Study on High Temperature Pyrolysis Light Cycle Oil to Acetylene and Carbon Black
Abstract
:1. Introduction
2. Experimental
2.1. Feedstock
2.2. Experimental Apparatus and Procedure
2.2.1. High Temperature Cracking Reaction Performance
2.2.2. Products Analysis and Characterization
2.2.3. Electrochemical Measurement
3. Results and Discussion
3.1. Effect of Temperature
3.2. Effect of Residence Time
3.3. Effect of Feedstock’s Composition
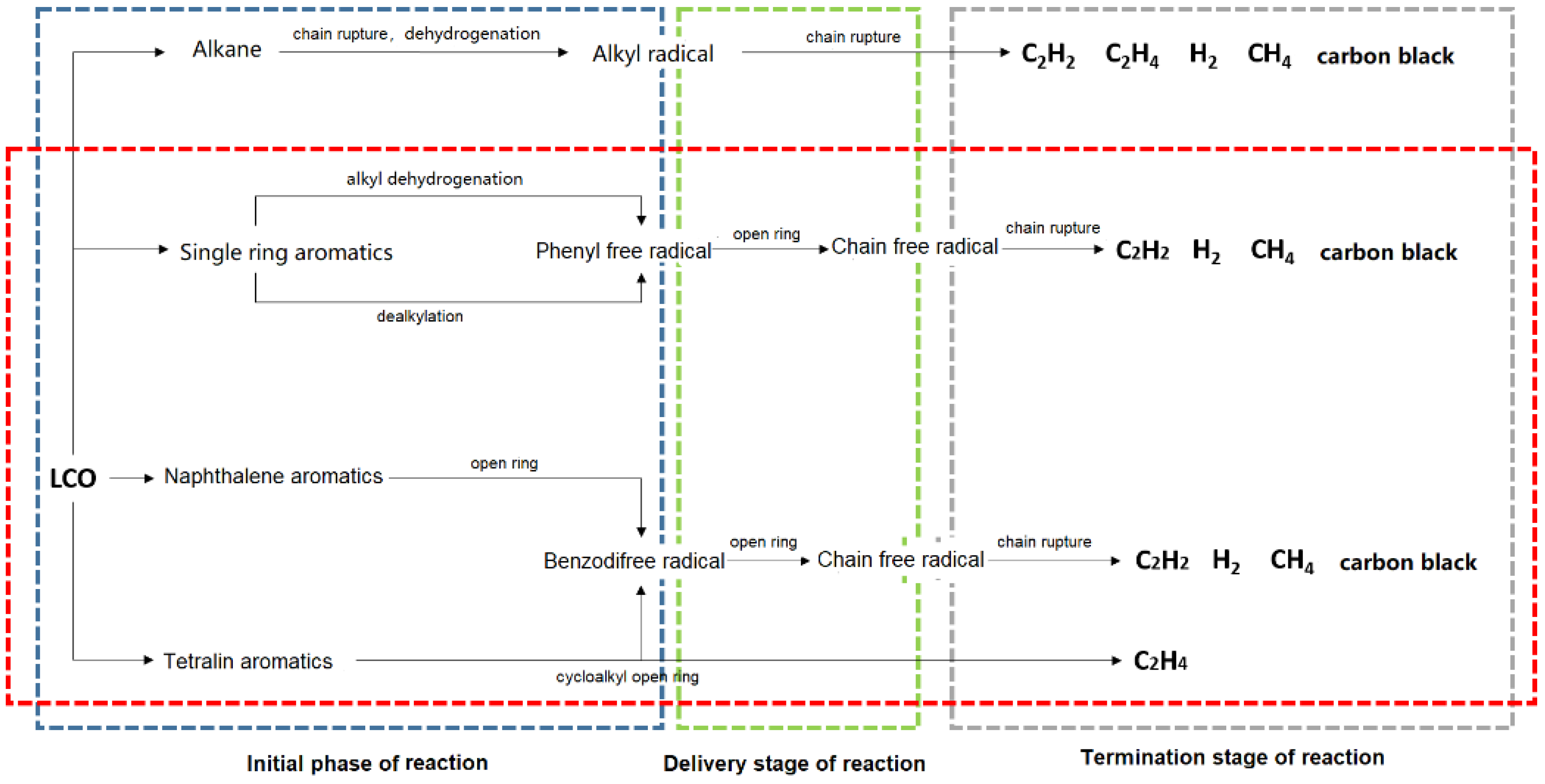
3.4. Proposed Mechanism of LCO High Temperature Pyrolysis
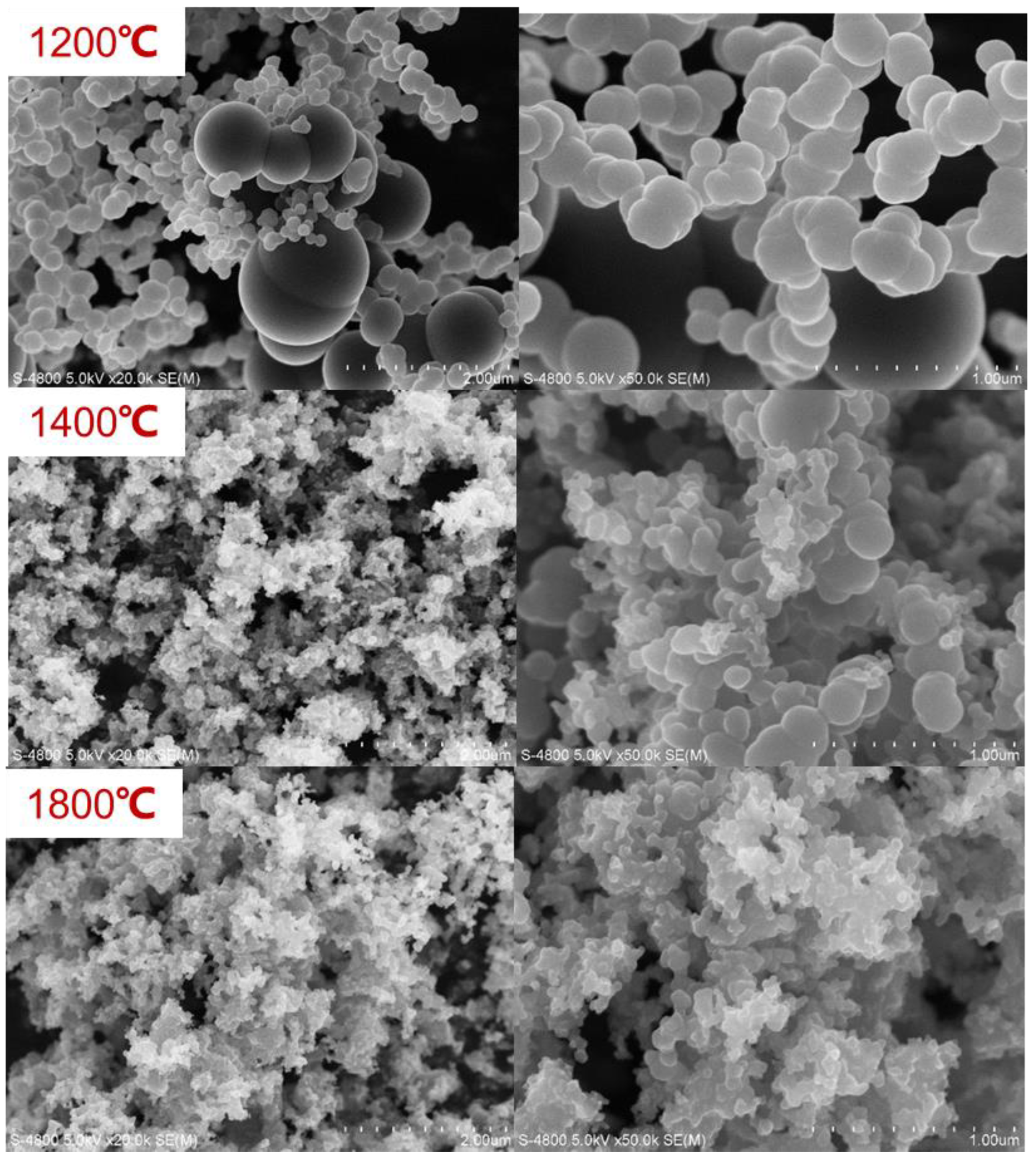
3.5. Characterization of the by-Product Carbon Black
3.6. Electrochemical Tests of the by-Product Carbon Black
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasiliki, D.; Stella, B.; Dimitrios, K. LCO upgrading via distillation and hydroprocessing technology. Energy Fuels 2019, 33, 1023–1028. [Google Scholar]
- Jin, N.; Wang, G.; Yao, L.B.; Hu, M.; Gao, J.S. Synergistic process for FCC light cycle oil efficient conversion to produce high-octane number gasoline. Ind. Eng. Chem. Res. 2016, 55, 5108–5115. [Google Scholar] [CrossRef]
- Georgina, C.L.; Vega Merino, P.M.; Hernández, P.S. Light cycle oil upgrading to high quality fuels and petrochemicals: A review. Ind. Eng. Chem. Res. 2018, 57, 7315–7321. [Google Scholar]
- Holmen, A. Direct conversion of methane to fuels and chemicals. Catal. Today. 2009, 142, 2–8. [Google Scholar] [CrossRef]
- Schobert, H. Production of acetylene and acetylene-based chemicals from coal. Chem. Rev. 2014, 114, 1743–1760. [Google Scholar] [CrossRef]
- Su, B.G.; Fang, J.W.; Wen, G.D.; Ma, J.; Xing, H.B.; Ren, Q.L. Development and process of pyrolysis of light alkanes to acetylene by thermal plasma. Chem. React. Eng. Technol. 2013, 29, 230–237. [Google Scholar]
- Chen, J.Q.; Cheng, Y.; Xiong, X.Y.; Wu, C.N.; Jin, Y. Research progress of coal pyrolysis to acetylene in thermal plasma reactor. Chem. Ind. Eng. 2009, 28, 361–367. [Google Scholar]
- Cheng, Y.; Li, T.Y.; Jin, Y. State-of-the-art development of research and applications of chemical conversion processes at ultra-high temperature in thermal plasma reactors. Chem. Ind. Eng. Prog. 2016, 35, 1676–1686. [Google Scholar]
- Cheng, Y.; Yan, B.H.; Li, T.Y.; Li, X.; Guo, C.Y. Experimental study on coal tar pyrolysis in thermal plasma. Plasma. Chem. Plasma. Process. 2015, 35, 401–413. [Google Scholar] [CrossRef]
- Wu, C.N.; Chen, J.Q.; Cheng, Y. Thermodynamic analysis of coal pyrolysis to acetylene in hydrogen plasma reactor. Fuel Process. Technol. 2010, 91, 823–830. [Google Scholar] [CrossRef]
- Tian, Y.L. The study of methane pyrolysis to acetylene in arc plasma. In Key Laboratory of Coal Science and Technology; Taiyuan University of Technology: Taiyuan, China, 2007. [Google Scholar]
- Heinz, G. How Hüels makes acetylene by DC arc. HydroCarb. Process. Pet. Refin. 1962, 41, 159–164. [Google Scholar]
- Haworth, J.W.; Grant, W.J. Acetylene Form Hydrocarbons. In Introduction to Petroleum Chemicals; Pergamon Press: Oxford, UK, 1961. [Google Scholar]
- Leutner, H.W. Producing acetylene in a plasma jet. Ind. Eng. Chem. 1961, 53, 341–342. [Google Scholar] [CrossRef]
- Anderson, J.E.; Case, L.K. Pyrolysis of methane in DC arc plasma. Ind. Eng. Chem. Process. Des. Dev. 1962, 54, 161–167. [Google Scholar] [CrossRef]
- Holmes, J.M. Evaluation of DuPont Arc Process for Acetylene and Vinyl Chloride Monomer Production; ORNL-TM-2725; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1969. [Google Scholar]
- Gehrmann, K.; Schmidt, H. Pyrolysis of Hydrocarbons Using a Hydrogen Plasma. In Proceedings of the 8th World Petroleum Congress, Moscow, Russia, 13 June 1971. [Google Scholar]
- Vursel, F.; Polak, L. Plasma chemical processing. In Reactions under Plasma Conditions; Venugopalan, M., Ed.; Wiley: New York, NY, USA, 1971. [Google Scholar]
- Plotczyk, W.W. Effect of Quenching Temperature of the Reaction on the Synthesis of Acetylene in Hydrogen Plasma Jet. In Proceedings of the Sixth International Symposium on Plasma Chemistry, Montreal, QC, Canada, 24–28 July1983; pp. 300–305. [Google Scholar]
- Plotczyk, W.W. Thermodynamic Models of Acetylene Synthesis in an Argon Plasma Jet. In Proceedings of the Seventh International Symposium on Plasma Chemistry, Eindhoven, The Netherlands, 1–5 July 1985; pp. 280–285. [Google Scholar]
- Show, D.K. Varner to make carbon black. Eur Rubber J. 1994, 9, 30–31. [Google Scholar]
- Zhang, X.F.; Zeng, D.Q. The plasma method for acetylene production from natural gas. Nat. Gas. Chem. Ind. 1998, 23, 39–43. [Google Scholar]
- Tao, X.M.; Dai, W.; Chen, Q.; Yin, Y.X.; Dai, X.Y. Laboratory test for conversion of natural gas to acetylene plasma jet. Nat. Gas. Ind. 2006, 26, 131–134. [Google Scholar]
- Lucia, O.; Maussion, P.; Dede, E.J. Induction heating technology and its applications: Past developments, current technology, and future challenges. IEEE Trans. Ind. Electron. 2014, 61, 2509–2520. [Google Scholar] [CrossRef]
- Hedayatanasab, Z.; Abnisa, F.; Daud, W.M.A.W. Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater. Des. 2017, 123, 174–196. [Google Scholar] [CrossRef]
- Leclercq, J.; Giraud, F.; Bianchi, D. Novel inductively heated catalytic system for fast VOCs abatement, application to IPA in air. Appl. Catal. B-Environ. 2014, 146, 131–137. [Google Scholar] [CrossRef]
- Ceylan, S.; Friese, C.; Lammel, C. Inductive heating for organic synthesis by using functionalized magnetic nanoparticles inside microreactors. Angew. Chem. Int. Ed. Engl. 2008, 47, 8950–8953. [Google Scholar] [CrossRef]
- Ahmad, Z. Investigating the performance of carbon black/Tin selenide composite as the counter electrode in dye sensitized solar cells. J. Electron. Mater. 2021, 50, 1544–1551. [Google Scholar]
- Butala, S.J.M.; Medina, J.C.; Taylor, T.Q. Mechanisms and kinetics of reactions leading to natural gas formation during coal maturation. Energy Fuels 2000, 14, 235–259. [Google Scholar] [CrossRef]
- Plooster, M.N.; Reed, T.B. Carbon-hydrogen-acetylene equilibriumat high temperatures. Chem. Phys. 1959, 31, 66–72. [Google Scholar]
- Klass, D.L. High Temperature Pyrolysis of Biomass. In Energy from Biomass & Wastes XV; Mracel Dekker Institute of Gas Technology: New York, NY, USA, 1991; pp. 877–894. [Google Scholar]
- Li, T.Y.; Rehmet, C.; Cheng, Y.; Jin, Y.; Cheng, Y. Experimental comparison of methane pyrolysis in thermal plasma. Plasma Chem. Plasma Process. 2017, 37, 1033–1049. [Google Scholar] [CrossRef]
- Yu, H.; Yin, Y.X.; Dai, X.Y. Numerical simulation of methane conversion to acetylene in plasmajet reactor. J. Chem. Ind. Eng. 2006, 57, 2319–2326. [Google Scholar]
- Mo, R.; Rooney, D.; Sun, K.; Yang, H.Y. 3D nitrogen-doped grapheme foam with encapsulated germanium/nitrogen-doped grapheme yolk-shell nano-architecture for high-performance flexible Li-ion battery. Nat. Commun. 2017, 8, 13949. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Z.; Tan, X.; Wang, H.; Holt, C.M.B.; Stephenson, T.; Olsen, B.C.; Mitlin, D. Mesoporous nitrogen-rich carbons derived from protein for ultra-high capacity battery anodes and supercapacitors. Energy Environ. Sci. 2013, 6, 871–878. [Google Scholar] [CrossRef]
- Zhou, J.; Lian, J.; Hou, L.; Zhang, J.; Gou, H.; Xia, M.; Zhao, Y.; Strobel, T.A.; Tao, L.; Gao, F. Ultrahigh volumetric capacitance and cyclic stability of fluorine and nitrogen co-doped carbon microspheres. Nat. Commun. 2015, 6, 8503. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, F.; Zhang, L.; Lu, Y.; Zhang, Y.; Yang, X.; Ma, Y.; Huang, Y. High energy density Li-ion capacitor assembled with all graphene-based electrodes. Carbon 2015, 92, 106–118. [Google Scholar] [CrossRef]






| Feedstocks | Yanshan LCO | Shijiazhuang HLCO | Yangzhou HCO | Methods |
|---|---|---|---|---|
| ρ20, (kg·m−3) | 948.6 | 888.7 | 894.7 | ISO12185 |
| w(C), % | 89.07 | 89.08 | 87.09 | ASTM D5291-0656 |
| w(H), % | 9.17 | 10.92 | 11.85 | ASTM D5291-0656 |
| w(S), % | 0.54 | 109 (1) | 0.34 | ASTM D5453-0689 |
| w(N), % | 0.036 | 0.90 (1) | 0.27 | ASTM D4629 |
| Composition of hydrocarbons, w% | ASTM D2425 | |||
| Chain alkane hydrocarbons | 13.3 | 12.5 | 53.5 | |
| Cycloalkanes | 4.1 | 19.6 | 8.3 | |
| Aromatics | 82.6 | 67.9 | 38.2 | |
| Monocyclic aromatics | 28.1 | 56.4 | 3.4 | |
| Indane/tetrahydronaphthalene | 10.1 | 35.4 | 1.8 | |
| Bicyclic aromatics | 54.4 | 10.5 | 8.0 | |
| Tricyclic aromatic hydrocarbons | 0.1 | 1.0 | 26.8 | |
| Temperature/°C | Main Gas Products | Toluene | Naphthalene | Tetrahydronaphthalene |
|---|---|---|---|---|
| Quality Yields/% | ||||
| 800 | CH4 | 0.03 | 0.05 | 4.15 |
| H2 | 0.00 | 0.00 | 2.09 | |
| C2H4 | 0.00 | 0.03 | 3.48 | |
| C2H2 | 0.00 | 0.00 | 0.03 | |
| C2H6 | 0.20 | 0.18 | 0.16 | |
| 1100 | CH4 | 2.71 | 1.45 | 6.84 |
| H2 | 1.50 | 2.19 | 5.20 | |
| C2H4 | 0.18 | 0.09 | 6.66 | |
| C2H2 | 0.05 | 0.04 | 1.70 | |
| C2H6 | 3.89 | 4.34 | 3.87 | |
| 1400 | CH4 | 2.44 | 0.59 | 1.59 |
| H2 | 5.44 | 5.97 | 7.86 | |
| C2H4 | 0.27 | 0.19 | 2.84 | |
| C2H2 | 5.70 | 4.62 | 5.91 | |
| C2H6 | 2.51 | 3.03 | 1.11 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Yuan, Q.; Tang, J.; Zhang, X.; Huang, S.; Gong, J. Study on High Temperature Pyrolysis Light Cycle Oil to Acetylene and Carbon Black. Processes 2022, 10, 1732. https://doi.org/10.3390/pr10091732
Li Z, Yuan Q, Tang J, Zhang X, Huang S, Gong J. Study on High Temperature Pyrolysis Light Cycle Oil to Acetylene and Carbon Black. Processes. 2022; 10(9):1732. https://doi.org/10.3390/pr10091732
Chicago/Turabian StyleLi, Zekun, Qimin Yuan, Jinlian Tang, Xiaoqiao Zhang, Shaobin Huang, and Jianhong Gong. 2022. "Study on High Temperature Pyrolysis Light Cycle Oil to Acetylene and Carbon Black" Processes 10, no. 9: 1732. https://doi.org/10.3390/pr10091732
APA StyleLi, Z., Yuan, Q., Tang, J., Zhang, X., Huang, S., & Gong, J. (2022). Study on High Temperature Pyrolysis Light Cycle Oil to Acetylene and Carbon Black. Processes, 10(9), 1732. https://doi.org/10.3390/pr10091732





