Catalytic Kinetics and Mechanisms of KCl with Different Concentrations on Gasification of Coal Char
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. TGA Experiments
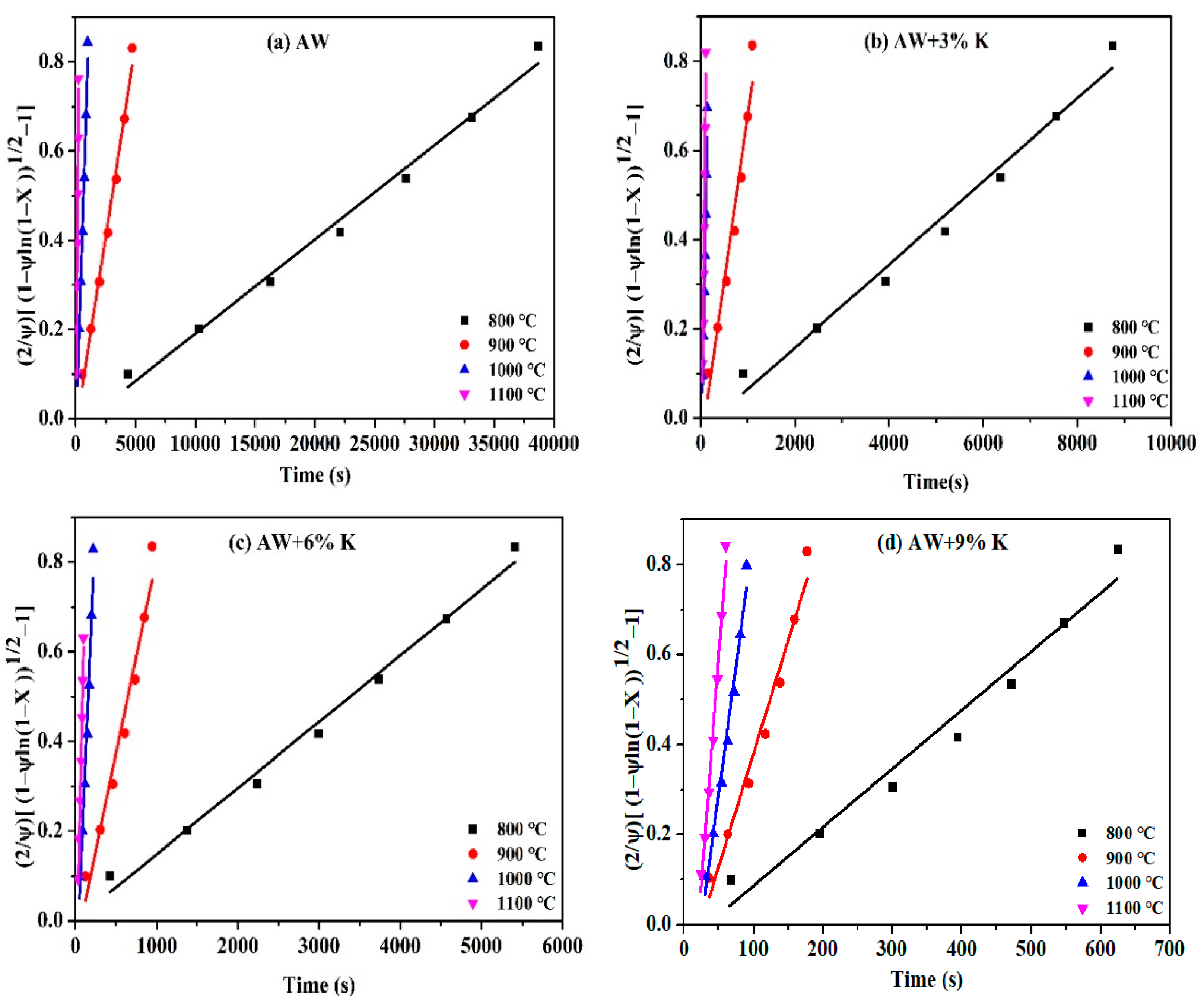
2.3. Random Pore Model (RPM)
3. Results and Discussion
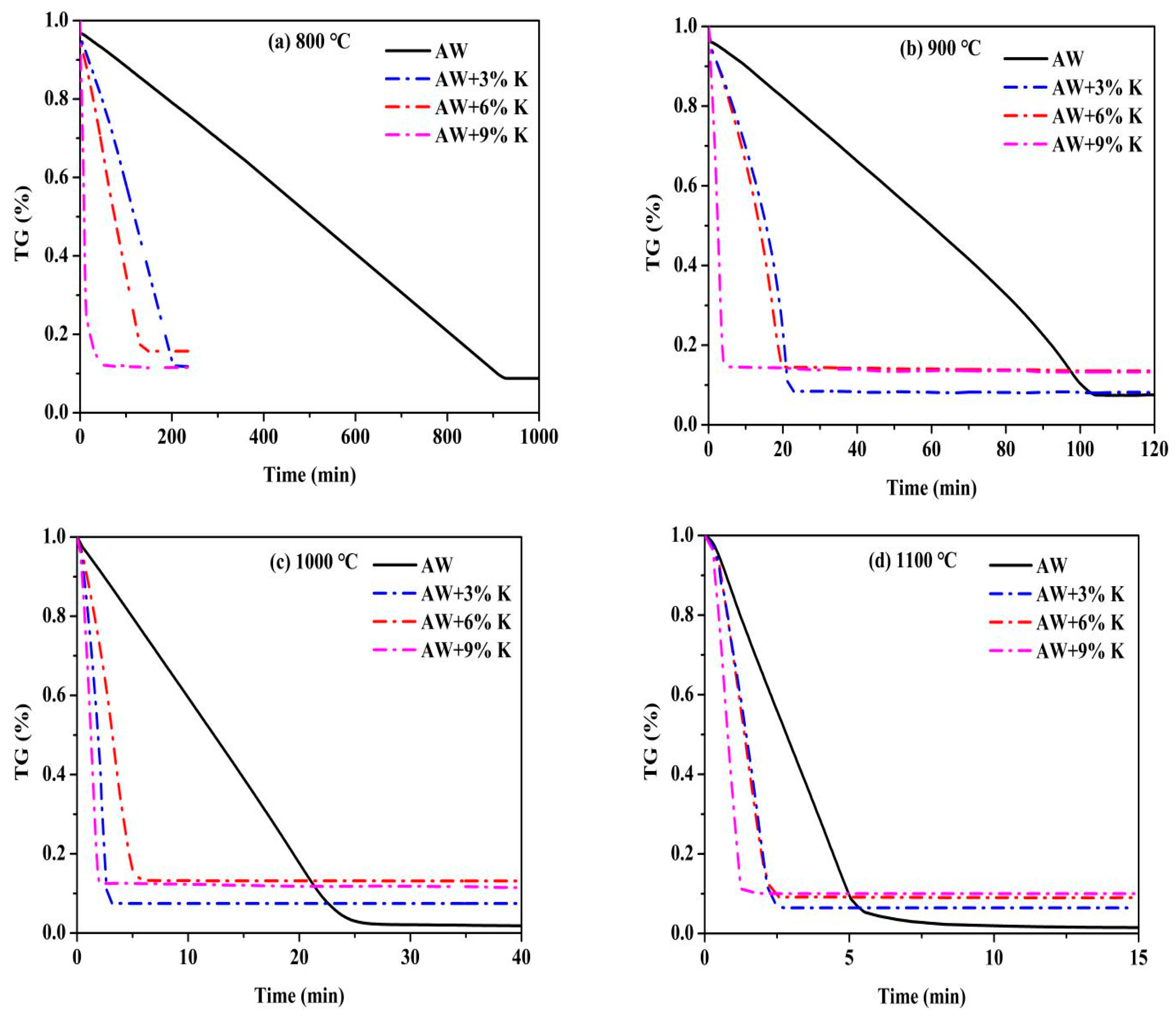
3.1. TGA (Temperature Programmed)
3.2. Gasification Characteristics
3.3. Kinetic Analysis
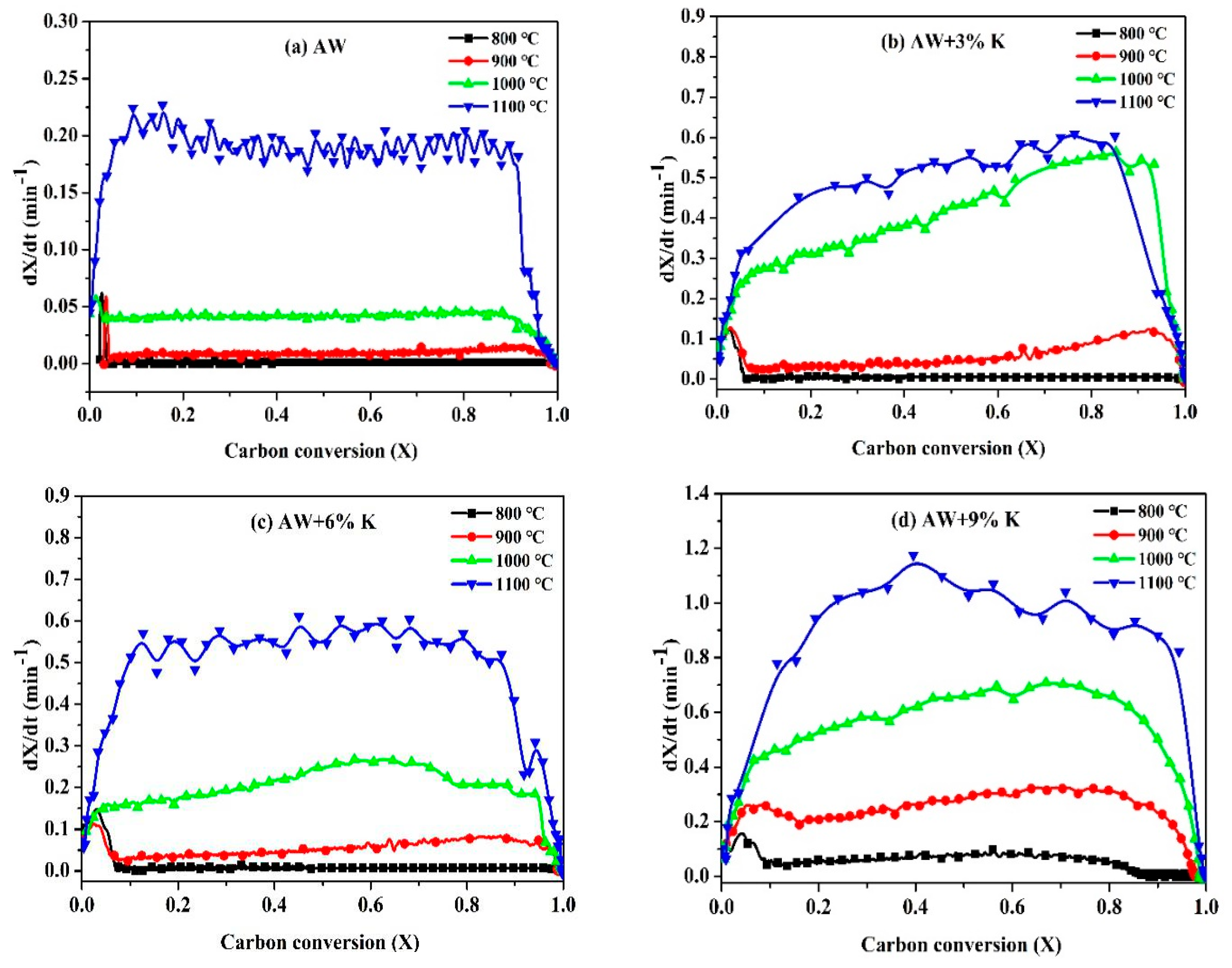
3.3.1. Reaction Rate
3.3.2. Kinetic Parameters
3.4. Mechanism of Catalytic Gasification
4. Conclusions
- Different catalyst concentration has different influence on the gasification of coal char. By increasing the concentration of KCl, the catalytic effect can be enhanced by shortening the half reaction time and increasing the gasification rate.
- The activation energy of AW-char is the highest, which can be reduced to some extent by adding KCl. The chemical bond between the metal cation and the carbon atom, as well as the gasifier in the coal char, is formed and broken continuously, so as to reduce the energy level required for the gasification reaction and achieve the effect of reducing the activation energy.
- The unified mechanism of catalytic gasification of alkali and alkaline earth metals is applicable for the KCl catalysis on coal char gasification.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| RAW | Raw coal |
| AW | Acid-washed coal |
| AW-char | Char from acid-washed coal |
| k | Apparent gasification reaction rate |
| E | Activation energy |
| T | Temperature |
| A | Pre-exponential factor |
| R | Universal gas constant |
| ad | Air dry basis |
| d | Dry basis |
| daf | Dry ash free basis |
| RPM | Random pore model |
| TGA | Thermal gravimetric analysis |
| XRD | X-ray diffraction |
References
- Tomaszewicz, M.; Łabojko, G.; Tomaszewicz, G.; Kotyczka-Morańska, M. The kinetics of CO2 gasification of coal chars. J. Anal. Calorim. 2013, 113, 1327–1335. [Google Scholar] [CrossRef]
- Chmielniak, T.; Sciazko, M.; Tomaszewicz, G.; Tomaszewicz, M. Pressurized CO2-enhanced gasification of coal. J. Anal. Calorim. 2014, 117, 1479–1488. [Google Scholar] [CrossRef]
- Zhao, H.; Cao, Y.; Orndorff, W.; Pan, W.-P. Gasification characteristics of coal char under CO2 atmosphere. J. Anal. Calorim. 2014, 116, 1267–1272. [Google Scholar] [CrossRef]
- Du, C.; Liu, L.; Qiu, P. Variation of Char Reactivity during Catalytic Gasification with Steam: Comparison among Catalytic Gasification by Ion-Exchangeable Na, Ca, and Na/Ca Mixture. Energy Fuel 2018, 32, 142–153. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, Y.; Zhang, K. Toward understanding the reactivity and catalytic mechanism of coal pyrolysis with metal chloride modification. J. Anal. Appl. Pyrol. 2019, 138, 196–202. [Google Scholar] [CrossRef]
- Kabir, K.; Tahmasebi, A.; Bhattacharya, S.; Yu, J. Intrinsic kinetics of CO2 gasification of a Victorian coal char. J. Anal. Calorim. 2016, 123, 1685–1694. [Google Scholar] [CrossRef]
- Mei, Y.; Wang, Z.; Fang, H.; Wang, Y.; Huang, J.; Fang, Y. Na-Containing Mineral Transformation Behaviors during Na2CO3-Catalyzed CO2 Gasification of High-Alumina Coal. Energy Fuel 2017, 31, 1235–1242. [Google Scholar] [CrossRef]
- Zhang, F.; Fan, M.; Huang, X.; Argyle, M.D.; Zhang, B.; Towler, B.; Zhang, Y. Catalytic gasification of a Powder River Basin coal with CO2 and H2O mixtures. Fuel Process. Technol. 2017, 161, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.J.; Hu, Q.J.; Liu, X.; Yu, G.S.; Wang, F.C. Effect of Iron Species and Calcium Hydroxide on High-Sulfur Petroleum Coke CO2 Gasification. Energy Fuel 2012, 26, 1489–1495. [Google Scholar] [CrossRef]
- Encinar, J.M. Catalysed and uncatalysed steam gasification of eucalyptus char: Influence of variables and kinetic study. Fuel Energy Abstr. 2002, 43, 267. [Google Scholar] [CrossRef]
- Liu, L.; Kumar, S.; Wang, Z.; He, Y.; Liu, J.; Cen, K. Catalytic effect of metal chlorides on coal pyrolysis and gasification part I. Combined TG-FTIR study for coal pyrolysis. Acta 2017, 655, 331–336. [Google Scholar] [CrossRef]
- Veraa, M.J.; Bell, A.T. Effect of alkali metal catalysts on gasification of coal char. Fuel 1978, 57, 194–200. [Google Scholar] [CrossRef]
- McKee, D.W. Mechanisms of the alkali metal catalysed gasification of carbon. Fuel 1983, 62, 170–175. [Google Scholar] [CrossRef]
- Jalan, B.; Rao, Y. A study of the rates of catalyzed Boudouard reaction. Carbon 1978, 16, 175–184. [Google Scholar] [CrossRef]
- Chen, S.G.; Yang, R.T. Unified mechanism of alkali and alkaline earth metals catalyzed gasification reactions of carbon by CO2 and H2O. Energy Fuel 1997, 11, 421–427. [Google Scholar] [CrossRef]


| Coal Sample | Proximate Analysis (wt.%, ad) | Ultimate Analysis (wt.%, daf) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vd | Vdaf | C | Hdaf | Hd | N | O a | S | |
| RAW | 29.14 | 13.00 | 41.92 | 48.19 | 71.32 | 4.13 | 3.59 | 1.36 | 22.4 | 0.79 |
| AW | 10.82 | 0.52 | 45.47 | 45.71 | 70.29 | 5.37 | 5.34 | 1.46 | 22.37 | 0.51 |
| Sample | Temperature (°C) | Half Reaction Time (τ0.5, min) | Reactivity Index (RI) | Gasification Rate (min−1) |
|---|---|---|---|---|
| AW | 800 | 460.30 | 0.0011 | 0.0011 |
| 900 | 56.20 | 0.0089 | 0.0087 | |
| 1000 | 12.20 | 0.0410 | 0.0415 | |
| 1100 | 2.85 | 0.1754 | 0.1845 | |
| AW + 3%K | 800 | 106.15 | 0.0047 | 0.0051 |
| 900 | 14.55 | 0.0344 | 0.0445 | |
| 1000 | 1.85 | 0.2703 | 0.4212 | |
| 1100 | 1.45 | 0.3448 | 0.5265 | |
| AW + 6%K | 800 | 62.30 | 0.0080 | 0.0071 |
| 900 | 12.30 | 0.0407 | 0.0490 | |
| 1000 | 2.95 | 0.1695 | 0.2476 | |
| 1100 | 1.35 | 0.3704 | 0.5469 | |
| AW + 9%K | 800 | 7.85 | 0.0637 | 0.0761 |
| 900 | 2.30 | 0.2174 | 0.2833 | |
| 1000 | 1.20 | 0.4167 | 0.6585 | |
| 1100 | 0.80 | 0.6250 | 1.0259 |
| Sample | T (°C) | RPM | ||
|---|---|---|---|---|
| k (s−1) | R2 | Ψ | ||
| AW | 800 | 0.00002111 | 0.9885 | 2.1117 |
| 900 | 0.0001739 | 0.9888 | 2.1555 | |
| 1000 | 0.0008424 | 0.9905 | 2.0497 | |
| 1100 | 0.00348 | 0.9962 | 3.0219 | |
| AW + 3% K | 800 | 0.00009308 | 0.9787 | 2.1155 |
| 900 | 0.0007373 | 0.9529 | 2.1145 | |
| 1000 | 0000596 | 0.9616 | 4.6752 | |
| 1100 | 0.0096 | 0.9805 | 2.4181 | |
| AW + 6% K | 800 | 0.0001476 | 0.9888 | 2.1406 |
| 900 | 0.0008754 | 0.9592 | 2.1109 | |
| 1000 | 0.00418 | 0.9669 | 2.1479 | |
| 1100 | 0.008 | 0.9950 | 6.1389 | |
| AW + 9% K | 800 | 0.0013 | 0.9610 | 2.1697 |
| 900 | 0.00502 | 0.9711 | 2.2405 | |
| 1000 | 0.01134 | 0.9782 | 2.4483 | |
| 1100 | 0.02037 | 0.9869 | 2.2305 | |
| Sample | RPM | ||
|---|---|---|---|
| E (kJ/mol) | A (s−1) | R2 | |
| AW | 207.53 | 2.79 × 105 | 0.9990 |
| AW + 3% K | 198.31 | 4.89 × 105 | 0.9527 |
| AW + 6% K | 167.46 | 2.35 × 104 | 0.9777 |
| AW + 9% K | 112.22 | 4.24 × 102 | 0.9796 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Q.; Pan, D.; Zhang, W.; Zeng, F.; Liu, L. Catalytic Kinetics and Mechanisms of KCl with Different Concentrations on Gasification of Coal Char. Processes 2022, 10, 1357. https://doi.org/10.3390/pr10071357
Qiu Q, Pan D, Zhang W, Zeng F, Liu L. Catalytic Kinetics and Mechanisms of KCl with Different Concentrations on Gasification of Coal Char. Processes. 2022; 10(7):1357. https://doi.org/10.3390/pr10071357
Chicago/Turabian StyleQiu, Qili, Danping Pan, Wendi Zhang, Fan Zeng, and Longlong Liu. 2022. "Catalytic Kinetics and Mechanisms of KCl with Different Concentrations on Gasification of Coal Char" Processes 10, no. 7: 1357. https://doi.org/10.3390/pr10071357
APA StyleQiu, Q., Pan, D., Zhang, W., Zeng, F., & Liu, L. (2022). Catalytic Kinetics and Mechanisms of KCl with Different Concentrations on Gasification of Coal Char. Processes, 10(7), 1357. https://doi.org/10.3390/pr10071357






