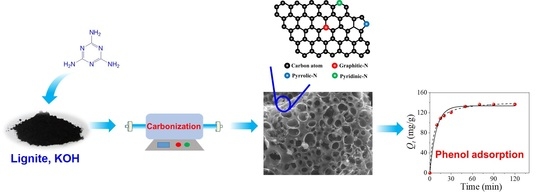
Lignite-Based N-Doped Porous Carbon as an Efficient Adsorbent for Phenol Adsorption
Abstract
:1. Introduction
2. Experimental
2.1. Materials
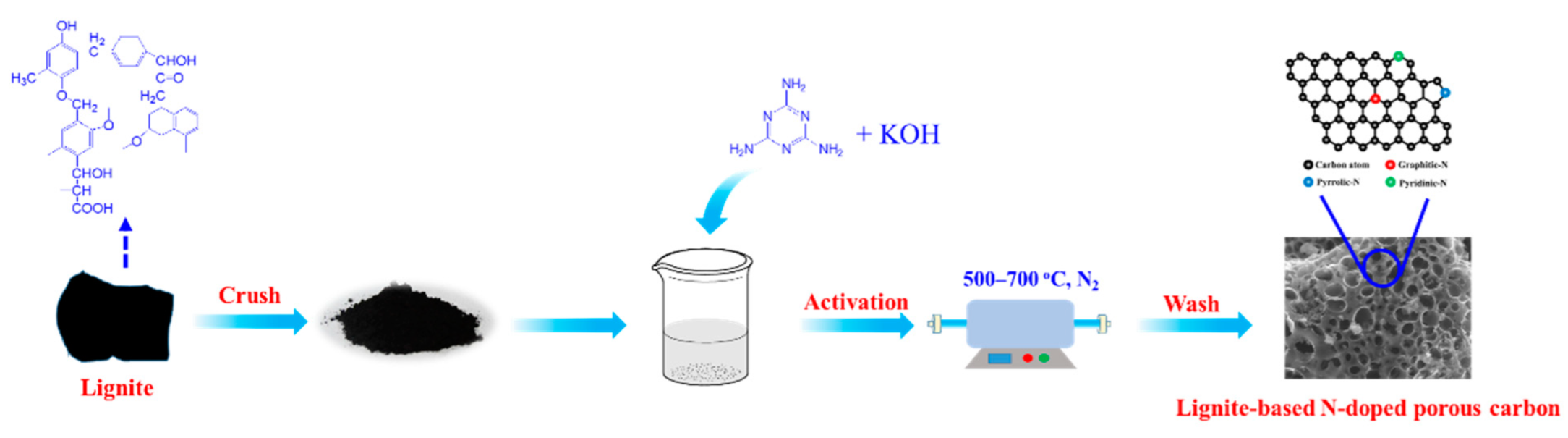
2.2. Preparation of Lignite-Based Porous Carbon
2.3. Sample Characterization
2.4. Adsorption Experiments
2.5. Models and Method
3. Results and Discussions
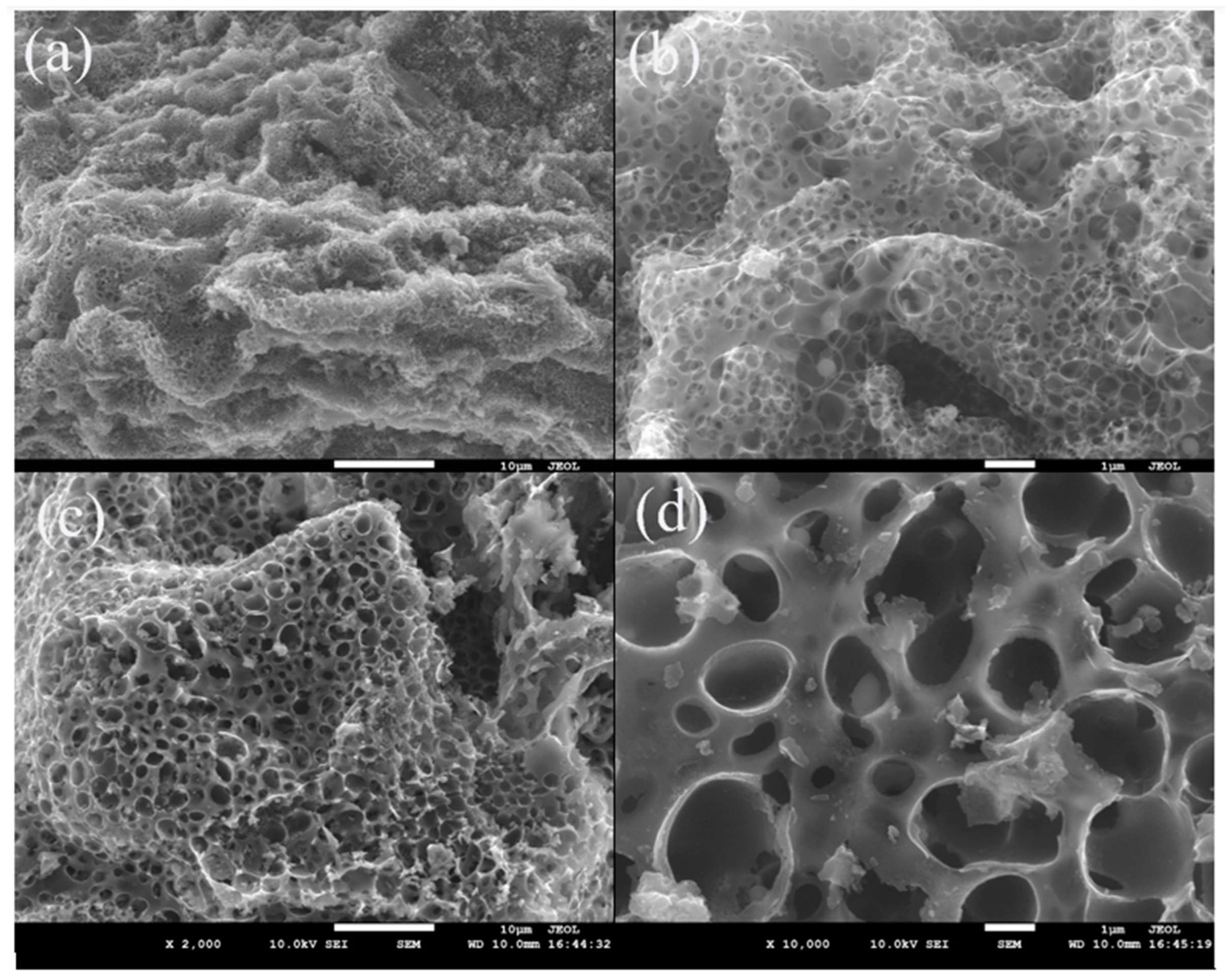
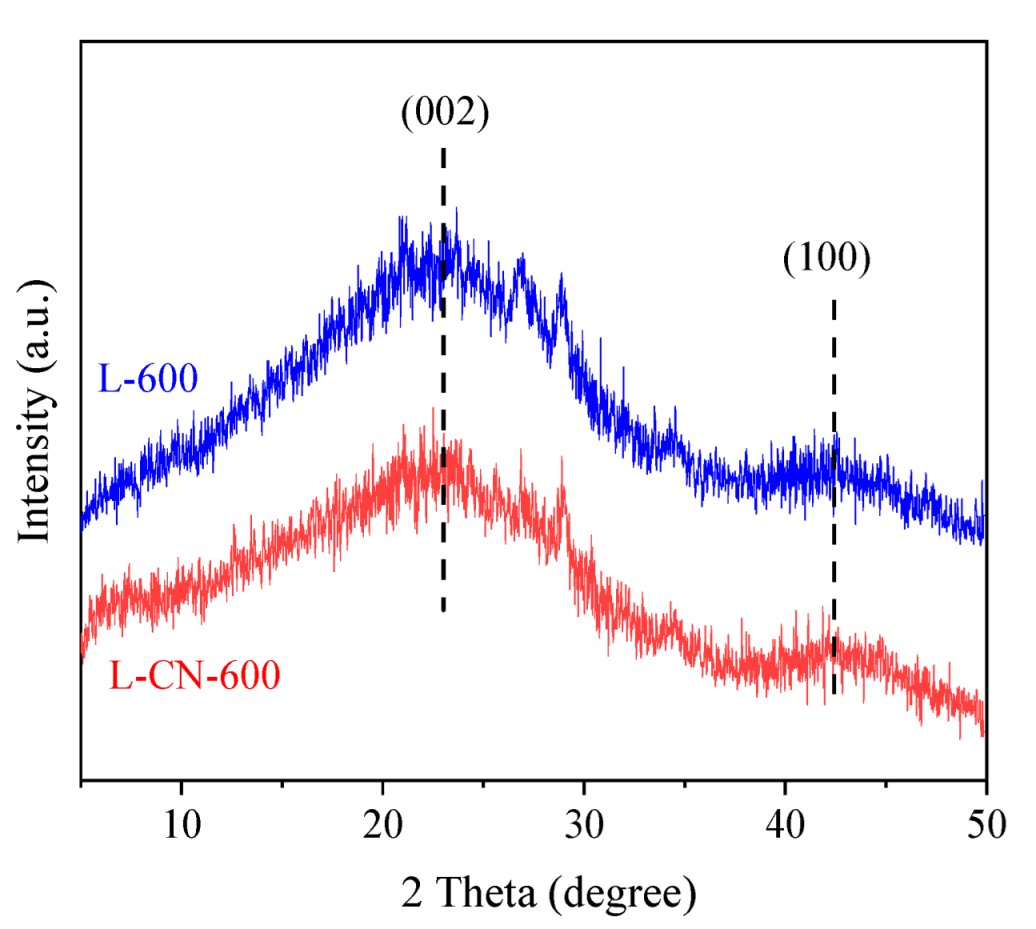
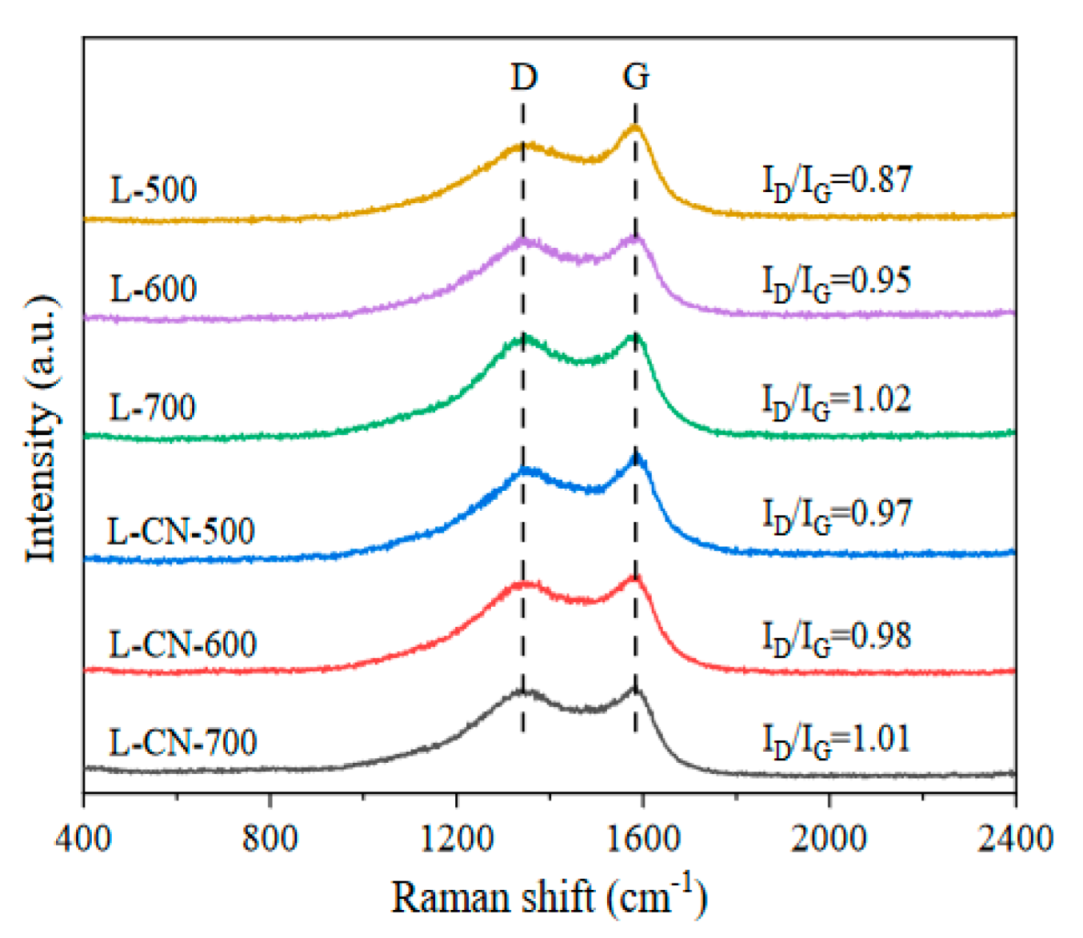
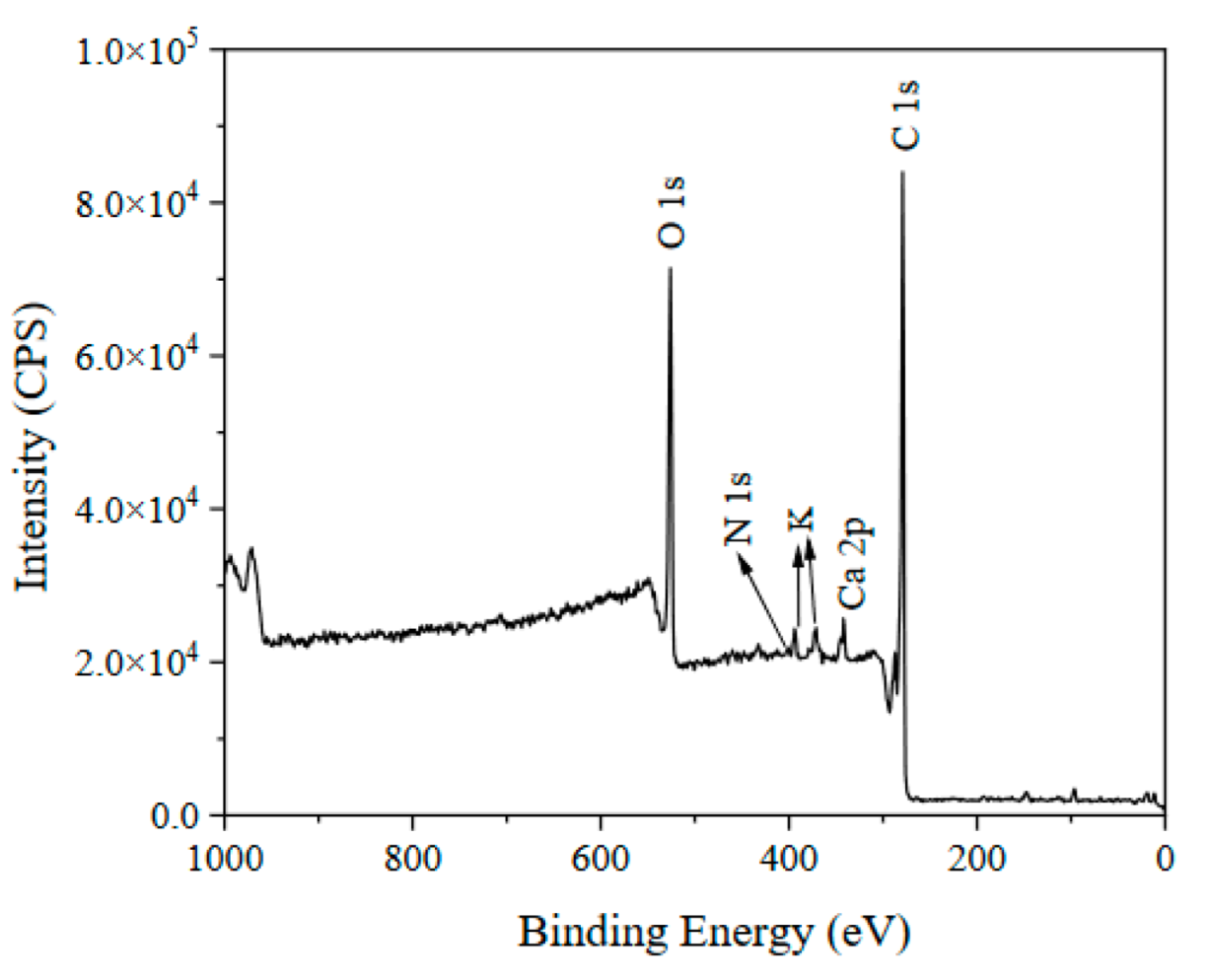
3.1. Characteristics of Lignite-Based Porous Carbon
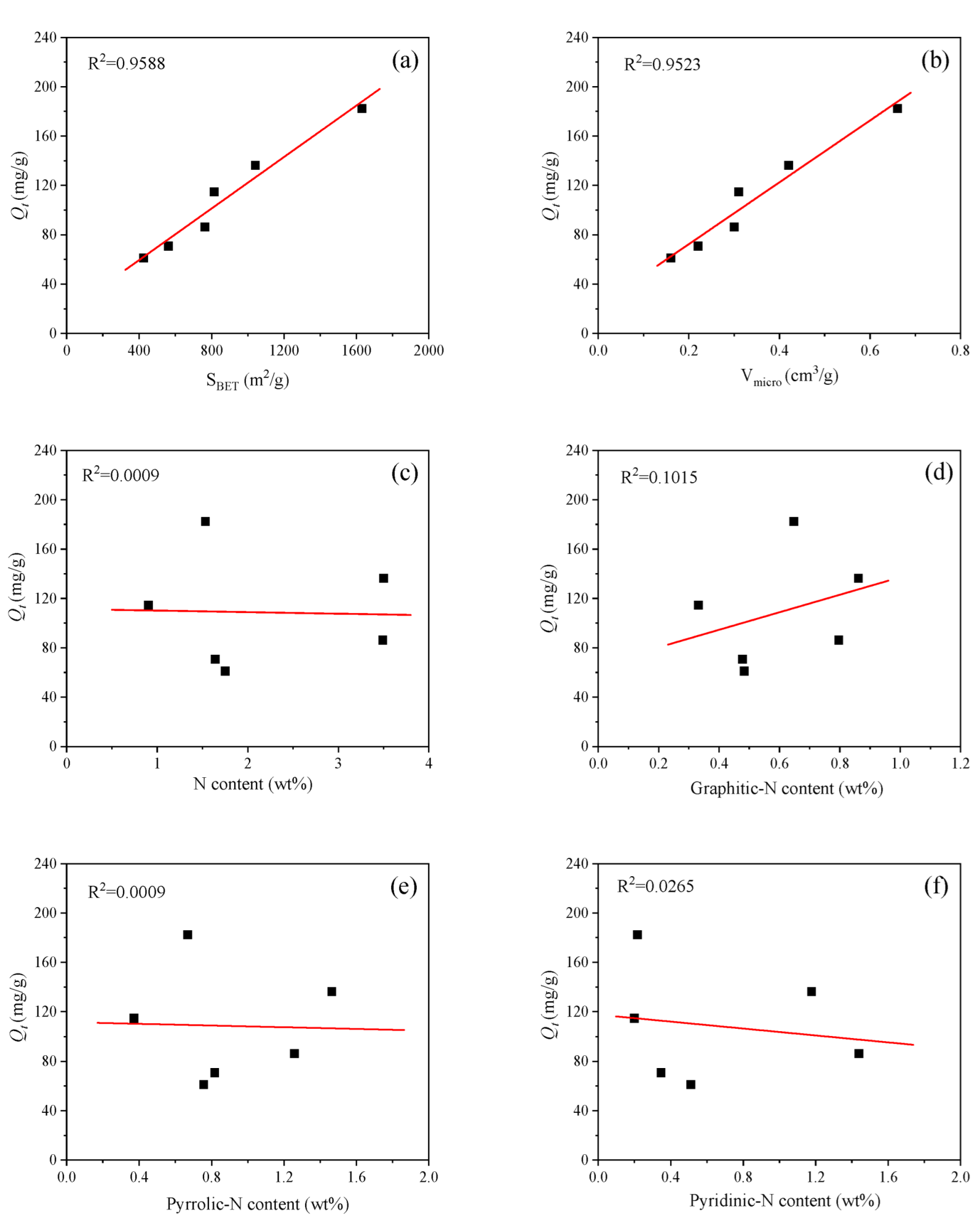
3.2. Phenol Adsorption Performance of Lignite-Based Porous Carbons
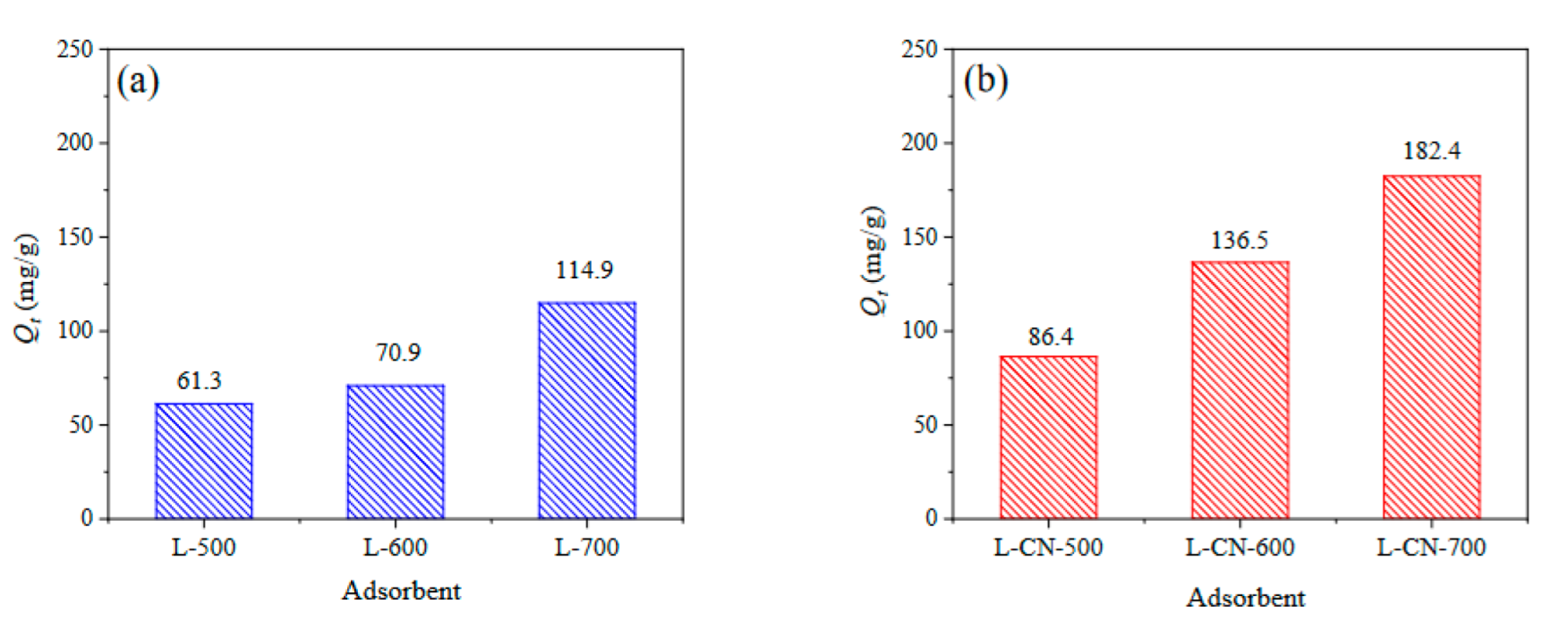
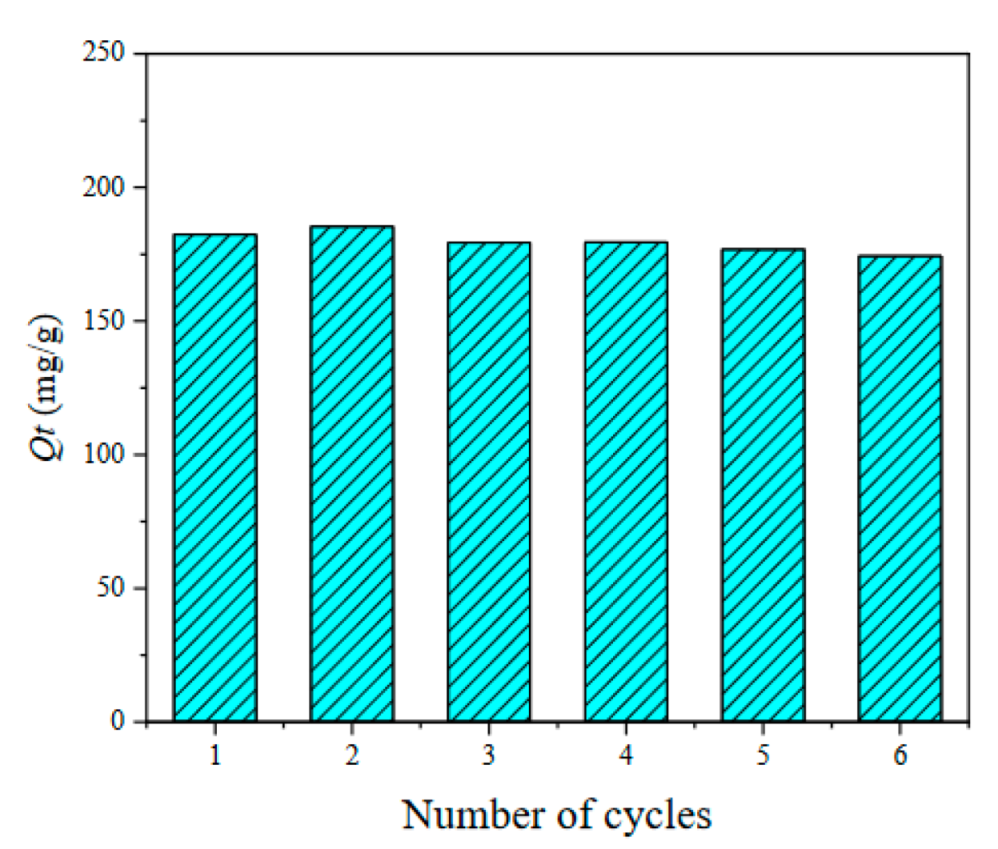
3.2.1. Adsorption Capacity of Different Lignite-Based Porous Carbons
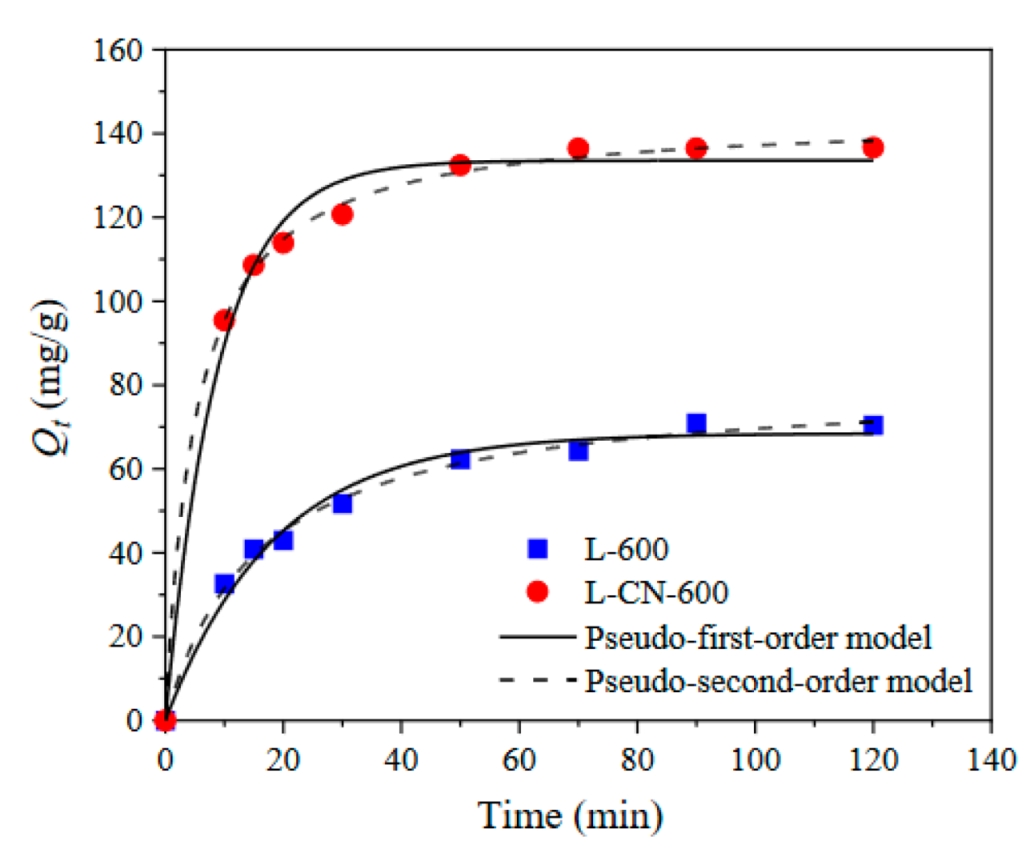
3.2.2. Adsorption Kinetics
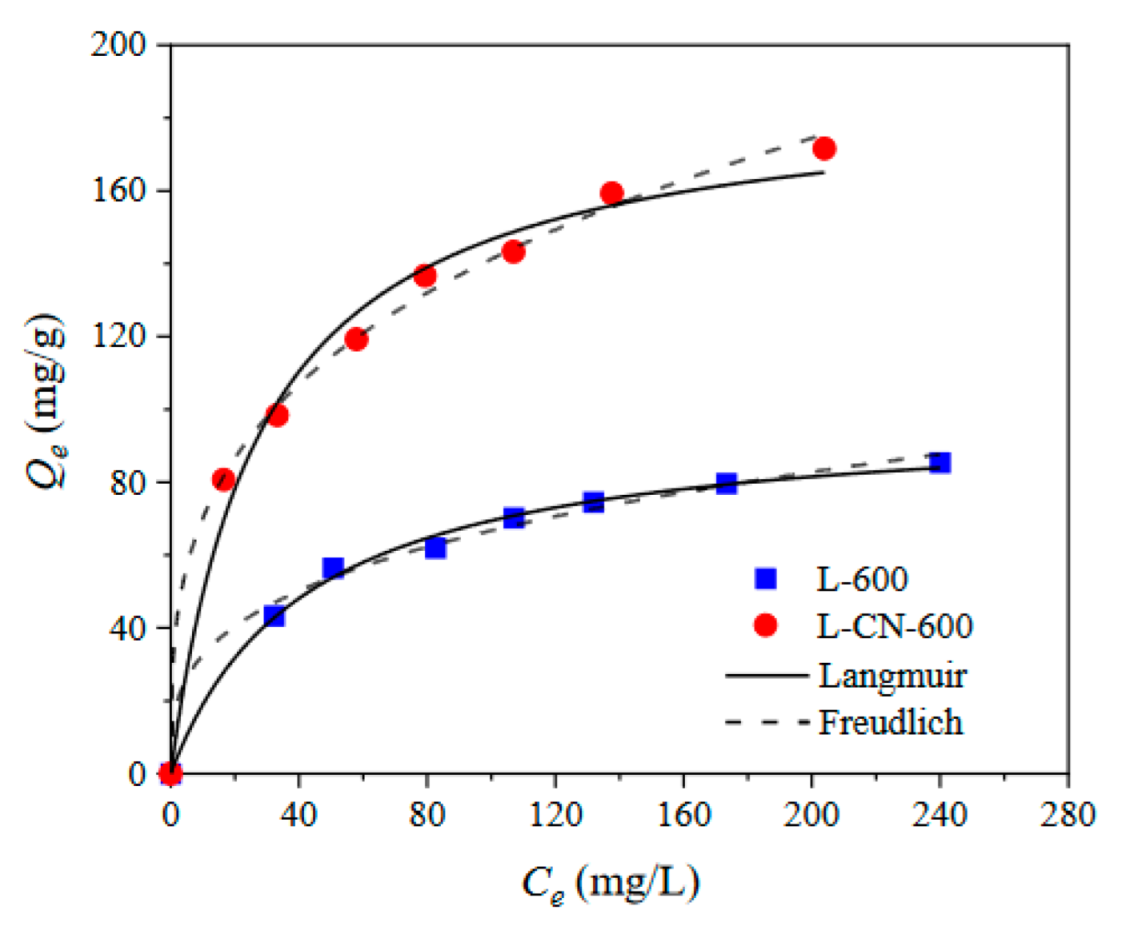
3.2.3. Adsorption Isotherms
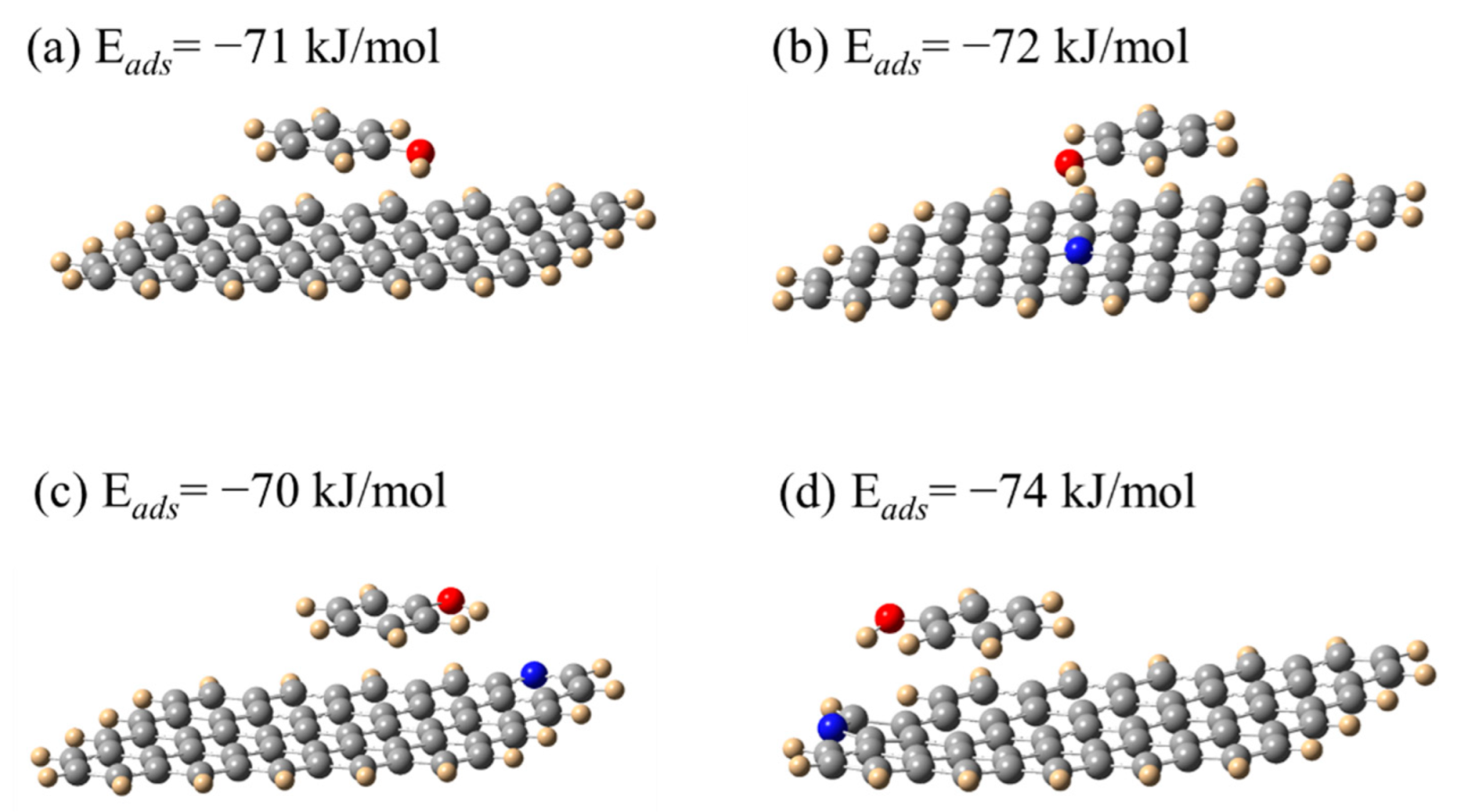
3.3. Theoretical Calculations and Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghafari, M.; Cui, Y.; Alali, A.; Atkinson, J.D. Phenol adsorption and desorption with physically and chemically tailored porous polymers: Mechanistic variability associated with hyper-cross-linking and amination. J. Hazard. Mater. 2019, 361, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Govindan, R.; Muthuchamy, M.; Cheng, S.; Li, X.; Ye, L.; Wang, L.; Guo, S.; Li, W.; Alharbi, N.S.; et al. Halophilic archaea and their extracellular polymeric compounds in the treatment of high salt wastewater containing phenol. Chemosphere 2022, 294, 133732. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Xu, Y.; Ren, L.; Shao, J.; He, Y. Improvement of phenol separation and biodegradation from saline wastewater in extractive membrane bioreactor (EMBR). Bioresour. Technol. Rep. 2022, 17, 100897. [Google Scholar] [CrossRef]
- Mohd, A. Presence of phenol in wastewater effluent and its removal: An overview. Int. J. Environ. Anal. Chem. 2020, 102, 1362–1384. [Google Scholar]
- Zhou, S.; Gu, P.; Wan, H.; Zhu, Y.; Li, N.; Chen, D.; Marcomini, A.; Xu, Q.; Lu, J. Preparation of new triptycene- and pentiptycene-based crosslinked polymers and their adsorption behavior towards aqueous dyes and phenolic organic pollutants. Sep. Purif. Technol. 2021, 278, 119495. [Google Scholar] [CrossRef]
- Qu, Y.; Qin, L.; Guo, M.; Liu, X.; Yang, Y. Multilayered molecularly imprinted composite membrane based on porous carbon nanospheres/pDA cooperative structure for selective adsorption and separation of phenol. Sep. Purif. Technol. 2022, 280, 119915. [Google Scholar] [CrossRef]
- Nady, H.; El-Rabiei, M.M.; Abd El-Hafez, G.M. Electrochemical oxidation behavior of some hazardous phenolic compounds in acidic solution. Egypt. J. Pet. 2017, 26, 669–678. [Google Scholar] [CrossRef]
- Wu, H.; Liu, R.; Sun, Y.; Wen, Y.; Zhao, Q.; Lin, S.; Wang, Y. Effect of MoS2 on phenol decomposition in water after high-voltage pulse discharge treatment. Chemosphere 2022, 294, 133808. [Google Scholar] [CrossRef]
- Dionisi, D.; Etteh, C.C. Effect of process conditions on the aerobic biodegradation of phenol and paracetamol by open mixed microbial cultures. J. Environ. Chem. Eng. 2019, 7, 103282. [Google Scholar] [CrossRef]
- Wu, Z.; Jing, J.; Zhang, K.; Li, W.; Yang, J.; Shen, J.; Zhang, S.; Xu, K.; Zhang, S.; Zhu, Y. Epitaxial BiP5O14 layer on BiOI nanosheets enhancing the photocatalytic degradation of phenol via interfacial internal-electric-field. Appl. Catal. B Environ. 2022, 307, 121153. [Google Scholar] [CrossRef]
- Bertoncini, C.; Raffaelli, J.; Fassino, L.; Odetti, H.S.; Bottani, E.J. Phenol adsorption on porous and non-porous carbons. Carbon 2003, 41, 1101–1111. [Google Scholar] [CrossRef]
- Liu, X.; Pinto, N.G. Ideal adsorbed phase model for adsorption of phenolic compounds on activated carbon. Carbon 1997, 35, 1387–1397. [Google Scholar] [CrossRef]
- Liu, H.; Kim, G.E.; Hong, C.O.; Song, Y.C.; Lee, W.K.; Liu, D.; Jang, S.H.; Park, Y.K. Treatment of phenol wastewater using nitrogen-doped magnetic mesoporous hollow carbon. Chemosphere 2021, 271, 129595. [Google Scholar] [CrossRef] [PubMed]
- Beker, U.; Ganbold, B.; Dertli, H.; Gülbayir, D.D. Adsorption of phenol by activated carbon: Influence of activation methods and solution pH. Energy Convers. Manag. 2010, 51, 235–240. [Google Scholar] [CrossRef]
- Su, F.; Lv, L.; Hui, T.M.; Zhao, X.S. Phenol adsorption on zeolite-templated carbons with different structural and surface properties. Carbon 2005, 43, 1156–1164. [Google Scholar] [CrossRef]
- Yang, G.; Chen, H.; Qin, H.; Feng, Y. Amination of activated carbon for enhancing phenol adsorption: Effect of nitrogen-containing functional groups. Appl. Surf. Sci. 2014, 293, 299–305. [Google Scholar] [CrossRef]
- Stavropoulos, G.G.; Samaras, P.; Sakellaropoulos, G.P. Effect of activated carbons modification on porosity, surface structure and phenol adsorption. J. Hazard. Mater. 2008, 151, 414–421. [Google Scholar] [CrossRef]
- Mojoudi, N.; Mirghaffari, N.; Soleimani, M.; Shariatmadari, H.; Belver, C.; Bedia, J. Phenol adsorption on high microporous activated carbons prepared from oily sludge: Equilibrium, kinetic and thermodynamic studies. Sci. Rep. 2019, 9, 19352. [Google Scholar] [CrossRef]
- Saleh, T.A.; Adio, S.O.; Asif, M.; Dafalla, H. Statistical analysis of phenols adsorption on diethylenetriamine-modified activated carbon. J. Clean. Prod. 2018, 182, 960–968. [Google Scholar] [CrossRef]
- Du, W.; Sun, J.; Zan, Y.; Zhang, Z.; Ji, J.; Dou, M.; Wang, F. Biomass-derived nitrogen-doped hierarchically porous carbon networks as efficient absorbents for phenol removal from wastewater over a wide pH range. RSC Adv. 2017, 7, 46629–46635. [Google Scholar] [CrossRef]
- Zhang, D.; Huo, P.; Liu, W. Behavior of phenol adsorption on thermal modified activated carbon. Chin. J. Chem. Eng. 2016, 24, 446–452. [Google Scholar] [CrossRef]
- Liu, S.; Wang, R. Modified activated carbon with an enhanced nitrobenzene adsorption capacity. J. Porous Mater. 2010, 18, 99–106. [Google Scholar] [CrossRef]
- Wang, T.; Cheng, Z.; Liu, Y.; Tang, W.; Fang, T.; Xing, B. Mechanistic understanding of highly selective adsorption of bisphenols on microporous-dominated nitrogen-doped framework carbon. Sci. Total Environ. 2021, 762, 143115. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.D.; Shao, D.D.; Ren, X.M.; Wang, X.Q.; Li, J.X.; Chen, Y.X.; Wang, X.K. Kinetics and thermodynamics of adsorption of ionizable aromatic compounds from aqueous solutions by as-prepared and oxidized multiwalled carbon nanotubes. J. Hazard. Mater. 2010, 178, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Cai, W. One-step hydrothermal preparation of N-doped carbon spheres from peanut hull for efficient removal of Cr(VI). J. Environ. Chem. Eng. 2020, 8, 104449. [Google Scholar]
- Zhang, F.; Zhang, S.; Chen, L.; Liu, Z.; Qin, J. Utilization of bark waste of Acacia mangium: The preparation of activated carbon and adsorption of phenolic wastewater. Ind. Crop. Prod. 2021, 160, 113157. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, D.; Chen, J.; Chen, Y.; Chen, W. Enhanced adsorption of aromatic chemicals on boron and nitrogen co-doped single-walled carbon nanotubes. Environ. Sci. Nano 2017, 4, 558–564. [Google Scholar] [CrossRef]
- Li, B.; Lei, Z.; Zhang, X.; Huang, Z. Adsorption of simple aromatics from aqueous solutions on modified activated carbon fibers. Catal. Today 2010, 158, 515–520. [Google Scholar] [CrossRef]
- Liu, X.; Tu, Y.; Liu, S.; Liu, K.; Zhang, L.; Li, G.; Xu, Z. Adsorption of ammonia nitrogen and phenol onto the lignite surface: An experimental and molecular dynamics simulation study. J. Hazard. Mater. 2021, 416, 125966. [Google Scholar] [CrossRef]
- Song, J.; Shen, W.; Wang, J.; Fan, W. Superior carbon-based CO2 adsorbents prepared from poplar anthers. Carbon 2014, 69, 255–263. [Google Scholar] [CrossRef]
- Ge, C.; Song, J.; Qin, Z.; Wang, J.; Fan, W. Polyurethane Foam-Based Ultramicroporous Carbons for CO2 Capture. ACS Appl. Mater. Inter. 2016, 8, 18849–18859. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Kumar, K.V. Pseudo-second order models for the adsorption of safranin onto activated carbon: Comparison of linear and non-linear regression methods. J. Hazard. Mater. 2007, 142, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, Q.; Liu, T.; Sun, J.; Jiao, Y.; Xia, Y.; Xia, L.; Wang, Z.; Zhang, W.; Wang, K.; et al. Equilibrium, kinetic and thermodynamic studies on the adsorption of phenol onto graphene. Mater. Res. Bull. 2012, 47, 1898–1904. [Google Scholar] [CrossRef]
- Jain, M.; Khan, S.A.; Sahoo, A.; Dubey, P.; Pant, K.K.; Ziora, Z.M.; Blaskovich, M.A.T. Statistical evaluation of cow-dung derived activated biochar for phenol adsorption: Adsorption isotherms, kinetics, and thermodynamic studies. Bioresour. Technol. 2022, 352, 127030. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09; Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Wang, B.; Gan, F.; Dai, Z.; Ma, S.; Chen, W.; Jiang, X. Air oxidation coupling NH3 treatment of biomass derived hierarchical porous biochar for enhanced toluene removal. J. Hazard. Mater. 2021, 403, 123995. [Google Scholar] [CrossRef]
- Franco, D.S.P.; Georgin, J.; Netto, M.S.; Allasia, D.; Oliveira, M.L.S.; Foletto, E.L.; Dotto, G.L. Highly effective adsorption of synthetic phenol effluent by a novel activated carbon prepared from fruit wastes of the Ceiba speciosa forest species. J. Environ. Chem. Eng. 2021, 9, 105927. [Google Scholar] [CrossRef]
- Song, J.; Shen, W.; Wang, J.; Fan, W. Synthesis of novel hollow graphitic vesicle-supported Pt nanoparticles for oxygen reduction reaction. Carbon 2016, 109, 505–516. [Google Scholar] [CrossRef]
- Xue, Y.; Li, J.; Wang, S.; Cui, X.; Dong, M.; Wang, G.; Qin, Z.; Wang, J.; Fan, W. Co-reaction of methanol with butene over a high-silica H-ZSM-5 catalyst. J. Catal. 2018, 367, 315–325. [Google Scholar] [CrossRef]
- Urbonaite, S.; Hälldahl, L.; Svensson, G. Raman spectroscopy studies of carbide derived carbons. Carbon 2008, 46, 1942–1947. [Google Scholar] [CrossRef]
- Shi, R.; Zhao, J.; Liu, S.; Sun, W.; Li, H.; Hao, P.; Li, Z.; Ren, J. Nitrogen-doped graphene supported copper catalysts for methanol oxidative carbonylation: Enhancement of catalytic activity and stability by nitrogen species. Carbon 2018, 130, 185–195. [Google Scholar] [CrossRef]
- Kong, X.; Gao, H.; Song, X.; Deng, Y.; Zhang, Y. Adsorption of phenol on porous carbon from Toona sinensis leaves and its mechanism. Chem. Phys. Lett. 2020, 739, 137046. [Google Scholar] [CrossRef]
- Lorenc-Grabowska, E.; Diez, M.A.; Gryglewicz, G. Influence of pore size distribution on the adsorption of phenol on PET-based activated carbons. J. Colloid Interface Sci. 2016, 469, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Deditius, A.; Ela, W.P.; Wiśniewski, M.; Gauden, P.A.; Terzyk, A.P.; Furmaniak, S.; Włoch, J.; Kaneko, K.; Neimark, A.V. Super-sieving effect in phenol adsorption from aqueous solutions on nanoporous carbon beads. Carbon 2018, 135, 12–20. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, L.; Yang, Y.; Liu, X. Porous carbon nanospheres aerogel based molecularly imprinted polymer for efficient phenol adsorption and removal from wastewater. Sep. Purif. Technol. 2021, 274, 119029. [Google Scholar] [CrossRef]
- Chen, A.; Li, Y.; Yu, Y.; Li, Y.; Xia, K.; Wang, Y.; Li, S.; Zhang, L. Synthesis of hollow mesoporous carbon spheres via “dissolution-capture” method for effective phenol adsorption. Carbon 2016, 103, 157–162. [Google Scholar] [CrossRef]
- Wang, T.; Huang, M.; Liu, X.; Zhang, Z.; Liu, Y.; Tang, W.; Bao, S.; Fang, T. Facile one-step hydrothermal synthesis of α-Fe2O3/g-C3N4 composites for the synergistic adsorption and photodegradation of dyes. RSC Adv. 2019, 9, 29109–29119. [Google Scholar] [CrossRef]
- Jun, L.Y.; Karri, R.R.; Mubarak, N.M.; Yon, L.S.; Bing, C.H.; Khalid, M.; Jagadish, P.; Abdullah, E.C. Modelling of methylene blue adsorption using peroxidase immobilized functionalized Buckypaper/polyvinyl alcohol membrane via ant colony optimization. Environ. Pollut. 2020, 259, 113940. [Google Scholar] [CrossRef]
- Cansado, I.P.P.; Mourão, P.A.M. Impact of the use of co-adjuvants agents during chemical activation on the performance of activated carbons in the removal of 4-chloro-2-methyl-phenoxyacetic acid. Environ. Technol. Inno. 2021, 24, 102058. [Google Scholar] [CrossRef]
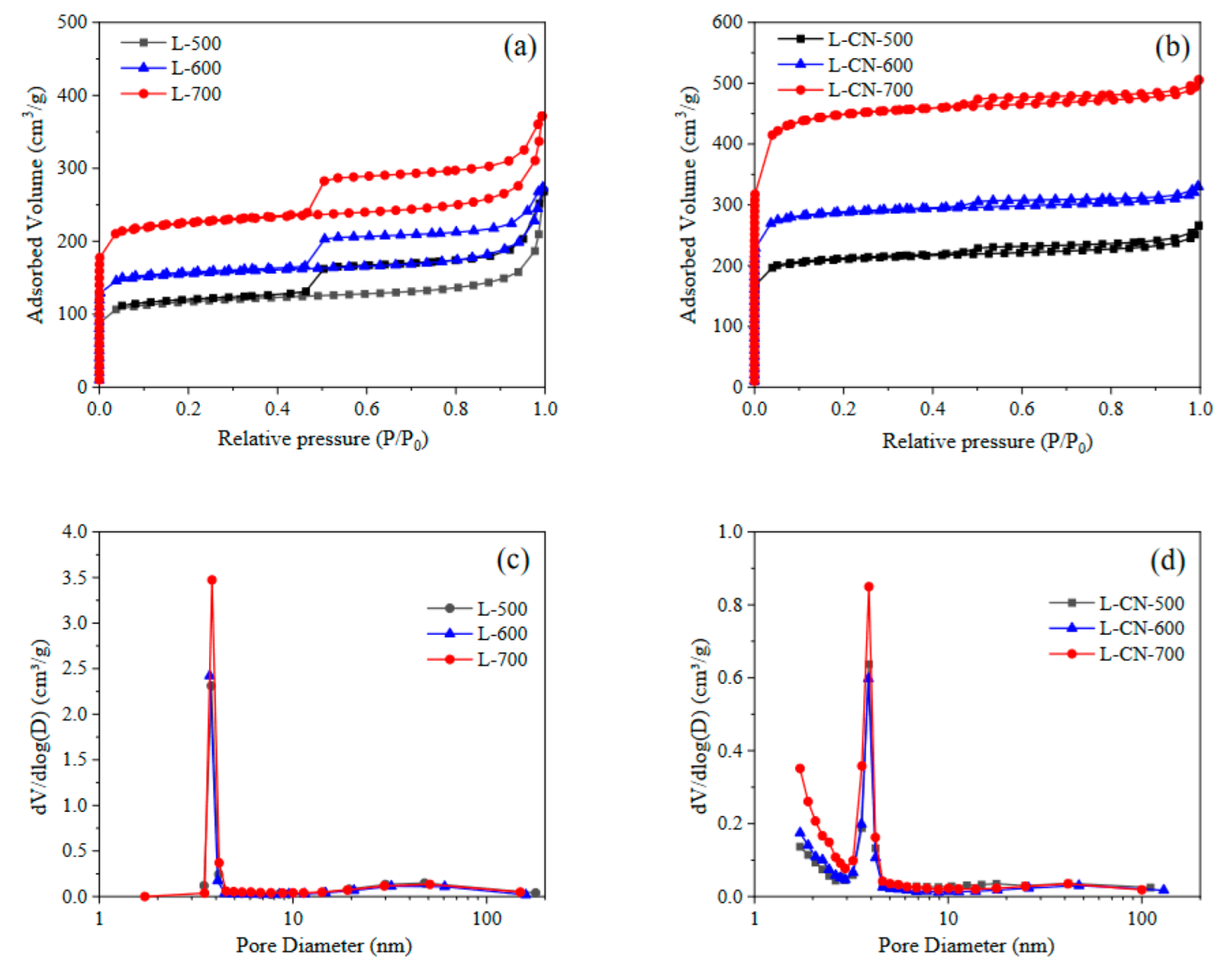
| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | ||
|---|---|---|---|---|---|
| SBET | Smeso | Vmicro | Vmeso | ||
| L-500 | 424 | 59 | 0.16 | 0.09 | 2.37 |
| L-600 | 561 | 55 | 0.22 | 0.09 | 2.22 |
| L-700 | 814 | 82 | 0.31 | 0.12 | 2.14 |
| L-CN-500 | 762 | 58 | 0.30 | 0.07 | 1.93 |
| L-CN-600 | 1041 | 59 | 0.42 | 0.06 | 1.85 |
| L-CN-700 | 1630 | 89 | 0.66 | 0.09 | 1.83 |
| Samples | Chemical Compositions (wt%) | N/C a | H/C a | ||||
|---|---|---|---|---|---|---|---|
| N | C | H | S | Other | |||
| L-500 | 1.75 | 67.53 | 2.24 | 0.38 | 28.11 | 0.02 | 0.40 |
| L-600 | 1.64 | 66.62 | 1.59 | 0.27 | 29.88 | 0.02 | 0.29 |
| L-700 | 0.90 | 65.58 | 1.41 | 0.30 | 31.81 | 0.01 | 0.26 |
| L-CN-500 | 3.49 | 64.77 | 2.08 | 0.29 | 29.37 | 0.05 | 0.39 |
| L-CN-600 | 3.50 | 68.37 | 1.58 | 0.16 | 26.39 | 0.04 | 0.28 |
| L-CN-700 | 1.53 | 75.60 | 0.85 | 0.22 | 21.80 | 0.02 | 0.14 |
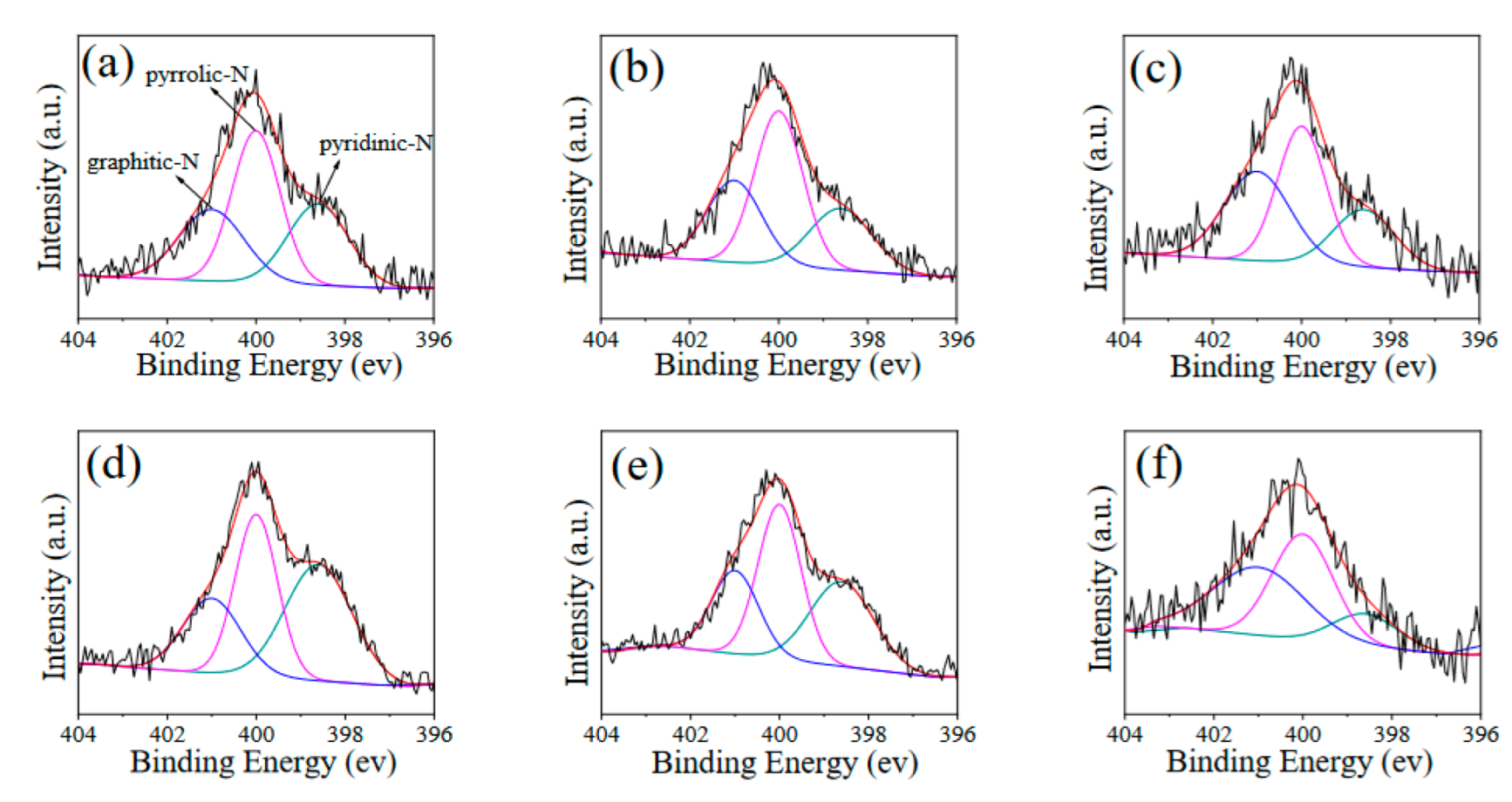
| Sample | Graphitic-N (%) | Pyrrolic-N (%) | Pyridinic-N (%) |
|---|---|---|---|
| L-500 | 27.6 | 43.2 | 29.2 |
| L-600 | 29.1 | 49.8 | 21.1 |
| L-700 | 36.8 | 41.2 | 22.0 |
| L-CN-500 | 22.8 | 36.0 | 41.2 |
| L-CN-600 | 24.6 | 41.8 | 33.6 |
| L-CN-700 | 42.3 | 43.6 | 14.1 |
| Adsorbent | Phenol Adsorption Capacity, (mg/g) | Reference |
|---|---|---|
| Molecularly imprinted composite membrane | 51.40 | [6] |
| Nitrogen-doped magnetic mesoporous hollow carbon | 55.86 | [13] |
| Oily sludge-based AC | 434 | [18] |
| Diethylenetriamine-modified activated carbon | 18.12 | [19] |
| Nitrogen-doped hierarchically porous carbon | 431 | [20] |
| Activated carbon | 96.92 | [26] |
| Graphene | 28.26 | [34] |
| Porous carbon from Toona sinensis leaves | 325 | [43] |
| Lignite-based N-doped porous carbon | 182.4 | This work |
| Adsorbent | Qe,exp a | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| Qe,cal b | k1 | R2 | Qe,cal b | k2 | R2 | ||
| L-600 | 70.44 | 68.49 | 0.0541 | 0.9849 | 80.44 | 0.0008 | 0.9953 |
| L-CN-600 | 136.64 | 133.54 | 0.1112 | 0.9898 | 144.19 | 0.0014 | 0.9988 |
| Adsorbent | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qm | kL | R2 | kF | n | R2 | |
| L-600 | 98.65 | 0.0238 | 0.9960 | 15.8861 | 3.2088 | 0.9938 |
| L-CN-600 | 187.73 | 0.0357 | 0.9870 | 34.7823 | 3.2859 | 0.9971 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Chen, Y.; Shi, L.; Wu, H.; Zhang, C.; Cheng, M.; Li, H.; Li, W.; Niu, Y. Lignite-Based N-Doped Porous Carbon as an Efficient Adsorbent for Phenol Adsorption. Processes 2022, 10, 1746. https://doi.org/10.3390/pr10091746
Xue Y, Chen Y, Shi L, Wu H, Zhang C, Cheng M, Li H, Li W, Niu Y. Lignite-Based N-Doped Porous Carbon as an Efficient Adsorbent for Phenol Adsorption. Processes. 2022; 10(9):1746. https://doi.org/10.3390/pr10091746
Chicago/Turabian StyleXue, Yanfeng, Yanyan Chen, Linxia Shi, Haotian Wu, Chao Zhang, Minghuang Cheng, Hongbin Li, Wanjun Li, and Yulan Niu. 2022. "Lignite-Based N-Doped Porous Carbon as an Efficient Adsorbent for Phenol Adsorption" Processes 10, no. 9: 1746. https://doi.org/10.3390/pr10091746
APA StyleXue, Y., Chen, Y., Shi, L., Wu, H., Zhang, C., Cheng, M., Li, H., Li, W., & Niu, Y. (2022). Lignite-Based N-Doped Porous Carbon as an Efficient Adsorbent for Phenol Adsorption. Processes, 10(9), 1746. https://doi.org/10.3390/pr10091746