The Influence of Cryogrinding on Essential Oil, Phenolic Compounds and Pigments Extraction from Myrtle (Myrtus communis L.) Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Cryogrinding (CG)
2.4. Hydrodistillation (HD)
2.5. Volatile Compounds Analysis
2.6. Agitation Assisted Extraction (AAE)
2.7. Pigment Analysis
2.8. Phenolic Compounds Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. The Influence of CG on the Composition of Myrtle EO
3.2. The Influence of CG on the Phenolic and Pigment Compositions of Myrtle
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sumbul, S.; Aftab Ahmad, M.; Asif, M.; Akhtar, M. Myrtus communis Linn—A review. Indian J. Nat. Prod. Resour. 2011, 2, 395–402. [Google Scholar]
- Jerkovic, I.; Radonic, A.; Borcic, I. Comparative study of leaf, fruit and flower essential oils of croatian Myrtus communis (L.) During a one-year vegetative cycle. J. Essent. Oil Res. 2002, 14, 266–270. [Google Scholar] [CrossRef]
- Aleksic, V.; Knezevic, P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Usai, M.; Mulas, M.; Marchetti, M. Chemical composition of essential oils of leaves and flowers from five cultivars of myrtle (Myrtus communis L.). J. Essent. Oil Res. 2015, 27, 465–476. [Google Scholar] [CrossRef]
- Maggio, A.; Loizzo, M.R.; Riccobono, L.; Bruno, M.; Tenuta, M.C.; Leporini, M.; Falco, T.; Leto, C.; Tuttolomondo, T.; Cammalleri, I.; et al. Comparative chemical composition and bioactivity of leaves essential oils from nine Sicilian accessions of Myrtus communis L. J. Essent. Oil Res. 2019, 31, 546–555. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Sandri, I.G.; Zacaria, J.; Fracaro, F.; Delamare, A.P.L.; Echeverrigaray, S. Antimicrobial activity of the essential oils of Brazilian species of the genus Cunila against foodborne pathogens and spoiling bacteria. Food Chem. 2007, 103, 823–828. [Google Scholar] [CrossRef]
- Buttner, M.P.; Willeke, K.; Grinshpun, S.A. Sampling and analysis of airborne microorganisms. In Manual of Environmental Microbiology; Hurst, C., Knudsen, G., McInerney, M., Stetzenbach, L., Walter, M., Eds.; ASM Press: Washington, DC, USA, 1997; pp. 629–640. [Google Scholar]
- Aidi Wannes, W.; Mhamdi, B.; Sriti, J.; Ben Jemia, M.; Ouchikh, O.; Hamdaoui, G.; Kchouk, M.E.; Marzouk, B. Antioxidant activities of the essential oils and methanol extracts from myrtle (Myrtus communis var. italica L.) leaf, stem and flower. Food Chem. Toxicol. 2010, 48, 1362–1370. [Google Scholar] [CrossRef]
- Bowles, E.J. The Chemistry of Aromatherapeutic Oils, 3rd ed.; Allen & Unwin: Crows Nest, Australia, 2003; ISBN 10:174114051X. [Google Scholar]
- Alipour, G.; Dashti, S.; Hosseinzadeh, H. Review of pharmacological effects of Myrtus communis L. and its active constituents. Phyther. Res. 2014, 28, 1125–1136. [Google Scholar] [CrossRef]
- Zanetti, S.; Cannas, S.; Molicotti, P.; Bua, A.; Cubeddu, M.; Porcedda, S.; Marongiu, B.; Sechi, L.A. Evaluation of the antimicrobial properties of the essential oil of Myrtus communis L. Against clinical strains of mycobacterium spp. Interdiscip. Perspect. Infect. Dis. 2010, 2010, 931530. [Google Scholar] [CrossRef]
- Harassi, Y.; Tilaoui, M.; Idir, A.; Frédéric, J.; Baudino, S.; Ajouaoi, S.; Mouse, H.A.; Zyad, A. Phytochemical analysis, cytotoxic and antioxidant activities of Myrtus communis essential oil from Morocco. J. Complement. Integr. Med. 2020, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Maxia, A.; Frau, M.A.; Falconieri, D.; Karchuli, M.S.; Kasture, S. Essential oil of Myrtus communis inhibits inflammation in rats by reducing serum IL-6 and TNF-α. Nat. Prod. Commun. 2011, 6, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.; Farhadi, N. Influence of environmental and genetic factors on resin yield, essential oil content and chemical composition of Ferula assa-foetida L. populations. J. Appl. Res. Med. Aromat. Plants 2015, 2, 69–76. [Google Scholar] [CrossRef]
- Djilani, A.; Dicko, A. The therapeutic benefits of essential oils. In Nutrition, Well-Being and Health; Bouayed, J., Bohn, T., Eds.; In Tech: Shanghai, China, 2012; pp. 155–178. [Google Scholar]
- Mulas, M.; Melis, R.A.M. Essential Oil Composition of Myrtle (Myrtus communis) Leaves. J. Herbs. Spices Med. Plants 2011, 17, 21–34. [Google Scholar] [CrossRef]
- Gardeli, C.; Vassiliki, P.; Athanasios, M.; Kibouris, T.; Komaitis, M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: Evaluation of antioxidant capacity of methanolic extracts. Food Chem. 2008, 107, 1120–1130. [Google Scholar] [CrossRef]
- Bradesi, P.; Tomi, F.; Casanova, J.; Costa, J.; Bemardini, A.F. Chemical composition of myrtle leaf essential oil from corsica (France). J. Essent. Oil Res. 1997, 9, 283–288. [Google Scholar] [CrossRef]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-free microwave extraction of essential oil from aromatic herbs: Comparison with conventional hydro-distillation. J. Chromatogr. A 2004, 1043, 323–327. [Google Scholar] [CrossRef]
- Akloul, R.; Benkaci-Ali, F.; Eppe, G. Kinetic study of volatile oil of Curcuma longa L. rhizome and Carum carvi L. fruits extracted by microwave-assisted techniques using the cryogrinding. J. Essent. Oil Res. 2014, 26, 473–485. [Google Scholar] [CrossRef]
- Mékaoui, R.; Benkaci-Ali, F.; Scholl, G.; Eppe, G. Effect of the Extraction Technique, Heating Time and Cryogenic Grinding (N2 at −196°C) on the Composition of Cumin Seeds Volatile Oil. J. Essent. Oil-Bear. Plants 2016, 19, 1903–1919. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Mulinacci, N.; Vincieri, F.F.; Tattini, M. Identification and quantitation of polyphenols in leaves of Myrtus communis L. Chromatographia 1999, 49, 17–20. [Google Scholar] [CrossRef]
- Čulina, P.; Cvitković, D.; Pfeifer, D.; Zorić, Z.; Repajić, M.; Garofulić, I.E.; Balbino, S.; Pedisić, S. Phenolic profile and antioxidant capacity of selected medicinal and aromatic plants: Diversity upon plant species and extraction technique. Processes 2021, 9, 2207. [Google Scholar] [CrossRef]
- Dairi, S.; Madani, K.; Aoun, M.; Him, J.L.K.; Bron, P.; Lauret, C.; Cristol, J.P.; Carbonneau, M.A. Antioxidative Properties and Ability of Phenolic Compounds of Myrtus communis Leaves to Counteract In Vitro LDL and Phospholipid Aqueous Dispersion Oxidation. J. Food Sci. 2014, 79, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Cvitković, D.; Lisica, P.; Zorić, Z.; Repajić, M.; Pedisić, S.; Dragović-Uzelac, V.; Balbino, S. Composition and antioxidant properties of pigments of mediterranean herbs and spices as affected by different extraction methods. Foods 2021, 10, 2477. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Mirzaei, H.; Emam-Djomeh, Z.; Sadeghi Mahoonak, A.R.; Khomeiri, M. Effect of harvest time on antioxidant activity of Glycyrrhiza glabra root extract and evaluation of its antibacterial activity. Int. Food Res. J. 2013, 20, 2951–2957. [Google Scholar]
- Zahir, A.; Abbasi, B.H.; Adil, M.; Anjum, S.; Zia, M. Ihsan-Ul-Haq Synergistic effects of drought stress and photoperiods on phenology and secondary metabolism of Silybum marianum. Appl. Biochem. Biotechnol. 2014, 174, 693–707. [Google Scholar] [CrossRef]
- Shoshtari, Z.V.; Rahimmalek, M.; Sabzalian, R. Essential Oil and Bioactive Compounds Variation in Myrtle (Myrtus communis L.) as Affected by Seasonal Variation and Salt Stress. Chem. Biodivers. 2017, 14, e1600365. [Google Scholar] [CrossRef]
- Amensour, M.; Sendra, E.; Abrini, J.; Bouhdid, S.; Pérez-Alvarez, J.A.; Fernández-López, J. Total Phenolic Content and Antioxidant Activity of Myrtle (Myrtus communis) Extracts. Nat. Prod. Commun. 2009, 4, 819–824. [Google Scholar] [CrossRef]
- Damjanović-Vratnica, B.; Perović, S.; Lu, T.; Santos, R. Effect of matrix pretreatment on the supercritical CO2 extraction of Satureja montana essential oil. Chem. Ind. Chem. Eng. Q. 2016, 22, 201–209. [Google Scholar] [CrossRef][Green Version]
- Saxena, V.; Patel, B.B.; Sutar, R.F.; Joshi, D.C. Improving quality of cumin powder through cryogenic grinding technology. J. Food Process. Preserv. 2018, 42, e13371. [Google Scholar] [CrossRef]
- Murthy, C.T.; Krishnamurthy, N.; Ramesh, T.; Srinivasa Rao, P.N. Effect of grinding methods on the retention of black pepper volatiles. J. Food Sci. Technol. 1996, 33, 299–301. [Google Scholar]
- Saxena, S.N.; Rathore, S.S.; Saxena, R.; Barnwal, P.; Sharma, L.K.; Singh, B. Effect of Cryogenic Grinding on Essential Oil Constituents of Coriander (Coriandrum sativum L.) Genotypes. J. Essent. Oil-Bear. Plants 2014, 17, 385–392. [Google Scholar] [CrossRef]
- Sharma, L.K.; Agarwal, D.; Sharma, Y.; Rathore, S.S.; Saxena, S.N. Cryogenic grinding technology enhances volatile oil, oleoresin and antioxidant activity of cumin (Cuminum cyminum L.). Int. J. Seed Spices 2014, 4, 68–72. [Google Scholar]
- Saxena, S.N.; Sharma, Y.K.; Rathore, S.S.; Singh, K.K.; Barnwal, P.; Saxena, R.; Upadhyaya, P.; Anwer, M.M. Effect of cryogenic grinding on volatile oil, oleoresin content and anti-oxidant properties of coriander (Coriandrum sativum L.) genotypes. J. Food Sci. Technol. 2015, 52, 568–573. [Google Scholar] [CrossRef]
- Balbino, S.; Dorić, M.; Vidaković, S.; Kraljić, K.; Škevin, D.; Drakula, S.; Voučko, B.; Čukelj, N.; Obranović, M.; Ćurić, D. Application of cryogenic grinding pretreatment to enhance extractability of bioactive molecules from pumpkin seed cake. J. Food Process Eng. 2019, 42, e13300. [Google Scholar] [CrossRef]
- Razumov, E.Y.; Safin, R.R.; Mukhametzyanov, S.R.; Baigildeeva, E.I.; Safina, A.V.; Lebedev, D.O. Studies of the composition of the cryogenic ground chaga. IOP Conf. Ser. Mater. Sci. Eng. 2020, 986, 012029. [Google Scholar] [CrossRef]
- Pharmacopoeia European Pharmacopoeia, 9th ed.; Council of Europe: Strasbourg, France, 2017; pp. 183–184.
- Castro-Puyana, M.; Pérez-Sánchez, A.; Valdés, A.; Ibrahim, O.H.M.; Suarez-Álvarez, S.; Ferragut, J.A.; Micol, V.; Cifuentes, A.; Ibáñez, E.; García-Cañas, V. Pressurized liquid extraction of Neochloris oleoabundans for the recovery of bioactive carotenoids with anti-proliferative activity against human colon cancer cells. Food Res. Int. 2016, 99, 1048–1055. [Google Scholar] [CrossRef]
- Almela, L.; Fernández-López, J.A.; Roca, M.J. High-performance liquid chromatographic screening of chlorophyll derivatives produced during fruit storage. J. Chromatogr. A 2000, 870, 483–489. [Google Scholar] [CrossRef]
- Lafeuille, J.L.; Lefèvre, S.; Lebuhotel, J. Quantitation of chlorophylls and 22 of their colored degradation products in culinary aromatic herbs by HPLC-DAD-MS and correlation with color changes during the dehydration process. J. Agric. Food Chem. 2014, 62, 1926–1935. [Google Scholar] [CrossRef]
- Edelenbos, M.; Christensen, L.P.; Grevsen, K. HPLC determination of chlorophyll and carotenoid pigments in processed green pea cultivars (Pisum sativum L.). J. Agric. Food Chem. 2001, 49, 4768–4774. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Zorić, Z.; Pedisić, S.; Brnčić, M.; Dragović-Uzelac, V. UPLC-MS2 Profiling of Blackthorn Flower Polyphenols Isolated by Ultrasound-Assisted Extraction. J. Food Sci. 2018, 83, 2782–2789. [Google Scholar] [CrossRef]
- Carboni Martins, C.; Rodrigues, R.C.; Domeneghini Mercali, G.; Rodrigues, E. New insights into non-extractable phenolic compounds analysis. Food Res. Int. 2022, 157, 111487. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, I.; Maslov, L. Simultaneous determination of phenolic compounds in different matrices using phenyl-hexyl stationary phase. Food Anal. Methods 2016, 9, 401–410. [Google Scholar] [CrossRef]
- Benkaci-Ali, F.; Akloul, R.; Boukenouche, A.; Pauw, E.D. Composition of the Essential Oil of Nigella sativa Seeds Extracted by Microwave Steam Distillation. J. Essent. Oil Bear. Plants 2013, 16, 781–794. [Google Scholar] [CrossRef]
- Pereira, P.C.; Cebola, M.J.; Bernardo-Gil, M.G. Evolution of the yields and composition of essential oil from Portuguese myrtle (Myrtus comunis L.) through the vegetative cycle. Molecules 2009, 14, 3094–3105. [Google Scholar] [CrossRef]
- Bekhechi, C.; Malti, C.E.W.; Boussaïd, M.; Achouri, I.; Belilet, K.; Gibernau, M.; Casanova, J.; Tomi, F. Composition and Chemical Variability of Myrtus communis Leaf Oil From Northwestern Algeria. Nat. Prod. Commun. 2019, 14, 1–6. [Google Scholar] [CrossRef]
- Tischer, B.; Vendruscolo, R.G.; Wagner, R.; Menezes, C.R.; Barin, C.S.; Giacomelli, S.R.; Budel, J.M.; Barin, J.S. Effect of grinding method on the analysis of essential oil from Baccharis articulata (Lam.) Pers. Chem. Pap. 2017, 71, 753–761. [Google Scholar] [CrossRef]
- Kratky, L.; Jirout, T. Mechanical disintegration of wheat straw using a roller-plate grinding system with sharp-edged segments. Acta Polytech. 2015, 55, 113–122. [Google Scholar] [CrossRef][Green Version]
- Čukelj Mustač, N.; Novotni, D.; Habuš, M.; Drakula, S.; Nanjara, L.; Voučko, B.; Benković, M.; Ćurić, D. Storage stability, micronisation, and application of nutrient-dense fraction of proso millet bran in gluten-free bread. J. Cereal Sci. 2020, 91, 102864. [Google Scholar] [CrossRef]
- Caputo, L.; Capozzolo, F.; Amato, G.; De Feo, V.; Fratianni, F.; Vivenzio, G.; Nazzaro, F. Chemical composition, antibiofilm, cytotoxic, and anti-acetylcholinesterase activities of Myrtus communis L. leaves essential oil. BMC Complement. Med. Ther. 2022, 22, 1–16. [Google Scholar] [CrossRef]
- Baydar, H.; Schulz, H.; Krüger, H.; Erbas, S.; Kineci, S. Influences of fermentation time, hydro-distillation time and fractions on essential oil composition of damask rose (Rosa damascena mill.). J. Essent. Oil-Bear. Plants 2008, 11, 224–232. [Google Scholar] [CrossRef]
- Berka-Zougali, B.; Ferhat, M.A.; Hassani, A.; Chemat, F.; Allaf, K.S. Comparative study of essential oils extracted from Algerian Myrtus communis L. leaves using microwaves and hydrodistillation. Int. J. Mol. Sci. 2012, 13, 4673–4695. [Google Scholar] [CrossRef] [PubMed]
- Taamalli, A.; Iswaldi, I.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrezc, A.; Zarrouk, M. UPLC–QTOF/MS for a Rapid Characterisation of Phenolic Compounds from Leaves of Myrtus. Phytochem. Anal. 2013, 25, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.N.; Rathore, S.S.; Saxena, R. Effect of cryogenic grinding on phenolic compounds and antioxidant properties of fenugreek (Trigonella foenum-graecum L.) seed extract. J. Spices Aromat. Crop. 2016, 25, 73–78. [Google Scholar]
- Jung, J.-Y.; Rhee, J.-K. Roasting and Cryogenic Grinding Enhance the Antioxidant Property of Sword Beans (Canavalia gladiata). J. Microbiol. Biotechnol. 2020, 30, 1706–1719. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, H.; Zhang, G.; Tang, W. Effect of superfine grinding on the physicochemical properties and antioxidant activity of red grape pomace powders. Powder Technol. 2015, 286, 838–844. [Google Scholar] [CrossRef]
- Gião, M.S.; Pereira, C.I.; Fonseca, S.C.; Pintado, M.E.; Malcata, F.X. Effect of particle size upon the extent of extraction of antioxidant power from the plants Agrimonia eupatoria, Salvia sp. and Satureja montana. Food Chem. 2009, 117, 412–416. [Google Scholar] [CrossRef]
- Sedem, C.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Herrero, M.; Urreta, I.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E.; Suárez-Alvarez, S. Optimization of clean extraction methods to isolate carotenoids from the microalga Neochloris oleoabundans and subsequent chemical characterization using liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 4607–4616. [Google Scholar] [CrossRef]
- Sánchez, C.; Baranda, A.B.; de Marañón, I.M. The effect of high pressure and high temperature processing on carotenoids and chlorophylls content in some vegetables. Food Chem. 2014, 163, 37–45. [Google Scholar] [CrossRef]
- Saxena, R.; Saxena, S.N.; Barnwal, P.; Rathore, S.S.; Sharma, Y.K.; Soni, A. Estimation of antioxidant activity, phenolic and flavonoid content of cryo and conventionally ground seeds of coriander (Coriandrum sativum L.) and fenugreek (Trigonella foenum-graecum L.). Inter. J. Seed Spices 2012, 2, 83–86. [Google Scholar]
- Ghodki, B.M.; Goswami, T.K. Optimization of cryogenic grinding process for cassia (Cinnamomum loureirii Nees L.). J. Food Process Eng. 2016, 39, 659–675. [Google Scholar] [CrossRef]
- Shmatchenko, N.; Artamonova, M.; Aksonova, O.; Oliinyk, S. Investigation of the Properties of Marmalade with Plant Cryoadditives During Storage. Food Sci. Technol. 2017, 11, 82–89. [Google Scholar] [CrossRef]
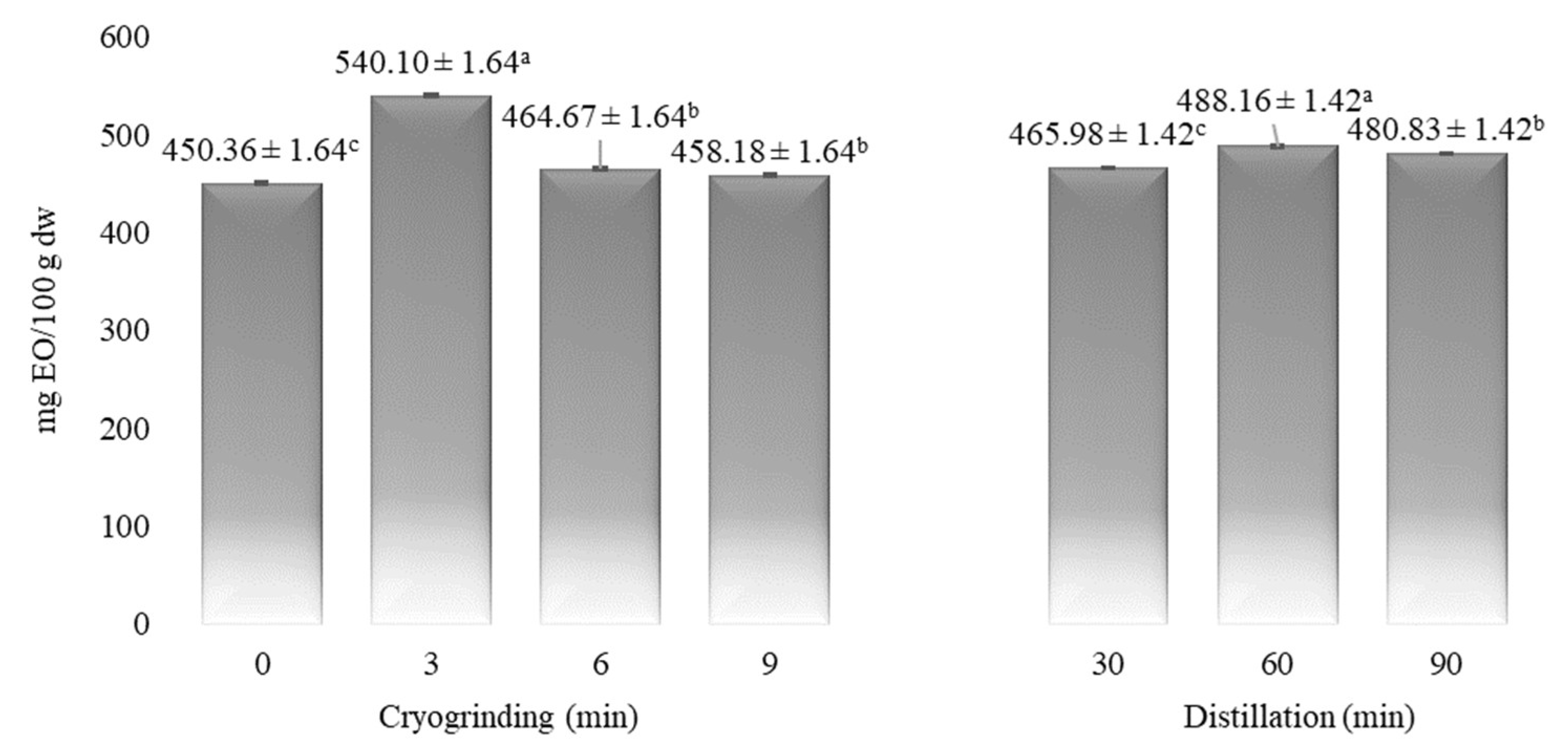
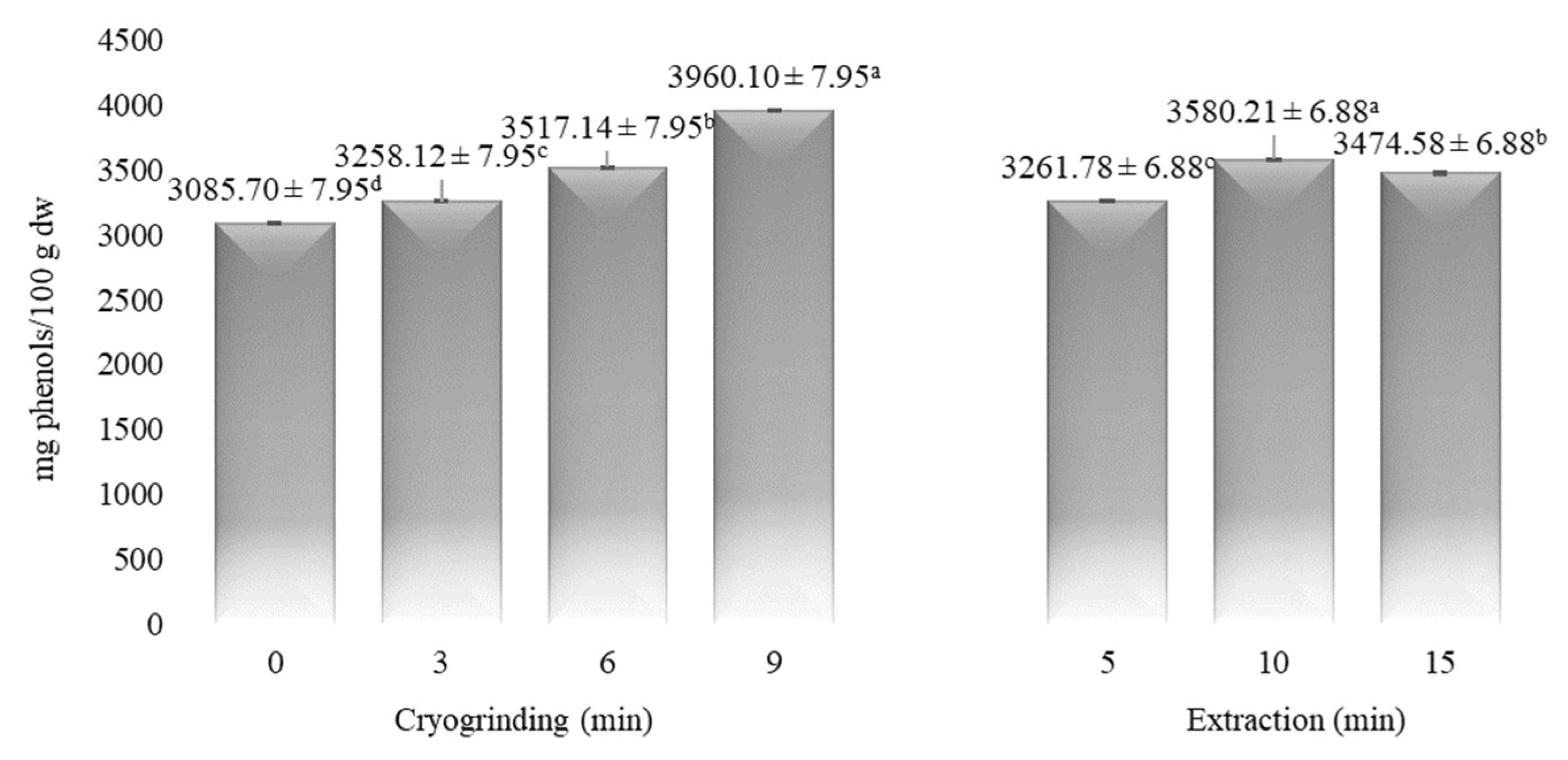
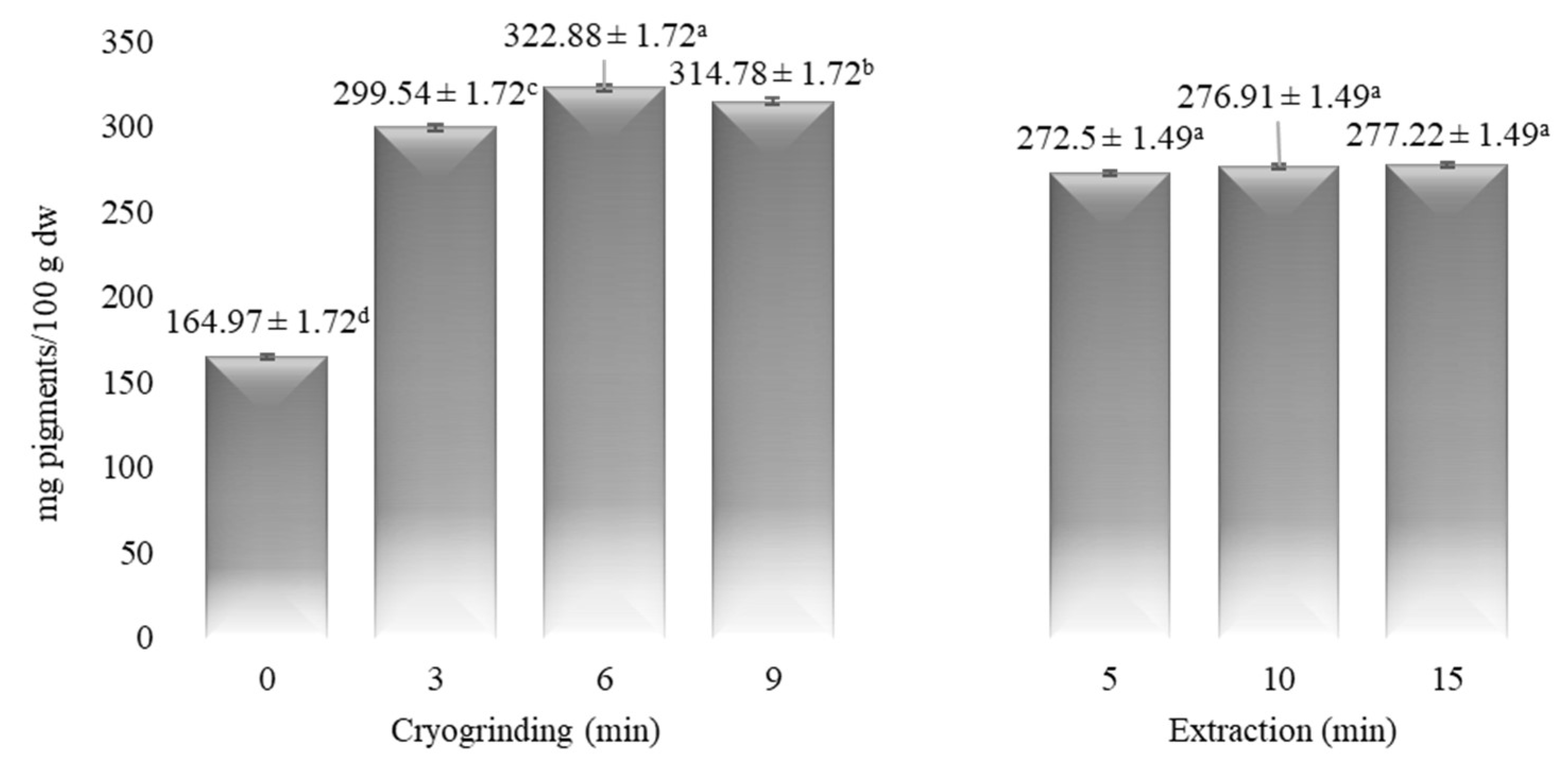
| Time (min) | α-Pinene | Myrcene | p-Cymene | Limonene | 1,8-Cineole | Linalool | α-Terpineol | Myrtenol | Geraniol | Myrtenyl Acetate | Eugenol |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cryogrinding | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * |
| 0 | 71.85 ± 0.35 d | 3.06 ± 0.02 c | 2.52 ± 0.01 c | 12.19 ± 0.07 d | 135.12 ± 0.63 b | 49.67 ± 0.26 c | 16.64 ± 0.08 c | 5.05 ± 0.02 a | 4.60 ± 0.02 d | 136.15 ± 0.44 b | 3.16 ± 0.01 d |
| 3 | 97.60 ± 0.35 a | 4.26 ± 0.02 a | 2.90 ± 0.01 a | 16.00 ± 0.07 a | 158.30 ± 0.63 a | 60.66 ± 0.26 a | 20.91 ± 0.08 a | 4.85 ± 0.02 b | 5.64 ± 0.02 a | 153.62 ± 0.44 a | 3.41 ± 0.01 a |
| 6 | 85.50 ± 0.35 b | 3.55 ± 0.02 b | 2.57 ± 0.01 b | 13.71 ± 0.07 b | 134.48 ± 0.63 b | 50.09 ± 0.26 c | 16.25 ± 0.08 d | 4.06 ± 0.02 d | 4.89 ± 0.02 c | 135.15 ± 0.44 b | 3.29 ± 0.01 c |
| 9 | 81.30 ± 0.35 c | 3.59 ± 0.02 b | 2.53 ± 0.01 c | 12.92 ± 0.07 c | 132.70 ± 0.63 b | 52.31 ± 0.26 b | 17.54 ± 0.08 b | 4.24 ± 0.02 c | 5.22 ± 0.02 b | 131.29 ± 0.44 c | 3.38 ± 0.01 b |
| Distillation | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p = 0.096 | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * |
| 30 | 78.20 ± 0.30 c | 3.54 ± 0.01 b | 2.53 ± 0.01 c | 12.90 ± 0.06 c | 141.89 ± 0.54 a | 54.09 ± 0.22 a | 17.90 ± 0.07 a | 4.32 ± 0.02 c | 4.81 ± 0.02 b | 132.03 ± 0.38 c | 3.14 ± 0.00 c |
| 60 | 86.19 ± 0.30 b | 3.80 ± 0.01 a | 2.74 ± 0.01 a | 14.25 ± 0.06 a | 141.43 ± 0.54 a | 53.67 ± 0.22 a | 17.90 ± 0.07 a | 4.59 ± 0.02 b | 5.24 ± 0.02 a | 143.56 ± 0.38 a | 3.38 ± 0.00 b |
| 90 | 87.79 ± 0.30 a | 3.51 ± 0.01 b | 2.62 ± 0.01 b | 13.97 ± 0.06 b | 137.13 ± 0.54 b | 51.78 ± 0.22 b | 17.70 ± 0.07 a | 4.74 ± 0.02 a | 5.22 ± 0.02 a | 141.57 ± 0.38 b | 3.41 ± 0.00 a |
| Interaction | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p = 0.003 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p = 0.01 * |
| 0 × 30 | 49.06 ± 0.60 g | 2.41 ± 0.03 f | 2.27 ± 0.01 f | 9.47 ± 0.13 f | 138.17 ± 1.08 b,c | 48.16 ± 0.45 e | 15.94 ± 0.14 e | 4.75 ± 0.04 b,c | 3.82 ± 0.04 g | 117.47 ± 0.76 g | 2.87 ± 0.01 e |
| 0 × 60 | 77.73 ± 0.60 e | 3.33 ± 0.03 e | 2.79 ± 0.01 b | 13.47 ± 0.13 c,d | 135.50 ± 1.08 b,c,d,e | 52.11 ± 0.45 d | 17.49 ± 0.14 c | 5.27 ± 0.04 a | 4.93 ± 0.04 e | 150.57 ± 0.76 b | 3.27 ± 0.01 c |
| 0 × 90 | 88.77 ± 0.60 c | 3.44 ± 0.03 d,e | 2.49 ± 0.01 d | 13.63 ± 0.13 c d | 131.69 ± 1.08 d,e | 48.74 ± 0.45 e | 16.50 ± 0.14 d,e | 5.13 ± 0.04 a | 5.05 ± 0.04 d,e | 140.42 ± 0.76 c,d | 3.35 ± 0.01 b |
| 3 × 30 | 105.98 ± 0.60 a | 4.77 ± 0.03 a | 3.00 ± 0.01 a | 16.72 ± 0.13 a | 160.43 ± 1.08 a | 64.83 ± 0.45 a | 21.99 ± 0.14 a | 4.81 ± 0.04 b | 5.76 ± 0.04 a | 157.82 ± 0.76 a | 3.31 ± 0.01 b,c |
| 3 × 60 | 94.40 ± 0.60 b | 4.56 ± 0.03 b | 2.95 ± 0.01 a | 16.30 ± 0.13 a | 159.93 ± 1.08 a | 60.38 ± 0.45 b | 20.57 ± 0.14 b | 4.62 ± 0.04 b,c | 5.82 ± 0.04 a | 150.81 ± 0.76 b | 3.45 ± 0.01 a |
| 3 × 90 | 92.42 ± 0.60 b | 3.45 ± 0.03 d,e | 2.76 ± 0.01 b | 14.98 ± 0.13 b | 154.53 ± 1.08 a | 56.76 ± 0.45 c | 20.16 ± 0.14 b | 5.12 ± 0.04 a | 5.35 ± 0.04 b,c | 152.21 ± 0.76 b | 3.48 ± 0.01 a |
| 6 × 30 | 83.51 ± 0.60 d | 3.44 ± 0.03 d,e | 2.47 ± 0.01 d | 13.21 ± 0.13 d | 139.09 ± 1.08 b | 50.63 ± 0.45 d,e | 15.89 ± 0.14 e | 3.75 ± 0.04 f | 4.48 ± 0.04 f | 127.44 ± 0.76 f | 3.19 ± 0.01 d |
| 6 × 60 | 84.18 ± 0.60 d | 3.65 ± 0.03 c | 2.63 ± 0.01 c | 14.05 ± 0.13 c | 134.26 ± 1.08 b,c,d,e | 50.44 ± 0.45 d,e | 16.40 ± 0.14 d,e | 4.26 ± 0.04 d | 5.08 ± 0.04 d,e | 143.75 ± 0.76 c | 3.34 ± 0.01 b |
| 6 × 90 | 88.80 ± 0.60 c | 3.57 ± 0.03 c,d | 2.61 ± 0.01 c | 13.86 ± 0.13 c,d | 130.09 ± 1.08 d,e | 49.19 ± 0.45 e | 16.46 ± 0.14 d,e | 4.17 ± 0.04 d,e | 5.1 ± 0.04 d,e | 134.27 ± 0.76 e | 3.34 ± 0.01 b |
| 9 × 30 | 74.26 ± 0.60 f | 3.55 ± 0.03 c,d | 2.37 ± 0.01 e | 12.19 ± 0.13 e | 129.86 ± 1.08 e | 52.73 ± 0.45 d | 17.78 ± 0.14 c | 3.97 ± 0.04 e,f | 5.16 ± 0.04 c,d | 125.39 ± 0.76 f | 3.2 ± 0.01 d |
| 9 × 60 | 88.44 ± 0.60 c | 3.65 ± 0.03 c | 2.60 ± 0.01 c | 13.16 ± 0.13 d | 136.02 ± 1.08 b,c,d | 51.76 ± 0.45 d | 17.15 ± 0.14 c,d | 4.20 ± 0.04 d | 5.12 ± 0.04 d,e | 129.11 ± 0.76 f | 3.44 ± 0.01 a |
| 9 × 90 | 81.19 ± 0.60 d | 3.58 ± 0.03 c,d | 2.61 ± 0.01 c | 13.40 ± 0.13 d | 132.22 ± 1.08 c,d,e | 52.43 ± 0.45 d | 17.69 ± 0.14 c | 4.54 ± 0.04 c | 5.38 ± 0.04 b | 139.38 ± 0.76 d | 3.49 ± 0.01 a |
| Time (min) | Myricetin 3-O-G | Myricetin 3-O-R | Q-3-G | EGKG | Quercitrin | Myricetin | Digalloylquinic A | Galloylquinic A | Caffeic A | Gallic A |
|---|---|---|---|---|---|---|---|---|---|---|
| Cryogrinding | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * |
| 0 | 1287.19 ± 2.89 d | 1081.19 ± 2.89 d | 16.14 ± 0.43 b | 21.68 ± 0.29 d | 12.32 ± 0.26 c | 186.97 ± 0.87 b | 95.50 ± 0.87 c | 280.26 ± 0.58 b | 15.61 ± 0.29 c | 59.93 ± 0.23 b |
| 3 | 1364.81 ± 2.89 c | 1179.41 ± 2.89 c | 17.84 ± 0.43 b | 24.55 ± 0.29 c | 13.52 ± 0.26 b | 190.26 ± 0.87 b | 121.55 ± 0.87 b | 244.73 ± 0.58 d | 18.51 ± 0.29 b | 66.36 ± 0.23 a |
| 6 | 1506.90 ± 2.89 b | 1256.85 ± 2.89 b | 21.18 ± 0.43 a | 26.57 ± 0.29 b | 16.94 ± 0.26 a | 229.06 ± 0.87 a | 124.94 ± 0.87 b | 248.91 ± 0.58 c | 21.80 ± 0.29 a | 52.94 ± 0.23 c |
| 9 | 1698.98 ± 2.89 a | 1396.04 ± 2.89 a | 21.15 ± 0.43 a | 28.09 ± 0.29 a | 16.40 ± 0.26 a | 228.43 ± 0.87 a | 139.76 ± 0.87 a | 334.84 ± 0.58 a | 18.22 ± 0.29 b | 66.32 ± 0.23 a |
| Extraction | p < 0.0001 * | p < 0.0001 * | p = 0.137 | p < 0.0001 * | p = 0.025 * | p = 0.176 | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * |
| 5 | 1383.56 ± 2.50 c | 1174.73 ± 2.50 c | 19.48 ± 0.37 a | 22.74 ± 0.25 c | 15.36 ± 0.23 a | 207.52 ± 0.75 a | 115.23 ± 0.75 b | 258.12 ± 0.50 c | 18.99 ± 0.25 b | 46.04 ± 0.20 c |
| 10 | 1532.05 ± 2.50 a | 1268.50 ± 2.50 a | 19.33 ± 0.37 a | 27.50 ± 0.25 a | 14.67 ± 0.23 a,b | 208.90 ± 0.75 a | 130.79 ± 0.75 a | 295.19 ± 0.50 a | 20.59 ± 0.25 a | 62.69 ± 0.20 b |
| 15 | 1477.80 ± 2.50 b | 1241.88 ± 2.50 b | 18.42 ± 0.37 a | 25.43 ± 0.25 b | 14.37 ± 0.23 b | 209.62 ± 0.75 a | 115.29 ± 0.75 b | 278.24 ± 0.50 b | 18.10 ± 0.25 b | 75.44 ± 0.20 a |
| Interaction | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * |
| 0 × 5 | 1323.18 ± 5.00 f | 1093.95 ± 5.00 h | 16.52 ± 0.75 c,d | 20.10 ± 0.50 e | 11.64 ± 0.45 e | 179.30 ± 1.50 g | 103.63 ± 1.50 g | 272.09 ± 1.00 e | 24.10 ± 0.50 d | 48.04 ± 0.40 g |
| 0 × 10 | 1475.54 ± 5.00 d | 1233.61 ± 5.00 e,f | 18.36 ± 0.75 b,c | 25.76 ± 0.50 c,d | 13.43 ± 0.45 d,e | 201.35 ± 1.50 f | 115.13 ± 1.50 e,f | 384.11 ± 1.00 b | 13.88 ± 0.50 f | 67.17 ± 0.40 d |
| 0 × 15 | 1062.86 ± 5.00 h | 916.01 ± 5.00 j | 13.56 ± 0.75 d | 19.17 ± 0.50 e | 11.88 ± 0.45 e | 180.27 ± 1.50 g | 67.75 ± 1.50 h | 184.59 ± 1.00 i | 61.16 ± 0.50 a | 64.59 ± 0.40 e |
| 3 × 5 | 1111.21 ± 5.00 g | 1051.48 ± 5.00 i | 16.18 ± 0.75 c,d | 20.42 ± 0.50 e | 12.84 ± 0.45 d,e | 181.74 ± 1.50 g | 109.99 ± 1.50 f,g | 335.02 ± 1.00 c | 45.89 ± 0.50 b | 50.69 ± 0.40 f |
| 3 × 10 | 1500.58 ± 5.00 d | 1258.00 ± 5.00 e | 18.80 ± 0.75 b,c | 25.77 ± 0.50 c,d | 13.65 ± 0.45 c,d,e | 181.63 ± 1.50 g | 127.40 ± 1.50 b,c,d | 165.71 ± 1.00 j | 13.49 ± 0.50 f | 67.70 ± 0.40 d |
| 3 × 15 | 1482.63 ± 5.00 d | 1228.76 ± 5.00 f | 18.54 ± 0.75 b,c | 27.45 ± 0.50 a,b,c,d | 14.08 ± 0.45 b,c,d,e | 207.41 ± 1.50 e,f | 127.27 ± 1.50 b,c,d | 233.45 ± 1.00 g | 7.29 ± 0.50 g | 80.69 ± 0.40 b |
| 6 × 5 | 1567.01 ± 5.00 b | 1259.66 ± 5.00 e | 25.76 ± 0.75 a | 25.22 ± 0.50 d | 20.79 ± 0.45 a | 249.94 ± 1.50 a | 119.07 ± 1.50 d,e | 191.45 ± 1.00 h | 33.01 ± 0.50 c | 45.01 ± 0.40 h |
| 6 × 10 | 1379.56 ± 5.00 e | 1165.40 ± 5.00 g | 18.37 ± 0.75 b,c | 28.22 ± 0.50 a,b,c | 15.04 ± 0.45 b,c,d | 222.37 ± 1.50 c,d | 132.90 ± 1.50 b | 251.82 ± 1.00 f | 7.44 ± 0.50 g | 45.42 ± 0.40 h |
| 6 × 15 | 1574.14 ± 5.00 b | 1345.50 ± 5.00 c | 19.42 ± 0.75 b,c | 26.28 ± 0.50 b,c,d | 15.00 ± 0.45 b,c,d | 214.88 ± 1.50 d,e | 122.86 ± 1.50 c,d,e | 303.46 ± 1.00 d | 24.94 ± 0.50 d | 68.40 ± 0.40 c,d |
| 9 × 5 | 1532.84 ± 5.00 c | 1293.84 ± 5.00 d | 19.48 ± 0.75 b,c | 25.20 ± 0.50 d | 16.15 ± 0.45 b,c | 219.12 ± 1.50 d | 128.25 ± 1.50 b,c | 233.93 ± 1.00 g | 12.95 ± 0.50 f | 40.42 ± 0.40 i |
| 9 × 10 | 1772.53 ± 5.00 a | 1417.01 ± 5.00 b | 21.79 ± 0.75 a,b | 30.23 ± 0.50 a | 16.54 ± 0.45 b | 230.26 ± 1.50 b,c | 147.73 ± 1.50 a | 379.12 ± 1.00 b | 23.56 ± 0.50 d | 70.47 ± 0.40 c |
| 9 × 15 | 1791.57 ± 5.00 a | 1477.26 ± 5.00 a | 22.17 ± 0.75 a,b | 28.84 ± 0.50 a,b | 16.51 ± 0.45 b | 235.91 ± 1.50 b | 143.29 ± 1.50 a | 391.46 ± 1.00 a | 19.01 ± 0.50 e | 88.07 ± 0.40 a |
| Time (min) | Chl b | Chl b’ | Chl a | Pheo a | Lutein | 9-cis Lutein | β-Carotene |
|---|---|---|---|---|---|---|---|
| Cryogrinding | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * |
| 0 | 36.04 ± 0.23 d | 3.68 ± 0.09 d | 11.15 ± 0.17 b | 57.77 ± 1.44 c | 35.88 ± 0.12 d | 6.06 ± 0.13 c | 14.39 ± 0.20 d |
| 3 | 65.67 ± 0.23 c | 5.29 ± 0.09 c | 13.92 ± 0.17 a | 108.67 ± 1.44 b | 68.99 ± 0.12 c | 9.86 ± 0.13 b | 27.14 ± 0.20 c |
| 6 | 69.46 ± 0.23 a | 6.48 ± 0.09 b | 8.84 ± 0.17 c | 121.99 ± 1.44 a | 75.93 ± 0.12 a | 10.19 ± 0.13 b | 29.99 ± 0.20 a |
| 9 | 67.13 ± 0.23 b | 7.24 ± 0.09 a | 8.15 ± 0.17 c | 120.36 ± 1.44 a | 72.77 ± 0.12 b | 10.85 ± 0.13 a | 28.28 ± 0.20 b |
| Extraction | p = 0.012 * | p < 0.0001 * | p < 0.0001 * | p = 0.006 * | p < 0.0001 * | p = 0.048 * | p = 0.454 |
| 5 | 59.19 ± 0.20 b | 5.35 ± 0.08 b | 12.43 ± 0.15 b | 99.61 ± 1.25 b | 62.00 ± 0.10 c | 9.13 ± 0.11 b | 24.78 ± 0.17 a |
| 10 | 60.16 ± 0.20 a | 5.37 ± 0.07 b | 13.41 ± 0.15 a | 100.78 ± 1.25 b | 63.14 ± 0.10 b | 9.09 ± 0.11 b | 24.96 ± 0.17 a |
| 15 | 59.37 ± 0.20 b | 6.30 ± 0.08 a | 5.71 ± 0.15 c | 106.20 ± 1.25 a | 65.04 ± 0.10 a | 9.50 ± 0.11 a | 25.10 ± 0.17 a |
| Interaction | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * | p = 0.000 * | p < 0.0001 * | p = 0.000 * | p < 0.0001 * |
| 0 × 5 | 41.77 ± 0.40 f | 4.427 ± 0.15 d | 12.01 ± 0.30 c | 69.68 ± 2.50 f | 42.08 ± 0.20 h | 6.94 ± 0.23 d | 16.40 ± 0.35 f |
| 0 × 10 | 32.94 ± 0.40 g | 3.159 ± 0.15 e | 14.03 ± 0.30 b | 48.38 ± 2.50 g | 31.96 ± 0.20 j | 5.39 ± 0.23 e | 13.01 ± 0.35 g |
| 0 × 15 | 33.41 ± 0.40 g | 3.468 ± 0.15 e | 7.42 ± 0.30 f | 55.26 ± 2.50 g | 33.61 ± 0.20 i | 5.84 ± 0.23 d,e | 13.74 ± 0.35 g |
| 3 × 5 | 65.19 ± 0.40 d | 4.671 ± 0.15 d | 18.74 ± 0.30 a | 102.48 ± 2.50 e | 66.98 ± 0.20 g | 9.84 ± 0.23 b,c | 27.04 ± 0.35 d,e |
| 3 × 10 | 66.98 ± 0.40 c,d | 5.094 ± 0.15 d | 17.71 ± 0.30 a | 109.33 ± 2.50 c,d,e | 69.25 ± 0.20 f | 9.62 ± 0.23 c | 27.24 ± 0.35 c,d,e |
| 3 × 15 | 64.85 ± 0.40 d | 6.102 ± 0.15 c | 5.30 ± 0.30 g | 114.19 ± 2.50 b,c,d,e | 70.74 ± 0.20 e | 10.11 ± 0.23 b,c | 27.14 ± 0.35 c,d,e |
| 6 × 5 | 67.96 ± 0.40 b,c | 6.281 ± 0.15 c | 10.31 ± 0.30 d,e | 117.62 ± 2.50 a,b,c,d | 72.28 ± 0.20 d | 10.01 ± 0.23 b,c | 29.02 ± 0.35 a,b,c |
| 6 × 10 | 71.31 ± 0.40 a | 5.988 ± 0.15 c | 11.02 ± 0.30 c,d | 122.91 ± 2.50 a,b,c | 77.10 ± 0.20 b | 10.36 ± 0.23 b,c | 30.93 ± 0.35 a |
| 6 × 15 | 69.11 ± 0.40 a,b,c | 7.176 ± 0.15 b | 5.20 ± 0.30 g | 125.46 ± 2.50 a,b | 78.40 ± 0.20 a | 10.22 ± 0.23 b,c | 30.01 ± 0.35 a,b |
| 9 × 5 | 61.86 ± 0.40 e | 6.021 ± 0.15 c | 8.67 ± 0.30 e,f | 108.68 ± 2.50 d,e | 66.66 ± 0.20 g | 9.72 ± 0.23 c | 26.67 ± 0.35 e |
| 9 × 10 | 69.40 ± 0.40 a,b | 7.241 ± 0.15 b | 10.86 ± 0.30 c,d | 122.51 ± 2.50 a,b,c,d | 74.25 ± 0.20 c | 11.01 ± 0.23 a,b | 28.67 ± 0.35 b,c,d |
| 9 × 15 | 70.12 ± 0.40 a,b | 8.460 ± 0.15 a | 4.93 ± 0.30 g | 129.90 ± 2.50 a | 77.41 ± 0.20 a,b | 11.82 ± 0.23 a | 29.51 ± 0.35 a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cvitković, D.; Lisica, P.; Zorić, Z.; Pedisić, S.; Repajić, M.; Dragović-Uzelac, V.; Balbino, S. The Influence of Cryogrinding on Essential Oil, Phenolic Compounds and Pigments Extraction from Myrtle (Myrtus communis L.) Leaves. Processes 2022, 10, 2716. https://doi.org/10.3390/pr10122716
Cvitković D, Lisica P, Zorić Z, Pedisić S, Repajić M, Dragović-Uzelac V, Balbino S. The Influence of Cryogrinding on Essential Oil, Phenolic Compounds and Pigments Extraction from Myrtle (Myrtus communis L.) Leaves. Processes. 2022; 10(12):2716. https://doi.org/10.3390/pr10122716
Chicago/Turabian StyleCvitković, Daniela, Patricija Lisica, Zoran Zorić, Sandra Pedisić, Maja Repajić, Verica Dragović-Uzelac, and Sandra Balbino. 2022. "The Influence of Cryogrinding on Essential Oil, Phenolic Compounds and Pigments Extraction from Myrtle (Myrtus communis L.) Leaves" Processes 10, no. 12: 2716. https://doi.org/10.3390/pr10122716
APA StyleCvitković, D., Lisica, P., Zorić, Z., Pedisić, S., Repajić, M., Dragović-Uzelac, V., & Balbino, S. (2022). The Influence of Cryogrinding on Essential Oil, Phenolic Compounds and Pigments Extraction from Myrtle (Myrtus communis L.) Leaves. Processes, 10(12), 2716. https://doi.org/10.3390/pr10122716











