Silica Particles Derived from Natural Kaolinite for the Removal of Rhodamine B from Polluted Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Silica Particles
2.2.2. XRPD Analysis
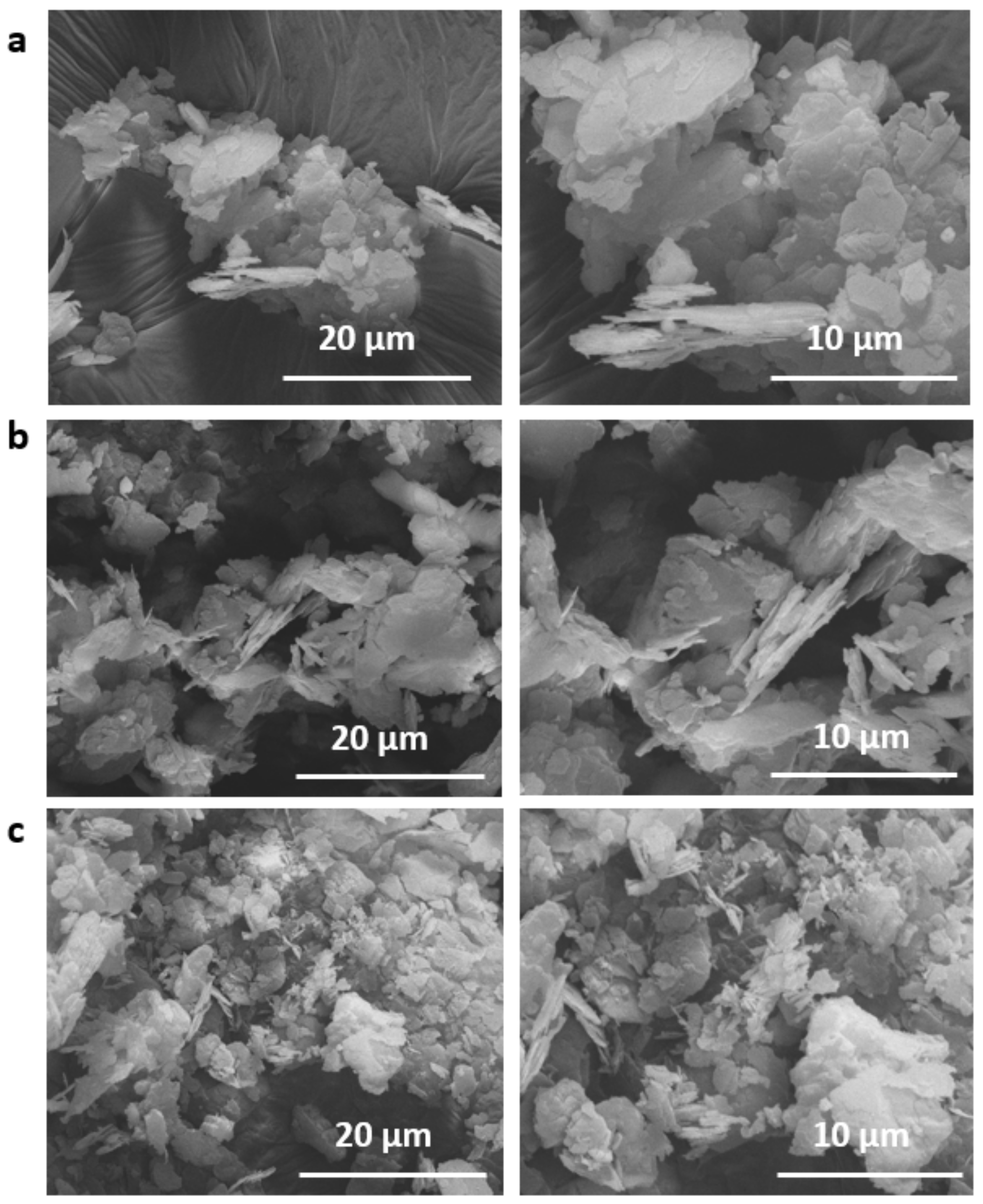
2.2.3. SEM-EDX
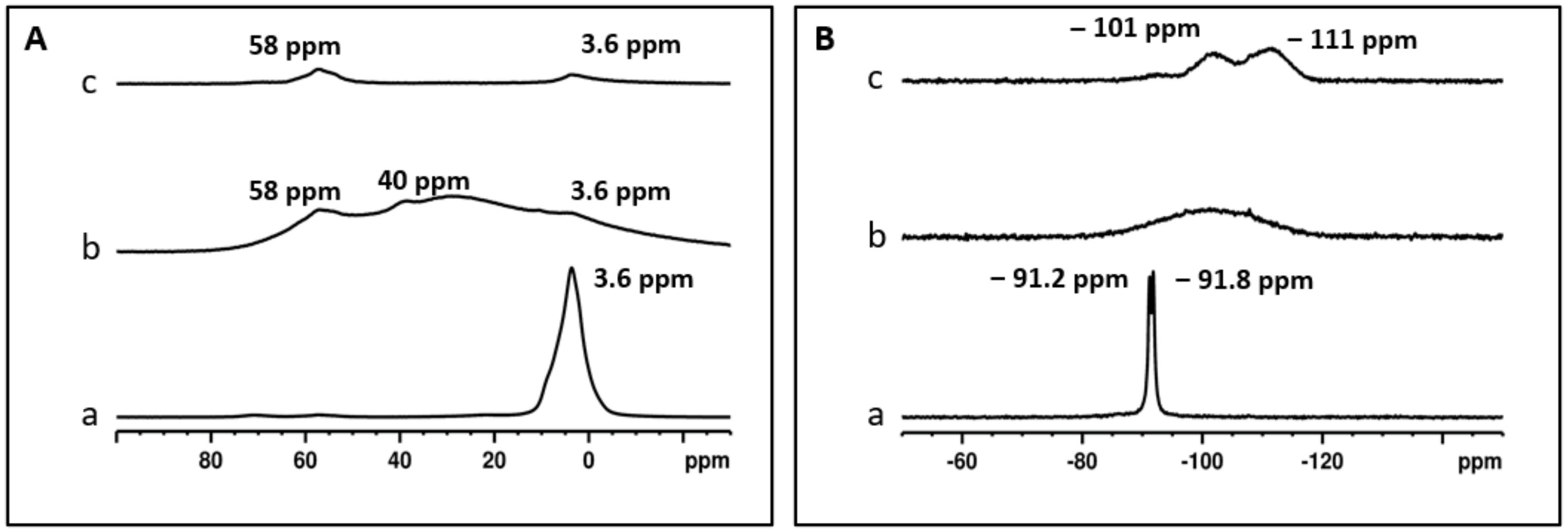
2.2.4. Solid-State NMR
2.2.5. FT-IR Spectroscopy
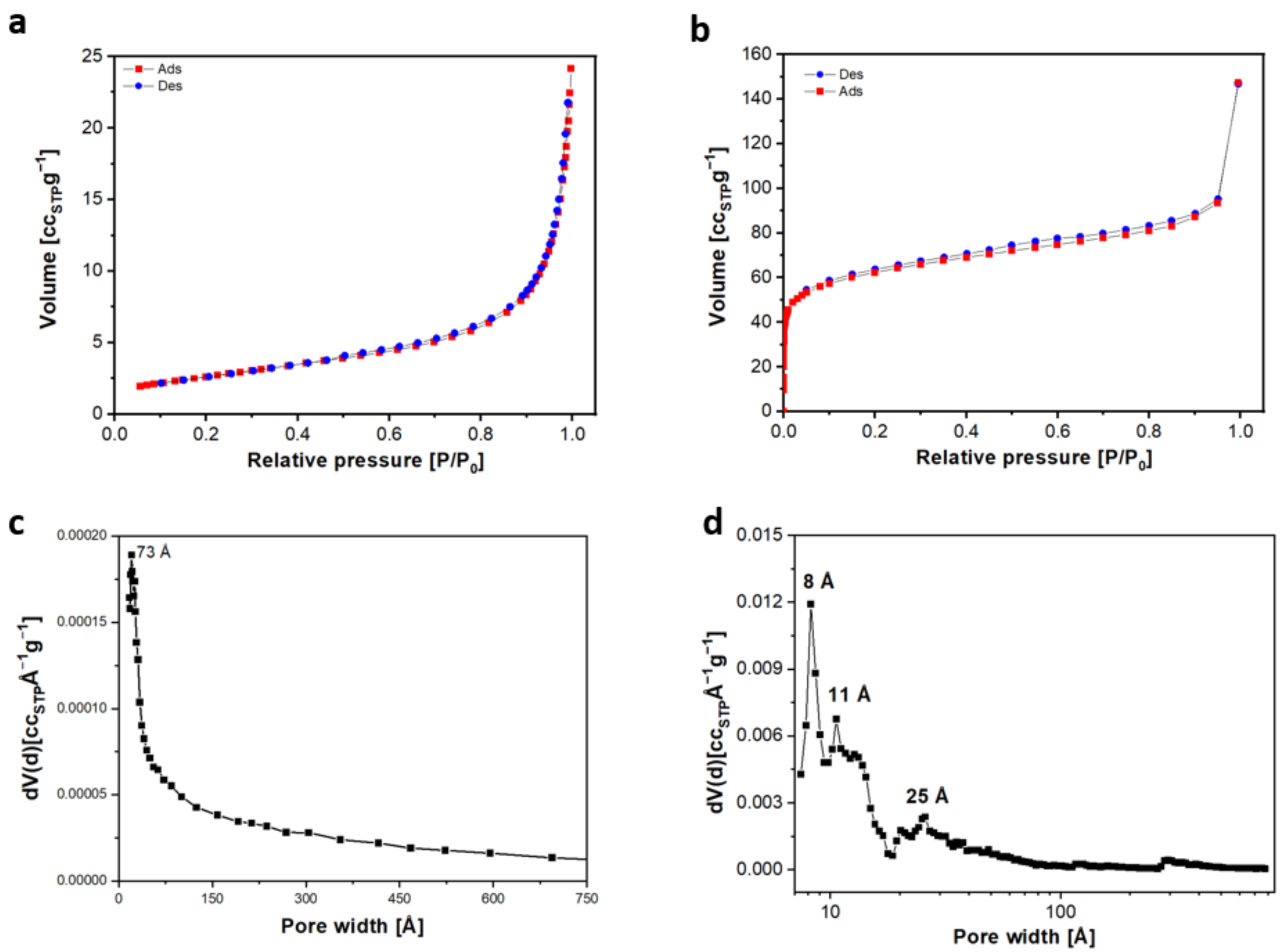
2.2.6. Nitrogen Physisorption
2.2.7. Z Potential
2.2.8. DLS
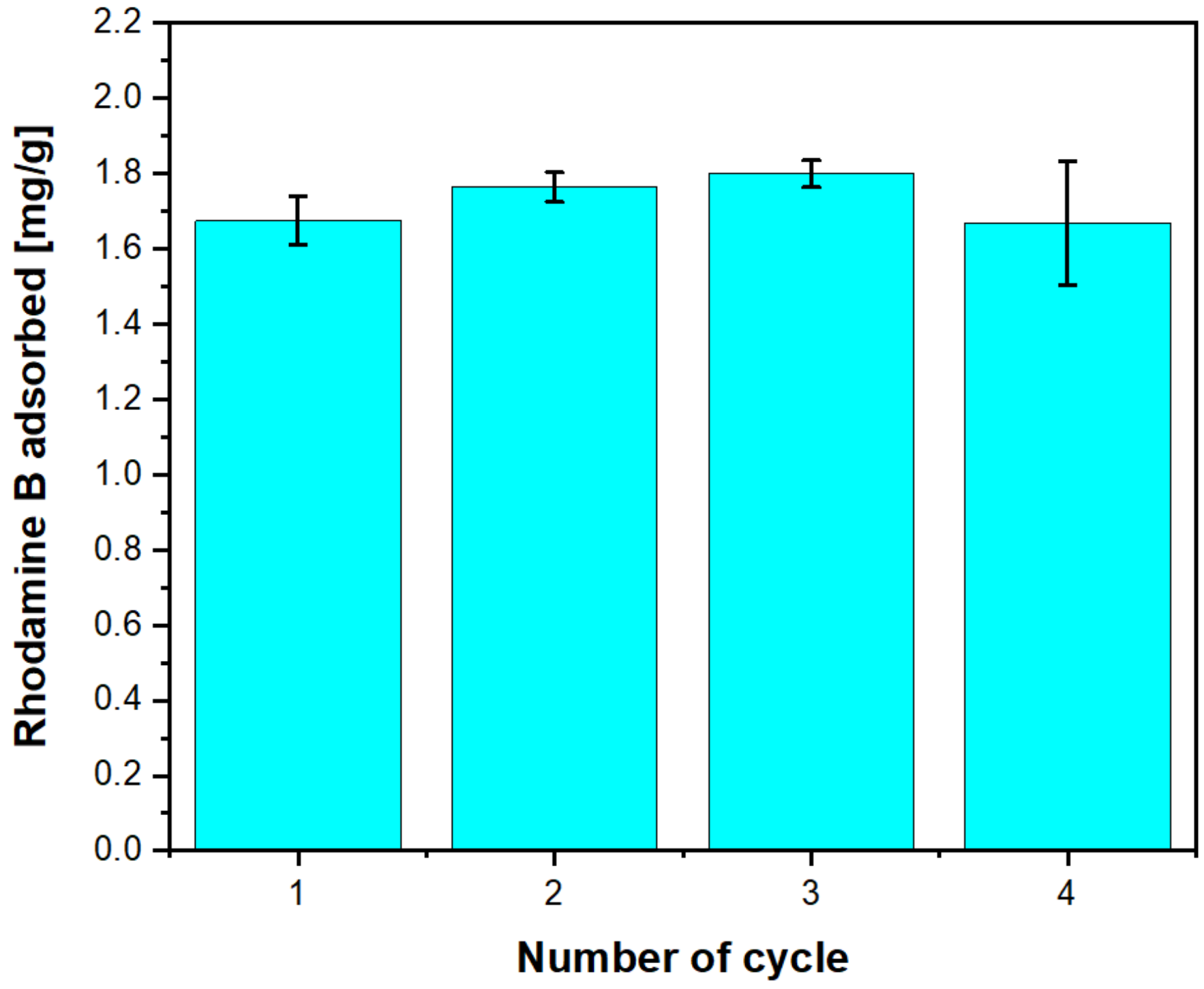
2.2.9. Rhodamine B Adsorption
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samanta, P.; Desai, A.V.; Let, S.; Ghosh, S.K. Advanced Porous Materials for Sensing, Capture and Detoxification of Organic Pollutants toward Water Remediation. ACS Sustain. Chem. Eng. 2019, 7, 7456–7478. [Google Scholar] [CrossRef]
- Boccaleri, E.; Marzetti, C.; Celoria, G.; Cassino, C.; Paul, G.; Miletto, I.; Gianotti, E. Adsorption Features of Various Inorganic Materials for the Drug Removal from Water and Synthetic Urine Medium: A Multi-Technique Time-Resolved In Situ Investigation. Materials 2021, 14, 6196. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro dos Santos, F.; de Oliveira Bruno, H.C.; Zelayaran Melgar, L. Use of Bentonite Calcined Clay as an Adsorbent: Equilibrium and Thermodynamic Study of Rhodamine B Adsorption in Aqueous Solution. Environ. Sci. Pollut. Res. 2019, 26, 28622–28632. [Google Scholar] [CrossRef] [PubMed]
- Gogate, P.R.; Pandit, A.B. A Review of Imperative Technologies for Wastewater Treatment II: Hybrid Methods. Adv. Environ. Res. 2004, 8, 553–597. [Google Scholar] [CrossRef]
- Ateia, M.; Alalm, M.G.; Awfa, D.; Johnson, M.S.; Yoshimura, C. Modeling the Degradation and Disinfection of Water Pollutants by Photocatalysts and Composites: A Critical Review. Sci. Total Environ. 2020, 698, 134197. [Google Scholar] [CrossRef]
- Al-Kahtani, A.A. Photocatalytic Degradation of Rhodamine B Dye in Wastewater Using Gelatin/CuS/PVA Nanocomposites under Solar Light Irradiation. JBNB 2017, 8, 66–82. [Google Scholar] [CrossRef] [Green Version]
- Miglio, V.; Zaccone, C.; Vittoni, C.; Braschi, I.; Buscaroli, E.; Golemme, G.; Marchese, L.; Bisio, C. Silica Monolith for the Removal of Pollutants from Gas and Aqueous Phases. Molecules 2021, 26, 1316. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; SenGupta, S.; Sarma, G.K. Interactions of the Dye, Rhodamine B with Kaolinite and Montmorillonite in Water. Appl. Clay Sci. 2014, 99, 7–17. [Google Scholar] [CrossRef]
- Al-Gheethi, A.A.; Azhar, Q.M.; Senthil Kumar, P.; Yusuf, A.A.; Al-Buriahi, A.K.; Radin Mohamed, R.M.S.; Al-shaibani, M.M. Sustainable Approaches for Removing Rhodamine B Dye Using Agricultural Waste Adsorbents: A Review. Chemosphere 2022, 287, 132080. [Google Scholar] [CrossRef]
- Gao, W.; Zhao, S.; Wu, H.; Deligeer, W.; Asuha, S. Direct Acid Activation of Kaolinite and Its Effects on the Adsorption of Methylene Blue. Appl. Clay Sci. 2016, 126, 98–106. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [Green Version]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Von Gunten, U.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Adeyemo, A.A.; Adeoye, I.O.; Bello, O.S. Adsorption of Dyes Using Different Types of Clay: A Review. Appl. Water Sci. 2017, 7, 543–568. [Google Scholar] [CrossRef] [Green Version]
- Anh Tran, V.; Vu, K.B.; Thi Vo, T.-T.; Thuan Le, V.; Do, H.H.; Bach, L.G.; Lee, S.-W. Experimental and Computational Investigation on Interaction Mechanism of Rhodamine B Adsorption and Photodegradation by Zeolite Imidazole Frameworks-8. Appl. Surf. Sci. 2021, 538, 148065. [Google Scholar] [CrossRef]
- Corda, N.C.; Kini, M.S. A Review on Adsorption of Cationic Dyes Using Activated Carbon. MATEC Web Conf. 2018, 144, 02022. [Google Scholar] [CrossRef]
- Mezohegyi, G.; Van der Zee, F.P.; Font, J.; Fortuny, A.; Fabregat, A. Towards Advanced Aqueous Dye Removal Processes: A Short Review on the Versatile Role of Activated Carbon. J. Environ. Manag. 2012, 102, 148–164. [Google Scholar] [CrossRef]
- Carniato, F.; Gatti, G.; Bisio, C. An Overview of the Recent Synthesis and Functionalization Methods of Saponite Clay. New J. Chem. 2020, 44, 9969–9980. [Google Scholar] [CrossRef]
- Gurses, A.; Dogar, C.; Yalcin, M.; Acikyildiz, M.; Bayrak, R.; Karaca, S. The Adsorption Kinetics of the Cationic Dye, Methylene Blue, onto Clay. J. Hazard. Mater. 2006, 131, 217–228. [Google Scholar] [CrossRef]
- Najafi, H.; Asasian-Kolur, N.; Sharifian, S. Adsorption of Chromium(VI) and Crystal Violet onto Granular Biopolymer-Silica Pillared Clay Composites from Aqueous Solutions. J. Mol. Liq. 2021, 344, 117822. [Google Scholar] [CrossRef]
- Farajfaed, S.; Sharifian, S.; Asasian-Kolur, N.; Sillanpää, M. Granular Silica Pillared Clay for Levofloxacin and Gemifloxacin Adsorption from Aqueous Systems. J. Environ. Chem. Eng. 2021, 9, 106306. [Google Scholar] [CrossRef]
- Awad, A.M.; Shaikh, S.M.R.; Jalab, R.; Gulied, M.H.; Nasser, M.S.; Benamor, A.; Adham, S. Adsorption of Organic Pollutants by Natural and Modified Clays: A Comprehensive Review. Sep. Purif. Technol. 2019, 228, 115719. [Google Scholar] [CrossRef]
- Belver, C.; Bañares Muñoz, M.A.; Vicente, M.A. Chemical Activation of a Kaolinite under Acid and Alkaline Conditions. Chem. Mater. 2002, 14, 2033–2043. [Google Scholar] [CrossRef]
- Sarma, G.K.; Sen Gupta, S.; Bhattacharyya, K.G. Removal of Hazardous Basic Dyes from Aqueous Solution by Adsorption onto Kaolinite and Acid-Treated Kaolinite: Kinetics, Isotherm and Mechanistic Study. SN Appl. Sci. 2019, 1, 211. [Google Scholar] [CrossRef] [Green Version]
- Daou, I.; Lecomte-Nana, G.; Tessier-Doyen, N.; Peyratout, C.; Gonon, M.; Guinebretiere, R. Probing the Dehydroxylation of Kaolinite and Halloysite by In Situ High Temperature X-Ray Diffraction. Minerals 2020, 10, 480. [Google Scholar] [CrossRef]
- Salahudeen, N.; Ahmed, A.S.; Al-Muhtaseb, A.H.; Dauda, M.; Waziri, S.M.; Jibril, B.Y. Synthesis and Characterization of Micro-Sized Silica from Kankara Kaolin. J. Eng. Res. 2014, 19, 27–32. [Google Scholar]
- Temuujin, J.; Burmaa, G.; Amgalan, J.; Okada, K. Preparation of Porous Silica from Mechanically Activated Kaolinite. J. Porous Mater. 2001, 8, 233–238. [Google Scholar] [CrossRef]
- Pinheiro, D.d.R.; Gonçalves, L.R.; De Sena, R.L.P.; Martelli, M.C.; Neves, R.d.F.; Ribeiro, N.F.d.P. Industrial Kaolin Waste as Raw Material in the Synthesis of the SAPO-34 Molecular Sieve. Mat. Res. 2020, 23, e20200043. [Google Scholar] [CrossRef]
- Tiozzo, C.; Bisio, C.; Carniato, F.; Gallo, A.; Scott, S.L.; Psaro, R.; Guidotti, M. Niobium–Silica Catalysts for the Selective Epoxidation of Cyclic Alkenes: The Generation of the Active Site by Grafting Niobocene Dichloride. Phys. Chem. Chem. Phys. 2013, 15, 13354. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Subhani, T.; Husain, S.W. Synthesis and Characterization of Silica Nanoparticles from Clay. J. Asian Ceram. Soc. 2016, 4, 91–96. [Google Scholar] [CrossRef]
- Marsh, A.; Heath, A.; Patureau, P.; Evernden, M.; Walker, P. A Mild Conditions Synthesis Route to Produce Hydrosodalite from Kaolinite, Compatible with Extrusion Processing. Microporous Mesoporous Mater. 2018, 264, 125–132. [Google Scholar] [CrossRef]
- Glid, M.; Sobrados, I.; Rhaiem, H.B.; Sanz, J.; Amara, A.B.H. Alkaline Activation of Metakaolinite-Silica Mixtures: Role of Dissolved Silica Concentration on the Formation of Geopolymers. Ceram. Int. 2017, 43, 12641–12650. [Google Scholar] [CrossRef]
- Hayashi, S.; Ueda, T.; Hayamizu, K.; Akiba, E. NMR Study of Kaolinite. 1. Silicon-29, Aluminum-27, and Proton Spectra. J. Phys. Chem. 1992, 96, 10922–10928. [Google Scholar] [CrossRef]
- Santos, E.; Costa, L.; Oliveira, E.; Bessa, R.; Freitas, A.; Oliveira, C.; Nascimento, R.; Loiola, A. Al-MCM-41 Synthesized from Kaolin via Hydrothermal Route: Structural Characterization and Use as an Efficient Adsorbent of Methylene Blue. J. Braz. Chem. Soc. 2018, 29, 2378–2386. [Google Scholar] [CrossRef]
- Paul, G.; Bisio, C.; Braschi, I.; Cossi, M.; Gatti, G.; Gianotti, E.; Marchese, L. Combined Solid-State NMR, FT-IR and Computational Studies on Layered and Porous Materials. Chem. Soc. Rev. 2018, 47, 5684–5739. [Google Scholar] [CrossRef] [Green Version]
- Protsak, I.S.; Morozov, Y.M.; Dong, W.; Le, Z.; Zhang, D.; Henderson, I.M. A 29Si, 1H, and 13C Solid-State NMR Study on the Surface Species of Various Depolymerized Organosiloxanes at Silica Surface. Nanoscale Res. Lett. 2019, 14, 160. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Yan, Z.; Ouyang, J.; Zhang, Y.; Yang, H.; Chen, D. Chemically Modified Kaolinite Nanolayers for the Removal of Organic Pollutants. Appl. Clay Sci. 2018, 157, 283–290. [Google Scholar] [CrossRef]
- Worasith, N.; Goodman, B.A.; Neampan, J.; Jeyachoke, N.; Thiravetyan, P. Characterization of Modified Kaolin from the Ranong Deposit Thailand by XRD, XRF, SEM, FTIR and EPR Techniques. Clay Miner. 2011, 46, 539–559. [Google Scholar] [CrossRef]
- Khan, A.S.; Khalid, H.; Sarfraz, Z.; Khan, M.; Iqbal, J.; Muhammad, N.; Fareed, M.A.; Rehman, I.U. Vibrational Spectroscopy of Selective Dental Restorative Materials. Appl. Spectrosc. Rev. 2017, 52, 507–540. [Google Scholar] [CrossRef]
- Darmakkolla, S.R.; Tran, H.; Gupta, A.; Rananavare, S.B. A Method to Derivatize Surface Silanol Groups to Si-Alkyl Groups in Carbon-Doped Silicon Oxides. RSC Adv. 2016, 6, 93219–93230. [Google Scholar] [CrossRef]
- Vittoni, C.; Gatti, G.; Paul, G.; Mangano, E.; Brandani, S.; Bisio, C.; Marchese, L. Non-Porous versus Mesoporous Siliceous Materials for CO 2 Capture. ChemistryOpen 2019, 8, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Cychosz, K.A.; Guillet-Nicolas, R.; García-Martínez, J.; Thommes, M. Recent Advances in the Textural Characterization of Hierarchically Structured Nanoporous Materials. Chem. Soc. Rev. 2017, 46, 389–414. [Google Scholar] [CrossRef]
- Jabłońska, B.; Busch, M.; Kityk, A.V.; Huber, P. Natural and Chemically Modified Post-Mining Clays—Structural and Surface Properties and Preliminary Tests on Copper Sorption. Minerals 2019, 9, 704. [Google Scholar] [CrossRef] [Green Version]
- Rao, W.; Piliouras, P.; Wang, X.; Guido, A.; Kugler, K.; Sieren, B.; Wang, L.; Lv, G.; Li, Z. Zwitterionic Dye Rhodamine B (RhB) Uptake on Different Types of Clay Minerals. Appl. Clay Sci. 2020, 197, 105790. [Google Scholar] [CrossRef]
- Čapková, P.; Malý, P.; Pospíšil, M.; Klika, Z.; Weissmannová, H.; Weiss, Z. Effect of Surface and Interlayer Structure on the Fluorescence of Rhodamine B–Montmorillonite: Modeling and Experiment. J. Colloid Interface Sci. 2004, 277, 128–137. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Adsorption of a Few Heavy Metals on Natural and Modified Kaolinite and Montmorillonite: A Review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef]
- Rasalingam, S.; Peng, R.; Koodali, R.T. An Insight into the Adsorption and Photocatalytic Degradation of Rhodamine B in Periodic Mesoporous Materials. Appl. Catal. B Environ. 2015, 174–175, 49–59. [Google Scholar] [CrossRef]



| Kaolinite | Silica Particles | |||
|---|---|---|---|---|
| Element | At % | SDev | At % | SDev |
| O | 63.407 | 1.594 | 67.737 | 0.931 |
| Na | 0.207 | 0.158 | 0.000 | 0.000 |
| Al | 16.463 | 0.801 | 2.673 | 0.542 |
| Si | 19.357 | 0.542 | 29.593 | 0.564 |
| K | 0.567 | 0.067 | 0.000 | 0.000 |
| Frequency (cm−1) | Vibration Mode |
|---|---|
| 3696, 3673 | Stretching mode of surface OH groups [36,37] |
| 3664, 3625 | Stretching mode of inner OH groups [36,37] |
| 1120–1000 | Stretching modes of siloxane (Si-O) [36,37] |
| 950–900 | Deformation modes of surface and inner Al-OH groups [36,37] |
| 798 | Translational bending modes of OH groups [36] |
| 754, 692 | Deformation vibration modes of Si-O [37] |
| Frequency (cm−1) | Vibration Mode |
|---|---|
| 3750–3200 | Stretching mode of OH groups (silanols) [7,38,39,40] |
| 1300–900 | Asymmetric stretching modes of siloxane (Si-O) [38,39] |
| 790 | Bending mode of OH groups (silanols) [38,39] |
| Cycle | Rhodamine B ads. [mg·g−1] | SDev |
|---|---|---|
| 1 | 1.70 | 0.06 |
| 2 | 1.80 | 0.04 |
| 3 | 1.80 | 0.03 |
| 4 | 1.70 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celoria, G.; Miglio, V.; Paul, G.; Bisio, C.; Golemme, G.; Boccaleri, E. Silica Particles Derived from Natural Kaolinite for the Removal of Rhodamine B from Polluted Water. Processes 2022, 10, 964. https://doi.org/10.3390/pr10050964
Celoria G, Miglio V, Paul G, Bisio C, Golemme G, Boccaleri E. Silica Particles Derived from Natural Kaolinite for the Removal of Rhodamine B from Polluted Water. Processes. 2022; 10(5):964. https://doi.org/10.3390/pr10050964
Chicago/Turabian StyleCeloria, Giorgio, Vanessa Miglio, Geo Paul, Chiara Bisio, Giovanni Golemme, and Enrico Boccaleri. 2022. "Silica Particles Derived from Natural Kaolinite for the Removal of Rhodamine B from Polluted Water" Processes 10, no. 5: 964. https://doi.org/10.3390/pr10050964
APA StyleCeloria, G., Miglio, V., Paul, G., Bisio, C., Golemme, G., & Boccaleri, E. (2022). Silica Particles Derived from Natural Kaolinite for the Removal of Rhodamine B from Polluted Water. Processes, 10(5), 964. https://doi.org/10.3390/pr10050964










