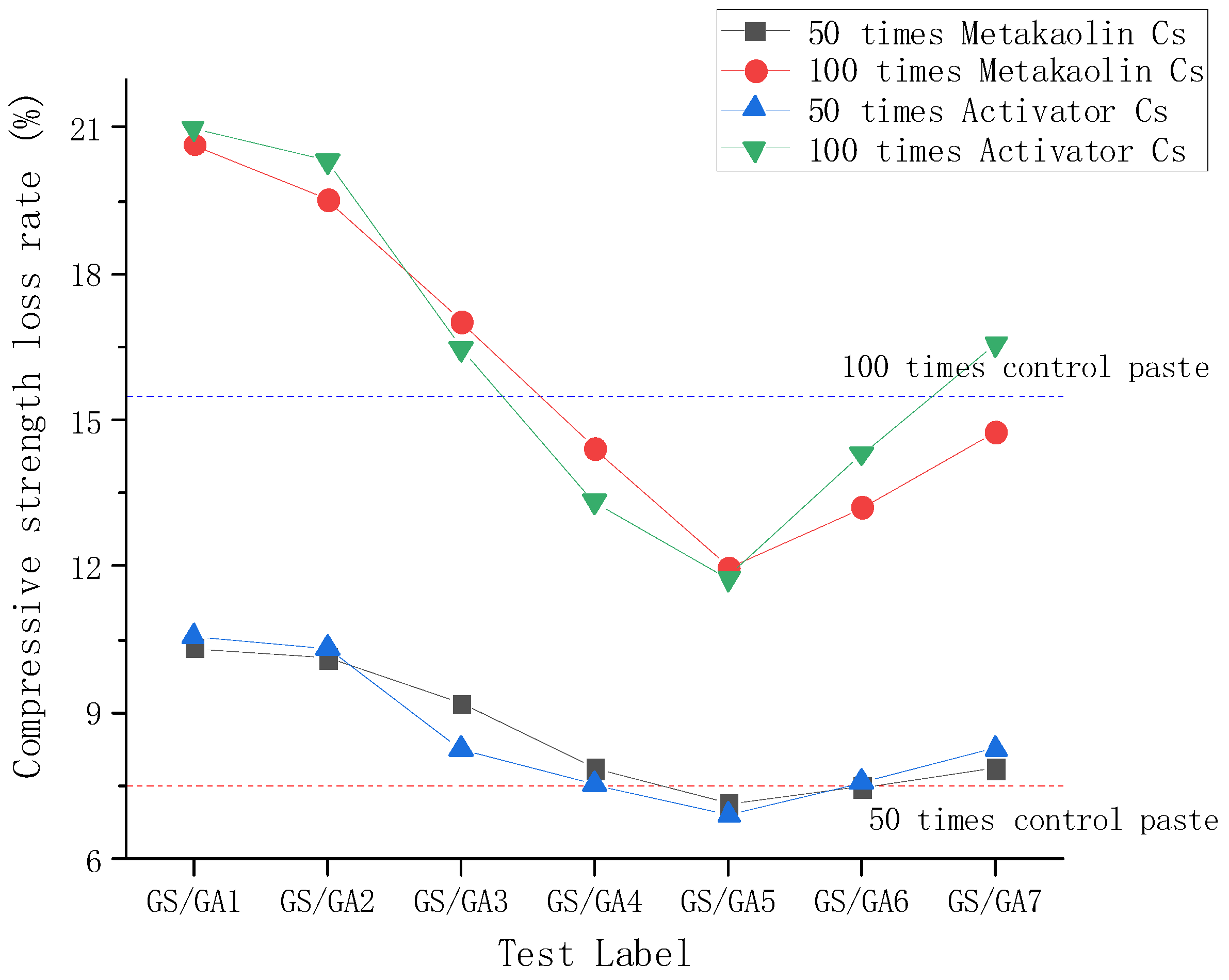
3.2. Resistance to Sulphate Corrosion
3.3. Freezing-Thawing Resistance and Appearance
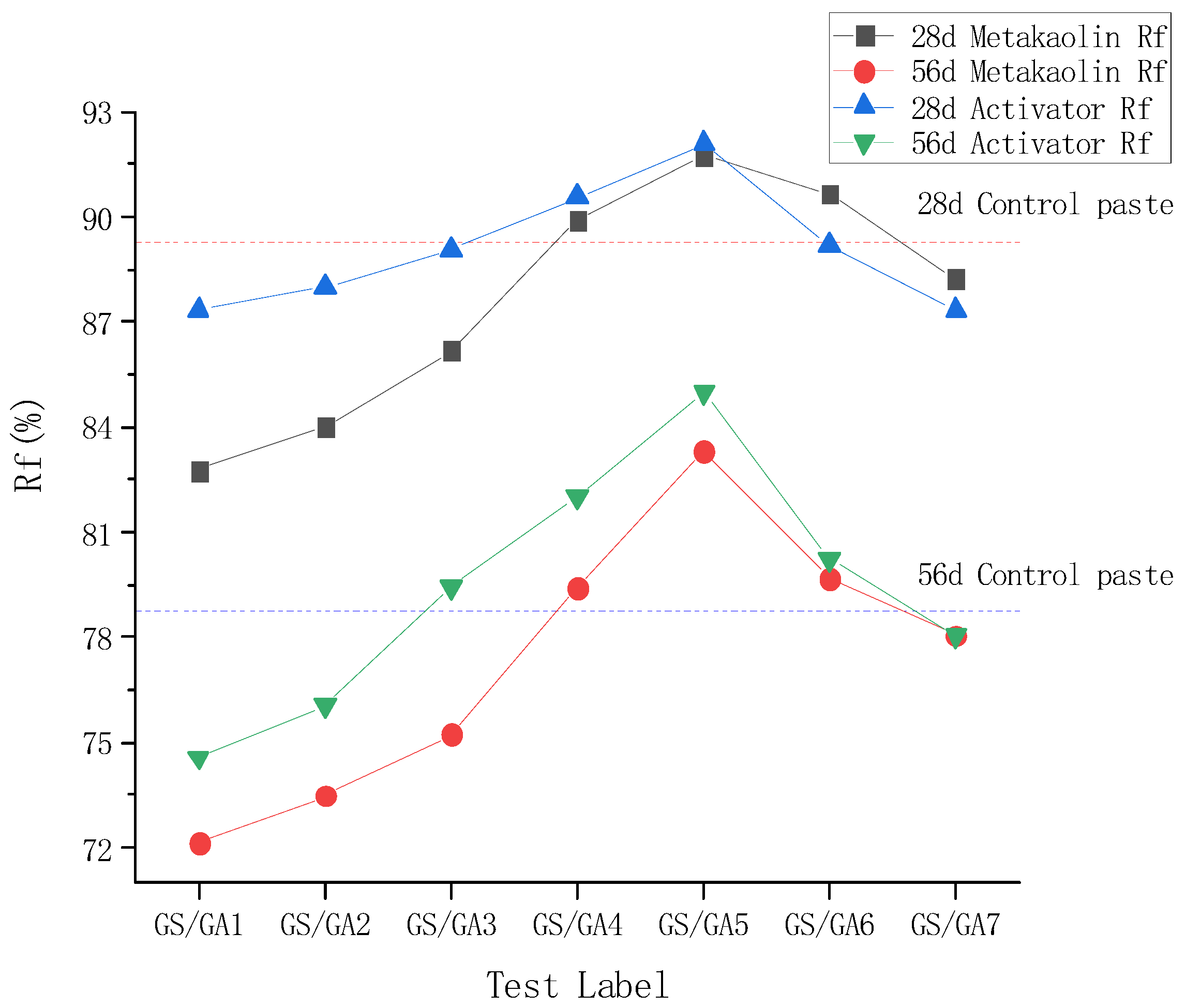
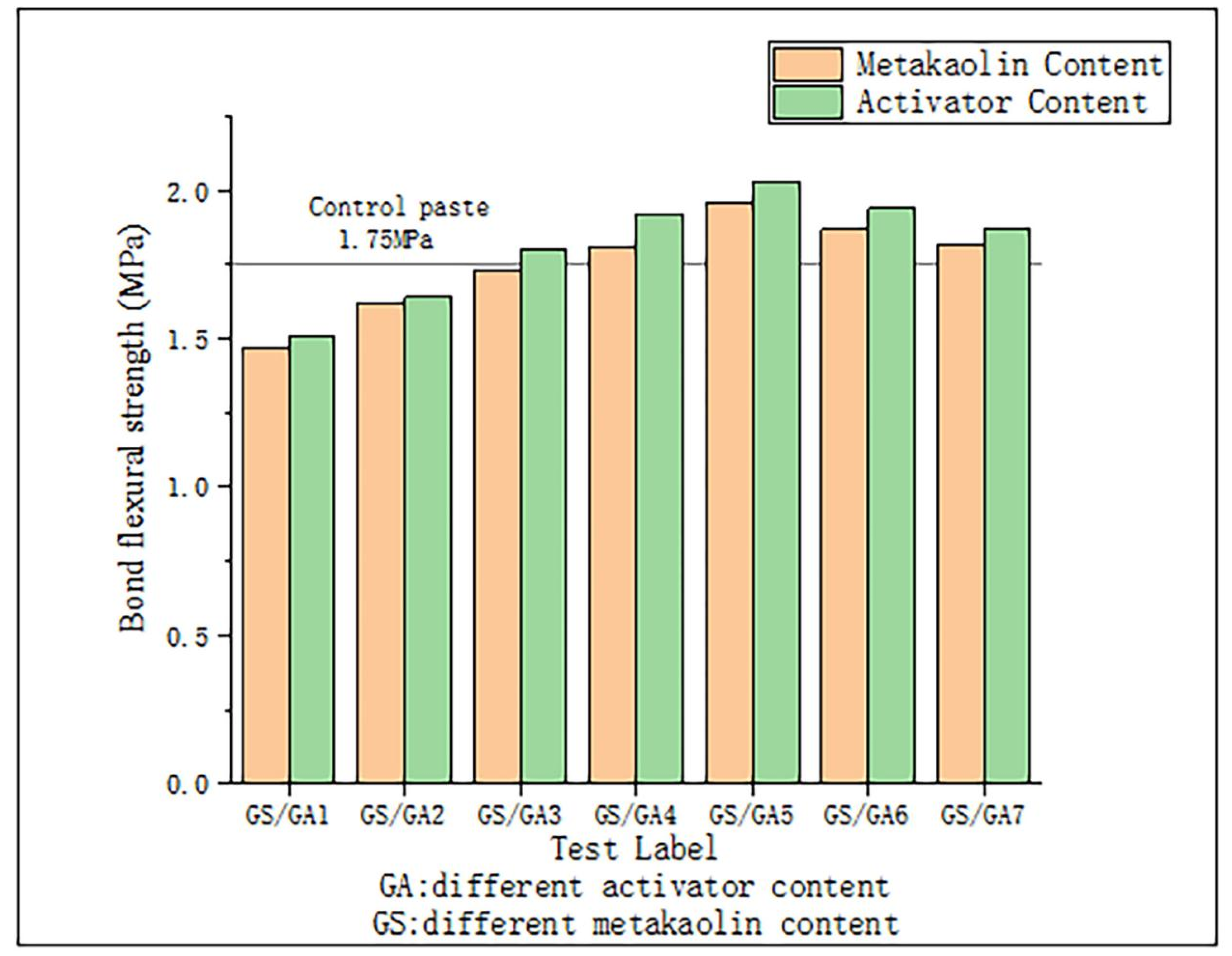
3.4. Bond Strength
3.5. Scanning Electron Microscopy
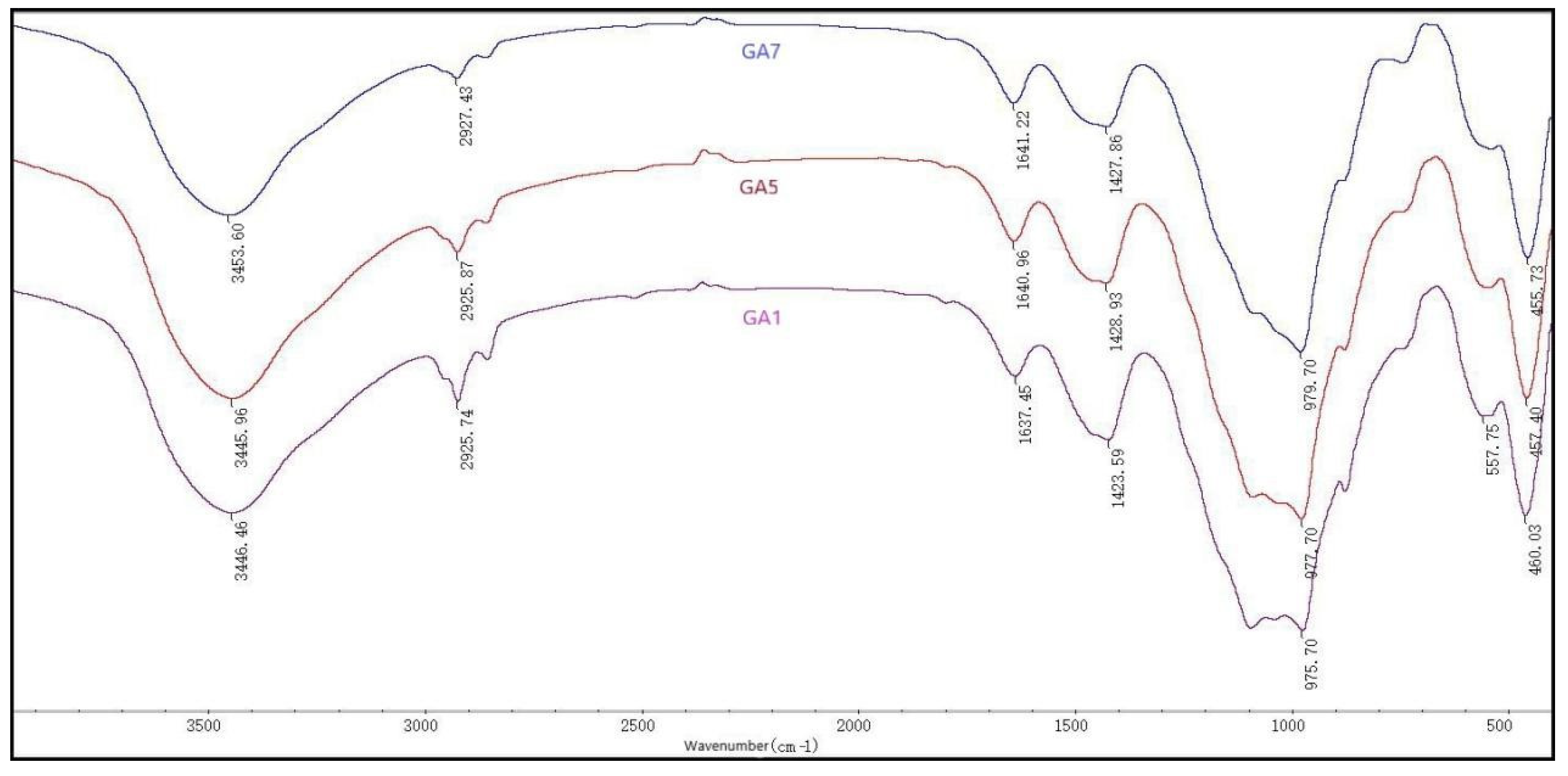
3.6. Fourier Transform-Infrared Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Madlool, N.; Saidur, R.; Hossain, M.; Rahim, N. A critical review on energy use and savings in the cement industries. Renew. Sustain. Energy Rev. 2011, 15, 2042–2060. [Google Scholar] [CrossRef]
- Yildirim, S.T.; Meyer, C.; Herfellner, S. Effects of internal curing on the strength, drying shrinkage and freeze-thaw resistance of concrete containing recycled concrete aggregates. Constr. Build. Mater. 2015, 91, 288–296. [Google Scholar] [CrossRef]
- Bossio, A.; Bellucci, F. Environmental degradation of reinforced concrete structures. Corros. Rev. 2019, 37, 1–2. [Google Scholar] [CrossRef]
- Wasim, M.; Ngo, T.D.; Law, D. A state-of-the-art review on the durability of geopolymer concrete for sustainable structures and infrastructure. Constr. Build. Mater. 2021, 291, 123381. [Google Scholar] [CrossRef]
- Wasim, M.; Ngo, T.D.; Abid, M. Investigation of long-term corrosion resistance of reinforced concrete structures constructed with various types of concretes in marine and various climate environments. Constr. Build. Mater. 2020, 20, 117701. [Google Scholar] [CrossRef]
- Amran, Y.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean production and properties of geopolymer concrete; a review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Xu, J.; Kang, A.; Wu, Z.; Xiao, P.; Gong, Y. Effect of high-calcium basalt fiber on the workability, mechanical properties and microstructure of slag-fly ash geopolymer grouting material. Constr. Build. Mater. 2021, 302, 124089. [Google Scholar] [CrossRef]
- Albitar, M.; Ali, M.S.M.; Visintin, P.; Drechsler, M. Durability evaluation of geopolymer and conventional concretes. Constr. Build. Mater. 2017, 136, 374–385. [Google Scholar] [CrossRef]
- Kuo, W.-T.; Liu, M.-Y.; Juang, C.-U. Bonding behavior of repair material using fly-ash/ground granulated blast furnace slag-based geopolymer. Materials 2019, 12, 1697. [Google Scholar] [CrossRef]
- Xing, Z.; He, D.; Wang, H.; Ye, Z.; Yang, S. Study on soil mechanics and frost resistance of fly ash–metakaolin geopolymer. Arab. J. Geosci. 2020, 13, 963. [Google Scholar] [CrossRef]
- Shehab El-Din, H.K.; Eisa, A.S.; Abdel Aziz, B.H.; Ibrahim, A. Mechanical performance of high strength concrete made from high volume of metakaolin and hybrid fibers. Constr. Build. Mater. 2017, 140, 203–209. [Google Scholar] [CrossRef]
- Pouhet, R. Formulation and Durability of Metakaolin-Based Geopolymers. Ph.D. Thesis, Université Toulouse III—Paul Sabatier, Toulouse, France, 2015. [Google Scholar] [CrossRef]
- Chen, K.; Wu, D.; Xia, L.; Cai, Q.; Zhang, Z. Geopolymer concrete durability subjected to aggressive environments—A review of influence factors and comparison with ordinary Portland cement. Constr. Build. Mater. 2021, 279, 122496. [Google Scholar] [CrossRef]
- Yu, G.; Jia, Y. Preparation of geopolymer composites based on alkali excitation. Arab. J. Geosci. 2021, 14, 600. [Google Scholar] [CrossRef]
- Bao, J.; Li, S.; Zhang, P.; Ding, X.; Xue, S.; Cui, Y.; Zhao, T. Influence of the incorporation of recycled coarse aggregate on water absorption and chloride penetration into concrete. Constr. Build. Mater. 2020, 239, 117845. [Google Scholar] [CrossRef]
- Chong, Z.; Li, X.; Yao, Q.; Zhang, J.; Chen, T. Anchorage behaviour of reinforced specimens containing a single fissure under uniaxial loading: A particle mechanics approach. Arab. J. Geosci. 2016, 9, 592. [Google Scholar] [CrossRef]
- Lingyu, T.; Dongpo, H.; Jianing, Z.; Hongguang, W. Durability of geopolymers and geopolymer concretes: A review. Rev. Adv. Mater. Sci. 2021, 60, 1–14. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers-Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Hanrahan, E.T. The Problem of Stress. In The Geotechnics of Real Materials: The εg, εk Method; Elsevier Science: Amsterdam, The Netherlands, 1985; ISBN 978-0-44459-817-2. [Google Scholar]
- Chen, K.; Wu, D.; Yi, M.; Cai, Q.; Zhang, Z. Mechanical and durability properties of metakaolin blended with slag geopolymer mortars used for pavement repair. Constr. Build. Mater. 2021, 281, 122566. [Google Scholar] [CrossRef]
- Hawa, A.; Tonnayopas, D.; Prachasaree, W.; Taneerananon, P. Development and performance evaluation of very high early strength geopolymer for rapid road repair. Adv. Mater. Sci. Eng. 2013, 2013, 764180. [Google Scholar] [CrossRef]
- Peng, X.Q.; Yang, T.; Wang, K.Y.; Meng, X.J. Preparation of geopolymeric concrete and its application to rapid repair of cement concrete pavement. J. Southwest Jiaotong Univ. 2011, 46, 205–210. [Google Scholar] [CrossRef]
- Jindal, B.B. Investigations on the properties of geopolymer mortar and concrete with mineral admixtures: A review. Constr. Build. Mater. 2019, 227, 116644. [Google Scholar] [CrossRef]
- GB/T 749-2008; Test Method for Determining Capability of Resisting Sulfate Corrode of Cement. China National Standards: Beijing, China, 2008. (In Chinese)
- JGJ/T70-2009; Standard for Test Method of Performance on Building Mortar. Ministry of Housing and Urban-Rural Development of the People Republic of China: Beijing, China, 2009. (In Chinese)
- Li, J.; Zhang, W.; Cao, Y. Laboratory evaluation of magnesium phosphate cement paste and mortar for rapid repair of cement concrete pavement. Constr. Build. Mater. 2014, 58, 122–128. [Google Scholar] [CrossRef]
- Ayeni, O.; Onwualu, A.P.; Boakye, E. Characterization and mechanical performance of metakaolin-based geopolymer for sustainable building applications. Constr. Build. Mater. 2021, 272, 121938. [Google Scholar] [CrossRef]
- Wang, S. Preparation of geopolymers from alkali-excited fly ash and its properties. Changsha University of Science and Technology. 2016. (In Chinese). Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201801&filename=1017299338.nh (accessed on 5 September 2022).
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Aboulayt, A.; Riahi, M.; Touhami, M.O.; Hannache, H.; Gomina, M.; Moussa, R. Properties of metakaolin based geopolymer incorporating calcium carbonate. Adv. Powder Technol. 2017, 28, 2393–2401. [Google Scholar] [CrossRef]
- Chen, X.; Sutrisno, A.; Struble, L.J. Effects of calcium on setting mechanism of metakaolin-based geopolymer. J. Am. Ceram. Soc. 2018, 101, 957–968. [Google Scholar] [CrossRef]
- Thokchom, S.; Ghosh, P.; Ghosh, S. Performance of fly ash based geopolymer mortars in sulphate solution. J. Eng. Sci. Technol. Rev. 2010, 3, 36–40. [Google Scholar] [CrossRef]
- Jinjiang, C. Durability of metakaolin based geopolymer under external sulfate attack. Low Temp. Archit. Technol. 2019, 41, 4–7. (In Chinese) [Google Scholar] [CrossRef]
- Aygörmez, Y.; Canpolat, O.; Al-Mashhadani, M.M.; Uysal, M. Elevated temperature, freezing-thawing and wetting-drying effects on polypropylene fiber reinforced metakaolin based geopolymer composites. Constr. Build. Mater. 2020, 235, 117502. [Google Scholar] [CrossRef]
- Pilehvar, S.; Szczotok, A.M.; Rodríguez, J.F.; Valentini, L.; Lanzón, M.; Pamies, R.; Kjøniksen, A.-L. Effect of freeze-thaw cycles on the mechanical behavior of geopolymer concrete and Portland cement concrete containing microencapsulated phase change materials. Constr. Build. Mater. 2019, 200, 94–103. [Google Scholar] [CrossRef]
- Allahverdi, A.; Abadi, M.M.B.R.; Hossain, K.M.A.; Lachemi, M. Resistance of chemically-activated high phosphorous slag content cement against freeze-thaw cycles. Cold Reg. Sci. Technol. 2014, 103, 107–114. [Google Scholar] [CrossRef]
- Basheer, L.; Kropp, J.; Cleland, D.J. Assessment of the durability of concrete from its permeation properties: A review. Constr. Build. Mater. 2001, 15, 93–103. [Google Scholar] [CrossRef]
- Mazaheri, A.; Bayat, A.; Hosinzadeh, F. Investigating the effect of alkali-activated slag on the strength of clay soil. Arab. J. Geosci. 2021, 14, 2662. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Characterisation of fly ashes: Potential reactivity as alkaline cements. Fuel 2003, 82, 2259–2265. [Google Scholar] [CrossRef]
- Sarker, P.K. Bond strength of reinforcing steel embedded in fly ash-based geopolymer concrete. Mater. Struct. 2011, 44, 1021–1030. [Google Scholar] [CrossRef]
- Nematollahzade, M.; Tajadini, A.; Afshoon, I.; Aslani, F. Influence of different curing conditions and water to cement ratio on properties of self-compacting concretes. Constr. Build. Mater. 2020, 237, 117570. [Google Scholar] [CrossRef]
- Chand, M.S.R.; Giri, P.S.N.R.; Kumar, P.R.; Kumar, G.R.; Raveena, C. Effect of self-curing chemicals in self compacting mortars. Constr. Build. Mater. 2016, 107, 356–364. [Google Scholar] [CrossRef]
- Gao, M.; Chen, B.; Lang, L.; Muhammad, R.A. Influence of Silica Fume on Mechanical Properties and Water Resistance of Magnesium-Ammonium Phosphate Cement. J. Mater. Civ. Eng. 2020, 32, 04019368. [Google Scholar] [CrossRef]
- Anantrao-Patil, A.; Vyawahare, M.R. Comparative study on durability of selfcured SCC and normally cured SCC. Int. J. Sci. Res. Eng. Technol. 2014, 3, 1201–1208. Available online: https://www.docin.com/p-1546931869.html (accessed on 5 September 2022).
- Bakharev, T. Durability of geopolymer materials in sodium and sodium sulfate solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]
- Hasnaoui, A.; Ghorbel, E.; Wardeh, G. Optimization approach of granulated blast furnace slag and metakaolin based geopolymer mortars. Constr. Build. Mater. 2019, 198, 10–26. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Rüscher, C.H. Mechanical and microstructural properties of metakaolin-based geopolymer cements from sodium water glass and phosphoric acid solution as hardeners: A comparative study. Appl. Clay Sci. 2017, 140, 81–87. [Google Scholar] [CrossRef]
- He, P.; Wang, M.; Fu, S.; Jia, D.; Yan, S.; Yuan, J.; Xu, J.; Wang, P.; Zhou, Y. Effects of Si/Al ratio on the structure and properties of metakaolin based geopolymer. Ceram. Int. 2016, 42, 14416–14422. [Google Scholar] [CrossRef]















| Components. | SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | K2O | Na2O | Whiteness | PH Value | Water Content | Loss on Ignition | Average Grain Size | Fineness |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 53.47% | 44.02% | 0.26% | 0.47% | 0.26% | 0.21% | 0.18% | 0.08% | 93.50% | 6.8 | 0.20% | 0.14% | 13μm | 10μm |
| Compositions | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | Loss on Ignition | Initial Time | Final Time | Fineness |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 55.30% | 26.04% | 6.61% | 4.32% | 2.44% | 2.33% | 3.12% | 130 min | 170 min | 20 μm |
| Components | SiO2 | Na2O | °Bé | Modulus |
|---|---|---|---|---|
| Content | 27.20% | 8.75% | 39 | 3.2 |
| Test Label | Cement (g) | Water (g) | Metakaolin (g) | Modulus | Activator (g) | Standard Sand (g) |
|---|---|---|---|---|---|---|
| GS1 | 160 | 320 | 640 | 1.5 | 200 | 1600 |
| GS2 | 200 | 320 | 600 | 1.5 | 200 | 1600 |
| GS3 | 240 | 320 | 560 | 1.5 | 200 | 1600 |
| GS4 | 280 | 320 | 520 | 1.5 | 200 | 1600 |
| GS5 | 320 | 320 | 480 | 1.5 | 200 | 1600 |
| GS6 | 360 | 320 | 440 | 1.5 | 200 | 1600 |
| GS7 | 400 | 320 | 400 | 1.5 | 200 | 1600 |
| GA1 | 320 | 320 | 480 | 1.5 | 80 | 1600 |
| GA2 | 320 | 320 | 480 | 1.5 | 120 | 1600 |
| GA3 | 320 | 320 | 480 | 1.5 | 160 | 1600 |
| GA4 | 320 | 320 | 480 | 1.5 | 200 | 1600 |
| GA5 | 320 | 320 | 480 | 1.5 | 240 | 1600 |
| GA6 | 320 | 320 | 480 | 1.5 | 280 | 1600 |
| GA7 | 320 | 320 | 480 | 1.5 | 320 | 1600 |
| Wavemunber Position [cm−1] | Bond Component | ||
|---|---|---|---|
| GA1 | GA5 | GA7 | |
| 460.03 | 457.40 | 455.73 | Bending of Si-O bonds [47] |
| 975.70 | 977.70 | 979.70 | Stretching of Si-OH bonds [47] |
| 1423.59 | 1428.93 | 1427.86 | Bending of O-C-O bonds ill CO32-ions [48] |
| 1637.45 | 1640.96 | 1641.22 | Bending of H-O-H bonds [47] |
| 2925.74 | 2925.87 | 2927.43 | Stretching of C-H bonds [49] |
| 3446.46 | 3445.96 | 3453.60 | Stretching of OH- groups [47] |
| Wavenumber Position [cm−1] | Bond Component | ||
|---|---|---|---|
| GS1 | GS5 | GS7 | |
| 462.05 | 458.31 | 457.58 | Bending of Si-O bonds [47] |
| 559.31 | 554.71 | 540.01 | Bending of Si-O-Al bonds [50] |
| 973.54 | 977.97 | 978.00 | Stretching of Si-OH bonds [47] |
| 1097.97 | 1103.35 | 1096.00 | Stretching of SiO42- units [49] |
| 1431.75 | 1428.33 | 1428.53 | Bending of O-C-O bonds in CO32- ions [48] |
| 2927.14 | 2925.61 | 2927.01 | Stretching of C-O bonds [49] |
| 3449.44 | 3456.17 | 3455.19 | Stretching of OH- groups [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, B.; Liu, J. Durability of Repair Metakaolin Geopolymeric Cement under Different Factors. Processes 2022, 10, 1818. https://doi.org/10.3390/pr10091818
Feng B, Liu J. Durability of Repair Metakaolin Geopolymeric Cement under Different Factors. Processes. 2022; 10(9):1818. https://doi.org/10.3390/pr10091818
Chicago/Turabian StyleFeng, Bowen, and Jiesheng Liu. 2022. "Durability of Repair Metakaolin Geopolymeric Cement under Different Factors" Processes 10, no. 9: 1818. https://doi.org/10.3390/pr10091818
APA StyleFeng, B., & Liu, J. (2022). Durability of Repair Metakaolin Geopolymeric Cement under Different Factors. Processes, 10(9), 1818. https://doi.org/10.3390/pr10091818





