Physicochemical Characterization of Santa Barbara Amorphous-15 (SBA-15) and Its Functionalization with Polyaniline for Phenol Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. SBA-15 Synthesis
2.2. Modification with Polyaniline
2.3. Textural Characterization
2.4. Chemical Characterization
2.5. Determination of Immersion Enthalpy
2.6. Adsorption Isotherms
3. Results
3.1. Textural Characterization
3.2. Chemical Characterization
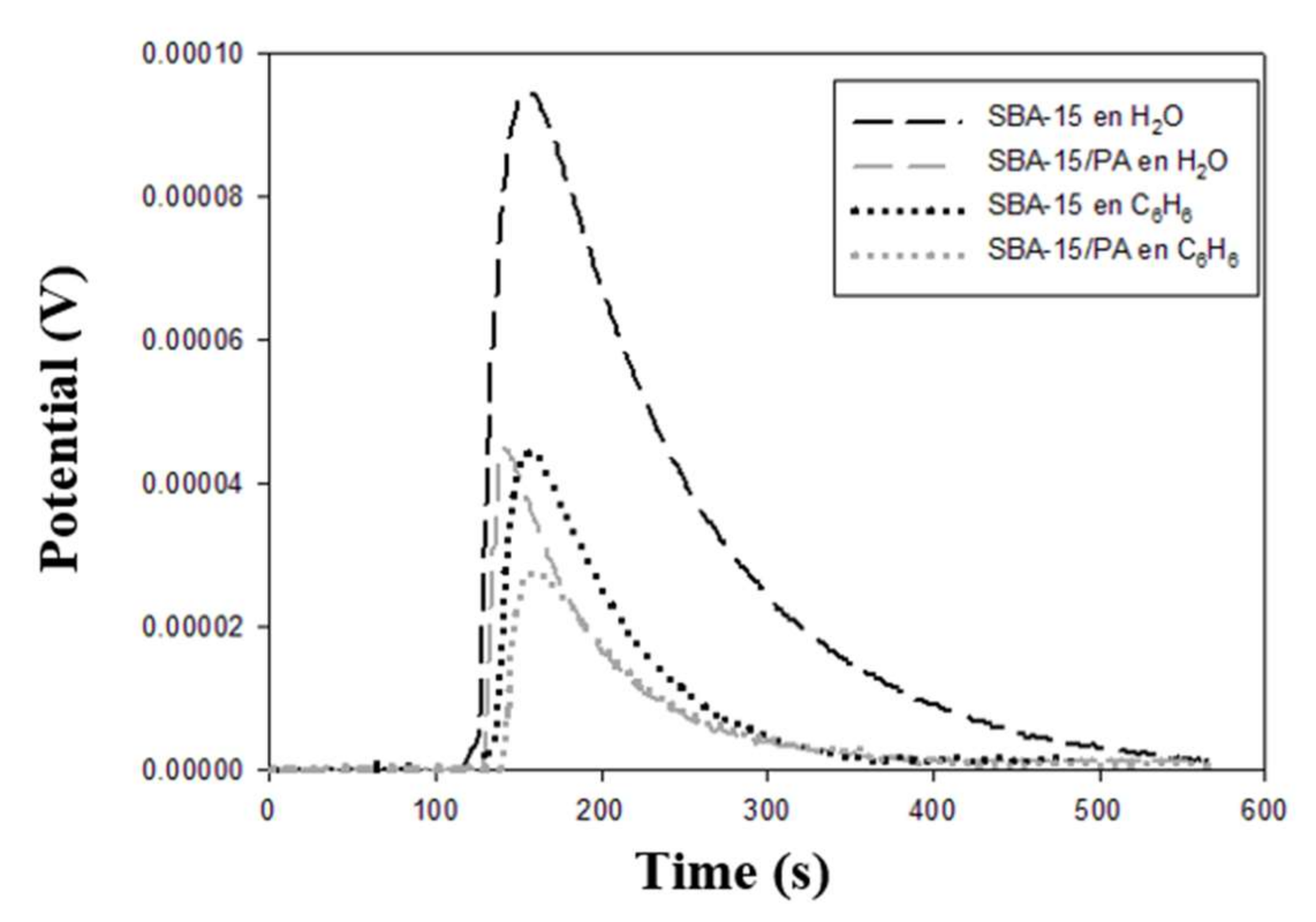
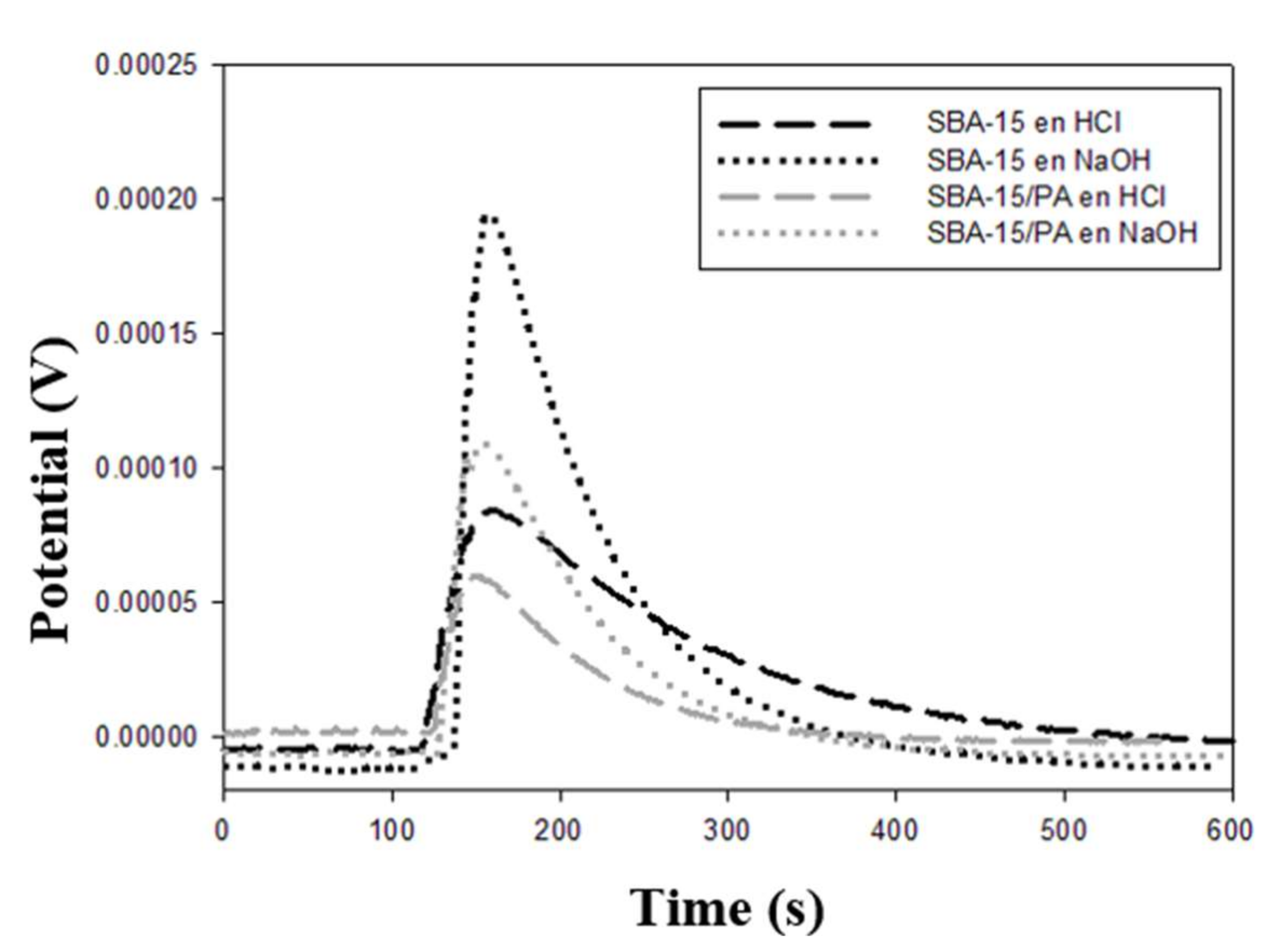
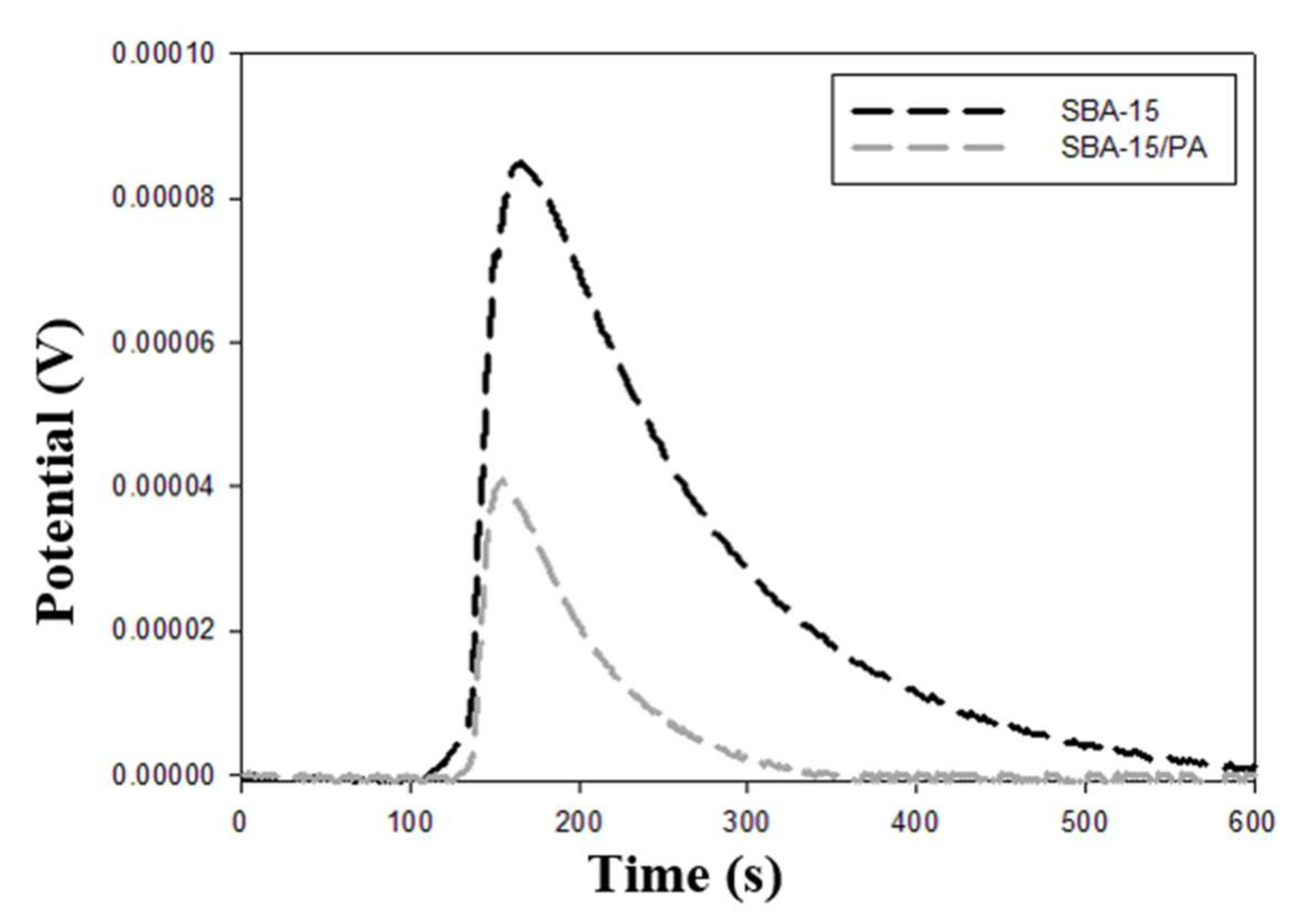
3.3. Immersion Enthalpy Measurements
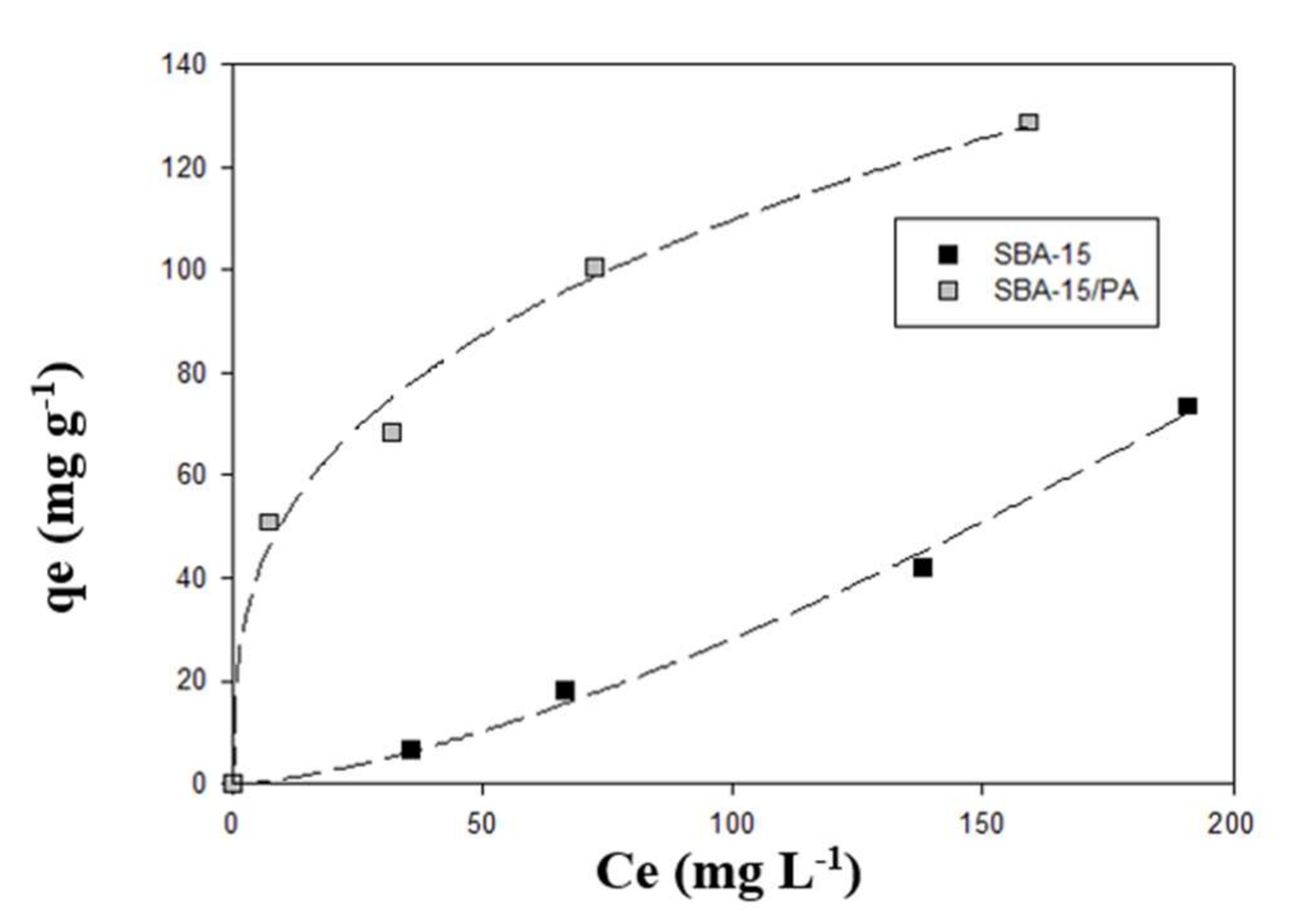
3.4. Adsorption Isotherms
3.4.1. Langmuir Model
3.4.2. Freundlich Model
3.4.3. Sips Model
3.4.4. Tóth Model
3.4.5. Redlich–Peterson Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biglari, H.; Afsharnia, M.; Alipour, V.; Khosravi, R.; Sharafi, K.; Mahvi, Y. A review and investigation of the effect of nanophotocatalytic ozonation process for phenolic compound removal from real effluent of pulp and paper industry. Environ. Sci. Pollut. Res. 2017, 24, 4105–4116. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, A.; Podkościelny, P.; Hubicki, Z.; Barczak, M. Adsorption of phenolic compounds by activated carbon—A critical review. Chemosphere 2005, 58, 1049–1070. [Google Scholar] [CrossRef] [PubMed]
- Kuśmierek, K.; Świątkowski, A.; Skrzypczyńska, K.; Błażewicz, S.; Hryniewicz, J. The effects of the thermal treatment of activated carbon on the phenols adsorption. Korean J. Chem. Eng. 2017, 34, 1081–1090. [Google Scholar] [CrossRef]
- Lorenc-Grabowska, E. Effect of micropore size distribution on phenol adsorption on steam activated carbons. Adsorption 2016, 22, 599–607. [Google Scholar] [CrossRef] [Green Version]
- Rincón-Silva, N.G.; Moreno-Piraján, J.C.; Giraldo, L. Equilibrium, kinetics and thermodynamics study of phenols adsorption onto activated carbon obtained from lignocellulosic material (Eucalyptus Globulus labill seed). Adsorption 2016, 22, 33–48. [Google Scholar] [CrossRef]
- Vargas, D.P.; Giraldo, L.; Moreno-Piraján, J.C. Effect of textural and chemical characteristics of activated carbons on phenol adsorption in aqueous solutions. Pol. J. Chem. Technol. 2017, 19, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Huo, P.; Liu, W. Behavior of phenol adsorption on thermal modified activated carbon. Chin. J. Chem. Eng. 2016, 24, 446–452. [Google Scholar] [CrossRef]
- Carvajal-Bernal, A.M.; Gómez-Granados, F.; Giraldo, L.; Moreno-Pirajan, J.C. Application of the Sips model to the calculation of maximum adsorption capacity and immersion enthalpy of phenol aqueous solutions on activated carbons. Eur. J. Chem. 2017, 8, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Gaber, D.; Haija, M.A.; Eskhan, A.; Banat, F. Graphene as an Efficient and Reusable Adsorbent Compared to Activated Carbons for the Removal of Phenol from Aqueous Solutions. Water Air Soil Pollut. 2017, 228, 320. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Deditius, A.; Ela, W.P.; Wiśniewski, M.; Gauden, P.A.; Terzyk, A.P.; Neimark, A.V. Super-sieving effect in phenol adsorption from aqueous solutions on nanoporous carbon beads. Carbon 2018, 135, 12–20. [Google Scholar] [CrossRef]
- Cheng, W.; Gao, W.; Cui, X.; Ma, J.; Li, R. Phenol adsorption equilibrium and kinetics on zeolite X/activated carbon composite. J. Taiwan Inst. Chem. Eng. 2016, 62, 192–198. [Google Scholar] [CrossRef]
- Toufaily, J.; Koubaissy, B.; Kafrouny, L.; Hamad, H.; Magnoux, P.; Ghannam, L.; Karout, A.; Hazimeh, H.; Nemra, G.; Hamieh, M.; et al. Functionalization of SBA-15 materials for the adsorption of phenols from aqueous solution. Cent. Eur. J. Eng. 2013, 3, 126–134. [Google Scholar] [CrossRef]
- Nakagawa, K.; Namba, A.; Ariayadejwanich, P.; Tanthapanichakoon, W. Adsorption of phenol and reactive dye from aqueous solution on activated carbons derived from solid wastes. Water Res. 2004, 38, 1791–1798. [Google Scholar] [CrossRef]
- Papadimas, S.P.; Sorial, G.A.; Suidan, M.T.; Speth, T.F. The effect of molecular oxygen on the activated carbon adsorption of natural organic matter in Ohio river water. Water Res. 1995, 29, 551–562. [Google Scholar]
- Su, F.; Lv, L.; Hui, T.M.; Zhao, X.S. Phenol adsorption on zeolite-templated carbons with different structural and surface properties. Carbon 2005, 43, 1156–1164. [Google Scholar] [CrossRef]
- Pan, B.C.; Zhang, X.; Zhang, W.M.; Zhang, Q.X. Adsorption of phenolic compounds from aqueous solution onto a macroporous polymer and its aminated derivative: Isotherm analysis. J. Hazard. Mater. 2005, 121, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.G.; Zhang, L.H.; Lin, Y. Thermodynamics of Adsorption of Organic Compounds at the Silica Gel/Nonpolar Solvent Interfaces. J. Colloid Interface Sci. 1994, 166, 23–28. [Google Scholar] [CrossRef]
- Parida, S.; Dash, S.; Patel, S.; Mishra, B. Adsorption of organic molecules on silica surface. Adv. Colloid Interface Sci. 2006, 121, 77–110. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Pan, B.; Zhang, Q. Modeling cooperative adsorption of aromatic compounds in aqueous solutions to nonpolar adsorbent. Sep. Purif. Technol. 2006, 49, 130–135. [Google Scholar] [CrossRef]
- Delval, F.; Crini, G.; Vebrel, J. Removal of organic pollutants from aqueous solutions by adsorbents prepared from an agroalimentary by-product. Bioresour. Technol. 2006, 97, 2173–2181. [Google Scholar] [CrossRef]
- Wang, S.; Boyjoo, Y.; Choueib, A.; Zhu, Z.H. Removal of dyes from aqueous solution using fly ash and red mud. Water Res. 2005, 39, 129–138. [Google Scholar] [CrossRef]
- Alkaram, U.F.; Mukhlis, A.A.; Al-Dujaili, A.H. The removal of phenol from aqueous solutions by adsorption using surfactant-modified bentonite and kaolinite. J. Hazard. Mater. 2005, 169, 324–332. [Google Scholar] [CrossRef]
- Koubaissy, B.; Toufaily, J.; Kafrouny, L.; Joly, G. Industrial water treatment, by adsorption, using organized mesoporous materials. Phys. Procedia 2011, 21, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Khalid, M.; Joly, G.; Renaud, A.; Magnoux, P. Removal of Phenol from Water by Adsorption Using Zeolites. Ind. Eng. Chem. Res. 2004, 43, 5275–5280. [Google Scholar] [CrossRef]
- Koubaissy, B.; Joly, G.; Magnoux, P. Adsorption and Competitive Adsorption on Zeolites of Nitrophenol Compounds Present in Wastewater. Ind. Eng. Chem. Res. 2008, 47, 9558–9565. [Google Scholar] [CrossRef]
- Koubaissy, B.; Joly, G.; Batonneau-Gene, I.; Magnoux, P. Adsorptive Removal of Aromatic Compounds Present in Wastewsssgater by Using Dealuminated Faujasite Zeolite. Ind. Eng. Chem. Res. 2011, 50, 5705–5713. [Google Scholar] [CrossRef]
- Koubaissy, B.; Toufaily, J.; El-Murr, M.; Daou, T.J.; Joly, G.; Magnoux, P.; Hamieh, T. Adsorption Kinetics and Equilibrium of Phenol Drifts on three Zeolites. Cent. Eur. J. Eng. 2012, 2, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.Y.; Feng, J.; Huo, Q.; Melosh, N. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.Y.; Huo, Q.; Feng, J.; Chmelka, B.F. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036, Erratum in J. Am. Chem. Soc. 2014, 136, 10546. [Google Scholar] [CrossRef]
- Zhang, L.X.; Yu, C.C.; Zhao, W.R.; Hua, Z.L. Preparation of multi-amine-grafted mesoporous silicas and their application to heavy metal ions adsorption. J. Non-Cryst. Solids 2007, 353, 4055–4061. [Google Scholar] [CrossRef]
- Brunel, D. Functionalized micelle-templated silicas (MTS) and their use as catalysts for fine chemicals. Microporous Mesoporous Mater. 1999, 27, 329–344. [Google Scholar] [CrossRef]
- Stein, A.; Melde, B.J.; Schroden, R.C. Hybrid Inorganic-Organic Mesoporous Silicates-Nanoscopic Reactors Coming of Age. Adv. Mater. 2000, 12, 1403–1419. [Google Scholar] [CrossRef]
- Mangrulkar, P.A.; Kamble, S.P.; Meshramb, J.; Rayalu, S.S. Adsorption of phenol and o-chlorophenol by mesoporous MCM-41. J. Hazard. Mater. 2008, 160, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Li, X. Efficient removal of cadmium (II) with SBA-15 nanoporous silica: Studies on equilibrium, isotherm, kinetics and thermodynamics. Appl. Water Sci. 2019, 9, 143. [Google Scholar] [CrossRef] [Green Version]
- Kjellman, T.; Reichhardt, N.; Sakeye, M.; Smått, J.H.; Lindén, M.; Alfredsson, V. Independent Fine-Tuning of the Intrawall Porosity and Primary Mesoporosity of SBA-15. Chem. Mater. 2013, 25, 1989–1997. [Google Scholar] [CrossRef]
- Weng, S.; Lin, Z.; Zhan, Y.; Chen, L.; Zhou, J. Facile synthesis of SBA-15/polyaniline nanocomposites with high electrochemical activity under neutral and acidic conditions. React. Funct. Polym. 2009, 69, 130–136. [Google Scholar] [CrossRef]
- Filip, A.; Macdonald, T.; Martis, V.; Parkin, I. Evaluation of the BET theory for the characterization of meso and microporous MOFs. Small Methods 2018, 2, 11. [Google Scholar]
- Boehm, H. Chemical Identification of Surface Groups. Adv. Catal. 1966, 16, 179–274. [Google Scholar]
- Babić, B.; Milonjić, S.; Polovina, M.; Kaludierović, B. Point of zero charge and intrinsic equilibrium constants of activated carbon cloth. Carbon 1999, 37, 477–481. [Google Scholar] [CrossRef]
- Moreno, J.; Giraldo, L. Determination of the immersion enthalpy of activated carbon by microcalorimetry of the heat conduction. Instrum. Sci. Technol. 2000, 28, 171–178. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1117. [Google Scholar] [CrossRef] [Green Version]
- Takamori, D.; Bizeto, M.; Fantini, A.; Rubinger, C.; Faez, R.; Martins, T. Polyaniline inclusion into ordered mesoporous silica matrices: Synthesis, characterization and electrical transport mechanism. Microporous Mesoporous Mater. 2019, 274, 212–219. [Google Scholar] [CrossRef]
- Boukoussa, B.; Hakiki, A.; Nunes-Beltrao, A.; Hamacha, R.; Azzouz, A. Assessment of the intrinsic interactions of nanocomposite polyaniline/SBA-15 with carbon dioxide: Correlation between the hydrophilic character and surface basicity. J. CO2 Util. 2018, 26, 171–178. [Google Scholar] [CrossRef]
- Santos, S.; Cecilia, J.; Vilarrasa-García, E.; Silva Junior, I.; Rodríguez-Castellón, E.; Azevedo, D. The effect of structure modifying agents in the SBA-15 for their application in the biomolecules adsorption. Microporous Mesoporous Mater. 2016, 232, 53–64. [Google Scholar] [CrossRef]
- Rodríguez-Estupiñán, P.; Giraldo, L.; Moreno-Piraján, J.C. Calorimetric study of amino-functionalised SBA-15. J. Therm. Anal. Calorim. 2015, 121, 127–134. [Google Scholar] [CrossRef]
- Navarrete, L.; Giraldo, L.; Moreno, J.C. Influence of surface chemistry on the immersion enthalpy of activated carbons in aqueous solutions of phenol and 4-nitro phenol. Rev. Colomb. Chem. 2006, 35, 215–224. [Google Scholar]
- Rodríguez-Estupiñán, F.; Giraldo, L.; Moreno-Piraján, J.C. Relationship between immersion enthalpies of activated carbons modified in their surface chemistry in different liquids and their physicochemical characteristics. Afinidad Rev. Quím. Teór. Apl. 2015, 72, 114–119. [Google Scholar]
- Vargas, D.; Giraldo, L.; Moreno-Piraján, J.C. Characterization of granular activated carbon prepared by activation with CaCl2 by means of gas adsorption and immersion calorimetry. Adsorption 2016, 22, 717–723. [Google Scholar] [CrossRef]
- Carvajal-Bernal, A.; Gómez-Granados, F.; Giraldo, L.; Moreno-Piraján, J.C. A study of the interactions of activated carbon-phenol in aqueous solution using the determination of immersion enthalpy. Appl. Sci. 2018, 8, 843. [Google Scholar] [CrossRef] [Green Version]
- Asmaly, H.; Ihsanullah; Abussaud, B.; Saleh, T.; Laoui, T.; Gupta, K.; Ali, M. Adsorption of phenol on aluminum oxide impregnated fly ash. Desalin. Water Treat. 2016, 57, 6801–6808. [Google Scholar] [CrossRef]
- Li, Q.; Yu, H.; Song, J.; Pan, X.; Liu, J.; Wang, Y.; Tang, L. Synthesis of SBA-15/polyaniline mesoporous composite for removal of resorcinol from aqueous solution. Appl. Surf. Sci. 2014, 290, 260–266. [Google Scholar] [CrossRef]
- Kundu, S.; Gupta, A.K. Arsenic adsorption onto iron oxide-coated cement (IOCC): Regression analysis of equilibrium data with several isotherm models and their optimization. Chem. Eng. J. 2006, 122, 93–106. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; Wiley Interscience: New York, NY, USA, 1997. [Google Scholar]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon. Part II. Models with more than two parameters. J. Hazard. Mater. 2007, 147, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Porter, J.F.; Mckay, G. Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: Copper, nickel and lead single component systems. Water Air Soil Pollut. 2002, 141, 1–33. [Google Scholar] [CrossRef]
- Prasad, R.K.; Srivastava, S.N. Sorption of distillery spent wash onto fly ash: Kinetics and mass transfer studies. Chem. Eng. J. 2009, 146, 90–97. [Google Scholar]
- Gimbert, F.; Morin-Crini, N.; Renault, F.; Badot, P.M.; Crini, G. Adsorption isotherm models for dye removal by cationized starch-based material in a single component system: Error analysis. J. Hazard. Mater. 2008, 157, 34–46. [Google Scholar] [CrossRef]
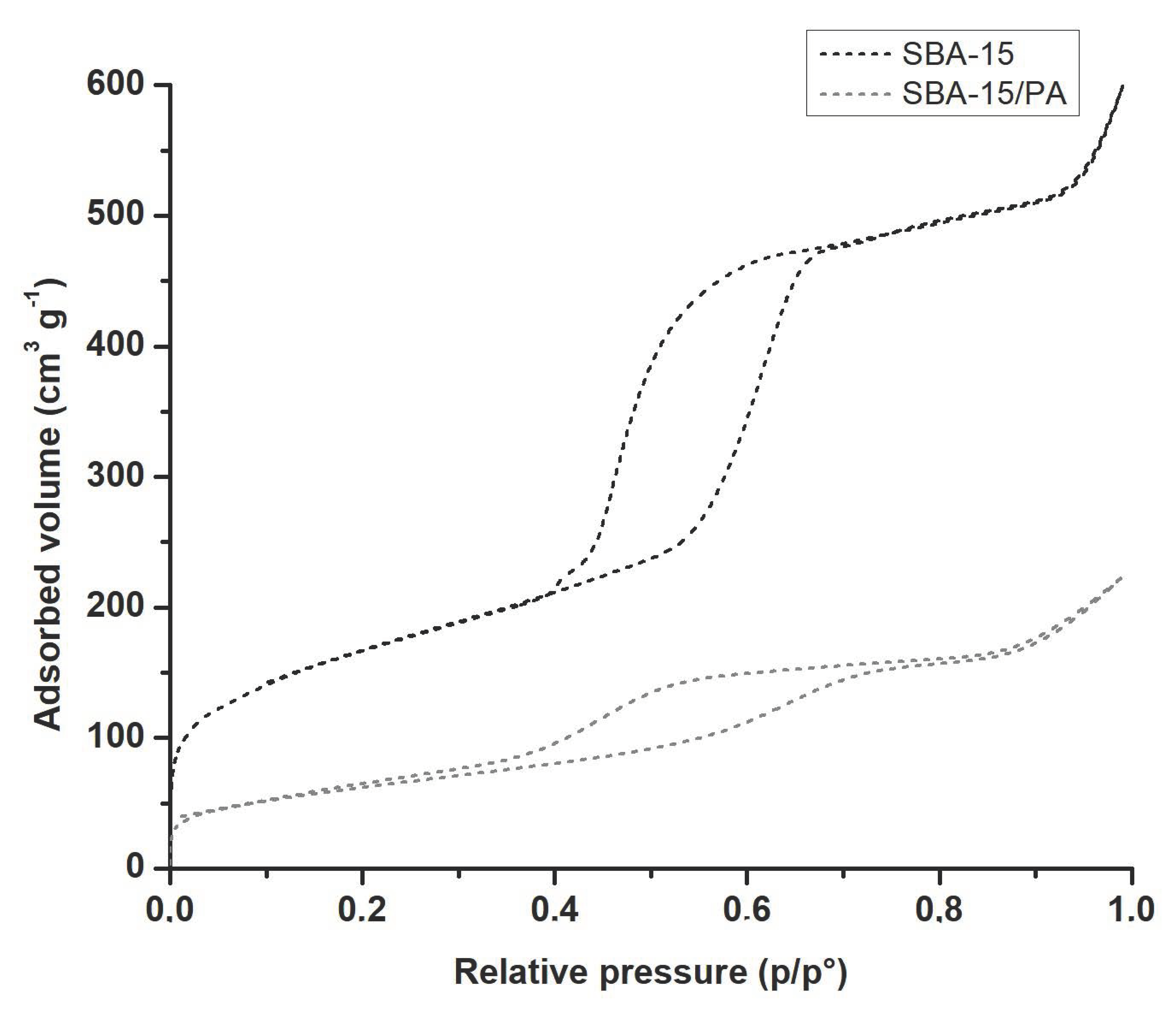
| Sample | Surface Area (m2 g−1) BET | Vo (cm3 g−1) | Vmeso (cm3 g−1) | Total V (cm3 g−1) | Average Pore Diameter (nm) |
|---|---|---|---|---|---|
| SBA-15 | 655 | 0.22 | 0.61 | 0.84 | 6.08 ± 0.06 |
| SBA-15/PA | 215 | 0.07 | 0.26 | 0.33 | 5.28 ± 0.05 |
| Sample | Acidic Sites (meq g−1) | Basic Sites (meq g−1) | pHpzc |
|---|---|---|---|
| SBA-15 | 0.026 ± 1.0 × 10−4 | 0.010 ± 1.2 × 10−4 | 6.5 ± 0.01 |
| SBA-15/PA | 0.011 ± 1.2 × 10−4 | 0.006 ± 0.8 × 10−4 | 3.0 ± 0.01 |
| Sample | −ΔHExp. Water (J g−1) | −ΔHExp. Benzene (J g−1) | −ΔHExp.HCl (J g−1) | −ΔHExp. NaOH (J g−1) | −ΔHExp. Phenol Solution (J g−1) |
|---|---|---|---|---|---|
| SBA-15 | 31.2 ± 0.5 | 28.1 ± 0.4 | 62.2 ± 0.8 | 174.1 ± 2.3 | 41.0 ± 0.6 |
| SBA-15/PA | 20.4 ± 0.3 | 15.1 ± 0.2 | 58.7 ± 0.8 | 167.7 ± 2.2 | 18.5 ± 0.3 |
| Model | Parameter | SBA-15 | SBA-15/PA |
|---|---|---|---|
| Langmuir | qm | 85.54 | 138 |
| KL | 0.0024 | 0.0463 | |
| R2 | 0.992 | 0.972 | |
| ε (%) | 2193 | 2929 | |
| Freundlich | KF | 0.0347 | 24.07 |
| 1/n | 1.4552 | 0.3296 | |
| R2 | 0.9971 | 0.9962 | |
| ε (%) | 1289 | 1376 |
| Model Isotherms Parameters | SBA-15 | SBA-15/PA |
|---|---|---|
| Sips | ||
| qms (mg g−1) | 67.37 | 78.97 |
| Ks (Lm mg−m) | 4.32 × 10−4 | 3.23 × 10−3 |
| ms | 1988 | 0.902 |
| R2 | 0.9769 | 0.9804 |
| Tòth | ||
| qmT (mg g−1) | 67.34 | 96.45 |
| KT | 0.467 | 0.134 |
| mT | 3.691 | 0.928 |
| R2 | 0.9587 | 0.9601 |
| Redlich–Peterson | ||
| KRP | 6.4326 | 3.6784 |
| α | 1.6785 | 1.7865 |
| β | 0.2745 | 0.3215 |
| R2 | 0.9587 | 0.9301 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cárdenas, L.J.; Giraldo, L.; Moreno-Piraján, J.C. Physicochemical Characterization of Santa Barbara Amorphous-15 (SBA-15) and Its Functionalization with Polyaniline for Phenol Adsorption. Processes 2022, 10, 188. https://doi.org/10.3390/pr10020188
Cárdenas LJ, Giraldo L, Moreno-Piraján JC. Physicochemical Characterization of Santa Barbara Amorphous-15 (SBA-15) and Its Functionalization with Polyaniline for Phenol Adsorption. Processes. 2022; 10(2):188. https://doi.org/10.3390/pr10020188
Chicago/Turabian StyleCárdenas, Lady Johana, Liliana Giraldo, and Juan Carlos Moreno-Piraján. 2022. "Physicochemical Characterization of Santa Barbara Amorphous-15 (SBA-15) and Its Functionalization with Polyaniline for Phenol Adsorption" Processes 10, no. 2: 188. https://doi.org/10.3390/pr10020188
APA StyleCárdenas, L. J., Giraldo, L., & Moreno-Piraján, J. C. (2022). Physicochemical Characterization of Santa Barbara Amorphous-15 (SBA-15) and Its Functionalization with Polyaniline for Phenol Adsorption. Processes, 10(2), 188. https://doi.org/10.3390/pr10020188







