Application of Hydrophobic Deep Eutectic Solvents in Extraction of Metals from Real Solutions Obtained by Leaching Cathodes from End-of-Life Li-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
Preparation of DESs
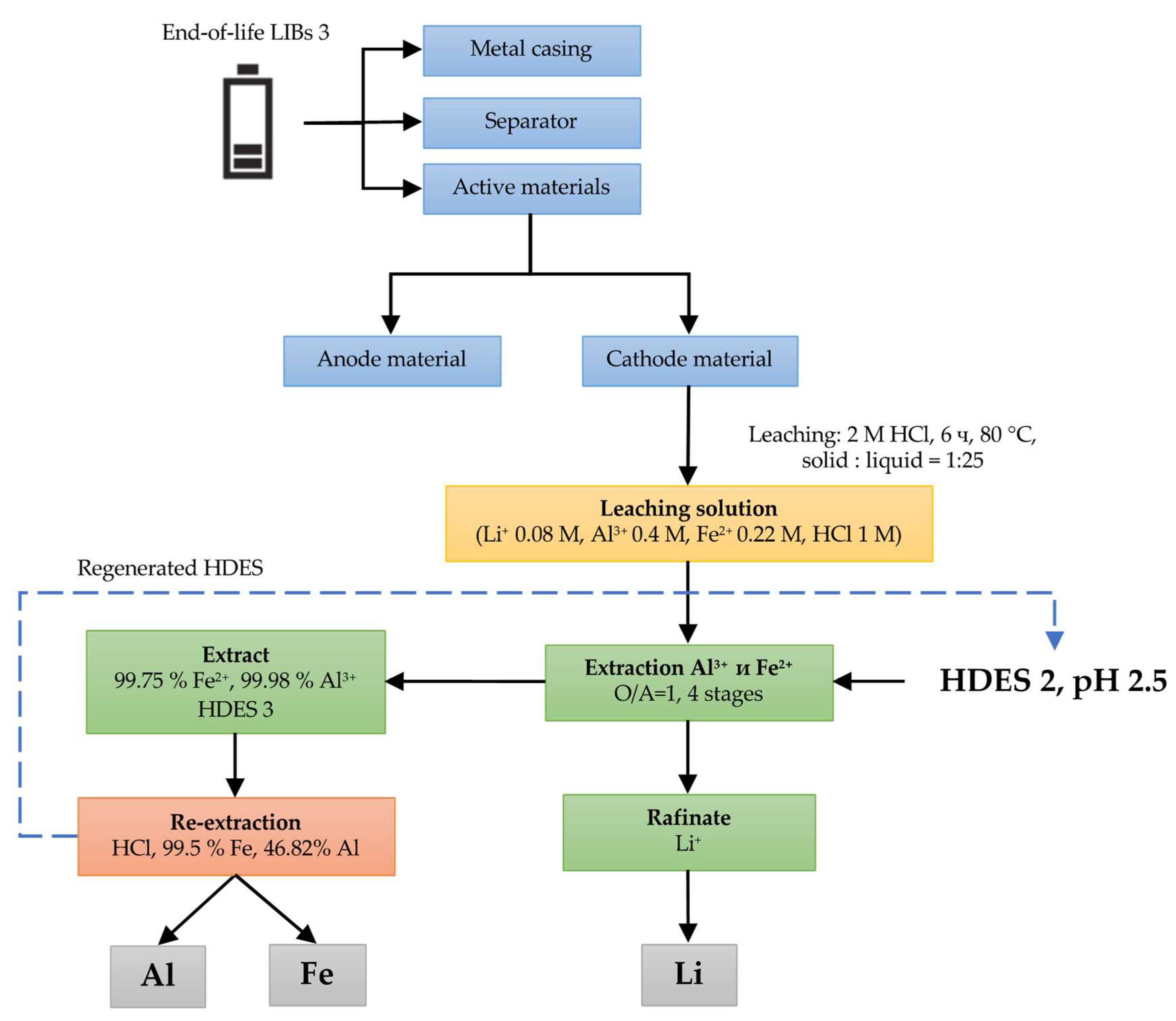
2.2. Leaching Experiments
2.3. Extraction Experiments with HDESs
3. Results and Discussion
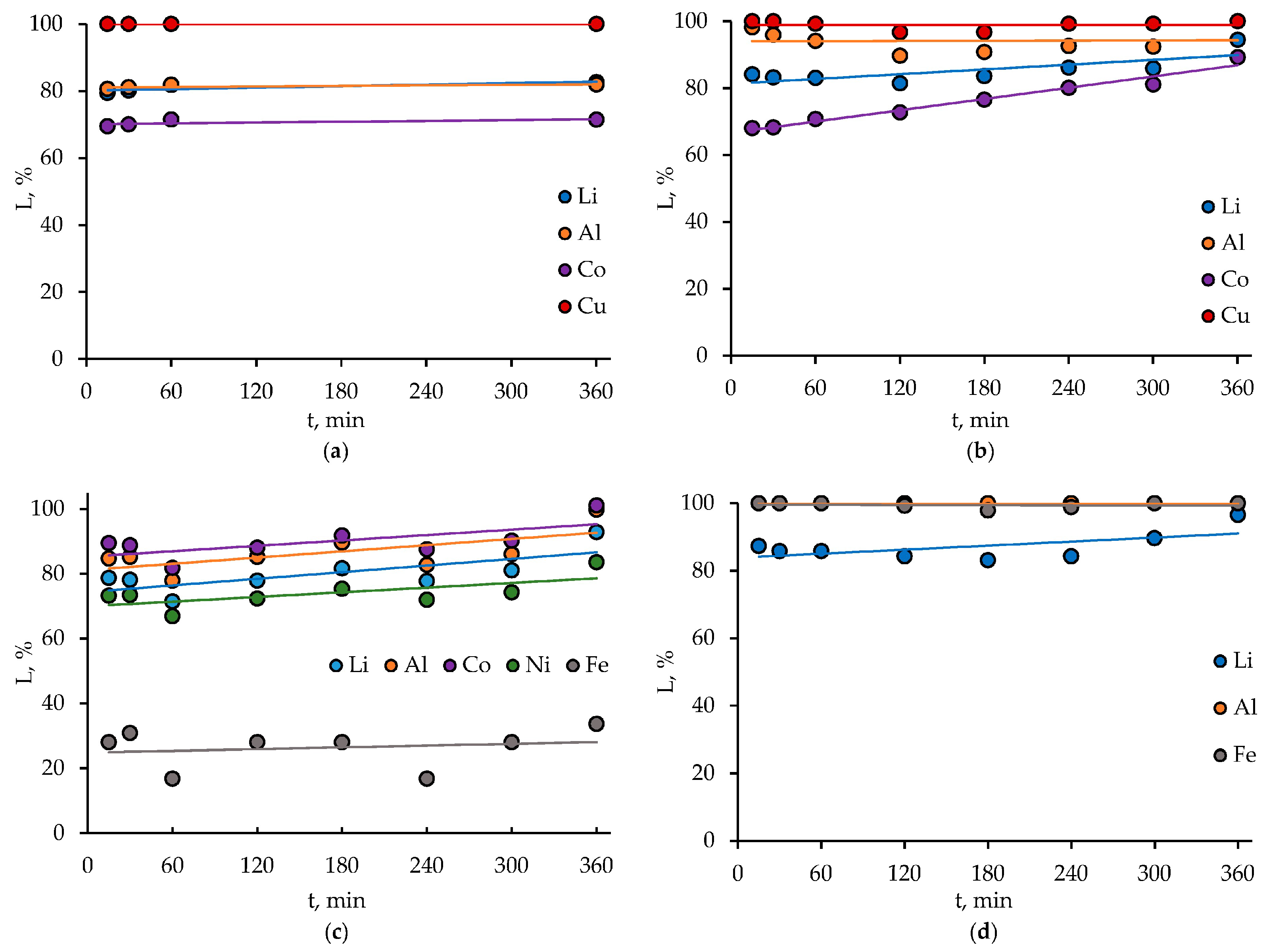
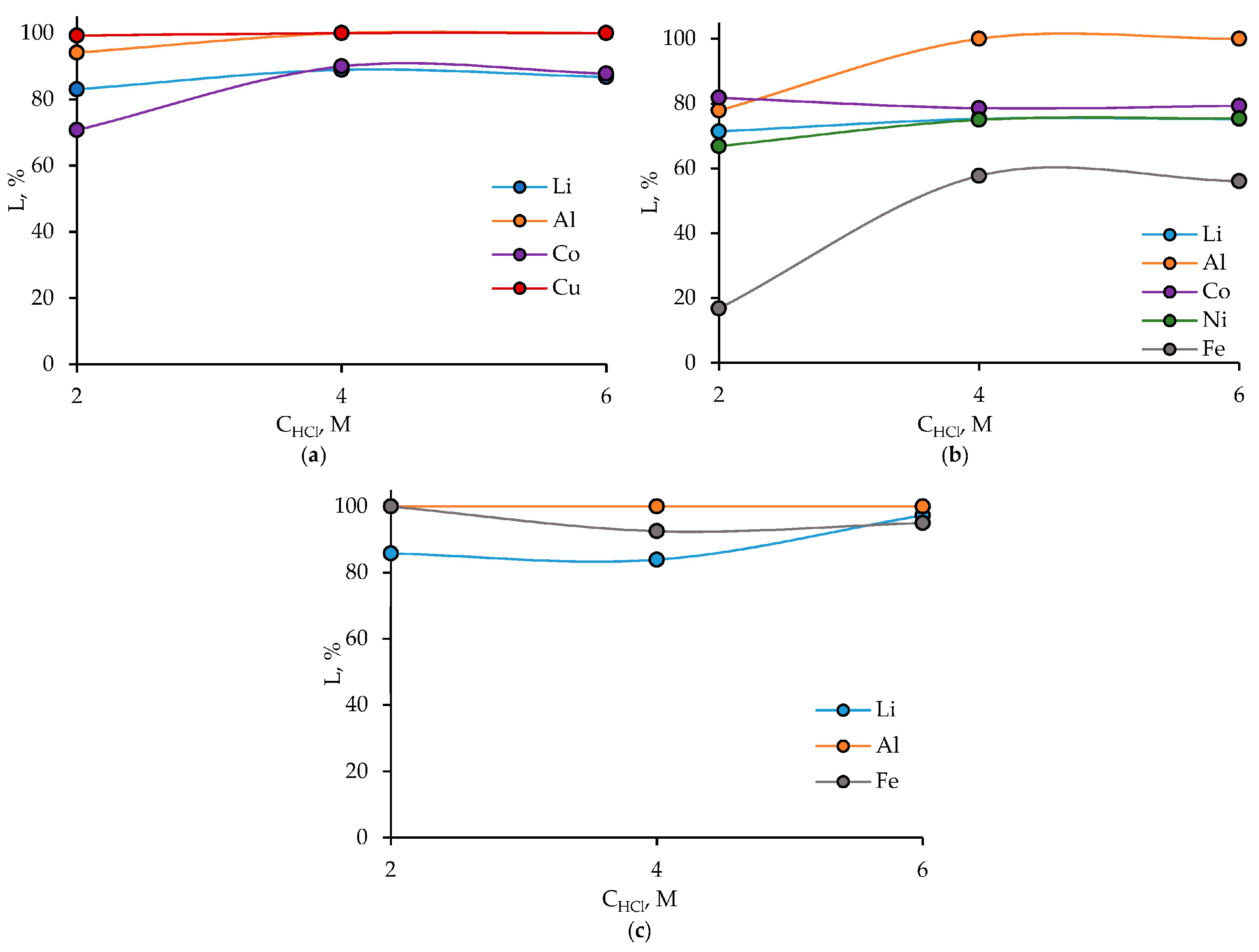
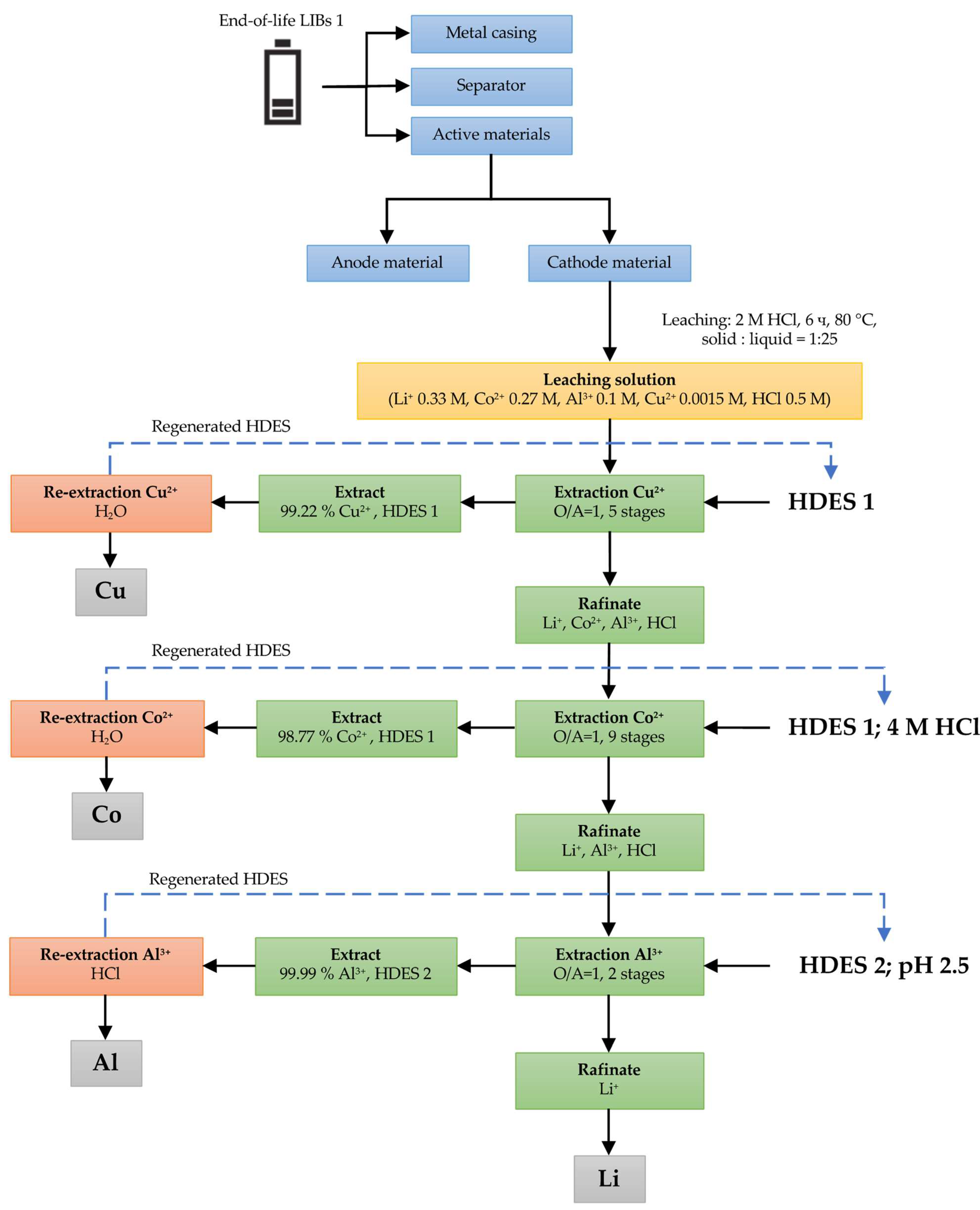
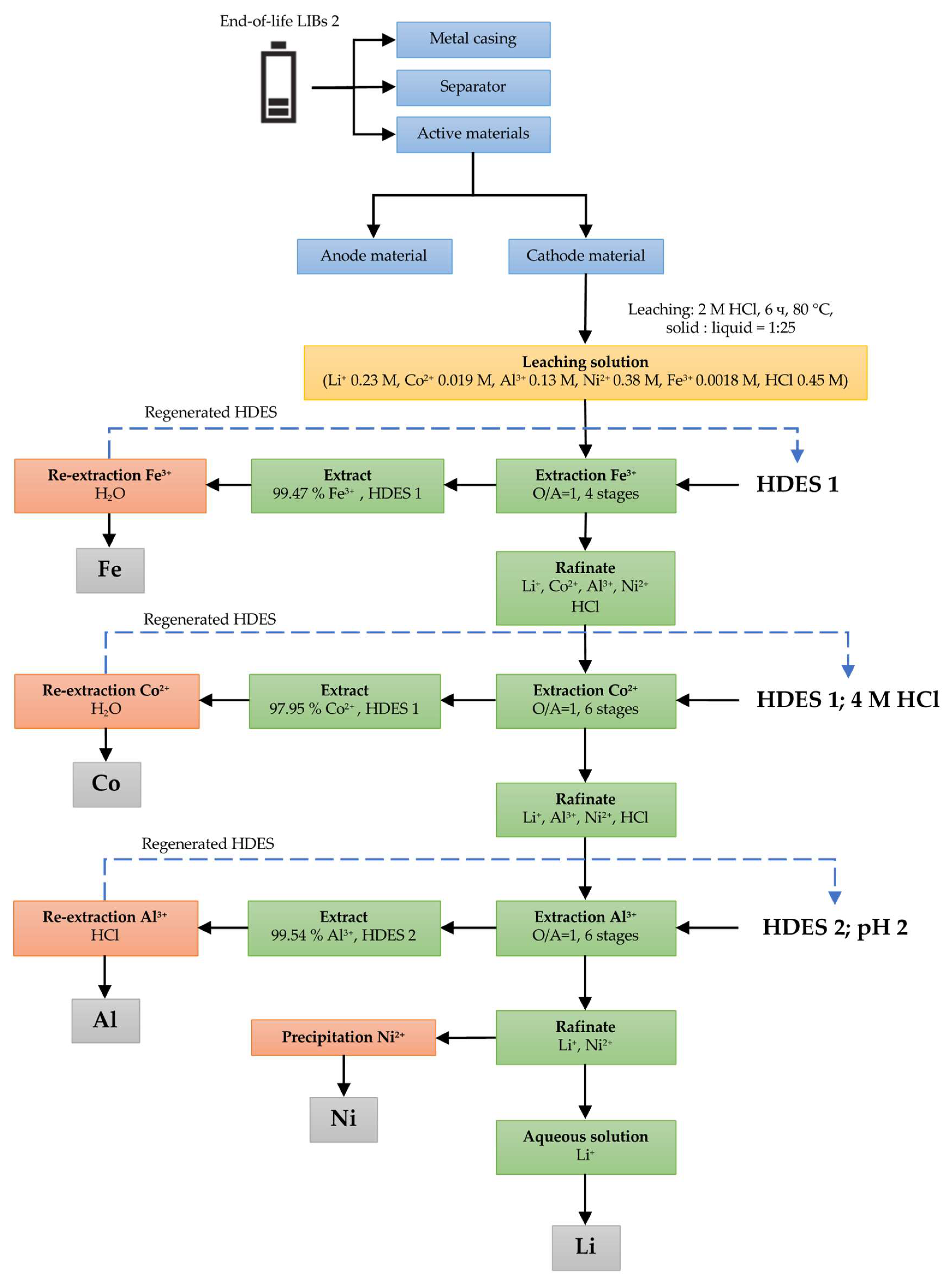
3.1. HCl Leaching of the Cathodes of Three Different LIB Types
3.2. Solvent Extraction
3.2.1. LIB 1
3.2.2. LIB 2
3.2.3. LIB 3
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xie, J.; Lu, Y.-C. A retrospective on lithium-ion batteries. Nat. Commun. 2020, 11, 2499. [Google Scholar] [CrossRef] [PubMed]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Jung, J.C.-Y.; Sui, P.-C.; Zhang, J. A review of recycling spent lithium-ion battery cathode materials using hydrometallurgical treatments. J. Energy Storage 2021, 35, 102217. [Google Scholar] [CrossRef]
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Svärd, M.; Xiao, X.; Gardner, J.M.; Olsson, R.T.; Forsberg, K. Precipitation and Crystallization Used in the Production of Metal Salts for Li-Ion Battery Materials: A Review. Metals 2020, 10, 1609. [Google Scholar] [CrossRef]
- Kim, K.; Raymond, D.; Candeago, R.; Su, X. Selective cobalt and nickel electrodeposition for lithium-ion battery recycling through integrated electrolyte and interface control. Nat. Commun. 2021, 12, 6554. [Google Scholar] [CrossRef]
- Lei, S.; Sun, W.; Yang, Y. Solvent extraction for recycling of spent lithium-ion batteries. J. Hazard. Mater. 2022, 424, 127654. [Google Scholar] [CrossRef] [PubMed]
- Torkaman, R.; Asadollahzadeh, M.; Torab-Mostaedi, M.; Maragheh, M.G. Recovery of cobalt from spent lithium-ion batteries by using acidic and basic extractants in solvent extraction process. Sep. Purif. Technol. 2017, 186, 318–325. [Google Scholar] [CrossRef]
- Jin, S.; Mu, D.; Lu, Z.; Li, R.; Liu, Z.; Wang, Y.; Tian, S.; Dai, C. A comprehensive review on the recycling of spent lithium-ion batteries: Urgent status and technology advances. J. Clean. Prod. 2022, 340, 130535. [Google Scholar] [CrossRef]
- Qin, L.; Di, J.; He, Y. Efficient Synthesis of Furfuryl Alcohol from Corncob in a Deep Eutectic Solvent System. Processes 2022, 10, 1873. [Google Scholar] [CrossRef]
- Zinov’Eva, I.V.; Fedorov, A.Y.; Milevskii, N.A.; Zakhodyaeva, Y.A.; Voshkin, A.A. Dissolution of Metal Oxides in a Choline Chloride–Sulphosalicylic Acid Deep Eutectic Solvent. Theor. Found. Chem. Eng. 2021, 55, 663–670. [Google Scholar] [CrossRef]
- Ijardar, S.P.; Singh, V.; Gardas, R.L. Revisiting the Physicochemical Properties and Applications of Deep Eutectic Solvents. Molecules 2022, 27, 1368. [Google Scholar] [CrossRef]
- Gradov, O.M.; Zinov’Eva, I.V.; Zakhodyaeva, Y.A.; Voshkin, A.A. Modelling of the Erosive Dissolution of Metal Oxides in a Deep Eutectic Solvent—Choline Chloride/Sulfosalicylic Acid—Assisted by Ultrasonic Cavitation. Metals 2021, 11, 1964. [Google Scholar] [CrossRef]
- Zinov’Eva, I.V.; Fedorov, A.Y.; Milevskii, N.A.; Zakhodyaeva, Y.A.; Voshkin, A.A. A Deep Eutectic Solvent Based on Choline Chloride and Sulfosalicylic Acid: Properties and Applications. Theor. Found. Chem. Eng. 2021, 55, 371–379. [Google Scholar] [CrossRef]
- Milevsky, N.A.; Zinovieva, I.V.; Zakhodyaeva, Y.A.; Voshkin, A.A. Extractive Separation of Co/Ni Pair With the Deep Eutectic Solvent Aliquat 336/Timol. Theor. Found. Chem. Eng. 2022, 56, 45–52. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Tang, N.; Liu, L.; Yin, C.; Zhu, G.; Huang, Q.; Dong, J.; Yang, X.; Wang, S. Environmentally benign hydrophobic deep eutectic solvents for palladium(II) extraction from hydrochloric acid solution. J. Taiwan Inst. Chem. Eng. 2021, 121, 92–100. [Google Scholar] [CrossRef]
- Yu, L.-Y.; Wu, K.-J.; He, C.-H. Tailoring hydrophobic deep eutectic solvent for selective lithium recovery from dilute aqueous solutions. Sep. Purif. Technol. 2022, 281, 119928. [Google Scholar] [CrossRef]
- Ni, S.; Su, J.; Zhang, H.; Zeng, Z.; Zhi, H.; Sun, X. A cleaner strategy for comprehensive recovery of waste SmCo magnets based on deep eutectic solvents. Chem. Eng. J. 2021, 412, 128602. [Google Scholar] [CrossRef]
- Schaeffer, N.; Martins, M.A.R.; Neves, C.M.S.S.; Pinho, S.P.; Coutinho, J.A.P. Sustainable hydrophobic terpene-based eutectic solvents for the extraction and separation of metals. Chem. Commun. 2018, 54, 8104–8107. [Google Scholar] [CrossRef] [PubMed]
- Hanada, T.; Goto, M. Synergistic Deep Eutectic Solvents for Lithium Extraction. ACS Sustain. Chem. Eng. 2021, 9, 2152–2160. [Google Scholar] [CrossRef]
- Milevskii, N.; Zinov’Eva, I.; Zakhodyaeva, Y.; Voshkin, A. Separation of Li(I), Co(II), Ni(II), Mn(II), and Fe(III) from hydrochloric acid solution using a menthol-based hydrophobic deep eutectic solvent. Hydrometallurgy 2022, 207, 105777. [Google Scholar] [CrossRef]
- Zinov’Eva, I.V.; Kozhevnikova, A.V.; Milevskii, N.A.; Zakhodyaeva, Y.A.; Voshkin, A.A. Extraction of Cu(II), Ni(II), and Al(III) with the Deep Eutectic Solvent D2EHPA/Menthol. Theor. Found. Chem. Eng. 2022, 56, 221–229. [Google Scholar] [CrossRef]
- Guo, Y.; Li, F.; Zhu, H.; Li, G.; Huang, J.; He, W. Leaching lithium from the anode electrode materials of spent lithium-ion batteries by hydrochloric acid (HCl). Waste Manag. 2016, 51, 227–233. [Google Scholar] [CrossRef]
- Wang, R.-C.; Lin, Y.-C.; Wu, S.-H. A novel recovery process of metal values from the cathode active materials of the lithium-ion secondary batteries. Hydrometallurgy 2009, 99, 194–201. [Google Scholar] [CrossRef]
- Barik, S.; Prabaharan, G.; Kumar, L. Leaching and separation of Co and Mn from electrode materials of spent lithium-ion batteries using hydrochloric acid: Laboratory and pilot scale study. J. Clean. Prod. 2017, 147, 37–43. [Google Scholar] [CrossRef]
- Drajlin, D.S.; Suarez, D.S.; Toro, N.; Gálvez, E.D.; Pinna, E.G.; Rodriguez, M.H. Comparative Study of the Dissolution of LCO in HCl Medium with and without H2O2. Metals 2022, 12, 727. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, Q.; Yin, X.; Zeng, D. Trace Amounts of Aqueous Copper(II) Chloride Complexes in Hypersaline Solutions: Spectrophotometric and Thermodynamic Studies. J. Solut. Chem. 2014, 43, 326–339. [Google Scholar] [CrossRef]
- Kozhevnikova, A.V.; Milevskii, N.A.; Zinov’eva, I.V.; Zakhodyaeva, Y.u.A.; Voshkin, A.A. A Flow-Chart for Processing of a Lithium-Manganese Battery Using HDES Aliquat 336/Menthol. Theor. Found. Chem. Eng. 2022, 56, 518–523. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, J.; Li, H.; Chen, Y.; Wang, C. A promising approach for the recovery of high value-added metals from spent lithium-ion batteries. J. Power Sources 2017, 351, 192–199. [Google Scholar] [CrossRef]
- Zhu, S.-G.; He, W.-Z.; Li, G.-M.; Zhou, X.; Zhang, X.-J.; Huang, J.-W. Recovery of Co and Li from spent lithium-ion batteries by combination method of acid leaching and chemical precipitation. Trans. Nonferrous Met. Soc. China 2012, 22, 2274–2281. [Google Scholar] [CrossRef]
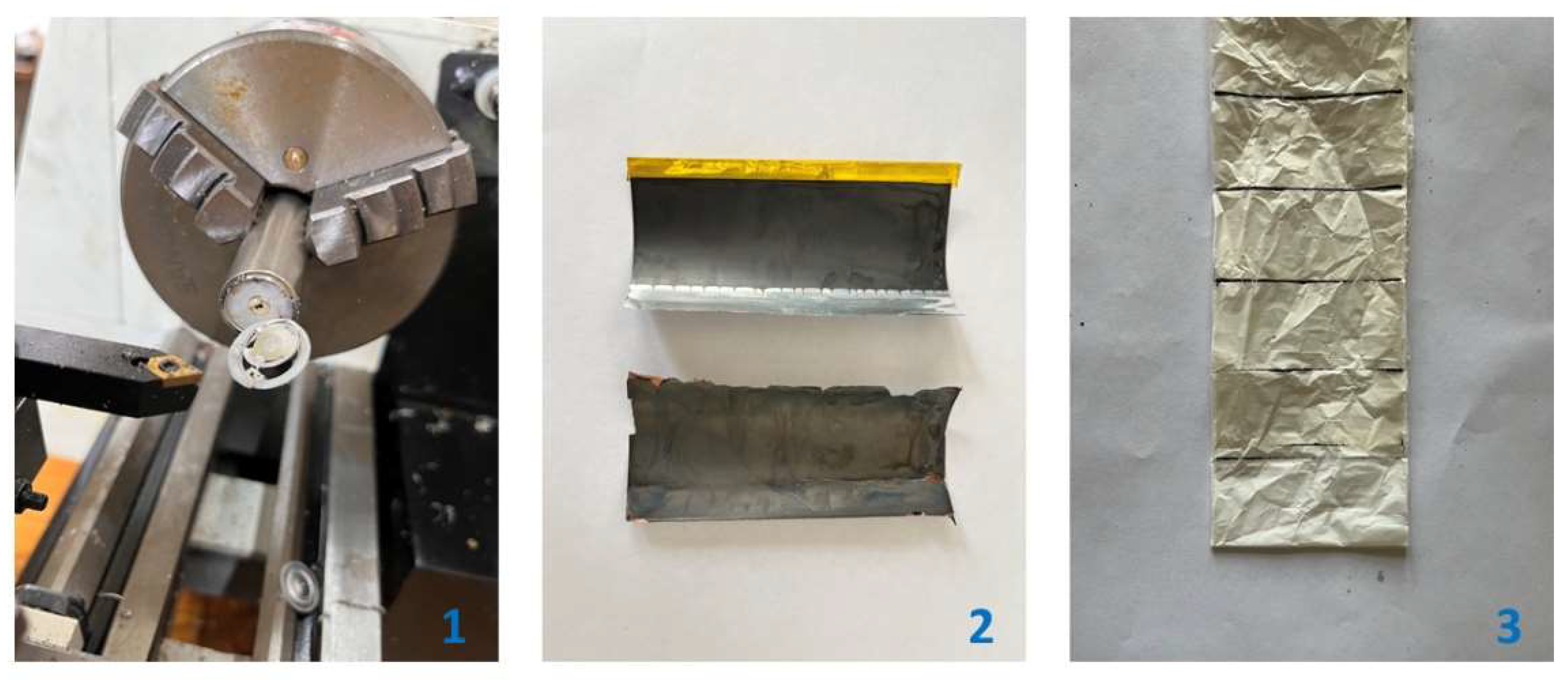
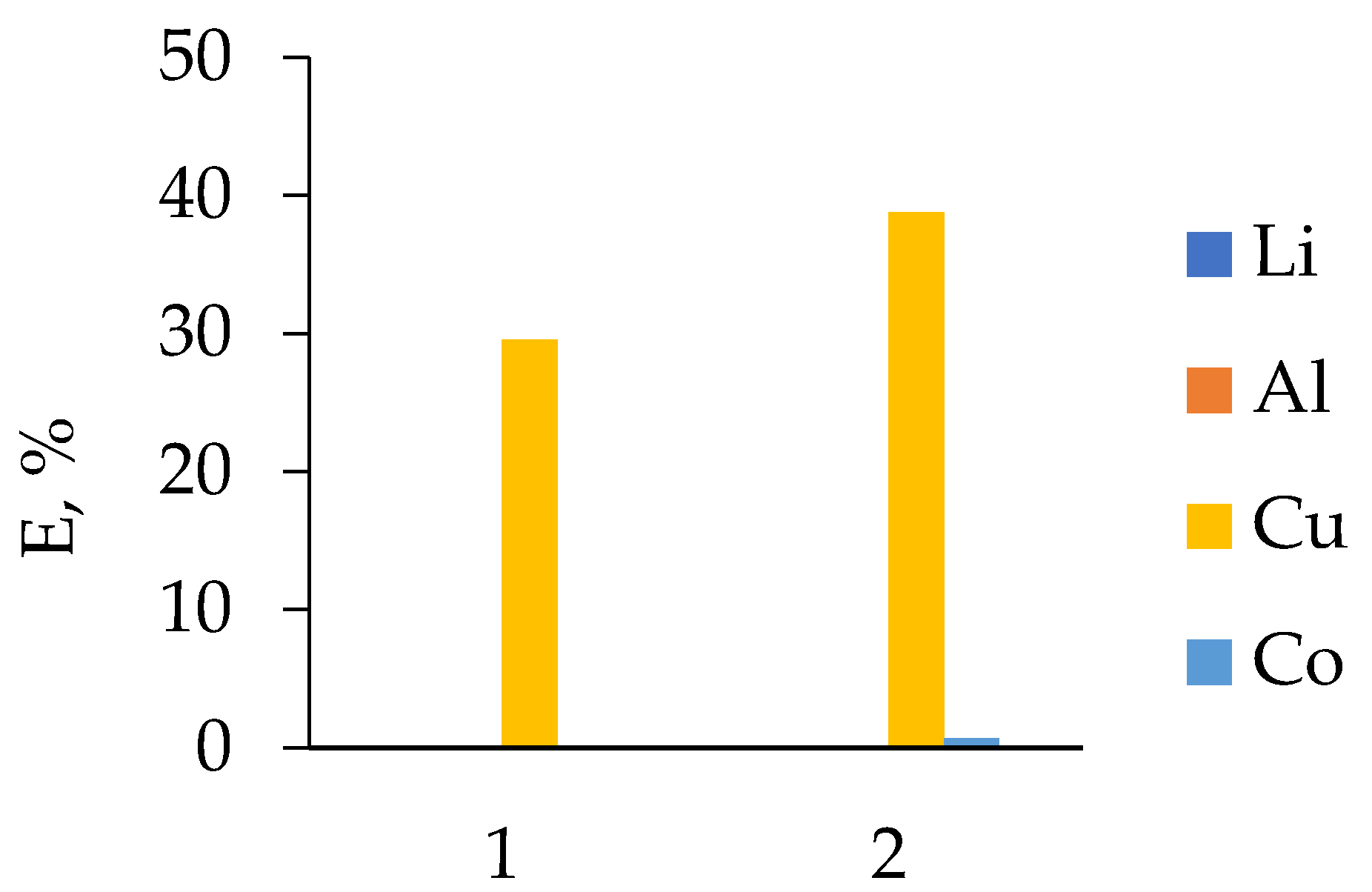

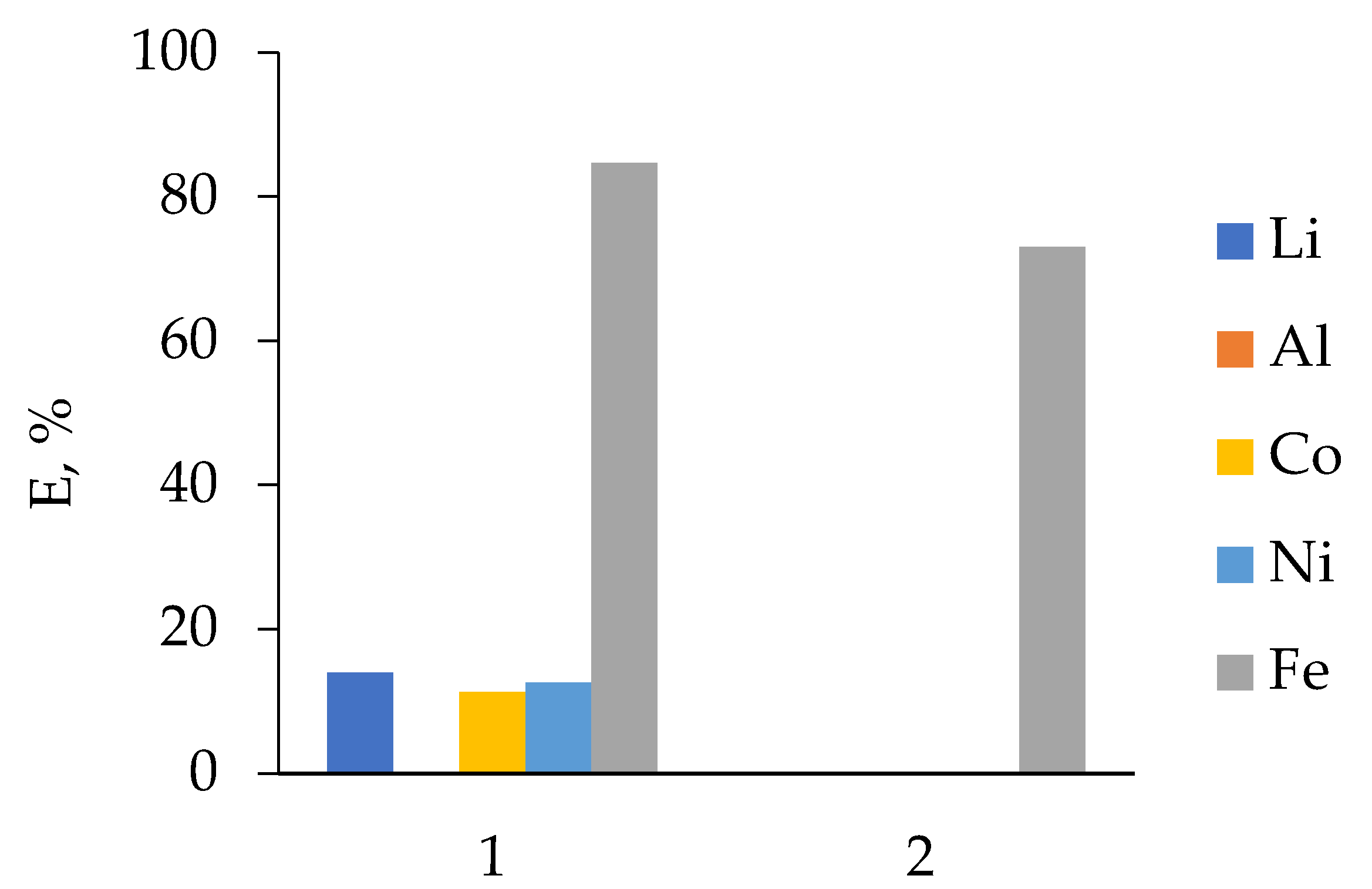
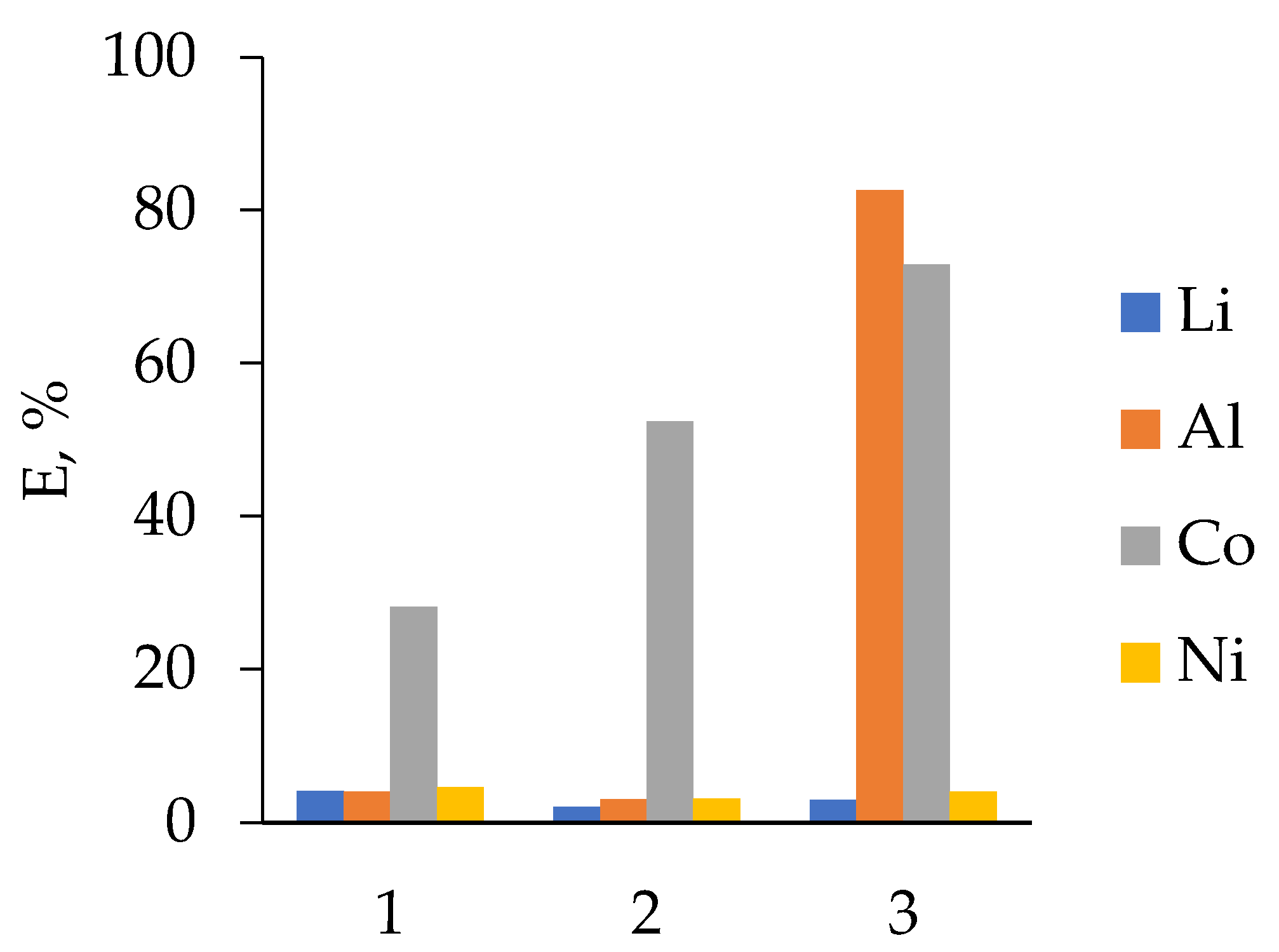
| Element | wt.% | ||
|---|---|---|---|
| LIB 1 | LIB 2 | LIB 3 | |
| Si | 0.09 | 0.09 | 0.07 |
| P | 0.49 | 0.44 | 36.68 |
| S | 0.28 | 0.07 | 0.41 |
| Cl | 0.14 | 0.12 | 0.04 |
| K | 0.12 | 0.13 | 0.19 |
| Ti | 0.19 | 0.03 | 0.02 |
| Cr | 0.05 | 0.02 | 0.03 |
| Mn | 0.07 | 0.06 | 0.07 |
| Fe | 0.08 | 0.06 | 62.12 |
| Co | 97.03 | 4.34 | 0.21 |
| Ni | 0.24 | 94.54 | 0.01 |
| Cu | 1.22 | 0.09 | 0.15 |
| Element | Wavelength, nm |
|---|---|
| Fe | 259.940 |
| 239.562 | |
| Al | 167.079 |
| 396.152 | |
| Li | 610.362 |
| 812.645 | |
| Ti | 334.941 |
| 323.452 | |
| Nb | 309.418 |
| 316.340 | |
| Co | 238.892 |
| 237.862 | |
| Ni | 300.249 |
| 221.647 | |
| Cu | 324.754 |
| 327.396 | |
| Mn | 257.610 |
| 259.373 | |
| K | 766.490 |
| Si | 251.611 |
| 212.412 |
| Element | Li | Al | Co | Fe | Ni | Cu | Battery |
|---|---|---|---|---|---|---|---|
| wt.% | 6.89 | 8.67 | 54.06 | - | - | 0.2 | LIB 1 |
| 4.25 | 8.72 | 2.8 | 0.18 | 64.42 | - | LIB 2 | |
| 1.31 | 14.83 | - | 25.32 | - | - | LIB 3 |
| Compound | Supplier | CAS | Purity, wt.% * |
|---|---|---|---|
| Aliquat 336 | Acros | 63393-96-4 | 98 |
| D2EHPA | Acros | 298-07-7 | 95 |
| L-Menthol | Acros | 2216-51-5 | 99 |
| HCl | Aldosa | 7647-01-0 | 99 |
| NaOH | Chimmed | 1310-73-2 | 98 |
| Distilled water | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozhevnikova, A.V.; Zinov’eva, I.V.; Zakhodyaeva, Y.A.; Baranovskaya, V.B.; Voshkin, A.A. Application of Hydrophobic Deep Eutectic Solvents in Extraction of Metals from Real Solutions Obtained by Leaching Cathodes from End-of-Life Li-Ion Batteries. Processes 2022, 10, 2671. https://doi.org/10.3390/pr10122671
Kozhevnikova AV, Zinov’eva IV, Zakhodyaeva YA, Baranovskaya VB, Voshkin AA. Application of Hydrophobic Deep Eutectic Solvents in Extraction of Metals from Real Solutions Obtained by Leaching Cathodes from End-of-Life Li-Ion Batteries. Processes. 2022; 10(12):2671. https://doi.org/10.3390/pr10122671
Chicago/Turabian StyleKozhevnikova, Arina V., Inna V. Zinov’eva, Yulia A. Zakhodyaeva, Vasilisa B. Baranovskaya, and Andrey A. Voshkin. 2022. "Application of Hydrophobic Deep Eutectic Solvents in Extraction of Metals from Real Solutions Obtained by Leaching Cathodes from End-of-Life Li-Ion Batteries" Processes 10, no. 12: 2671. https://doi.org/10.3390/pr10122671
APA StyleKozhevnikova, A. V., Zinov’eva, I. V., Zakhodyaeva, Y. A., Baranovskaya, V. B., & Voshkin, A. A. (2022). Application of Hydrophobic Deep Eutectic Solvents in Extraction of Metals from Real Solutions Obtained by Leaching Cathodes from End-of-Life Li-Ion Batteries. Processes, 10(12), 2671. https://doi.org/10.3390/pr10122671









