Characterization of Refining the Morphology of Al–Fe–Si in A380 Aluminum Alloy due to Ca Addition
Abstract
:1. Introduction
2. Materials and Methods
3. Results
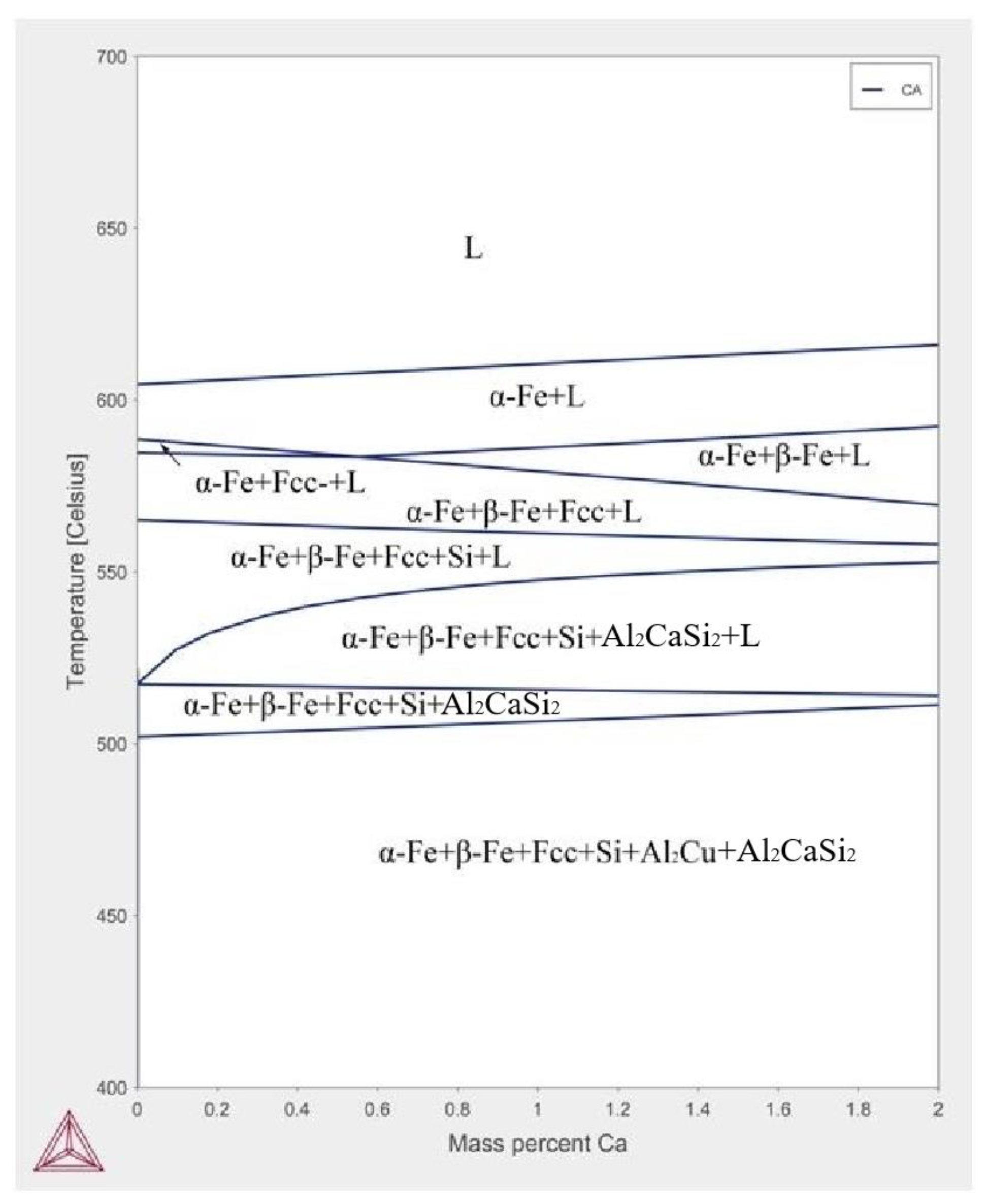
3.1. A380–Ca Equilibrium Phase Diagram
3.2. The Effect of Ca Addition on the Morphology and Phase Fraction of A380 at Different Cooling Rates
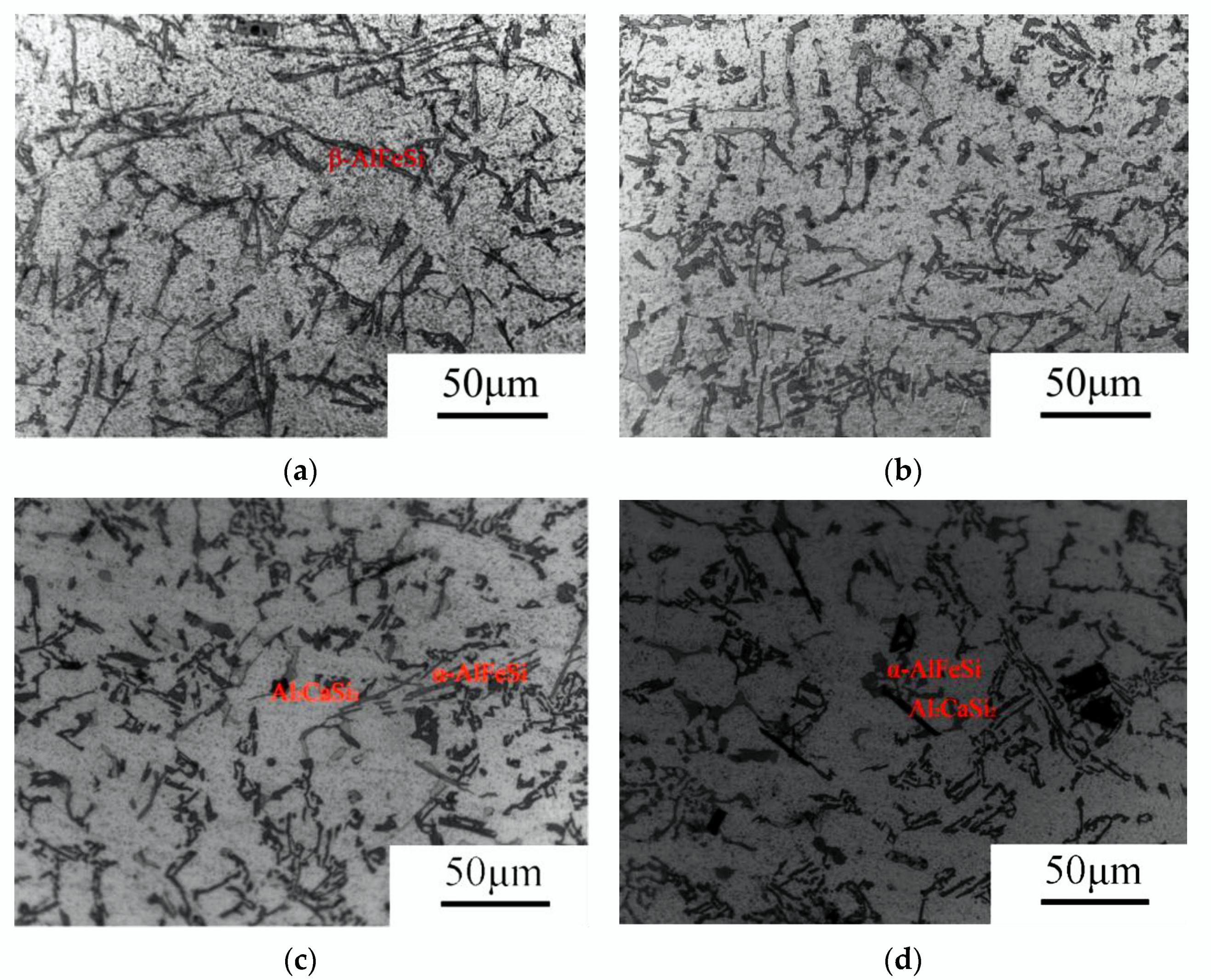
3.2.1. Equilibrium Situation (Low Cooling Rate)
3.2.2. Moderate Cooling Rates
3.2.3. High Cooling Rates
4. Discussion
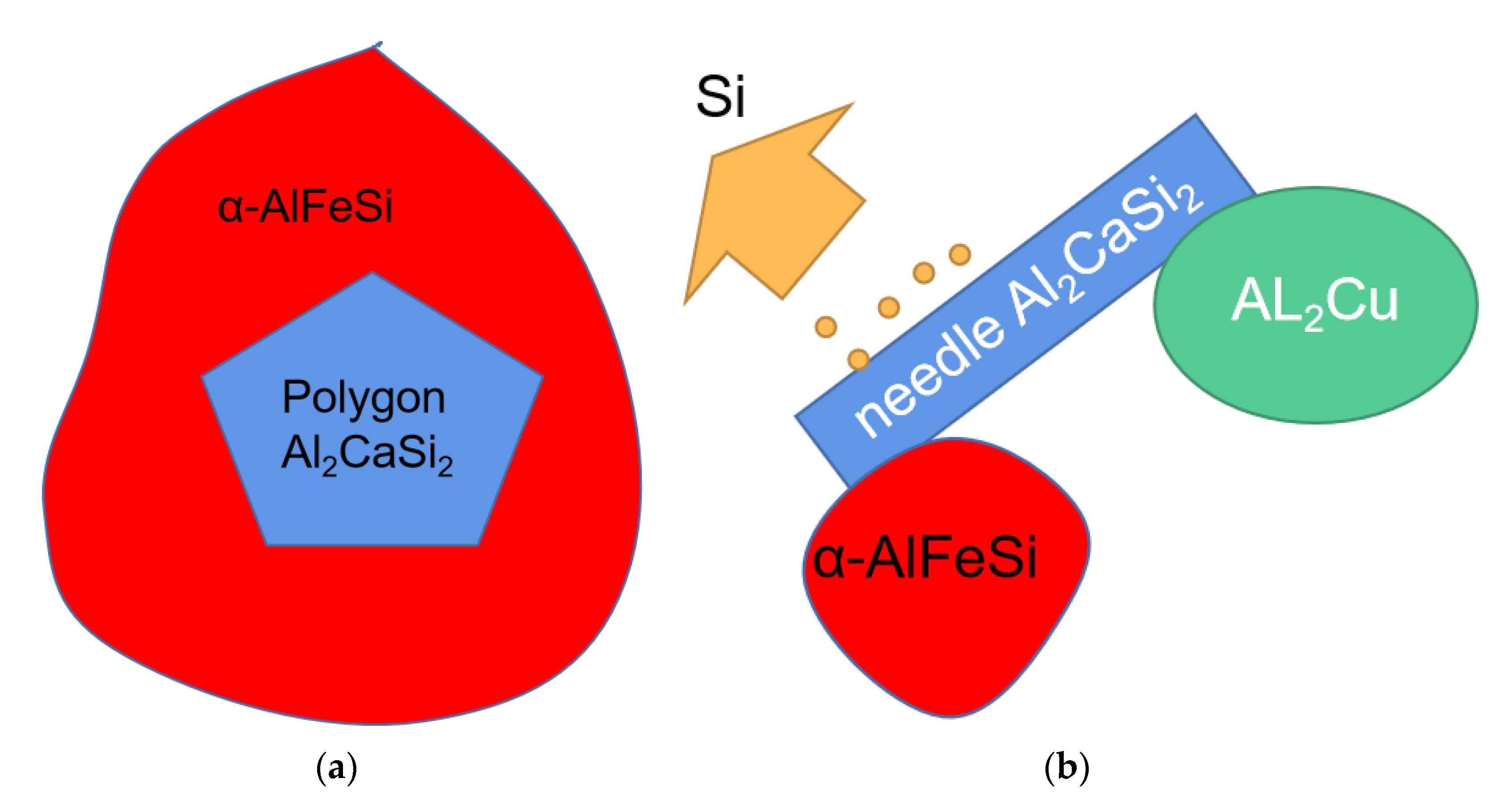
4.1. The Refinement Effect of Ca on the AlFeSi Phase
4.2. Ca Refinement Effect on Si
4.3. Al2CaSi2 Morphology
5. Conclusions
- (1)
- Ca can accelerate the fragmentation of the β-AlFeSi phase and introduce Al2CaSi2, which acts as the nucleation substrate of α-AlFeSi. At a low cooling rate, Al2CaSi2 can transform primary β-AlFeSi to secondary α-AlFeSi. At a high cooling rate, primary Al2CaSi2 can directly nucleate secondary α-AlFeSi.
- (2)
- A high cooling rate can facilitate the transformation of β-AlFeSi to α-AlFeSi with lower Ca addition. Therefore, the best Ca addition for A380 alloy at the cooling rates of 0.05, 5, and 50 °C/s are 0.1 wt.%, 0.05 wt.%, and 0.01 wt.%, respectively.
- (3)
- Ca itself and Al2CaSi2 can serve as the modification agents of eutectic silicon, and high cooling rates can only refine eutectic Si.
- (4)
- At low cooling rates, polygon-shaped Al2CaSi2 form in a 3D growth manner, while at high cooling rates, needle-shaped Al2CaSi2 form in a 2D growth manner, and both can serve as a nucleation substrate for α-AlFeSi.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, J.; Fang, X.; Chen, D. The effect of heat treatments on the microstructural evolution of twin-roll-cast Al-Fe-Si alloys. J. Materi. Eng. Perform. 2021, 30, 4401–4410. [Google Scholar] [CrossRef]
- Li, G.; Qu, W.-Y.; Luo, M.; Cheng, L.; Guo, C.; Li, X.-G.; Xu, Z.; Hu, X.-G.; Li, D.-Q.; Lu, H.-X.; et al. Semi-solid processing of aluminum and magnesium alloys: Status, opportunity, and challenge in China. Trans. Nonferrous Met. Soc. China 2021, 31, 3255–3280. [Google Scholar] [CrossRef]
- Liu, X.; Jia, H.-L.; Wang, C.; Wu, X.; Zha, M.; Wang, H.-Y. Enhancing mechanical properties of twin-roll cast Al–Mg–Si–Fe alloys by regulating Fe-bearing phases and macro-segregation. Mater. Sci. Eng. A 2021, 831, 142256. [Google Scholar] [CrossRef]
- Bardziński, P.J. New Al3Si7 phase with tetragonal silicon structure in quasicrystal-forming near-eutectic Al-Cu-Fe-Si alloys. J. Alloy. Compd. 2021, 869, 159349. [Google Scholar] [CrossRef]
- Gan, J.; Du, J.; Wen, C.; Zhang, G.; Shi, M.; Yuan, Z. The Effect of Fe Content on the Solidification Pathway, Microstructure and Thermal Conductivity of Hypoeutectic Al–Si Alloys. Int. J. Met. 2021, 16, 178–190. [Google Scholar] [CrossRef]
- Gao, T.; Li, Z.; Zhang, Y. Phase Evolution of β-Al5FeSi During Recycling of Al–Si–Fe Alloys by Mg Melt. Int. J. Metalcast. 2019, 13, 473–478. [Google Scholar] [CrossRef]
- Lu, L.; Dahle, A.K. Iron-rich intermetallic phases and their role in casting defect formation in hypoeutectic Al-Si alloys. Metall. Mater. Trans. A 2005, 36, 819–835. [Google Scholar]
- Dinnis, C.M.; Taylor, J.A.; Dahle, A.K. As-cast morphology of iron-intermetallics in Al–Si foundry alloys. Scr. Mater. 2005, 53, 955–958. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, J.; Damoah, L.; Robertson, D.G. Removal of Iron From Aluminum: A Review. Miner. Process. Extr. Met. Rev. 2012, 33, 99–157. [Google Scholar] [CrossRef]
- Becker, H.; Bergh, T.; Vullum, P.E.; Leineweber, A.; Li, Y. Effect of Mn and cooling rates on α-, β- and δ-Al–Fe–Si intermetallic phase formation in a secondary Al–Si alloy. Materialia 2019, 5, 100198. [Google Scholar] [CrossRef]
- Becker, H.; Bergh, T.; Vullum, P.; Leineweber, A.; Li, Y. β- and δ-Al-Fe-Si intermetallic phase, their intergrowth and polytype formation. J. Alloy. Compd. 2019, 780, 917–929. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Peng, H.; Huang, H.; Li, J.C. Abnormal Grain Growth Mechanism in the Twin-Roller Cast Al-Fe-Si Alloy in the Annealing Process. Adv. Mater. Sci. Eng. 2020, 2020, 1056274. [Google Scholar] [CrossRef]
- Gao, T.; Li, Z.-Q.; Liu, X.-F.; Zhang, Y.-X. Evolution Behavior of γ-Al3.5FeSi in Mg Melt and a Separation Method of Fe from Al–Si–Fe Alloys. Acta Met. Sin. Engl. Lett. 2017, 31, 48–54. [Google Scholar] [CrossRef]
- Samuel, M.; Samuel, F.H. Effect of alloying elements and dendrite arm spacing on the microstructure and hardness of an Al-Si-Cu-Mg-Mg-Fe-Mn (380). J. Mater. Sci. 1995, 301, 1698–1708. [Google Scholar] [CrossRef]
- Wang, M.; Xu, W.; Han, Q.Y. Effect of heat treatment on controlling the morphology of AlFeSi phase in A380 alloy. Int. J. Metalcast. 2016, 10, 516–523. [Google Scholar] [CrossRef]
- Wang, M.; Xu, W.; Han, Q. The Influence of Sr Addition on the Microstructure of A380 Alloy. Int. J. Met. 2017, 11, 321–327. [Google Scholar] [CrossRef]
- Wang, P.; Deng, Y.; Dai, Q.; Jiang, K.; Chen, J.; Guo, X. Microstructures and strengthening mechanisms of high Fe containing Al–Mg–Si–Mn–Fe alloys with Mg, Si and Mn modified. Mat. Sci. Eng. A-Struct. 2021, 803, 140477. [Google Scholar] [CrossRef]
- Kumari, S.S.; Pillai, R.; Pai, B. A study on the structural, age hardening and mechanical characteristics of Mn and Ca added Al–7Si–0.3Mg–0.6Fe alloy. J. Alloy. Compd. 2008, 453, 167–173. [Google Scholar] [CrossRef]
- Kumari, S.S.; Pillai, R.; Rajan, T.; Pai, B. Effects of individual and combined additions of Be, Mn, Ca and Sr on the solidification behaviour, structure and mechanical properties of Al–7Si–0.3Mg–0.8Fe alloy. Mater. Sci. Eng. A 2007, 460–461, 561–573. [Google Scholar] [CrossRef]
- Belov, N.; Naumova, E.; Akopyan, T. Effect of 0.3% Sc on microstructure, phase composition and hardening of Al-Ca-Si eutectic alloys. Trans. Nonferrous Met. Soc. China 2017, 27, 741–746. [Google Scholar] [CrossRef]
- Wang, M.; Xu, W.; Han, Q. Study of Refinement and Morphology Change of AlFeSi Phase in A380 Alloy due to Addition of Ca, Sr/ Ca, Mn and Mn, Sr. Mater. Trans. 2016, 57, 1509–1513. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Campbell, J. Morphology of Beta-Al5FeSi Phase in Al-Si Cast Alloys. Mater. Trans. 2006, 4, 1303–1312. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J. Casting; Butterworth-Heinemann: Oxford, UK, 1991. [Google Scholar]
- Korotkova, N.O.; Belov, N.A.; Avxentieva, N.N. Effect of Calcium additives on the phase composition and physicomechanical properties of a conductive alloy Al-0.5% Fe-0.2% Si-0.2% Zr-0.1% Sc. Phys. Met. Metallogr. 2020, 121, 95–101. [Google Scholar] [CrossRef]
- Kumari, S.S.S.; Pillai, R.M.; Pai, B.C. Role of calcium in aluminium based alloys and composites. Int. Mater. Rev. 2005, 50, 216–238. [Google Scholar] [CrossRef]



| Material | Major | Minor Element Composition (% by weight) | Al | ||||
|---|---|---|---|---|---|---|---|
| Si | Fe | Mg | Others Each | Others Total | |||
| Ca10 | Ca = 10% | ≤0.20 | ≤0.30 | ≤0.05 | ≤0.05 | ≤0.10 | Balance |
| Element | Si | Fe | Cu | Mn | Mg | Ni | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| wt.% | 9.0 | 1.0 | 3.5 | 0.4 | 0.2 | 0.3 | 0.35 | 0.08 | Balance |
| Match Planes | [hkl] s | [hkl] n | d[hkl] s (nm) | d[hkl] n (nm) | θ | δ (Pct) |
|---|---|---|---|---|---|---|
| (0001)s//(001)n | 2.146011 | 2.53 | 0 deg | 7.90 | ||
| 3.651899 | 3.57796 | 4.10 deg | ||||
| 2.700338 | 2.53 | 0 deg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Guo, Y.; Wang, H.; Zhao, S. Characterization of Refining the Morphology of Al–Fe–Si in A380 Aluminum Alloy due to Ca Addition. Processes 2022, 10, 672. https://doi.org/10.3390/pr10040672
Wang M, Guo Y, Wang H, Zhao S. Characterization of Refining the Morphology of Al–Fe–Si in A380 Aluminum Alloy due to Ca Addition. Processes. 2022; 10(4):672. https://doi.org/10.3390/pr10040672
Chicago/Turabian StyleWang, Meng, Yu Guo, Hongying Wang, and Shengsheng Zhao. 2022. "Characterization of Refining the Morphology of Al–Fe–Si in A380 Aluminum Alloy due to Ca Addition" Processes 10, no. 4: 672. https://doi.org/10.3390/pr10040672
APA StyleWang, M., Guo, Y., Wang, H., & Zhao, S. (2022). Characterization of Refining the Morphology of Al–Fe–Si in A380 Aluminum Alloy due to Ca Addition. Processes, 10(4), 672. https://doi.org/10.3390/pr10040672






