Efficacy of Supercritical Fluid Decellularized Porcine Acellular Dermal Matrix in the Post-Repair of Full-Thickness Abdominal Wall Defects in the Rabbit Hernia Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Acellular Dermal Matrix
2.2. Predicate Device
2.3. Hematoxylin and Eosin Staining
2.4. DAPI (4,6-Diamidino-2-phenylindole, Dihydrochloride) Staining
2.5. DNA Quantification
2.6. Alpha-Gal (α-Gal) Staining
2.7. Surgery for the Repair of Full-Thickness Abdominal Wall Defects
2.8. Mechanical Testing
2.9. Suture Retention Strength Test
2.9.1. Tear Strength Test
2.9.2. Burst Strength Test
2.9.3. Specimen Tissue Section Preparation and Staining
3. Results
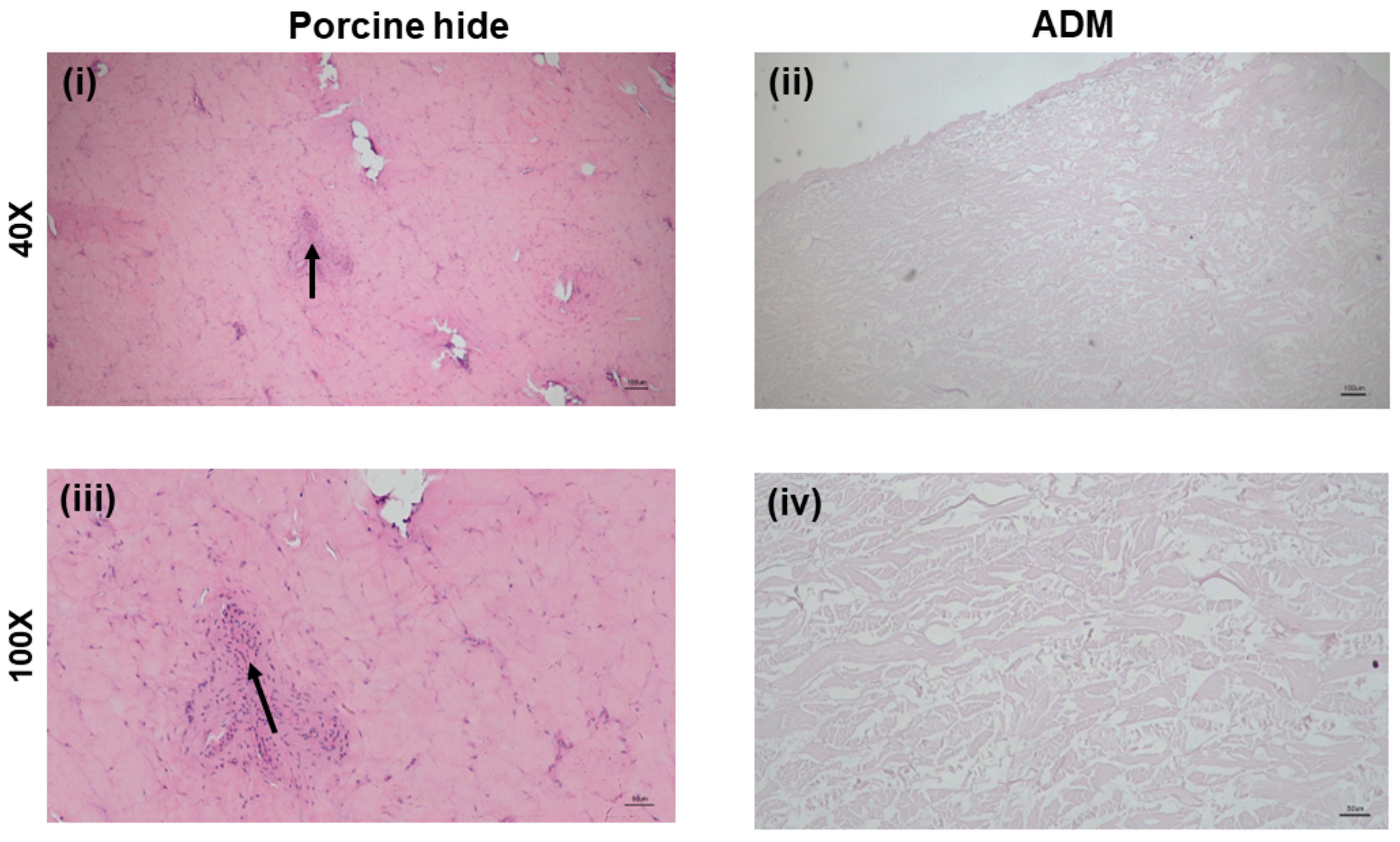
3.1. H&E Staining of SCCO2 Decellularized ADM
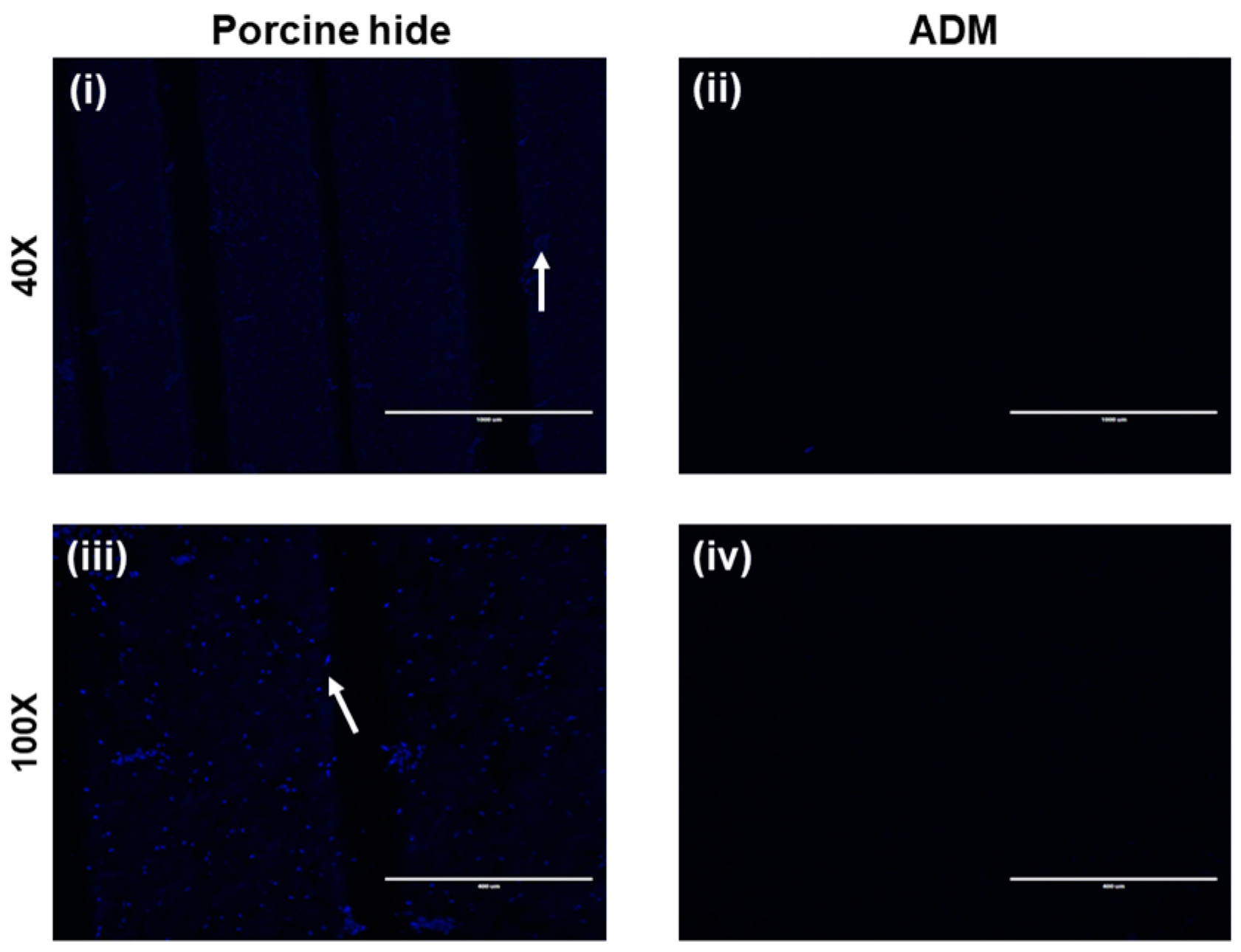
3.2. DAPI Staining of SCCO2 Decellularized ADM
3.3. Residual DNA Content of SCCO2 Decellularized ADM
3.4. Alpha-Gal Staining of SCCO2 Decellularized ADM
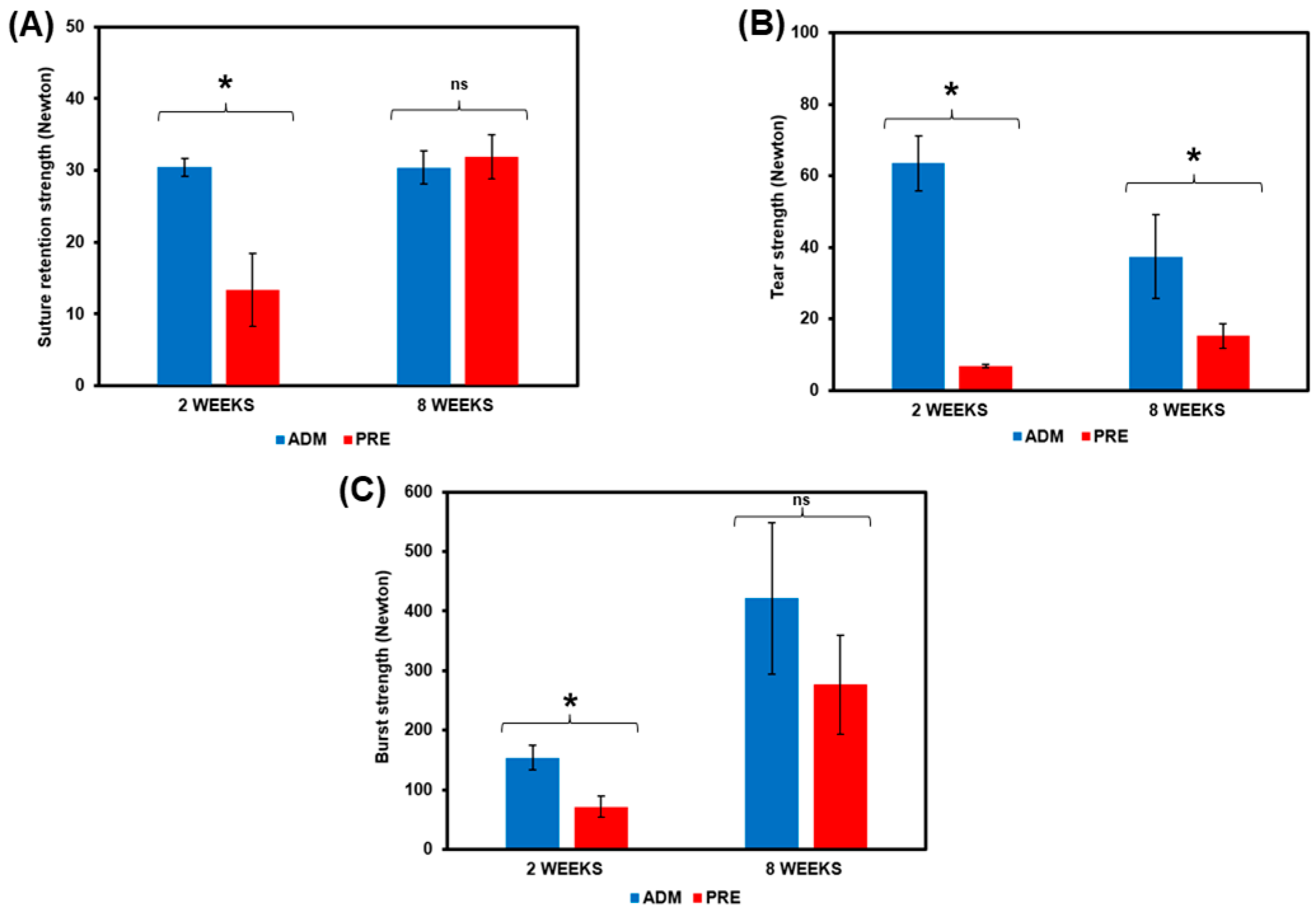
3.5. The Mechanical Strength of SCCO2 Decellularized ADM
3.6. Histological Evaluation of the Abdominal Wall Defect Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kingsnorth, A.; LeBlanc, K. Hernias: Inguinal and incisional. Lancet 2003, 362, 1561. [Google Scholar] [CrossRef]
- Luijendijk, R.W.; Hop, W.C.; van del Tol, M.P.; de Lange, D.C.; Braaksma, M.M.; Ijzermans, J.N.; Boelhouwer, R.U.; de Vriers, B.C.; Salu, M.K.; Wereldsma, J.C.; et al. A comparison of suture repair with mesh repair for incisional hernia. N. Engl. J. Med. 2000, 343, 392. [Google Scholar] [CrossRef] [PubMed]
- Ventral Hernia Working Group; Breuing, K.; Butler, C.; Ferzoso, S.; Franz, M.; Hultman, C.S.; Kilbridge, J.F.; Rosen, M.; Silverman, R.P.; Vargo, D. Incisional ventral hernias: Review of the literature and recommendations regarding the grading and technique or repair. Surgery 2010, 148, 544. [Google Scholar] [CrossRef] [PubMed]
- Bellón, J.M.; Rodríguez, M.; Pérez-Köhler, B.; Pérez-López, P.; Pascual, G. The New Zealand White Rabbit as a Model for Preclinical Studies Addressing Tissue Repair at the Level of the Abdominal Wall. Tissue Eng. Part C Methods 2017, 23, 863–880. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, L.E.; Gerecht, S. Engineered Biopolymeric Scaffolds for Chronic Wound Healing. Front. Physiol. 2016, 7, 341. [Google Scholar] [CrossRef] [PubMed]
- Eweida, A.M.; Marei, M.K. Naturally Occurring Extracellular Matrix Scaffolds for Dermal Regeneration: Do They Really Need Cells? BioMed Res. Int. 2015, 2015, 839694. [Google Scholar] [CrossRef]
- Gelse, K.; Poschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Schonfelder, U.; Abel, M.; Ruth, P.; Kaatz, M.; Hipler, U.C. Protease and pro-inflammatory cytokine concentrations are elevated in chronic compared to acute wounds and can be modulated by collagen type I in vitro. Arch. Dermatol. Res. 2010, 302, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Silverman, R.P. Acellular dermal matrix in abdominal wall reconstruction. Aesthet. Surg. J. 2011, 31 (Suppl. 7), 24S–29S. [Google Scholar] [CrossRef]
- Gowda, A.U.; Chang, S.M.; Chopra, K.; Matthews, J.A.; Sabino, J.; Stromberg, J.A.; Zahiri, H.R.; Pinczewski, J.; Holton, L.H., 3rd; Silverman, R.P.; et al. Porcine acellular dermal matrix (PADM) vascularises after exposure in open necrotic wounds seen after complex hernia repair. Int. Wound J. 2016, 13, 972–976. [Google Scholar] [CrossRef]
- Carlson, T.L.; Lee, K.W.; Pierce, L.M. Effect of cross-linked and non-cross-linked acellular dermal matrices on the expression of mediators involved in wound healing and matrix remodeling. Plast. Reconstr. Surg. 2013, 131, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Flynn, L.; Semple, J.L.; Woodhouse, K.A. Decellularized placental matrices for adipose tissue engineering. J. Biomed. Mater. Res. A 2006, 79, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.K.; Luo, B.; Guneta, V.; Li, L.; Foo, S.E.M.; Dai, Y.; Tan, T.T.Y.; Tan, N.S.; Choong, C.; Wong, M.T.C. Supercritical carbon dioxide extracted extracellular matrix material from adipose tissue. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, E.M.; Beltrán, S.; Sanz, M.T. Extraction of fat from pigskin with supercritical carbon dioxide. J. Supercrit. Fluids 2006, 37, 142–150. [Google Scholar] [CrossRef]
- Jenkins, C.L.; Raines, R.T. Insights on the conformational stability of collagen. Nat. Prod. Rep. 2002, 19, 49–59. [Google Scholar]
- Chou, P.R.; Lin, Y.N.; Wu, S.H.; Lin, S.D.; Srinivasan, P.; Hsieh, D.J.; Huang, S.H. Supercritical Carbon Dioxide-decellularized Porcine Acellular Dermal Matrix combined with Autologous Adipose-derived Stem Cells: Its Role in Accelerated Diabetic Wound Healing. Int. J. Med. Sci. 2020, 17, 354–367. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Zhang, X.; Lu, W.; Huang, X.; Xie, H.; Zhou, J.; Wang, W.; Zhang, Y.; Liu, Y.; et al. Synergistic angiogenesis promoting effects of extracellular matrix scaffolds and adipose-derived stem cells during wound repair. Tissue Eng. Part A 2011, 17, 725–739. [Google Scholar] [CrossRef]
- Carvalho-Júnior, J.D.C.; Zanata, F.; Aloise, A.C.; Ferreira, L.M. Acellular dermal matrix in skin wound healing in rabbits—Histological and histomorphometric analyses. Clinics 2021, 76, e2066. [Google Scholar] [CrossRef]
- Wu, C.C.; Tarng, Y.W.; Hsu, D.Z.; Srinivasan, P.; Yeh, Y.C.; Lai, Y.P.; Hsieh, D.J. Supercritical carbon dioxide decellularized porcine cartilage graft with PRP attenuated OA progression and regenerated articular cartilage in ACLT-induced OA rats. J. Tissue Eng. Regen. Med. 2021, 15, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, C.; Long, G.; Wolf, J.; Okerberg, C.; Herbert, R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol. Pathol. 2002, 30, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Jardelino, C.; Takamori, E.R.; Hermida, L.F.; Lenharo, A.; Castro-Silva, I.I.; Granjeiro, J.M. Porcine peritoneum as source of biocompatible collagen in mice. Acta Cir. Bras. 2010, 25, 332. [Google Scholar] [CrossRef] [PubMed]
- McPherson, T.B.; Liang, H.; Record, R.D.; Badylak, S.F. Galalpha(1,3)Gal epitope in porcine small intestinal submucosa. Tissue Eng. 2000, 6, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Daly, K.A.; Stewart-Akers, A.M.; Hara, H.; Ezzelarab, M.; Long, C.; Cordero, K.; Johnson, S.A.; Ayares, D.; Cooper, D.K.; Badylak, S.F. Effect of the alphaGal epitope on the response to small intestinal submucosa extracellular matrix in a nonhuman primate model. Tissue Eng. Part A 2009, 15, 3877–3888. [Google Scholar] [CrossRef]
- Campbell, K.T.; Burns, N.K.; Rios, C.N.; Mathur, A.B.; Butler, C.E. Human versus non-cross-linked porcine acellular dermal matrix used for ventral hernia repair: Comparison of in vivo fibrovascular remodelling and mechanical repair strength. Plast. Reconstr. Surg. 2011, 127, 2321–2332. [Google Scholar] [CrossRef]
- Smart, N.J.; Marshall, M.; Daniels, I.R. Biological meshes: A review of their use in abdominal wall hernia repairs. Surgeon 2012, 10, 159–171. [Google Scholar] [CrossRef]
- Meintjes, J.; Yan, S.; Zhou, L.; Zheng, S.; Zheng, M. Synthetic, biological and composite scaffolds for abdominal wall reconstruction. Expert Rev. Med. Devices 2011, 8, 275–288. [Google Scholar] [CrossRef]
- Sandor, M.; Xu, H.; Connor, J.; Lombardi, J.; Harper, J.R.; Silverman, R.P.; McQuillan, D.J. Host response to implanted porcine-derived biologic materials in a primate model of abdominal wall repair. Tissue Eng. Part A 2008, 14, 2021–2031. [Google Scholar] [CrossRef]
- Monteiro, G.A.; Rodriguez, N.L.; Delossantos, A.I.; Wagner, C.T. Short-term in vivo biological and mechanical remodeling of porcine acellular dermal matrices. J. Tissue Eng. 2013, 4, 2041731413490182. [Google Scholar] [CrossRef]
- Förstemann, T.; Trzewik, J.; Holste, J.; Batke, B.; Konerding, M.A.; Wolloscheck, T.; Hartung, C. Forces and deformations of the abdominal wall—A mechanical and geometrical approach to the linea alba. J. Biomech. 2011, 44, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Konerding, M.A.; Bohn, M.; Wolloscheck, T.; Batke, B.; Holste, J.L.; Wohlert, S.; Trzewik, J.; Förstemann, T.; Hartung, C. Maximum forces acting on the abdominal wall: Experimental validation of a theoretical modeling in a human cadaver study. Med. Eng. Phys. 2011, 33, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Burns, N.K.; Jaffari, M.V.; Rios, C.N.; Mathur, A.B.; Butler, C.E. Non-cross-linked porcine acellular dermal matrices for abdominal wall reconstruction. Plast. Reconstr. Surg. 2010, 125, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.E.; Burns, N.K.; Campbell, K.T.; Mathur, A.B.; Jaffari, M.V.; Rios, C.N. Comparison of cross-linked and non-cross-linked porcine acellular dermal matrices for ventral hernia repair. J. Am. Coll. Surg. 2010, 211, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Sacks, J.M.; Butler, C.E. Outcomes of complex abdominal wall reconstruction with bioprosthetic mesh in cancer patients. Plast. Reconstr. Surg. 2008, 121, 39. [Google Scholar]
- Glasberg, S.B.; D’Amico, R.A. Use of regenerative human acellular tissue (AlloDerm) to reconstruct the abdominal wall following pedicle TRAM flap breast reconstruction surgery. Plast. Reconstr. Surg. 2006, 118, 8–15. [Google Scholar] [CrossRef]
- Ge, L.; Zheng, S.; Wei, H. Comparison of histological structure and biocompatibility between human acellular dermal matrix (ADM) and porcine ADM. Burns 2009, 35, 46–50. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, Y.-L.; Lin, Y.-N.; Chen, Y.-J.; Periasamy, S.; Yen, K.-C.; Hsieh, D.-J. Efficacy of Supercritical Fluid Decellularized Porcine Acellular Dermal Matrix in the Post-Repair of Full-Thickness Abdominal Wall Defects in the Rabbit Hernia Model. Processes 2022, 10, 2588. https://doi.org/10.3390/pr10122588
Chiu Y-L, Lin Y-N, Chen Y-J, Periasamy S, Yen K-C, Hsieh D-J. Efficacy of Supercritical Fluid Decellularized Porcine Acellular Dermal Matrix in the Post-Repair of Full-Thickness Abdominal Wall Defects in the Rabbit Hernia Model. Processes. 2022; 10(12):2588. https://doi.org/10.3390/pr10122588
Chicago/Turabian StyleChiu, Yen-Lung, Yun-Nan Lin, Yun-Ju Chen, Srinivasan Periasamy, Ko-Chung Yen, and Dar-Jen Hsieh. 2022. "Efficacy of Supercritical Fluid Decellularized Porcine Acellular Dermal Matrix in the Post-Repair of Full-Thickness Abdominal Wall Defects in the Rabbit Hernia Model" Processes 10, no. 12: 2588. https://doi.org/10.3390/pr10122588
APA StyleChiu, Y.-L., Lin, Y.-N., Chen, Y.-J., Periasamy, S., Yen, K.-C., & Hsieh, D.-J. (2022). Efficacy of Supercritical Fluid Decellularized Porcine Acellular Dermal Matrix in the Post-Repair of Full-Thickness Abdominal Wall Defects in the Rabbit Hernia Model. Processes, 10(12), 2588. https://doi.org/10.3390/pr10122588







