Temperature-Related N2O Emission and Emission Potential of Freshwater Sediment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set Up
2.2. Physicochemical Analysis
2.3. Calculating N2O Exchange Flux
2.4. Measurements of N2O Emission and Dissolved N2O
2.5. Measurement of Denitrification Rate in Sediment
2.6. Statistical Analysis
3. Results
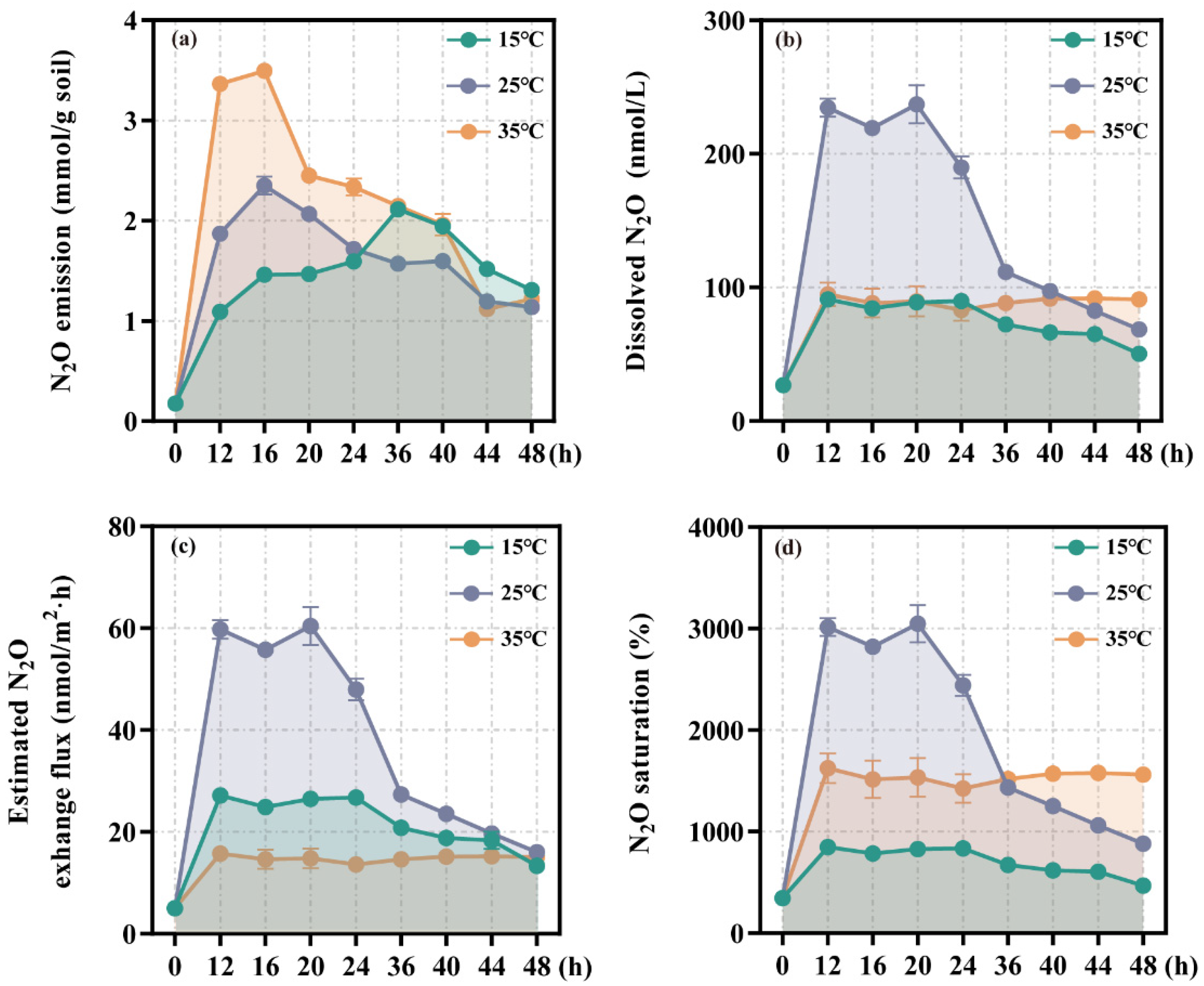
3.1. N2O Emission and Dissolved N2O
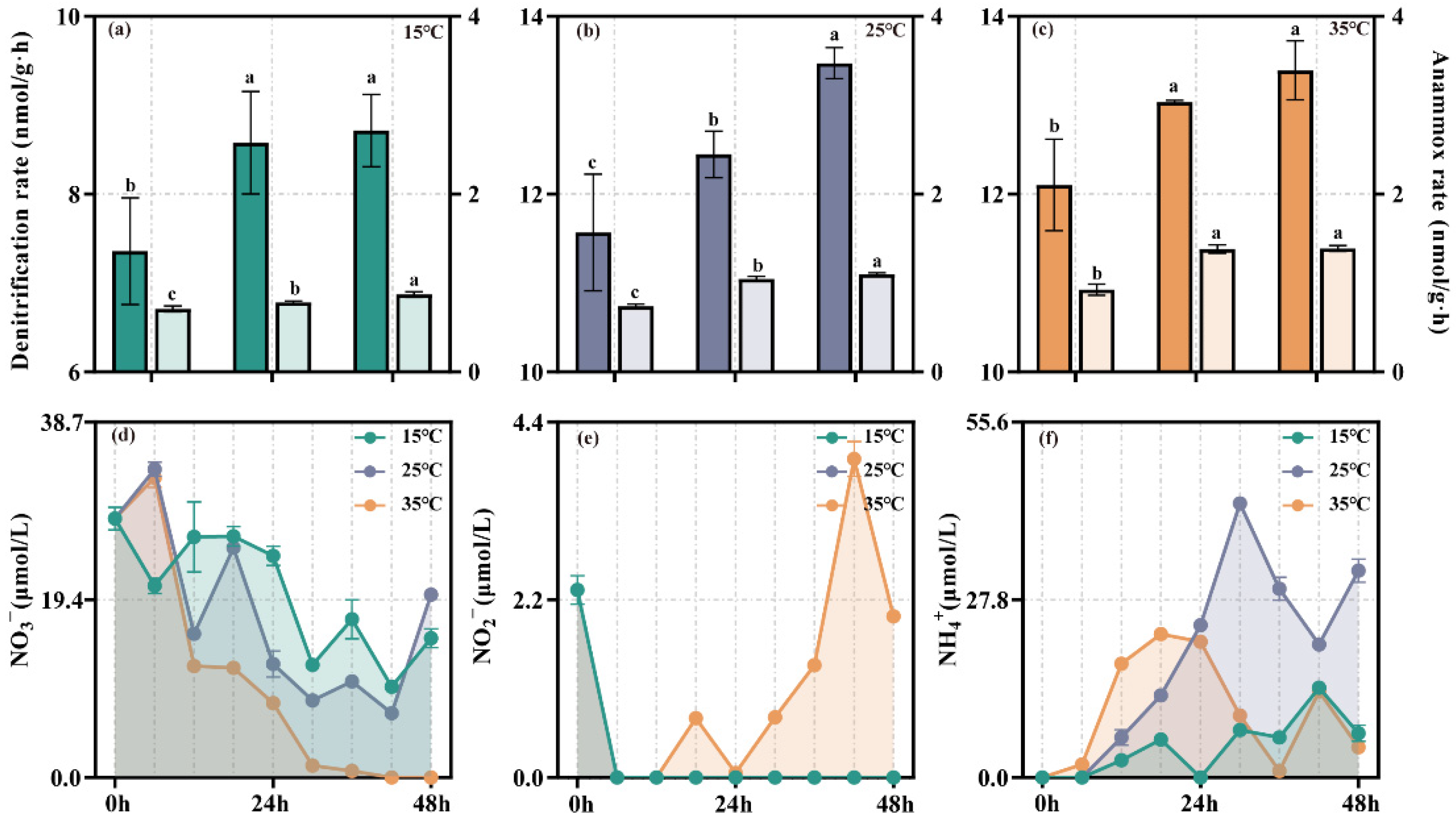
3.2. Denitrification Rate in Sediment and the Concentration of Inorganic Nitrogen in Water
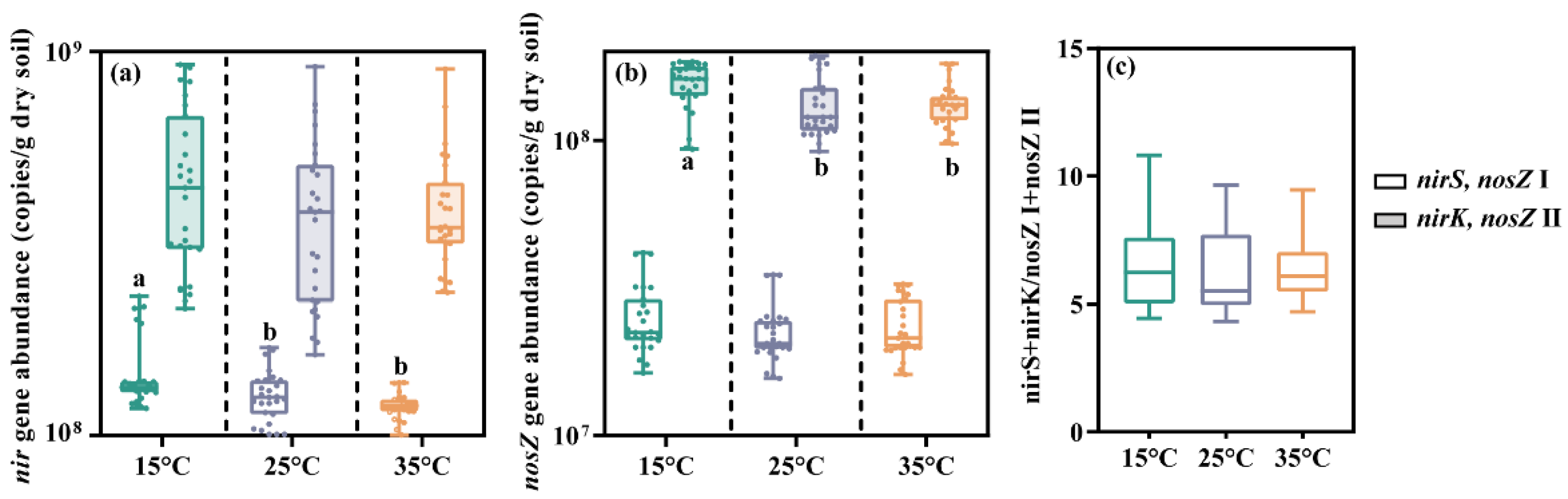
3.3. Abundance of N2O-Related Functional Gene in Sediment
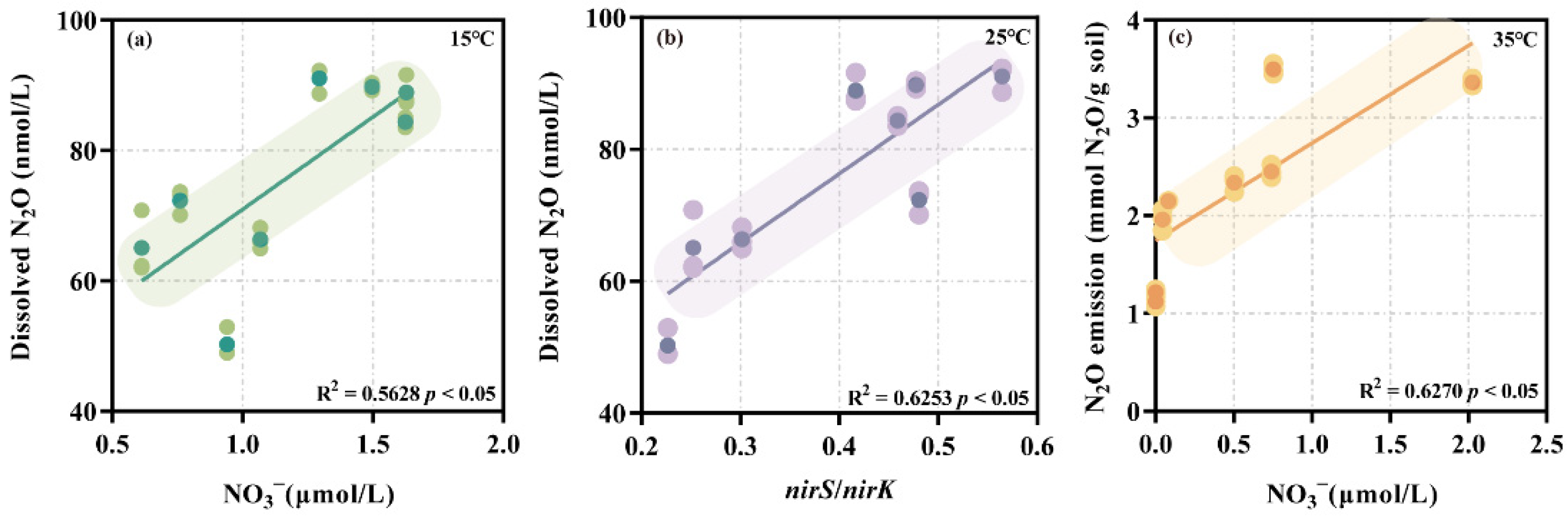
3.4. Factors Determining the N2O Generation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neubauer, S.C.; Megonigal, J.P. Moving Beyond Global Warming Potentials to Quantify the Climatic Role of Ecosystems. Ecosystems 2015, 18, 1000–1013. [Google Scholar] [CrossRef]
- Seitzinger, S.P.; Styles, R.V.; Kroeze, C. Global distribution of N2O emissions from aquatic systems: Natural emissions and anthropogenic effects. Chemosphere Glob. Chang. Sci. 2000, 2, 267–279. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef]
- Crutzen, P.J. The influence of nitrogen oxides on the atmospheric ozone content. Q. J. R. Meteorol. Soc. 1970, 96, 320–325. [Google Scholar] [CrossRef]
- Rütting, T.; Boeckx, P.; Müller, C.; Klemedtsson, L. Assessment of the importance of dissimilatory nitrate reduction to ammonium for the terrestrial nitrogen cycle. Biogeosciences 2011, 8, 1779–1791. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.L.; Beusichem, M.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Skiba, U.; Smith, K.A. Nitrification and denitrification as sources of nitric oxide and nitrous oxide in a sandy loam soil. Soil Biol. Biochem. 1993, 25, 1527–1536. [Google Scholar] [CrossRef]
- Bradley, R.L.; Whalen, J.; Chagnon, P.L.; Lanoix, M.; Alves, M.C. Nitrous oxide production and potential denitrification in soils from riparian buffer strips: Influence of earthworms and plant litter. Appl. Soil Ecol. 2011, 47, 6–13. [Google Scholar] [CrossRef]
- Islam, S.M.M.; Gaihre, Y.K.; Islam, M.R.; Khatun, A.; Islam, A. Integrated Plant Nutrient Systems Improve Rice Yields without Affecting Greenhouse Gas Emissions from Lowland Rice Cultivation. Sustainability 2022, 14, 11338. [Google Scholar] [CrossRef]
- Islam, S.M.M.; Gaihre, Y.K.; Islam, M.R.; Ahmed, M.N.; Akter, M.; Singh, U.; Sander, B.O. Mitigating greenhouse gas emissions from irrigated rice cultivation through improved fertilizer and water management. J. Environ. Manag. 2022, 307, 114520. [Google Scholar] [CrossRef]
- Vilain, G.; Garnier, J.; Decuq, C.; Lugnot, M. Nitrous oxide production from soil experiments: Denitrification prevails over nitrification. Nutr. Cycl. Agroecosystems 2014, 98, 169–186. [Google Scholar] [CrossRef]
- Seitzinger, S.P.; Kroeze, C. Global distribution of nitrous oxide production and N inputs in freshwater and coastal marine ecosystems. Glob. Biogeochem. Cycles 1998, 12, 93–113. [Google Scholar] [CrossRef]
- De Klein, C.A.M.; Sherlock, R.R.; Cameron, K.C.; Van der Weerden, T.J. Nitrous oxide emissions from agricultural soils in New Zealand—A review of current knowledge and directions for future research. J. R. Soc. N. Z. 2001, 31, 543–574. [Google Scholar] [CrossRef]
- Saggar, S.; Jha, N.; Deslippe, J.; Bolan, N.S.; Luo, J.; Giltrap, D.L.; Kim, D.G.; Zaman, M.; Tillman, R.W. Denitrification and N2O:N2 production in temperate grasslands: Processes, measurements, modelling and mitigating negative impacts. Sci. Total Environ. 2013, 465, 173–195. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Rees, R.M.; Tarsitano, D.; Zhan, X.; Jone, S.K.; Whitmor, A.P. Simulation of nitrous oxide emissions at field scale using the SPACSYS model. Sci. Total Environ. 2015, 530, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Mosier, A.R. Nitrous oxide emissions from agricultural soils. Fert. Res. 1994, 37, 191–200. [Google Scholar] [CrossRef]
- Abdalla, M.; Smith, P.; Williams, M. Emissions of nitrous oxide from agriculture: Responses to management and climate change. ACS Sym. Ser. 2011, 1072, 343–370. [Google Scholar]
- Wang, H.; Yang, L.; Wang, W.; Lu, J.; Yin, C. Nitrous oxide (N2O) fluxes and their relationships with water-sediment characteristics in a hyper-eutrophic shallow lake, China. J. Geophys. Res. B 2007, 112, G01005. [Google Scholar] [CrossRef]
- Hinshaw, S.E.; Dahlgren, R.A. Dissolved nitrous oxide concentrations and fluxes from the eutrophic San Joaquin River, California. Environ. Sci. Technol. 2013, 47, 1313–1322. [Google Scholar] [CrossRef]
- Soued, C.; Giorgio, P.A.d.; Maranger, R. Nitrous oxide sinks and emissions in boreal aquatic networks in Québec. Nat. Geosci. 2015, 9, 116–120. [Google Scholar] [CrossRef]
- Hallin, S.; Philippot, L.; Löffler, F.E.; Sanford, R.A.; Jones, C.M. Genomics and Ecology of Novel N2O-Reducing Microorganisms. Trends Microbiol. 2018, 26, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Denton, M. N2O and N2 emissions from denitrification respond differently to temperature and nitrogen supply. J. Soils Sediment 2018, 18, 1548–1557. [Google Scholar] [CrossRef]
- Tomaszek, J.A.; Gardner, W.S.; Johengen, T.H. Denitrification in sediments of a Lake Erie coastal wetland. J. Great Lakes Res. 1997, 23, 403–415. [Google Scholar] [CrossRef]
- Avrahami, S.; Liesack, W.; Conrad, R. Effects of temperature and fertilizer on activity and community structure of soil ammonia oxidizers. Environ. Microbiol. 2003, 5, 691–705. [Google Scholar] [CrossRef]
- Jiapeng, W.; Yiguo, H.; Fengjie, G.; Yan, W.; Yehui, T.; Weizhong, Y.; Meilin, W.; Liying, B.; Jiaping, W.; Jiali, W. A rapid and high-throughput microplate spectrophotometric method for field measurement of nitrate in seawater and freshwater. Sci. Rep. 2016, 6, 20165. [Google Scholar]
- Clough, T.J.; Buckthought, L.E.; Kelliher, F.M.; Sherlock, R.R. Diurnal fluctuations of dissolved nitrous oxide (N2O) concentrations and estimates of N2O emissions from a spring-fed river: Implications for IPCC methodology. Glob. Chang. Biol. 2007, 13, 1016–1027. [Google Scholar] [CrossRef]
- Wanninkhof, R. Relationship between wind speed and gas exchange over the ocean. J. Geophys. Res. 1992, 97, 7373–7382. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, X.; Pan, H.; Wang, Y.; Zhu, G. High N2O reduction potential by denitrification in the nearshore site of a riparian zone. Sci. Total Environ. 2022, 813, 152458. [Google Scholar] [CrossRef]
- Vitenberg, A.G. Equilibrium model in the description of gas extraction and headspace analysis. J. Anal. Chem. 2002, 58, 6–21. [Google Scholar]
- Hashimoto, S.; Gojo, K.; Hikota, S.; Sendai, N.; Otsuki, A. Nitrous oxide emissions from coastal waters in tokyo bay. Mar. Environ. Res. 1998, 47, 213–223. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, G.-L.; Zheng, L.-X.; Zhang, F.; Zhao, J. Seasonal variation of fluxes and distributions of dissolved methane in the North Yellow Sea. Cont. Shelf Res. 2010, 30, 187–192. [Google Scholar] [CrossRef]
- Nils, R.-P.; Meyer, R.L.; Schmid, M.; Mike, S.M.J.; Enrich-Prast, A.; Rysgaard, S.; Revsbech, N.P. Anaerobic ammonium oxidation in an estuarine sediment. Aquat. Microb. Ecol. 2004, 36, 293–304. [Google Scholar]
- Thamdrup, B.; Dalsgaard, T. Production of N2O through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl. Environ. Microbiol. 2002, 68, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, J.T.; Juutinen, S.; Alm, J.; Larmola, T.; Hammar, T.; Silvola, J.; Martikainen, P.J. Nitrous oxide flux to the atmosphere from the littoral zone of a boreal lake. J. Geophys. Res. Atmos. 2003, 108, D14. [Google Scholar] [CrossRef]
- Song, A.; Liang, Y.; Zeng, X.; Yin, H.; Xu, D.; Wang, B.; Wen, S.; Li, D.; Fan, F. Substrate-driven microbial response: A novel mechanism contributes significantly to temperature sensitivity of N2O emissions in upland arable soil. Soil Biol. Biochem. 2018, 118, 18–26. [Google Scholar] [CrossRef]
- Poh, L.S.; Jiang, X.; Zhang, Z.; Liu, Y.; Ng, W.J.; Zhou, Y. N2O accumulation from denitrification under different temperatures. Appl. Microbiol. Biotechnol. 2015, 99, 9215–9226. [Google Scholar] [CrossRef]
- Maltais-Landry, G.; Maranger, R.; Brisson, J.; Chazarenc, F. Nitrogen transformations and retention in planted and artificially aerated constructed wetlands. Water Res. 2009, 43, 535–545. [Google Scholar] [CrossRef]
- Velthuis, M.; Veraart, A.J. Temperature sensitivity of freshwater denitrification and N2O emission—A meta-analysis. Glob. Biogeochem. Cycles 2022, 36, e2022GB007339. [Google Scholar] [CrossRef]
- Farquharson, R.; Baldock, J. Concepts in modelling N2O emissions from land use. Plant Soil 2007, 309, 147–167. [Google Scholar] [CrossRef]
- Dong, L.F.; Nedwell, D.B.; Colbeck, I.; Finch, J. Nitrous oxide emission from some English and Welsh rivers and Estuaries. Water Air Soil Poll. 2004, 4, 127–134. [Google Scholar] [CrossRef]
- Xia, Y.; Li, Y.; Ti, C.; Li, X.; Zhao, Y.; Yan, X. Is indirect N2O emission a significant contributor to the agricultural greenhouse gas budget? A case study of a rice paddy-dominated agricultural watershed in eastern China. Atmos. Environ. 2013, 77, 943–950. [Google Scholar] [CrossRef]
- Xiong, Z.; Xing, G.; Shen, G.; Shi, S.; Du, L. Dissolved N2O concentrations and N2O emissions from aquatic systems of lake and river in Taihu Lake region. Eur. PMC 2002, 23, 26–30. [Google Scholar]
- Hartono, A.; Juliussen, O.; Svendsen, H.F. Solubility of N2O in aqueous solution of diethylenetriamine. J. Chem. Eng. Data 2008, 53, 2696–2700. [Google Scholar] [CrossRef]
- Weiss, R.F.; Price, B.A. Nitrous oxide solubility in water and seawater. Mar. Chem. 1979, 8, 347–359. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Tian, P. Carbon quality and soil microbial property control the latitudinal pattern in temperature sensitivity of soil microbial respiration across Chinese forest ecosystems. Glob. Chang. Biol. 2018, 24, 2841–2849. [Google Scholar] [CrossRef]
- Liu, X.S.; Bai, J.; Sun, J.J.; Hou, R.; Zhao, Y.G. The study of denitrification rate and N2O release rate in Shuangtaizi Estuary Wetland. Appl. Mech. Mater. 2014, 665, 416–419. [Google Scholar] [CrossRef]
- Wang, X.; Hu, M.; Ren, H.; Li, J.; Tong, C.; Musenze, R.S. Seasonal variations of nitrous oxide fluxes and soil denitrification rates in subtropical freshwater and brackish tidal marshes of the Min River estuary. Sci. Total Environ. 2018, 616–617, 1404–1413. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Zhao, S.; Wang, X.; Hefting, M.M.; Schwark, L.; Zhu, G. Anammox and denitrification separately dominate microbial N-loss in water saturated and unsaturated soils horizons of riparian zones. Water Res. 2019, 162, 139–150. [Google Scholar] [CrossRef]
- Beaulieu, J.J.; Tank, J.L.; Hamilton, S.K.; Wollheim, W.M.; Hall, R.O., Jr.; Mulholland, P.J.; Peterson, B.J.; Ashkenas, L.R.; Cooper, L.W.; Dahm, C.N.; et al. Nitrous oxide emission from denitrification in stream and river networks. Int. J. Biol. Sci. 2011, 108, 214–219. [Google Scholar] [CrossRef]
- Weymann, D.; Well, R.; Flessa, H.; von der Heide, C.; Deurer, M.; Meyer, K.; Konrad, C.; Walther, W. Groundwater N2O emission factors of nitrate-contaminated aquifers as derived from denitrification progress and N2O accumulation. Biogeosciences 2008, 5, 1215–1226. [Google Scholar] [CrossRef]
- Hefting, M.M.; Bobbink, R.; de Caluwe, H. Nitrous oxide emission and denitrification in chronically nitrate-loaded riparian buffer zones. J. Environ. Qual. 2003, 32, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Saarenheimo, J.; Rissanen, A.J.; Arvola, L.; Nykanen, H.; Lehmann, M.F.; Tiirola, M. Genetic and environmental controls on nitrous oxide accumulation in lakes. PLoS ONE. 2015, 10, 0121201. [Google Scholar] [CrossRef] [PubMed]
- Hergoualc’h, K.; Akiyama, o.; Bernoux, M.; Chirinda, N.; Prado, A.d.; Kasimir, Å.; MacDonald, J.D.; Ogle, S.M.; Regina, K.; Weerden, T.J.v.d. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; IPCC: Geneva, Switzerland, 2006. [Google Scholar]
- Domeignoz-Horta, L.A.; Spor, A.; Bru, D.; Breuil, M.C.; Bizouard, F.; Leonard, J.; Philippot, L. The diversity of the N2O reducers matters for the N2O:N2 denitrification end-product ratio across an annual and a perennial cropping system. Front. Microbiol. 2015, 6, 971. [Google Scholar] [CrossRef] [PubMed]
- Yergeau, E.; Kang, S.; He, Z.; Zhou, J.; Kowalchuk, G.A. Functional microarray analysis of nitrogen and carbon cycling genes across an Antarctic latitudinal transect. ISME J. 2007, 1, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Hallin, S.; Lindgren, P.-E. PCR detection of genes encoding nitrite reductase in denitrifying bacteria. Appl. Environ. Microbiol. 1999, 65, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.; Bru, D.; Stres, B.; Hallet, S.; Philippot, L. Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl. Environ. Microbiol. 2006, 72, 5181–5189. [Google Scholar] [CrossRef]
- Jones, C.M.; Graf, D.R.H.; Bru, D.; Philippot, L.; Hallin, S. The unaccounted yet abundant nitrous oxide-reducing microbial community: A potential nitrous oxide sink. ISME J. 2013, 7, 417–426. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Yue, A.; Moore, S.S.; Ye, F.; Wu, J.; Hong, Y.; Wang, Y. Temperature-Related N2O Emission and Emission Potential of Freshwater Sediment. Processes 2022, 10, 2728. https://doi.org/10.3390/pr10122728
Li S, Yue A, Moore SS, Ye F, Wu J, Hong Y, Wang Y. Temperature-Related N2O Emission and Emission Potential of Freshwater Sediment. Processes. 2022; 10(12):2728. https://doi.org/10.3390/pr10122728
Chicago/Turabian StyleLi, Shuai, Ang Yue, Selina Sterup Moore, Fei Ye, Jiapeng Wu, Yiguo Hong, and Yu Wang. 2022. "Temperature-Related N2O Emission and Emission Potential of Freshwater Sediment" Processes 10, no. 12: 2728. https://doi.org/10.3390/pr10122728
APA StyleLi, S., Yue, A., Moore, S. S., Ye, F., Wu, J., Hong, Y., & Wang, Y. (2022). Temperature-Related N2O Emission and Emission Potential of Freshwater Sediment. Processes, 10(12), 2728. https://doi.org/10.3390/pr10122728







