The Dynamic Shift of Bacterial Communities in Hybrid Anaerobic Baffled Reactor (ABR)—Aerobic Granules Process for Berberine Pharmaceutical Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Hybrid ABR–AGS System
2.2. Inoculum
2.3. Medium
2.4. Analytical Methods
2.5. Sampling and DNA Extraction
2.6. PCR Amplification and 16S rDNA Cloning
3. Results
3.1. The Performance of Hybrid ABR–AGS System
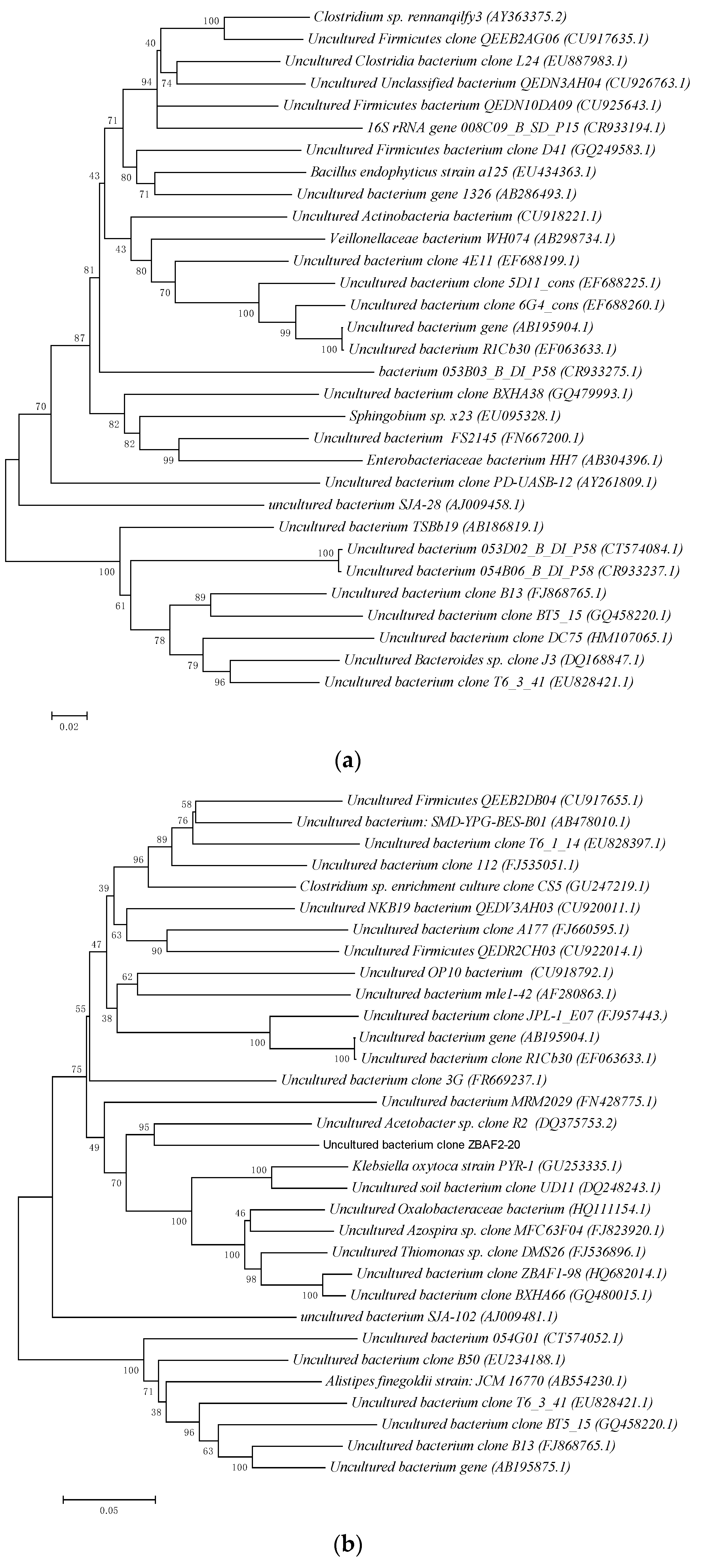
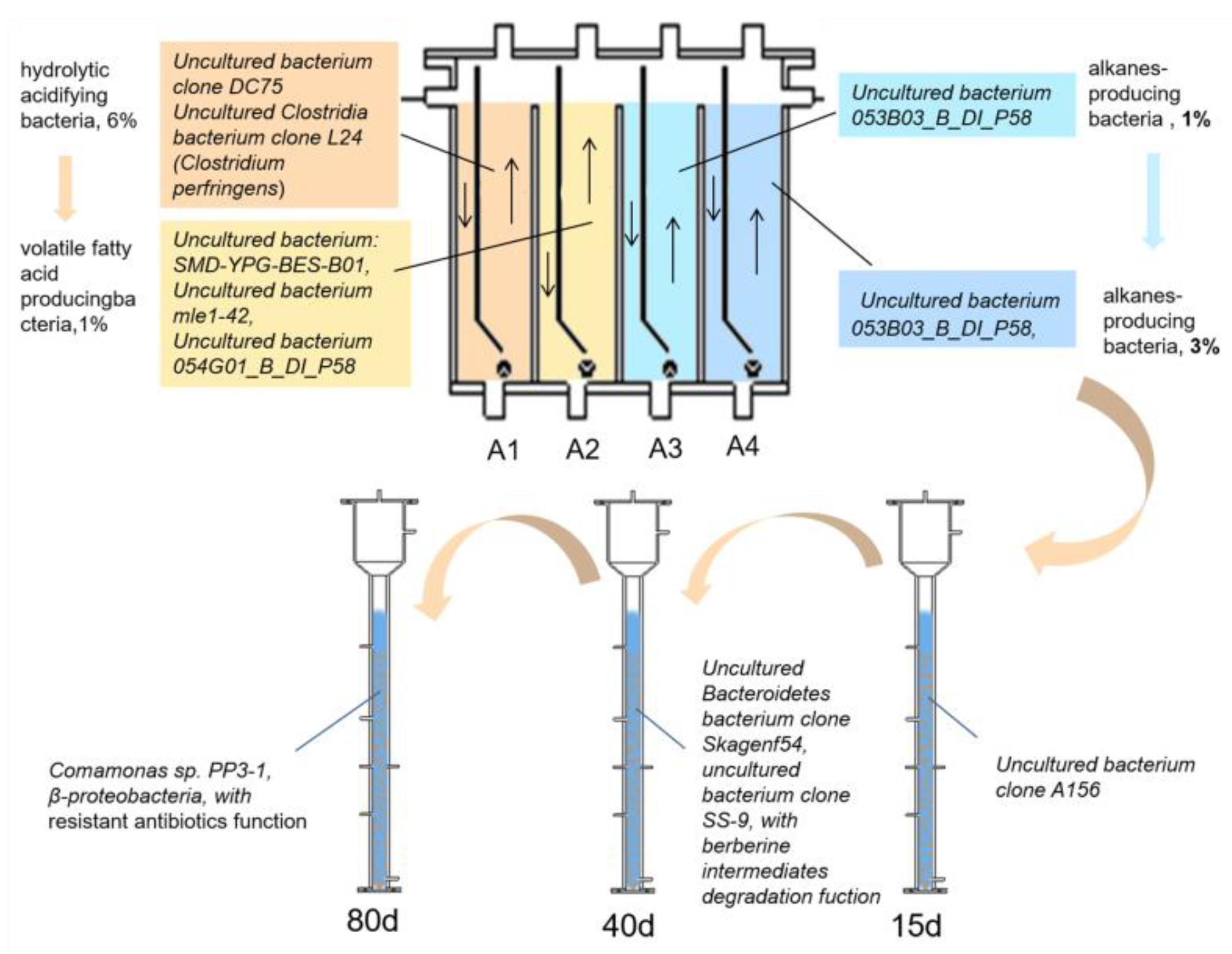
3.2. Differences in the Structure of Total Bacterial Communities in ABR Reactor Chambers
- (1)
- The total bacteria in chamber A1 of the ABR reactor:
- (2)
- The total bacteria in the A2 chamber of the ABR reactor:
- (3)
- The total bacteria in chamber A3 of the ABR reactor:
- (4)
- The total bacteria in chamber A4 of the ABR reactor:
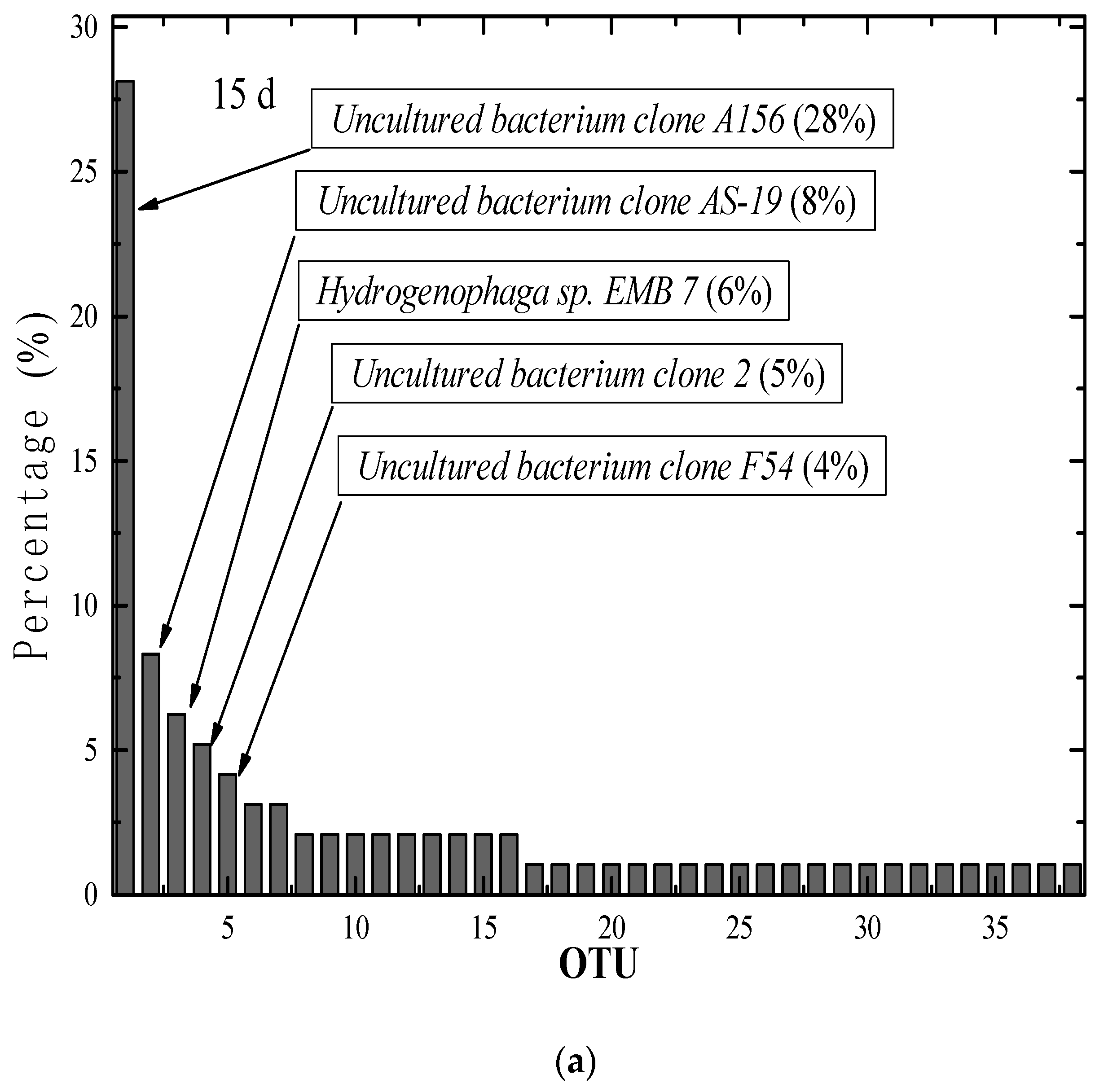
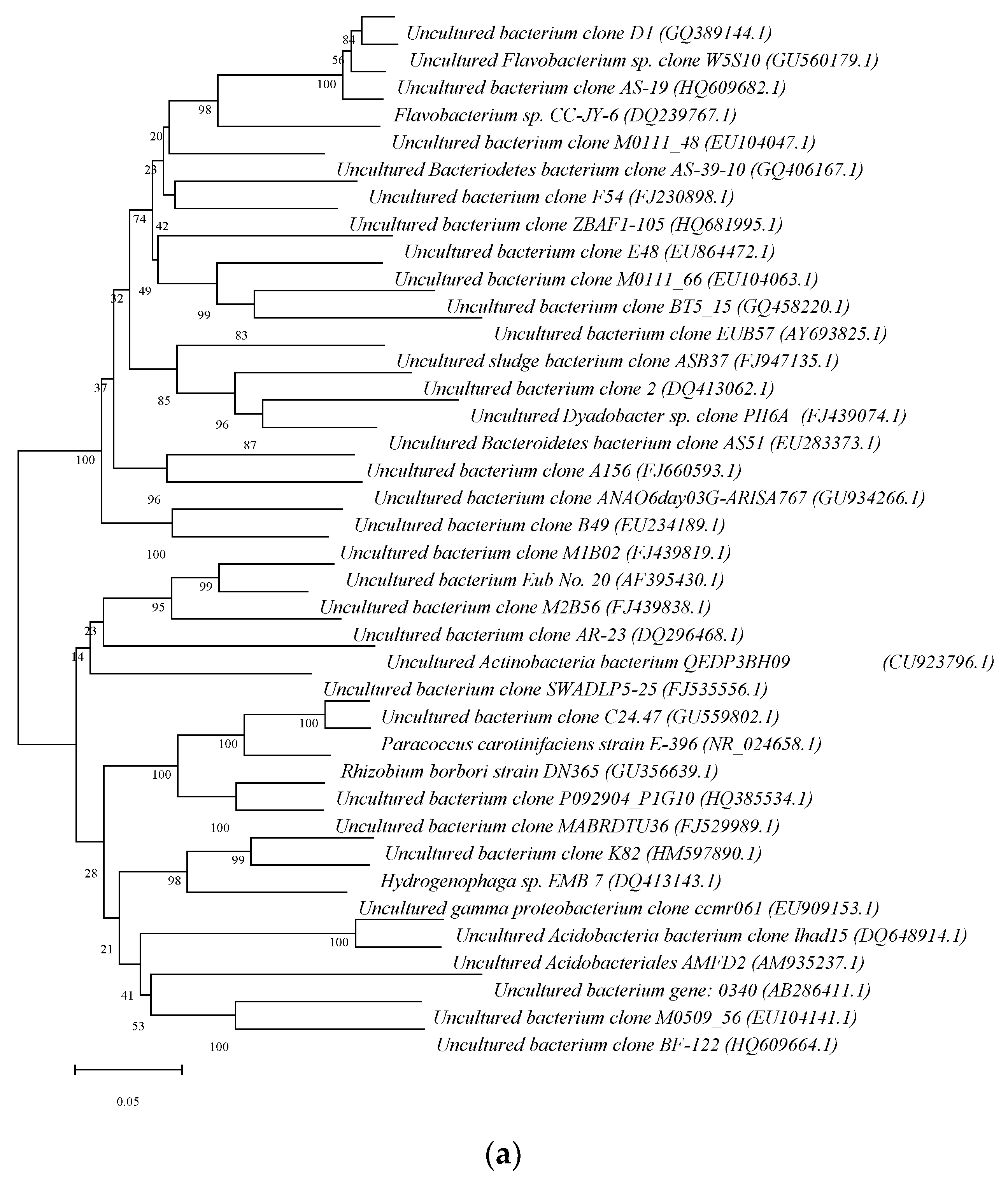
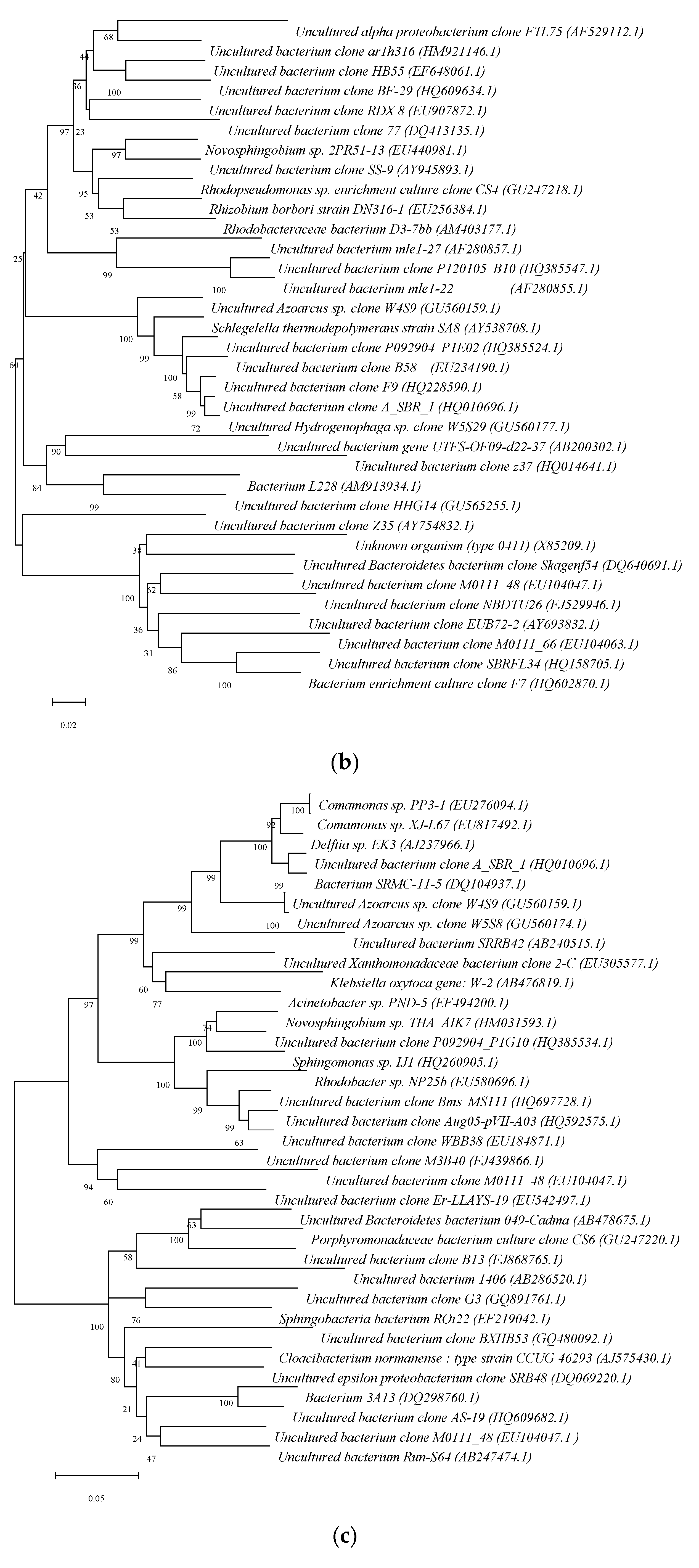
3.3. Differences in the R3 Reactor Sludge Granulation Process’s Overall Bacterial Community Structure
- (1)
- The AGS reactor with 15 days of operation:
- (2)
- The AGS reactor with 40 days of operation:
- (3)
- The AGS reactor with 80 days of operation:
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wojtyczka, R.D.; Arkadiusz, D.; MalGorzata, K.; Robert, K.; Agata, K.-D.; Tomasz, M.; Danuta, I. Berberine enhances the antibacterial activity of selected antibiotics against coagulase-negative Staphylococcus strains in vitro. Molecules 2014, 19, 6583–6596. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yin, Y.M.; Wang, X.Y.; Wu, H.; Ge, X.Z. Evaluation of berberine as a natural fungicide: Biodegradation and antimicrobial mechanism. J. Asian Nat. Prod. Res. 2017, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.S.; Safwat, G.; Aboulkhair, M.; Moneim, A.E.A. The potential effect of berberine in mercury-induced hepatorenal toxicity in albino rats. Food Chem. Toxicol. 2014, 69, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Čerňakova, M.; Košťálová, D. Antimicrobial activity of berberine—A constituent of mahonia aquifolium. Folia Microbiol. 2002, 47, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, L.J.; Liu, Y.Q.; Zeng, P.; Wang, Y.; Zhang, Y.Z. Removal of berberine from wastewater by MIL-101(Fe): Performance and mechanism. ACS Omega 2020, 5, 27962–27971. [Google Scholar] [CrossRef]
- Liu, S.Y.; Zhang, Y.; Zeng, P.; Wang, H.L.; Song, Y.H.; Li, J. Isolation and Characterization of a Bacterial Strain Capable of Efficient Berberine Degradation. Int. J. Environ. Res. Public Health 2019, 16, 646. [Google Scholar] [CrossRef]
- Shan, Y.P.; Song, Y.H.; Liu, Y.Q.; Liu, R.X.; Du, J.J.; Zeng, P. Adsorption of berberine by polymeric resin H103: Kinetics and thermodynamics. Environ. Earth Sci. 2015, 73, 4989–4994. [Google Scholar] [CrossRef]
- Qin, W.; Song, Y.; Dai, Y.; Qiu, G.; Ren, M.; Zeng, P. Treatment of berberine hydrochloride pharmaceutical wastewater by O3/UV/H2O2 advanced oxidation process. Environ. Earth Sci. 2015, 73, 4939–4946. [Google Scholar] [CrossRef]
- Ren, M.; Song, Y.; Xiao, S.; Ping, Z.; Peng, J. Treatment of berberine hydrochloride wastewater by using pulse electro-coagulation process with Fe electrode. Chem. Eng. J. 2011, 169, 84–90. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of People’s Republic of China. Guideline on Available Techniques of Pollution Prevention and Control for Pharmaceutical Industry Active Pharmaceutical Ingredient (Fermentation, Chemical Synthesis, Extraction) and Preparations Categories; Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 2022.
- Krishna, G.G.; Kumar, P.; Kumar, P. Treatment of low-strength soluble wastewater using an anaerobic baffled reactor (ABR). J. Environ. Manag. 2009, 90, 166–176. [Google Scholar] [CrossRef]
- Wang, J.L.; Huang, Y.H.; Zhao, X. Performance and Characteristics of an anaerobic baffled reactor. Bioresour. Technol. 2004, 93, 205–208. [Google Scholar] [CrossRef]
- Zeng, P.; Moy, B.; Song, Y.; Tay, J. Biodegradation of dimethyl phthalate by Sphingomonas sp. isolated from phthalic-acid-degrading aerobic granules. Appl. Microbiol. Biotechnol. 2008, 80, 899–905. [Google Scholar] [CrossRef]
- Zhang, Y.; Tay, J. Toxic and inhibitory effects of trichloroethylene aerobic co-metabolism on phenol-grown aerobic granules. J. Hazard. Mater. 2015, 286, 204–210. [Google Scholar] [CrossRef]
- Jiang, H.L.; Tay, J.H.; Tay, S.T.L. Changes in structure, activity and metabolism of aerobic granules as a microbial response to high phenol loading. Appl. Microbiol. Biotechnol. 2004, 63, 602–608. [Google Scholar] [CrossRef]
- Qiu, G.; Song, Y.H.; Zeng, P.; Duan, L.; Xiao, S. Characterization of bacterial communities in hybrid upflow anaerobic sludge blanket (UASB)-membrane bioreactor (MBR) process for berberine antibiotic wastewater treatment. Bioresour. Technol. 2013, 142, 52–62. [Google Scholar] [CrossRef]
- Liu, F.H. The Characteristics of Microbial Molecular Ecology of Hybrid ABR-Aerobic Granulation Reactor for Pharmaceutical Wastewater Treatment. Ph.D. Thesis, University of Science and Technology, Beijing, China, 2011. [Google Scholar]
- Liu, Y.Q.; Kong, Y.H.; Zhang, R.; Zhang, X.; Wong, F.S.; Tay, J.H.; Zhu, J.R.; Jiang, W.J. Microbial population dynamics of granular aerobic sequencing batch reactors during start-up and steady state periods. Water Sci. Technol. 2010, 62, 1281–1287. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Gao, Y.; Li, D.; Liu, R.; Liu, M.; Zhang, H.; Hu, B.; Yu, T.; Yang, M. Microbial community compositional analysis for series reactors treating high level antibiotic wastewater. Environ. Sci. Technol. 2012, 46, 795–801. [Google Scholar] [CrossRef]
- Ma, L.L.; Tank, Y.K.; Zhang, H.B. Development and design anaerobic baffled reactor for wastewater treatment. J. Guangxi Univ. 2008, 33, 378–380. [Google Scholar]
- Nachaiyasit, S.S.; Stuckey, D.C. The effect of shock loads on the performance of an anaerobic fabled reactor (ABR):2.step and transient hydraulic shocks at constant feed strength. Water Res. 1997, 31, 2747–2754. [Google Scholar] [CrossRef]
- Zhou, H. Analysis of ABR’s engineering design consideration. Water Wastewater Eng. 2009, 35, 226–229. [Google Scholar]
- Hu, X.Q.; Liu, D.Y.; Cai, H.S. Influence of Structure on Hydrodynamic Characteristics of Anaerobic Baffled Reactor. Earth Sci. 2004, 29, 369–374. [Google Scholar]
- Geng, Y.G.; Zhang, X.; Zhang, H.Q.; Liu, J.D. Discussion on Engineering design of anaerobic baffled reactor. Technol. Water Treat. 2009, 35, 103–107. [Google Scholar]
- Qiu, G.; Song, Y.H.; Zeng, P.; Duan, L.; Xiao, S. Combination of upflow anaerobic sludge blanket (UASB) and membrane bioreactor (MBR) for berberine reduction from wastewater and the effects of berberine on bacterial community dynamics. J. Hazard. Mater. 2013, 246, 34–43. [Google Scholar] [CrossRef] [PubMed]
- State Environmental Protection Administration Monitoring (Ed.) Analytical Methods of Water and Wastewater, 4th ed.; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Qiu, G. Hybrid UASB-MBR Process for Berberine Pharmaceutical Wastewater Treatment and the Process Mechanisms. Ph.D. Thesis, Beijing Normal University, Beijing, China, 2011. [Google Scholar]
- Winter, J.; Popoff, M.R.; Grimont, P.; Bokkenheuser, V.D. Clostridium orbiscindens sp. nov., a human intestinal bacterium capable of cleaving the flavonoid C-ring. Int. J. Syst. Evol. Microbiol. 1991, 41, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Kasmi, A.E.; Rajasekharan, S.; Ragsdale, S.W. Anaerobic pathway for conversion of the methyl group of aromatic methyl ethers to acetic acid by Clostridium thermoaceticum. Biochemistry 1994, 33, 11217–11224. [Google Scholar] [CrossRef]
- Narihiro, T.; Terada, T.; Kikuchi, K.; Iguchi, A.; Ikeda, M.; Yamauchi, T.; Shiraishi, K.; Kamagata, Y.; Nakamura, K.; Sekiguchi, Y. Comparative analysis of bacterial and archaeal communities in methanogenic sludge granules from upflow anaerobic sludge blanket reactors treating various food-processing, high strength organic wastewaters. Microbes Environ. 2009, 24, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Jia, R.; Feng, F.; Xie, K.; Li, H.; Jing, D.; Xu, X. Effect of solids retention time on antibiotics removal performance and microbial communities in an A/OMBR process. Bioresour. Technol. 2012, 106, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H. Experimental and Theoretical Studies of Protoberberine Alkaloids on the Mechanism of Multiple Activities. Ph.D. Thesis, Tianjin Medical University, Tianjin, China, 2004. [Google Scholar]




| Influent (mg/L) | ABR Effluent (mg/L) | AGS Effluent (mg/L) | ABR Removal Efficiency (%) | AGS Removal Efficiency (%) | Overall Removal Efficiency (%) | |
|---|---|---|---|---|---|---|
| Berberine | 121.6 ± 2.4 | 52.3 ± 1.3 | 9.5 ± 0.4 | 57.0 ± 0.2 | 81.8 ± 0.3 | 92.2 ± 0.7 |
| COD | 4253 ± 104 | 1193.1 ± 46 | 219.7 ± 2.4 | 71.9 ± 1.0 | 81.6 ± 0.5 | 94.8 ± 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, Y.; Li, J.; Ma, R.; Zeng, P.; Ng, C.A.; Liu, F. The Dynamic Shift of Bacterial Communities in Hybrid Anaerobic Baffled Reactor (ABR)—Aerobic Granules Process for Berberine Pharmaceutical Wastewater Treatment. Processes 2022, 10, 2506. https://doi.org/10.3390/pr10122506
Wang Y, Liu Y, Li J, Ma R, Zeng P, Ng CA, Liu F. The Dynamic Shift of Bacterial Communities in Hybrid Anaerobic Baffled Reactor (ABR)—Aerobic Granules Process for Berberine Pharmaceutical Wastewater Treatment. Processes. 2022; 10(12):2506. https://doi.org/10.3390/pr10122506
Chicago/Turabian StyleWang, Yan, Yongqiang Liu, Juan Li, Ruirui Ma, Ping Zeng, Choon Aun Ng, and Fenghua Liu. 2022. "The Dynamic Shift of Bacterial Communities in Hybrid Anaerobic Baffled Reactor (ABR)—Aerobic Granules Process for Berberine Pharmaceutical Wastewater Treatment" Processes 10, no. 12: 2506. https://doi.org/10.3390/pr10122506
APA StyleWang, Y., Liu, Y., Li, J., Ma, R., Zeng, P., Ng, C. A., & Liu, F. (2022). The Dynamic Shift of Bacterial Communities in Hybrid Anaerobic Baffled Reactor (ABR)—Aerobic Granules Process for Berberine Pharmaceutical Wastewater Treatment. Processes, 10(12), 2506. https://doi.org/10.3390/pr10122506








