Investigation of Catalytic and Photocatalytic Degradation of Methyl Orange Using Doped LaMnO3 Compounds
Abstract
1. Introduction
2. Materials and Method
3. Results and Discussion
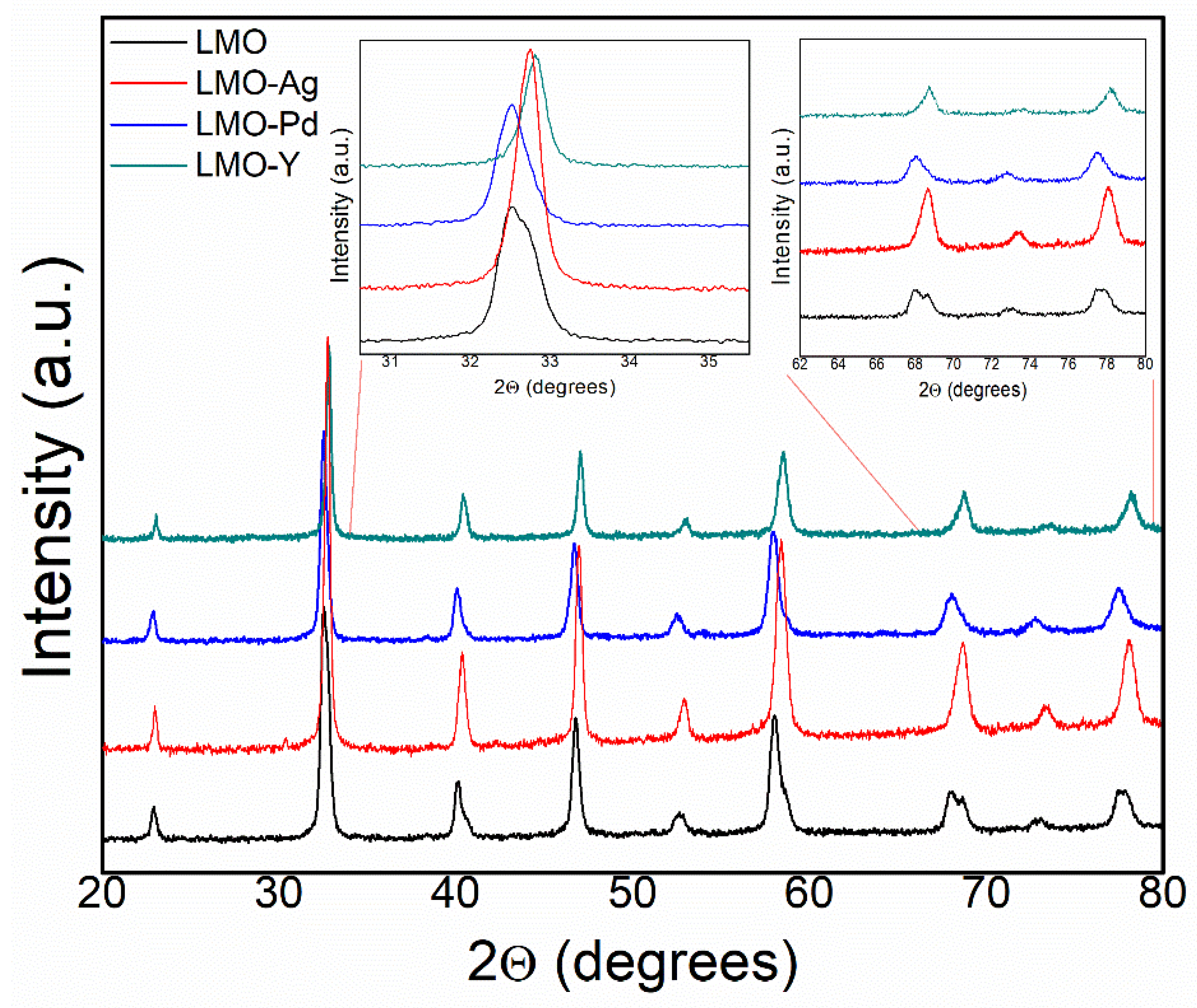
3.1. XRD Analysis
3.2. FT-IR Analysis
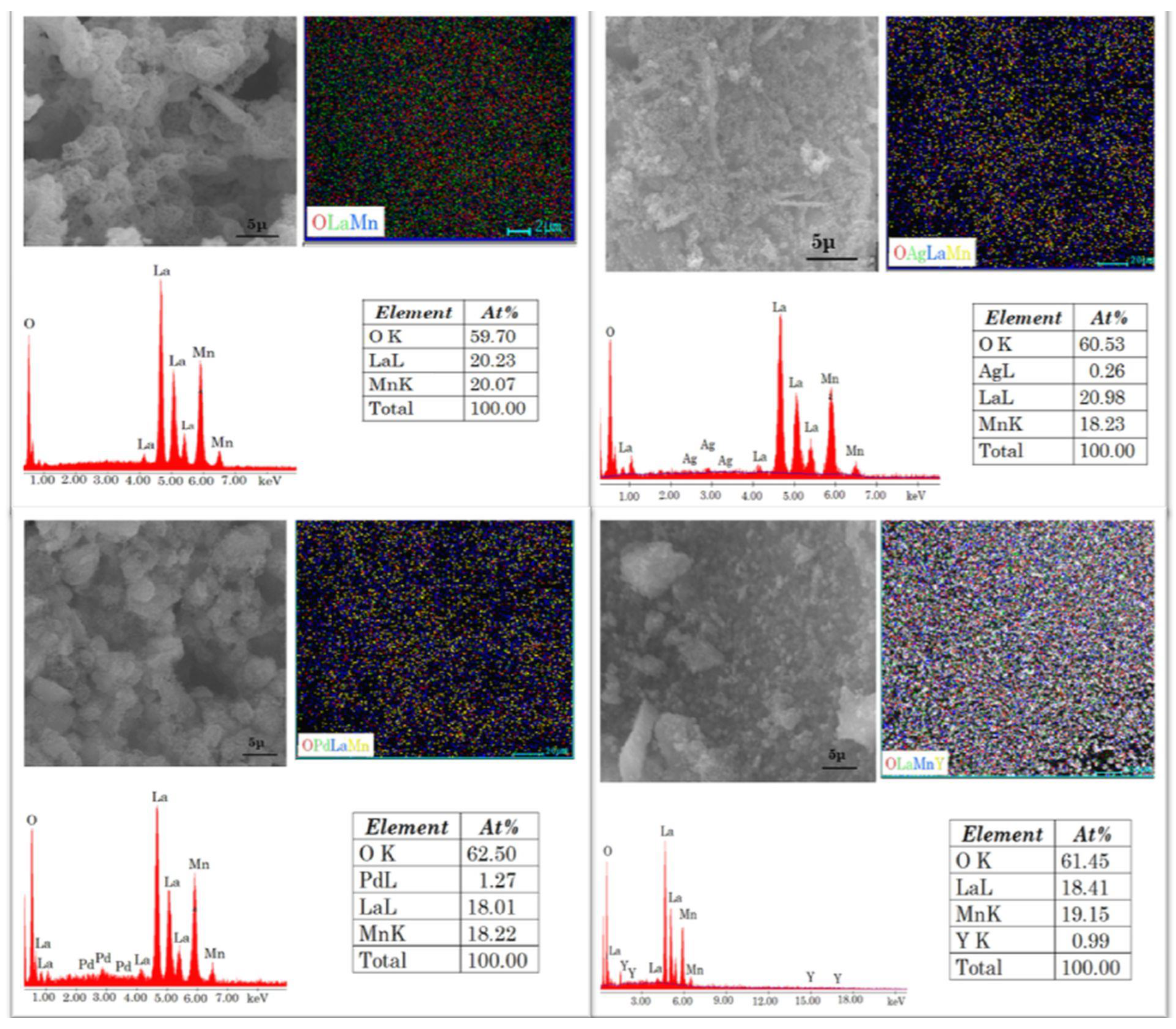
3.3. SEM-EDX/ Element Mapping
3.4. Catalytic Activity of the LaMnO3 Nanomaterials
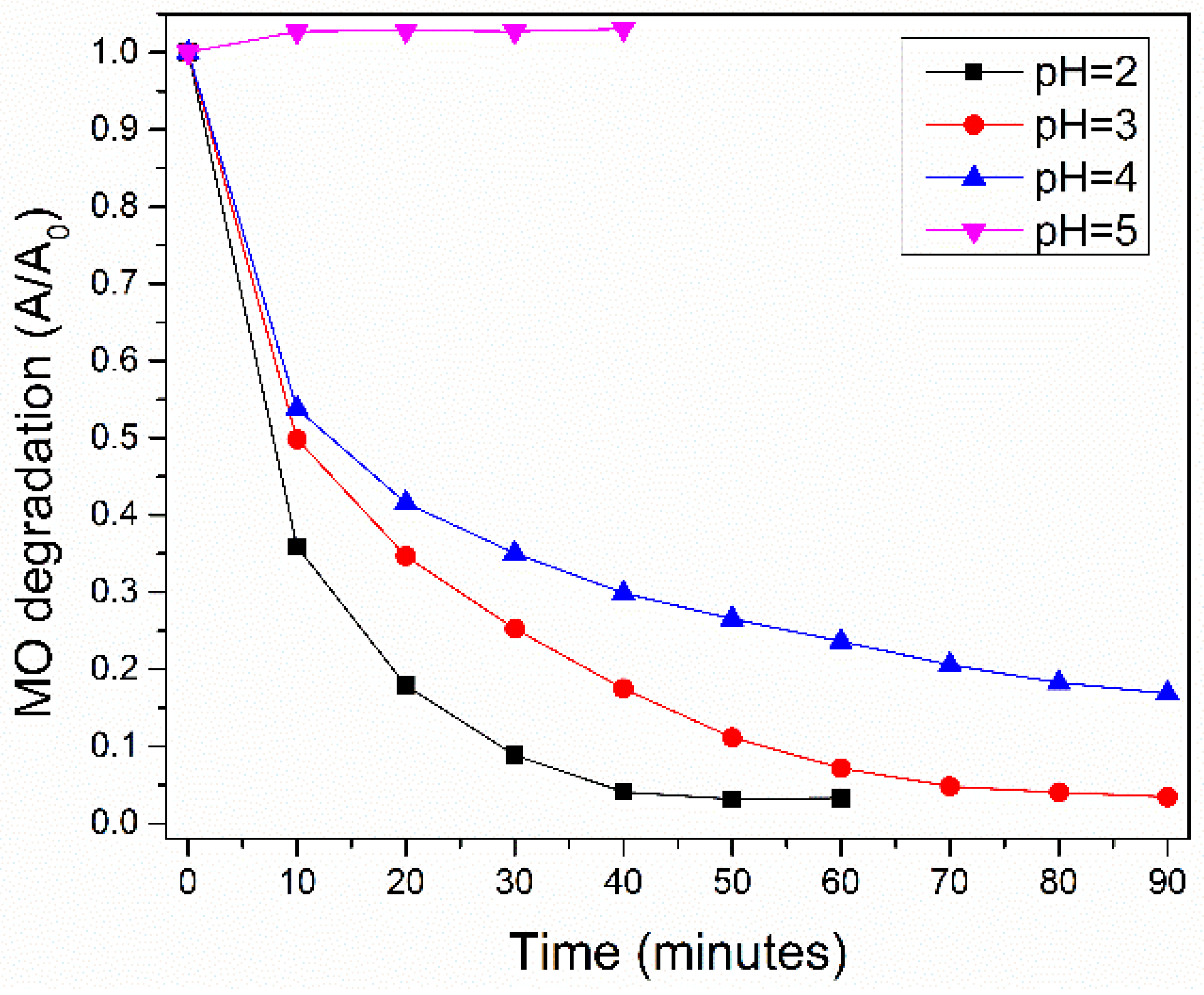
3.4.1. The Effect of the pH on the MO Absorption and on the Catalytic MO Degradation over Undoped LMO; Kinetic Parameters Determination
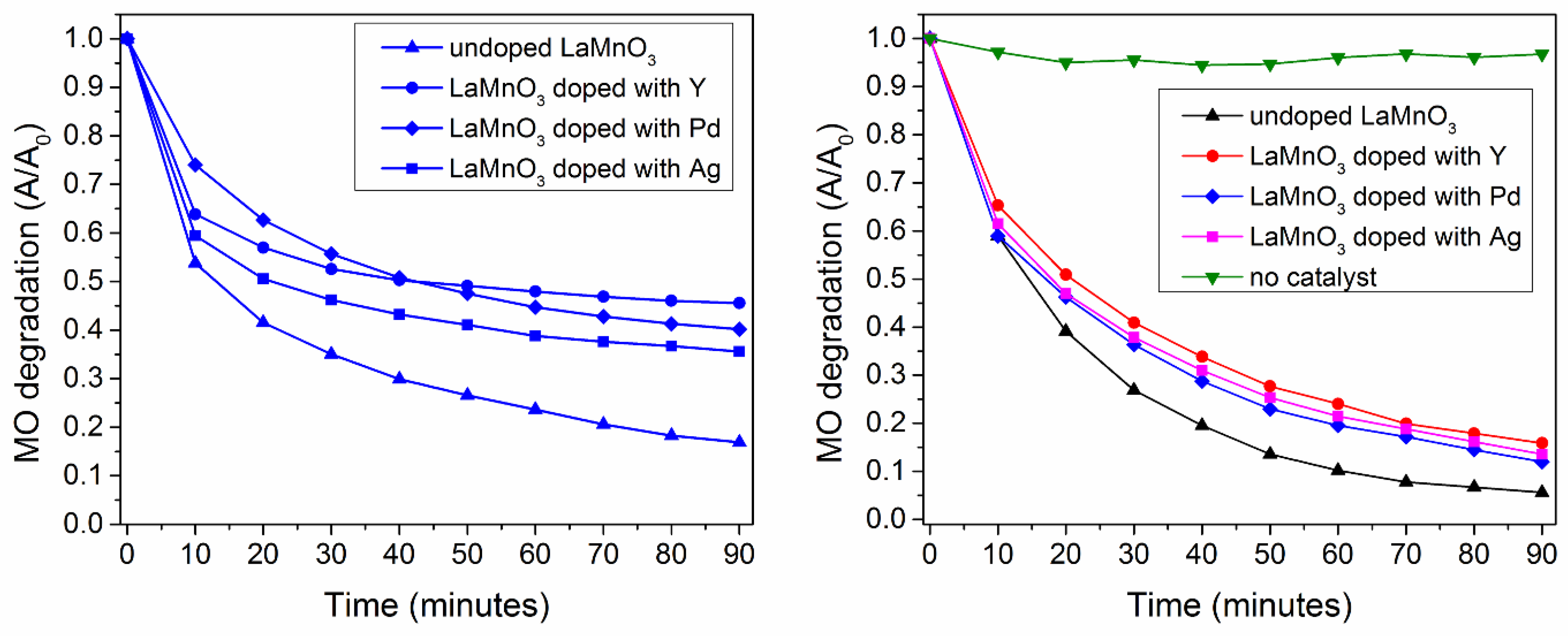
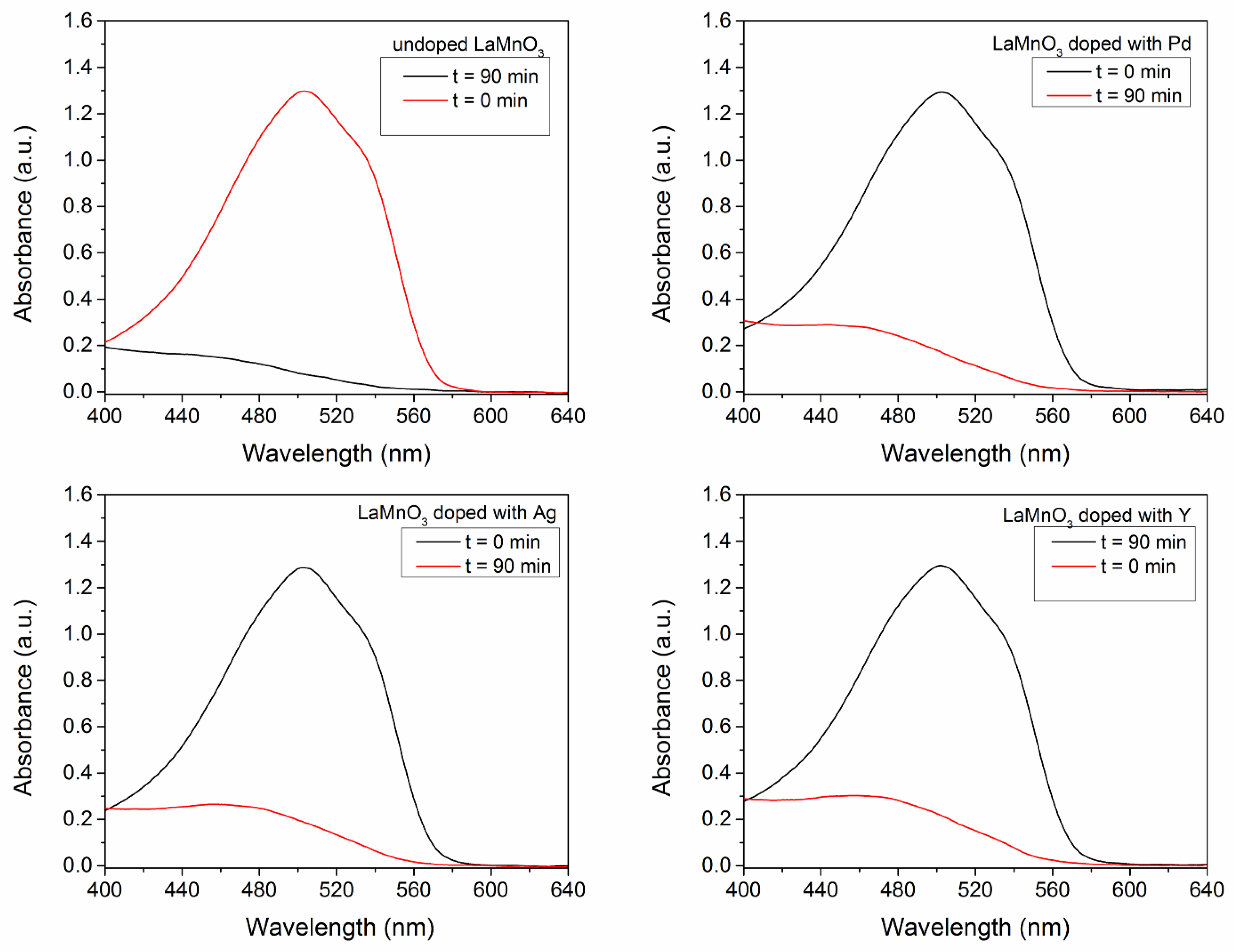
3.4.2. The Dopant Effect on the Catalytic and Photocatalytic Properties of LaMnO3—Comparative Approach
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rojas-Cervantes, M.L.; Castillejos, E. Perovskites as Catalysts in Advanced Oxidation Processes for Wastewater Treatment. Catalyst 2019, 9, 230. [Google Scholar] [CrossRef]
- Hu, J.; Men, J.; Ma, J.; Huang, H. Preparation of LaMnO3/graphene thin films and their photocatalytic activity. J. Rare Earths 2014, 32, 1126–1134. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y.; Men, J.; Zhang, L.; Huang, H. Ag modified LaMnO3 nanorods-reduced graphene oxide composite applied in the photocatalytic discoloration of direct green. Solid State Sci. 2016, 61, 239–245. [Google Scholar] [CrossRef]
- Gao, P.; Li, N.; Wang, A.; Wang, X.; Zhang, T. Perovskite LaMnO3 hollow nanospheres: The synthesis and the application in catalytic wet air oxidation of phenol. Mater. Lett. 2013, 92, 173–176. [Google Scholar] [CrossRef]
- Andrei, F.; Zavoianu, R.; Marcu, I.C. Complex Catalytic Materials Based on the Perovskite-Type Structure for Energy and Environmental Applications. Materials 2020, 13, 5555. [Google Scholar] [CrossRef]
- Labhasetwar, N.; Saravanan, G.; Megarajan, S.K.; Manwar, N.; Khobragade, R.; Doggali, P.; Grasset, F. Perovskite-type catalytic materials for environmental applications. Sci. Technol. Adv. Mater. 2015, 16, 036002. [Google Scholar] [CrossRef]
- Dhinesh Kumar, R.; Thangappan, R.; Jayavel, R. Enhanced visible light photocatalytic activity of LaMnO3 nanostructures for water purification. Res. Chem. Intermed. 2018, 44, 4323–4337. [Google Scholar] [CrossRef]
- Priyatharshni, S.; Kumar, S.R.; Viswanathan, C.; Ponpandian, N. Morphologically tuned LaMnO3 as an efficient nanocatalyst for the removal of organic dye from aqueous solution under sunlight. J. Environ. Chem. Eng. 2020, 8, 104146. [Google Scholar] [CrossRef]
- Tran, T.H.; Phi, T.H.; Nguyen, H.N.; Pham, N.H.; Nguyen, C.V.; Ho, K.H.; Doan, Q.K.; Le, V.Q.; Nguyen, T.T.; Nguyen, V.T. Sr doped LaMnO3 nanoparticles prepared by microwave combustion method: A recyclable visible light photocatalyst. Results Phys. 2020, 19, 103417. [Google Scholar] [CrossRef]
- Rekavandi, N.; Malekzadeh, A.; Ghiasi, E. Methyl orange degradation using nano-LaMnO3 as a green catalyst under the mild conditions. Nanochem. Res. 2019, 4, 1–10. [Google Scholar]
- Dhiman, T.K.; Singh, S. Enhanced catalytic and photocatalytic degradation of organic pollutans Rhodamine -B by LaMnO3 nanoparticles synthesized by non -aqueous sol-gel route. Phys. Status Solidi 2019, 216, 1900012. [Google Scholar] [CrossRef]
- Hu, J.; Men, J.; Liu, Y.; Huang, H.; Jiao, T. One-pot synthesis of Ag-modified LaMnO3-graphene hybrid photocatalysts and application in the photocatalytic discoloration of an azo-dye. RSC Adv. 2015, 5, 54028–54036. [Google Scholar] [CrossRef]
- Jauhar, S.; Dhiman, M.; Bansal, S.; Singhal, S. Mn3+ ion in perovskite lattice: A potential Fenton’s reagent exhibiting remarkably enhanced degradation of cationic and anionic dyes. J. Sol-Gel Sci. Technol. 2015, 75, 124–133. [Google Scholar] [CrossRef]
- Šojić Merkulov, D.; Vlazan, P.; Poienar, M.; Bognár, S.; Ianasi, C.; Sfirloaga, P. Sustainable removal of 17α-ethynylestradiol from aqueous environment using rare earth doped lanthanum manganite nanomaterials. Catal. Today 2022. [Google Scholar] [CrossRef]
- Sfirloaga, P.; Poienar, M.; Malaescu, I.; Lungu, A.; Mihali, C.V.; Vlazan, P. Electrical conductivity of Ca-substituted lanthanum manganites. Ceram. Int. 2018, 44, 5823–5828. [Google Scholar] [CrossRef]
- Sfirloaga, P.; Sebarchievici, I.; Taranu, B.; Poienar, M.; Vlase, G.; Vlase, T.; Vlazan, P. Investigation of physico-chemical features of lanthanum manganite with nitrogen addition. J. Alloys Compd. 2020, 843, 155854. [Google Scholar] [CrossRef]
- Sfirloaga, P.; Poienar, M.; Malaescu, I.; Lungu, A.; Vlazan, P. Perovskite type lanthanum manganite: Morpho-structural analysis and electrical investigations. J. Rare Earths 2018, 36, 499–504. [Google Scholar] [CrossRef]
- El-Moez, A.; Mohamed, A.; Alvarez-Alonso, P.; Hernando, B. The intrinsic exchange bias effect in the LaMnO3 and LaFeO3 compounds. J. Alloys. Compd. 2021, 850, 156713. [Google Scholar] [CrossRef]
- Elsiddig, Z.A.; Xu, H.; Wang, D.; Zhang, W.; Guo, X.; Zhang, Y.; Sun, Z.; Chen, J. Modulating Mn4+ Ions and Oxygen Vacancies in Nonstoichiometric LaMnO3 Perovskite by a Facile Sol-Gel Method as High-Performance Supercapacitor Electrodes. Electrochim. Acta 2017, 253, 422–429. [Google Scholar] [CrossRef]
- Li, Y.; Xue, L.; Fan, L.; Yan, Y. The effect of citric acid to metal nitrates molar ratio on sol–gel combustion synthesis of nanocrystalline LaMnO3 powders. J. Alloys Compd. 2009, 478, 493–497. [Google Scholar] [CrossRef]
- Onrubia, J.A.; Pereda-Ayo, B.; De-La-Torre, U.; González-Velasco, J.R. Key factors in Sr-doped LaBO3 (B = Co or Mn) perovskites for NO oxidation in efficient diesel exhaust purification. Appl. Catal. B Environ. 2017, 213, 198–210. [Google Scholar] [CrossRef]
- Shaterian, M.; Enhessari, M.; h Rabbani, D.; Asghari, M.; Salavati-Niasari, M. Synthesis, Characterization and Photocatalytic Activity of LaMnO3 Nanoparticles. Appl. Surf. Sci. 2014, 318, 213–217. [Google Scholar] [CrossRef]
- Yin, X.; Wang, S.; Wang, B.; Shen, L. Perovskite-type LaMn1−xBxO3+δ (B = Fe, Co and Ni) as oxygen carriers for chemical looping steam methane reforming. Chem. Eng. J. 2021, 422, 128751. [Google Scholar] [CrossRef]
- Sfirloaga, P.; Malaescu, I.; Marin, C.N.; Poienar, M.; Vlazan, P. Effect of Fe-Doping on the Structural, Morphological and Electrical Properties of LaMnO3. AIP Conf. Proc. 2020, 2218, 040003. [Google Scholar]
- Sfirloaga, P.; Vlase, G.; Vlase, T.; Malaescu, I.; Marin, C.N.; Vlazan, P. Silver doping in lanthanum manganite materials: Structural and electrical properties. J. Therm. Anal. Calorim. 2020, 142, 1817–1823. [Google Scholar] [CrossRef]
- Sfirloaga, P.; Malaescu, I.; Marin, C.N.; Vlazan, P. The effect of partial substitution of Pd in LaMnO3 polycrystalline materials synthesized by sol–gel technique on the electrical performance. J. Sol-Gel Sci. Technol. 2019, 92, 537–545. [Google Scholar] [CrossRef]
- Navas, D.; Fuentes, S.; Castro-Alvarez, A.; Chavez-Angel, E. Review on Sol-Gel Synthesis of Perovskite and Oxide Nanomaterials. Gels 2021, 7, 275. [Google Scholar] [CrossRef]
- Taranu, B.O.; Ivanovici, M.-G.; Svera, P.; Vlazan, P.; Sfirloaga, P.; Poienar, M. Ni11(HPO3)8(OH)6 multifunctional materials: Electrodes for oxygen evolution reaction and potential visible-light active photocatalysts. J. Alloys Compd. 2020, 848, 156595. [Google Scholar] [CrossRef]
- Najjar, H.; Bâtis, H.; Lamonier, J.-F.; Mentré, O.; Giraudon, J.-M. Effect of praseodymium and europium doping in La1−xLnxMnO3+δ (Ln: Pr or Eu, 0 ≤ x ≤ 1) perovskite catalysts for total methane oxidation. Appl. Catal. A: Gen. 2014, 469, 98–107. [Google Scholar] [CrossRef]
- Malavasi, L.; Ritter, C.; Mozzati, M.C.; Tealdi, C.; Saiful Islam, M.; Azzoni, C.B.; Flor, G. Effects of cation vacancy distribution in doped LaMnO3+δ perovskites. J. Solid State Chem. 2005, 178, 2042–2049. [Google Scholar] [CrossRef]
- Taranu, B.-O.; Vlazan, P.; Svera, P.; Poienar, M.; Sfirloaga, P. New functional hybrid materials based on clay minerals for enhanced electrocatalytic activity. J. Alloys Compd. 2022, 892, 162239. [Google Scholar] [CrossRef]
- Gao, F.; Lewis, R.A.; Wang, X.L.; Dou, S.X. Infrared absorption of lanthanum manganites. Phys. C. Supercond. Appl. 2000, 341, 2235–2236. [Google Scholar] [CrossRef]
- Afify, M.S.; El Faham, M.M.; Eldemerdash, U.; El Rouby, W.M.A.; El-Dek, S.I. Room temperature ferromagnetism in Ag doped LaMnO3 nanoparticles. J. Alloys Compd. 2021, 861, 158570. [Google Scholar] [CrossRef]
- Ansari, A.A.; Ahmad, N.; Alam, M.; Adil, S.; Shahid, F.; Ramay, M.; Albadri, A.; Ahmad, A.; Al-Enizi, A.M.; Alrayes, B.F.; et al. Physico-chemical properties and catalytic activity of the sol-gel prepared Ce-ion doped LaMnO3 perovskites. Sci. Rep. 2019, 9, 7747. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.M.; Bassler, G.C.; Morrill, T.C. Spectrometric Identification of Organic Compounds, 4th ed.; John Wiley and Sons: New York, NY, USA, 1981. [Google Scholar]
- Aal, A.A.; Hammad, T.R.; Zawrah, M.; Battisha, I.K.; Abou Hammad, A.B. FTIR study of nanostructure perovskite BaTiO3 doped with both Fe3+ and Ni2+ ions prepared by sol-gel technique. Acta Phys. Pol. A 2014, 126, 1318–1322. [Google Scholar]
- Del Nero, J.; de Araujo, R.E.; Gomes, A.S.L.; de Melo, C.P. Theoretical and experimental investigation of the second hyperpolarizabilities of methyl orange. J. Chem. Phys. 2005, 122, 104506. [Google Scholar] [CrossRef]
- Elumalai, S.; Muthuraman, G. Recovery of Methyl Orange and Congo Red from aqueous solutions using tri-octyl amine (TOA) in benzene as carrier. Process Saf. Environ. Prot. 2015, 96, 177–183. [Google Scholar] [CrossRef]
- Rahman, M.; Pinky, T.A.; Mondal, D.C.; Abedin, M.; Hasan, M. The Study of the Photocatalytic Degradation of Methyl Orange in the Presence of Zinc Oxide (ZnO) Suspension. J. Mater. Sci. Res. Rev. 2020, 5, 1–14. [Google Scholar]
- Youssef, N.A.; Shaban, S.A.; Ibrahim, F.A.; Mahmoud, A.S. Degradation of methyl orange using Fenton catalytic reaction. Egypt. J. Pet. 2016, 25, 317–321. [Google Scholar] [CrossRef]
- Bala, S.S.; Alkhatib, A.J.; Bashir, S.S.; Abdulhadi, M. Photocatalytic Degradation of Indigo Carmine in Aqueous Solutions by the Antibacterial Agent Pefloxacin and UVA. Biomed. J. Sci. Tech. Res. 2018, 5, 4903–4909. [Google Scholar]
- Olajire, A.A.; Olajide, A.J. Kinetic Study of Decolorization of Methylene Blue with Sodium Sulphite in Aqueous Media: Influence of Transition Metal Ions. J. Phys. Chem. Biophys. 2014, 4, 2. [Google Scholar]
- Coates, E. Aggregation of Dyes in Aqueous Solutions. J. Soc. Dye. Colour. 1969, 85, 355–368. [Google Scholar] [CrossRef]
- Barresi, A.A.; Mazza, D.; Ronchetti, S.; Spinicci, R.; Vallino, M. Non-stoichiometry and catalytic activity in ABO3 perovskites: LaMnO3 and LaFeO3. Stud. Surf. Sci. Catal. 2000, 130, 1223–1228. [Google Scholar]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M. A Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Lin, X.; Huang, F.; Wang, W.; Shan, Z.; Shi, J. Methyl orange degradation over a novel Bi-based photocatalyst Bi 3SbO 7: Correlation of crystal structure to photocatalytic activity. Dye. Pigment. 2008, 78, 39–47. [Google Scholar] [CrossRef]
- Ghiasi, M.; Malekzadeh, A. Solar photocatalytic degradation of methyl orange over La0.7Sr0.3MnO3 nano-perovskite. Sep. Purif. Technol. 2014, 134, 12–19. [Google Scholar] [CrossRef]
- Guo, J.; Chen, X.; Shi, Y.; Lan, Y.; Qin, C. Rapid Photodegradation of Methyl Orange (MO) Assisted with Cu(II) and Tartaric Acid. PLoS ONE 2015, 10, e0134298. [Google Scholar] [CrossRef]
- Verduzco, L.E.; Garcia-Díaz, R.; Martinez, A.I.; Almanza Salgado, R.; Mendez-Arriaga, F.; Lozano-Morales, S.A.; Avendano-Alejo, M.; Padmasree, K.P. Degradation efficiency of methyl orange dye by La0.5Sr0.5CoO3 perovskite oxide under dark and UV irradiated conditions. Dye. Pigment. 2020, 183, 108743. [Google Scholar] [CrossRef]
- Naikwade, A.G.; Jagadale, M.B.; Kale, D.P.; Gophane, A.D.; Garadkar, K.M.; and Rashinkar, G.S. Photocatalytic Degradation of Methyl Orange by Magnetically Retrievable Supported Ionic Liquid Phase Photocatalyst. ACS Omega 2020, 5, 131–144. [Google Scholar]
- Huang, H.; Sun, G.; Hu, J.; Jiao, T. Single-Step Synthesis of LaMnO3/MWCNT Nanocomposites and Their Photocatalytic Activities. Nanomater. Nanotechnol. 2014, 4, 27. [Google Scholar] [CrossRef]
- Zhong, W.; Jiang, T.; Dang, Y.; He, J.; Chen, S.-Y.; Kuo, C.-H.; Kriz, D.; Meng, Y.; Meguerdichian, A.; Sui, S.L. Mechanism studies on methyl orange dye degradation by perovskite-type LaNiO3-δ under dark ambient conditions. Appl. Catal. A Gen. 2018, 549, 302–309. [Google Scholar] [CrossRef]


| Fitting Parameters (for Zero, First and Second Order Reaction) | Reaction 1 | Reaction 2 | Reaction 3 | |
|---|---|---|---|---|
| pH = 2 | pH = 3 | pH = 4 | ||
| zero order reaction | k0 (mg L−1 min−1) | 0.20 | 0.10 | 0.08 |
| R2 | 0.72 | 0.72 | 0.67 | |
| first order reaction | k1 (min−1) | 0.07 | 0.04 | 0.02 |
| R2 | 0.98 | 0.98 | 0.91 | |
| second order reaction | k2 (L mg−1 min−1) | 0.05 | 0.03 | 0.01 |
| R2 | 0.91 | 0.914 | 0.99 | |
| Perovskite Type | Catalyst Concentration (ppm) | Aqueous Solution Volume (mL) | MO Concentration (ppm) | pH | MO Degradation Percent [%] | Dark or Illumination | Source |
|---|---|---|---|---|---|---|---|
| LaMnO3 | 200 | 50 | 3 | - | 90% after 60 min | Visible light | [7] |
| LaMnO3 | 60 | 40 | 6 | 2 | 98% after 90 min | Visible light | [22] |
| LaMnO3 | 500 | 100 | 20 | - | ~57% after 90 min | Visible light | [51] |
| LaMnO3 | 500 | 20 | 6.5 | 2.6 | ~90% in 90 min | dark | [10] |
| LaMnO3 | 500 | 20 | 6.5 | 3.3 | ~87% in 90 min | dark | [10] |
| LaMnO3 | 500 | 20 | 6.5 | 3.6 | ~73% in 90 min | dark | [10] |
| LaMnO3 | 500 | 20 | 6.5 | 3.1 | ~100% in 60 min | solar | [10] |
| LaNiO3 | 1500 | 100 | 5 | - | 94.3% in 4 h | dark | [52] |
| La0.5Sr0.5CoO3 | 25 | 100 | 20 | 2.5 | 99.5% in 25 min | dark | [49] |
| La0.5Sr0.5CoO3 | 25 | 100 | 20 | 2.5 | 99.7% in 25 min | UV radiation | [49] |
| LaMnO3 | 120 | 40 | 12 | 4 | 83% in 90 min | dark | This work |
| LaMnO3 | 120 | 40 | 12 | 4 | 94.7% in 90 min | Solar radiation | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfirloaga, P.; Ivanovici, M.-G.; Poienar, M.; Ianasi, C.; Vlazan, P. Investigation of Catalytic and Photocatalytic Degradation of Methyl Orange Using Doped LaMnO3 Compounds. Processes 2022, 10, 2688. https://doi.org/10.3390/pr10122688
Sfirloaga P, Ivanovici M-G, Poienar M, Ianasi C, Vlazan P. Investigation of Catalytic and Photocatalytic Degradation of Methyl Orange Using Doped LaMnO3 Compounds. Processes. 2022; 10(12):2688. https://doi.org/10.3390/pr10122688
Chicago/Turabian StyleSfirloaga, Paula, Madalina-Gabriela Ivanovici, Maria Poienar, Catalin Ianasi, and Paulina Vlazan. 2022. "Investigation of Catalytic and Photocatalytic Degradation of Methyl Orange Using Doped LaMnO3 Compounds" Processes 10, no. 12: 2688. https://doi.org/10.3390/pr10122688
APA StyleSfirloaga, P., Ivanovici, M.-G., Poienar, M., Ianasi, C., & Vlazan, P. (2022). Investigation of Catalytic and Photocatalytic Degradation of Methyl Orange Using Doped LaMnO3 Compounds. Processes, 10(12), 2688. https://doi.org/10.3390/pr10122688







