Comparative Study on UV-AOPs for Efficient Continuous Flow Removal of 4-tert-Butylphenol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
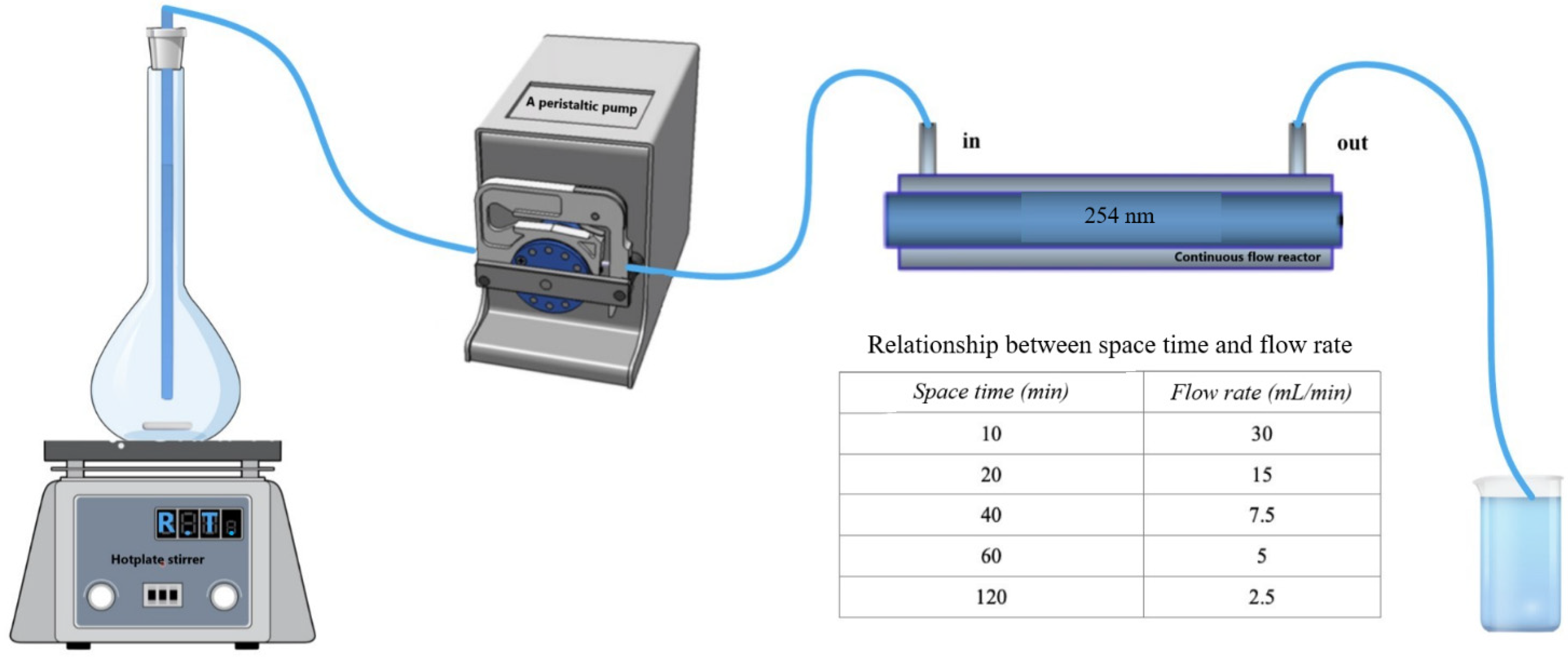
2.2. Experimental Procedures
2.3. Analytical Methods
3. Results and Discussion
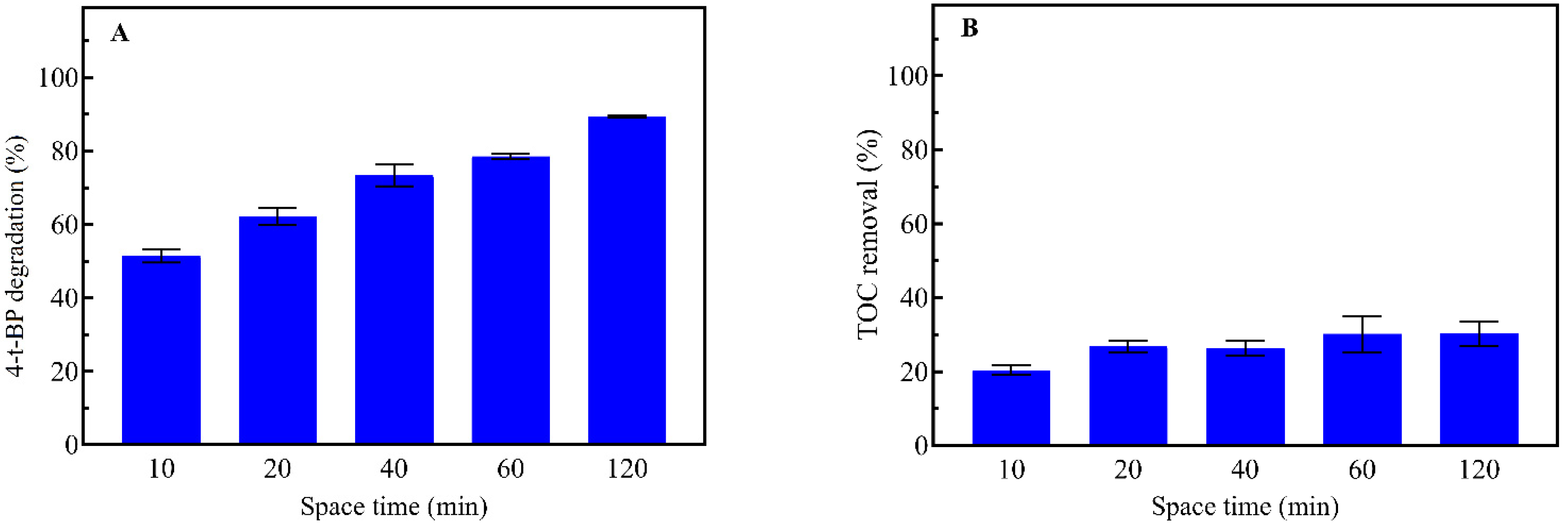
3.1. UV-Photolysis
3.2. UV/H2O2
3.3. UV/Fe 2+/H2O2 and UV/Fe 3+/H2O2
3.4. UV/TiO2 and UV/Fe-TiO2
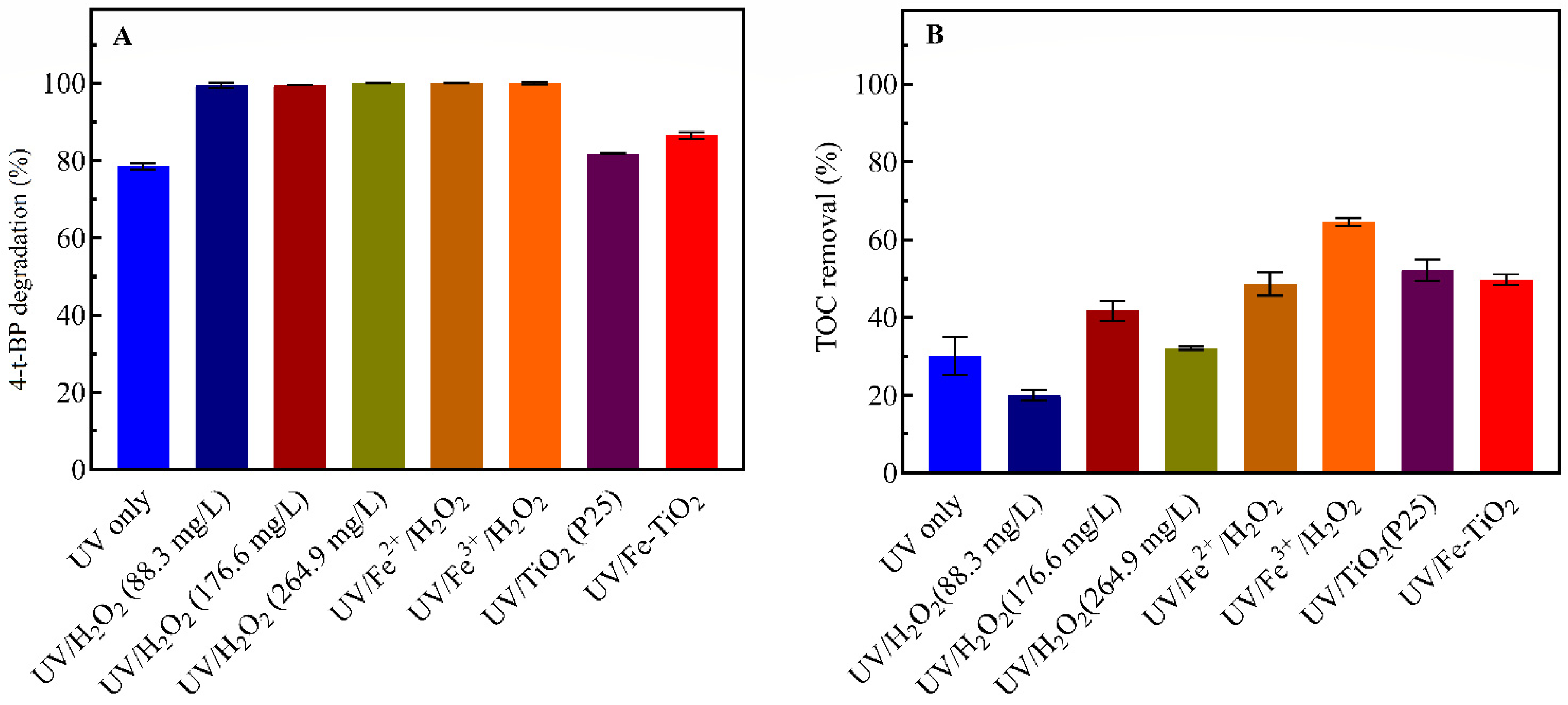
3.5. Comparison of UV-Induced Processes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heemken, O.P.; Reincke, H.; Stachel, B.; Theobald, N. The Occurrence of Xenoestrogens in the Elbe River and the North Sea. Chemosphere 2001, 45, 245–259. [Google Scholar] [CrossRef]
- Inoue, K.; Yoshie, Y.; Kondo, S.; Yoshimura, Y.; Nakazawa, H. Determination of Phenolic Xenoestrogens in Water by Liquid Chromatography with Coulometric-Array Detection. J. Chromatogr. A 2002, 946, 291–294. [Google Scholar] [CrossRef]
- Koh, C.-H.; Khim, J.S.; Villeneuve, D.L.; Kannan, K.; Giesy, J.P. Characterization of Trace Organic Contaminants in Marine Sediment from Yeongil Bay, Korea: 1. Instrumental Analyses. Environ. Pollut. 2006, 142, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-S.; Choo, K.-H.; Lee, B.; Choi, S.-J. The Methods of Identification, Analysis, and Removal of Endocrine Disrupting Compounds (EDCs) in Water. J. Hazard. Mater. 2009, 172, 1–12. [Google Scholar] [CrossRef]
- Tang, Y.; Yin, M.; Yang, W.; Li, H.; Zhong, Y.; Mo, L.; Liang, Y.; Ma, X.; Sun, X. Emerging Pollutants in Water Environment: Occurrence, Monitoring, Fate, and Risk Assessment. Water Environ. Res. Res. Publ. Water Environ. Fed. 2019, 91, 984–991. [Google Scholar] [CrossRef] [Green Version]
- Annamalai, J.; Namasivayam, V. Endocrine Disrupting Chemicals in the Atmosphere: Their Effects on Humans and Wildlife. Environ. Int. 2015, 76, 78–97. [Google Scholar] [CrossRef]
- Acir, I.-H.; Guenther, K. Endocrine-Disrupting Metabolites of Alkylphenol Ethoxylates—A Critical Review of Analytical Methods, Environmental Occurrences, Toxicity, and Regulation. Sci. Total Environ. 2018, 635, 1530–1546. [Google Scholar] [CrossRef] [PubMed]
- Balaguer, P.; Delfosse, V.; Grimaldi, M.; Bourguet, W. Structural and Functional Evidences for the Interactions between Nuclear Hormone Receptors and Endocrine Disruptors at Low Doses. Comptes Rendus Biol. 2017, 340, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Toyama, T.; Momotani, N.; Ogata, Y.; Miyamori, Y.; Inoue, D.; Sei, K.; Mori, K.; Kikuchi, S.; Ike, M. Isolation and Characterization of 4-tert-Butylphenol-Utilizing Sphingobium Fuliginis Strains from Phragmites Australis Rhizosphere Sediment. Appl. Environ. Microbiol. 2010, 76, 6733–6740. [Google Scholar] [CrossRef] [Green Version]
- Webster, F.; Gagné, M.; Patlewicz, G.; Pradeep, P.; Trefiak, N.; Judson, R.S.; Barton-Maclaren, T.S. Predicting Estrogen Receptor Activation by a Group of Substituted Phenols: An Integrated Approach to Testing and Assessment Case Study. Regul. Toxicol. Pharmacol. 2019, 106, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Wirasnita, R.; Mori, K.; Toyama, T. Effect of Activated Carbon on Removal of Four Phenolic Endocrine-Disrupting Compounds, Bisphenol A, Bisphenol F, Bisphenol S, and 4-tert-Butylphenol in Constructed Wetlands. Chemosphere 2018, 210, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhu, X.; Chen, H.; Dong, W.; Zhao, J. Photodegradation of 4-tert-Butylphenol in Aqueous Solution by UV-C, UV/H2O2 and UV/S2O82− System. J. Environ. Sci. Health Part A 2016, 51, 440–445. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, J.; Chen, H.; Zhao, J.; Dong, W. Aqueous Photodegradation of 4-tert-Butylphenol: By-Products, Degradation Pathway and Theoretical Calculation Assessment. Sci. Total Environ. 2016, 566–567, 86–92. [Google Scholar] [CrossRef]
- Olsen, C.M.; Meussen-Elholm, E.T.M.; Hongslo, J.K.; Stenersen, J.; Tollefsen, K.-E. Estrogenic Effects of Environmental Chemicals: An Interspecies Comparison. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2005, 141, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Barse, A.V.; Chakrabarti, T.; Ghosh, T.; Pal, A.; Jadhao, S. One-Tenth Dose of LC50 of 4-tert-Butylphenol Causes Endocrine Disruption and Metabolic Changes in Cyprinus Carpio. Pestic. Biochem. Physiol. 2006, 86, 172–179. [Google Scholar] [CrossRef]
- Meier, S.; Andersen, T.E.; Norberg, B.; Thorsen, A.; Taranger, G.L.; Kjesbu, O.S.; Dale, R.; Morton, H.C.; Klungsøyr, J.; Svardal, A. Effects of Alkylphenols on the Reproductive System of Atlantic Cod (Gadus Morhua). Aquat. Toxicol. 2007, 81, 207–218. [Google Scholar] [CrossRef]
- EUR-Lex-32019D1194-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/dec_impl/2019/1194/oj (accessed on 4 November 2021).
- Fan, D.; Liu, J.; Wang, L.; Yang, X.; Zhang, S.; Zhang, Y.; Shi, L. Development of Quantitative Structure–Activity Relationship Models for Predicting Chronic Toxicity of Substituted Benzenes to Daphnia Magna. Bull. Environ. Contam. Toxicol. 2016, 96, 664–670. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Liu, J.; Shi, L.; Wang, Z. Application of Microcosm and Species Sensitivity Distribution Approaches in the Ecological Hazard Assessment of 4-tert-Butylphenol. Chem. Ecol. 2017, 34, 108–125. [Google Scholar] [CrossRef]
- Janousek, R.M.; Müller, J.; Knepper, T.P. Combined Study of Source, Environmental Monitoring and Fate of Branched Alkylphenols: The Chain Length Matters. Chemosphere 2020, 241, 124950. [Google Scholar] [CrossRef] [PubMed]
- Makhatova, A.; Ulykbanova, G.; Sadyk, S.; Sarsenbay, K.; Atabaev, T.S.; Inglezakis, V.J.; Poulopoulos, S.G. Degradation and Mineralization of 4-tert-Butylphenol in Water Using Fe-Doped TiO2 Catalysts. Sci. Rep. 2019, 9, 19284. [Google Scholar] [CrossRef]
- Dan, A.; Fujii, D.; Soda, S.; Machimura, T.; Ike, M. Removal of Phenol, Bisphenol A, and 4-tert-Butylphenol from Synthetic Landfill Leachate by Vertical Flow Constructed Wetlands. Sci. Total Environ. 2017, 578, 566–576. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Yang, S.; Kang, J.; Leusch, F.D.L.; Roddick, F.; Price, W.E.; Nghiem, L.D. Removal of Pharmaceuticals, Steroid Hormones, Phytoestrogens, UV-Filters, Industrial Chemicals and Pesticides by Trametes Versicolor: Role of Biosorption and Biodegradation. Int. Biodeterior. Biodegrad. 2014, 88, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.N.; Hai, F.I.; Price, W.E.; Kang, J.; Leusch, F.D.L.; Roddick, F.; van de Merwe, J.P.; Magram, S.F.; Nghiem, L.D. Degradation of a Broad Spectrum of Trace Organic Contaminants by an Enzymatic Membrane Reactor: Complementary Role of Membrane Retention and Enzymatic Degradation. Int. Biodeterior. Biodegrad. 2015, 99, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Ogata, Y.; Toyama, T.; Yu, N.; Wang, X.; Sei, K.; Ike, M. Occurrence of 4-tert-Butylphenol (4-t-BP) Biodegradation in an Aquatic Sample Caused by the Presence of Spirodela Polyrrhiza and Isolation of a 4-t-BP-Utilizing Bacterium. Biodegradation 2013, 24, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Li, C.; Zhang, G.; Meng, Y.; Yin, B.; Zhao, Y.; Shi, W. Efficient and Stable Nb2O5 Modified G-C3N4 Photocatalyst for Removal of Antibiotic Pollutant. Chem. Eng. J. 2016, 299, 74–84. [Google Scholar] [CrossRef]
- Lee, Y.; von Gunten, U. Oxidative Transformation of Micropollutants during Municipal Wastewater Treatment: Comparison of Kinetic Aspects of Selective (Chlorine, Chlorine Dioxide, FerrateVI, and Ozone) and Non-Selective Oxidants (Hydroxyl Radical). Water Res. 2010, 44, 555–566. [Google Scholar] [CrossRef]
- Muruganandham, M.; Suri, R.; Jafari, S.; Sillanpää, M.; Lee, G.-J.; Wu, J.; Swaminathan, M. Recent Developments in Homogeneous Advanced Oxidation Processes for Water and Wastewater Treatment. Int. J. Photoenergy 2014, 2014, 821674. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Brigante, M.; Dong, W.; Claire, P.; Mailhot, G. Toward a Better Understanding of Fe(III)-EDDS Photochemistry: Theoretical Stability Calculation and Experimental Investigation of 4-tert-Butylphenol Degradation. J. Phys. Chem. A 2013, 118, 396–403. [Google Scholar] [CrossRef]
- Wu, Y.; Prulho, R.; Brigante, M.; Dong, W.; Hanna, K.; Mailhot, G. Activation of Persulfate by Fe(III) Species: Implications for 4-tert-Butylphenol Degradation. J. Hazard. Mater. 2016, 322, 380–386. [Google Scholar] [CrossRef]
- Xiao, X.; Xing, C.; He, G.; Zuo, X.; Nan, J.; Wang, L. Solvothermal Synthesis of Novel Hierarchical Bi4O5I2 Nanoflakes with Highly Visible Light Photocatalytic Performance for the Degradation of 4-tert-Butylphenol. Appl. Catal. B Environ. 2014, 148–149, 154–163. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, J.; Li, C.; Zhou, P.; Liu, J.; Ou, H. Degradation of 1 H -Benzotriazole by UV/H2O2 and UV/TiO2 : Kinetics, Mechanisms, Products and Toxicology. Environ. Sci. Water Res. Technol. 2018, 4, 1282–1294. [Google Scholar] [CrossRef]
- Peternel, I.T.; Koprivanac, N.; Božić, A.M.L.; Kušić, H.M. Comparative Study of UV/TiO2, UV/ZnO and Photo-Fenton Processes for the Organic Reactive Dye Degradation in Aqueous Solution. J. Hazard. Mater. 2007, 148, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Costa, J.I.; Rivera-Utrilla, J.; Leyva-Ramos, R.; Sánchez-Polo, M.; Velo-Gala, I.; Mota, A.J. Individual and Simultaneous Degradation of the Antibiotics Sulfamethoxazole and Trimethoprim in Aqueous Solutions by Fenton, Fenton-like and Photo-Fenton Processes Using Solar and UV Radiations. J. Photochem. Photobiol. Chem. 2018, 360, 95–108. [Google Scholar] [CrossRef]
- Chang, S.; Yang, X.; Sang, Y.; Liu, H. Highly Efficient Photocatalysts and Continuous-Flow Photocatalytic Reactors for Degradation of Organic Pollutants in Wastewater. Chem. Asian J. 2016, 11, 2352–2371. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, S.; Sugihara, M.N.; Strathmann, T.J. Continuous-Flow Photocatalytic Treatment of Pharmaceutical Micropollutants: Activity, Inhibition, and Deactivation of TiO2 Photocatalysts in Wastewater Effluent. Appl. Catal. B Environ. 2013, 129, 1–12. [Google Scholar] [CrossRef]
- Petala, A.; Spyrou, D.; Frontistis, Z.; Mantzavinos, D.; Kondarides, D.I. Immobilized Ag3PO4 Photocatalyst for Micro-Pollutants Removal in a Continuous Flow Annular Photoreactor. Catal. Today 2019, 328, 223–229. [Google Scholar] [CrossRef]
- Silva, C.P.; Oliveira, C.; Ribeiro, A.; Osório, N.; Otero, M.; Esteves, V.I.; Lima, D.L.D. Sulfamethoxazole Exposure to Simulated Solar Radiation under Continuous Flow Mode: Degradation and Antibacterial Activity. Chemosphere 2020, 238, 124613. [Google Scholar] [CrossRef] [PubMed]
- Shojaeimehr, T.; Tasbihi, M.; Acharjya, A.; Thomas, A.; Schomäcker, R.; Schwarze, M. Impact of Operating Conditions for the Continuous-Flow Degradation of Diclofenac with Immobilized Carbon Nitride Photocatalysts. J. Photochem. Photobiol. Chem. 2019, 388, 112182. [Google Scholar] [CrossRef]
- Jaganathan, S.; Philip, L. Elimination of Pesticides and Their Formulation Products from Drinking Water Using Thin Film Continuous Photoreactor under Solar Radiation. Sol. Energy 2012, 86, 2735–2745. [Google Scholar] [CrossRef]
- Piña-Pérez, Y.; Aguilar-Martínez, O.; Oros-Ruíz, S.; Gómez, R.; Tzompantzi, F. Commercial Aluminum Oxides with Different Crystalline Structures Efficient for the Mineralization of Phenolic Pollutants. J. Photochem. Photobiol. Chem. 2018, 353, 409–415. [Google Scholar] [CrossRef]
- Chen, J.; He, Z.; Ji, Y.; Li, G.; An, T.; Choi, W. OH Radicals Determined Photocatalytic Degradation Mechanisms of Gaseous Styrene in TiO2 System under 254 Nm versus 185 Nm Irradiation: Combined Experimental and Theoretical Studies. Appl. Catal. B Environ. 2019, 257, 117912. [Google Scholar] [CrossRef]
- Poulopoulos, S.G.; Yerkinova, A.; Ulykbanova, G.; Inglezakis, V.J. Photocatalytic Treatment of Organic Pollutants in a Synthetic Wastewater Using UV Light and Combinations of TiO2, H2O2 and Fe(III). PLoS ONE 2019, 14, e0216745. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.-S.; Ye, J.-S.; Ma, S.; Wei, C.-H.; Gao, N.-Y.; He, J. Degradation of Ciprofloxacin by UV and UV/H2O2 via Multiple-Wavelength Ultraviolet Light-Emitting Diodes: Effectiveness, Intermediates and Antibacterial Activity. Chem. Eng. J. 2016, 289, 391–401. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Xiao, Y.; Chang, V.; Lim, T. Kinetic and Mechanistic Investigation of Azathioprine Degradation in Water by UV, UV/H2O2 and UV/Persulfate. Chem. Eng. J. 2016, 302, 526–534. [Google Scholar] [CrossRef]
- Afzal, T.; Isa, M.H.; UI Mustafa, M.R. Removal of Organic Pollutants from Produced Water Using Fenton Oxidation. E3S Web Conf. 2018, 34, 02035. [Google Scholar] [CrossRef] [Green Version]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic Degradation of Azo Dye Acid Red 14 in Water on ZnO as an Alternative Catalyst to TiO2. J. Photochem. Photobiol. Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Watts, R.J.; Sarasa, J.; Loge, F.J.; Teel, A.L. Oxidative and Reductive Pathways in Manganese-Catalyzed Fenton’s Reactions. J. Environ. Eng. 2005, 131, 158–164. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Lu, S.; Wang, Z.; Wang, Y.; Zhang, G.; Guo, X.; Guo, W.; Zhang, T.; Xi, B. Degradation Difference of Ofloxacin and Levofloxacin by UV/H2O2 and UV/PS (Persulfate): Efficiency, Factors and Mechanism. Chem. Eng. J. 2020, 385, 123987. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Dargahi, A.; Zolghadr Nasab, H.; Ghahramani, E.; Salehi, S. Degradation of Azo Dye Acid Red 14 (AR14) from Aqueous Solution Using H2O2/NZVI and S2O82−/NZVI Processes in the Presence of UV Irradiation. Water Environ. Res. Res. Publ. Water Environ. Fed. 2020, 92, 1173–1183. [Google Scholar] [CrossRef]
- Rivas, F.J.; Beltrán, F.J.; Frades, J.; Buxeda, P. Oxidation of P-Hydroxybenzoic Acid by Fenton’s Reagent. Water Res. 2001, 35, 387–396. [Google Scholar] [CrossRef]
- Augugliaro, V.; Litter, M.; Palmisano, L.; Soria, J. The Combination of Heterogeneous Photocatalysis with Chemical and Physical Operations: A Tool for Improving the Photoprocess Performance. J. Photochem. Photobiol. C Photochem. Rev. 2006, 7, 127–144. [Google Scholar] [CrossRef] [Green Version]
- Hemmati Borji, S.; Nasseri, S.; Mahvi, A.H.; Nabizadeh, R.; Javadi, A.H. Investigation of Photocatalytic Degradation of Phenol by Fe(III)-Doped TiO2 and TiO2 Nanoparticles. J. Environ. Health Sci. Eng. 2014, 12, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- JIménez, D.H.; Triviño, G.M.H.; Villaseñor, E.A.; Mesa, E.B.; Hernández, J.A.; Zepeda, T.A.; Zapata, R.B.; Arias, A.N.A. Phenol Photocatalytic Degradation over Fe-TiO2 Materials Synthesized by Different Methods. Sci. Tech. 2019, 24, 523–531. [Google Scholar] [CrossRef]
- Jamil, T.S.; Gad-Allah, T.A.; Ghaly, M.Y. Parametric Study on Phenol Photocatalytic Degradation under Pure Visible and Solar Irradiations by Fe-Doped TiO2. Desalination Water Treat. 2012, 50, 264–271. [Google Scholar] [CrossRef]
- Moradi, V.; Ahmed, F.; Jun, M.B.G.; Blackburn, A.; Herring, R.A. Acid-Treated Fe-Doped TiO2 as a High Performance Photocatalyst Used for Degradation of Phenol under Visible Light Irradiation. J. Environ. Sci. 2019, 83, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Nahar, M.S.; Hasegawa, K.; Kagaya, S.; Kuroda, S. Comparative Assessment of the Efficiency of Fe-Doped TiO2 Prepared by Two Doping Methods and Photocatalytic Degradation of Phenol in Domestic Water Suspensions. Sci. Technol. Adv. Mater. 2007, 8, 286–291. [Google Scholar] [CrossRef]
- Shawabkeh, R.; Khashman, O.; Bisharat, G. Photocatalytic Degradation of Phenol Using Fe-TiO2 by Different Illumination Sources. Int. J. Chem. 2010, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Yodsomnuk, P.; Junjeam, K.; Termtanun, M. Photoactivity of Fe and Zn-Doped TiO2 in Phenol Degradation under Visible Light. MATEC Web Conf. 2018, 192, 03047. [Google Scholar] [CrossRef]
- Crişan, M.; Ianculescu, A.-C.; Crișan, D.; Drăgan, N.; Todan, L.; Niţoi, I.; Oancea, P. Chapter 10—Fe-Doped TiO2 Nanomaterials for Water Depollution. In Nanotechnology in the Beverage Industry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 265–313. [Google Scholar] [CrossRef]
- Sun, T.; Liu, E.; Fan, J.; Hu, X.; Wu, F.; Hou, W.; Yang, Y.; Kang, L. High Photocatalytic Activity of Hydrogen Production from Water over Fe Doped and Ag Deposited Anatase TiO2 Catalyst Synthesized by Solvothermal Method. Chem. Eng. J. 2013, 228, 896–906. [Google Scholar] [CrossRef]
- Moradi, H.; Eshaghi, A.; Hosseini, S.R.; Ghani, K. Fabrication of Fe-Doped TiO2 Nanoparticles and Investigation of Photocatalytic Decolorization of Reactive Red 198 under Visible Light Irradiation. Ultrason. Sonochem. 2016, 32, 314–319. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mergenbayeva, S.; Poulopoulos, S.G. Comparative Study on UV-AOPs for Efficient Continuous Flow Removal of 4-tert-Butylphenol. Processes 2022, 10, 8. https://doi.org/10.3390/pr10010008
Mergenbayeva S, Poulopoulos SG. Comparative Study on UV-AOPs for Efficient Continuous Flow Removal of 4-tert-Butylphenol. Processes. 2022; 10(1):8. https://doi.org/10.3390/pr10010008
Chicago/Turabian StyleMergenbayeva, Saule, and Stavros G. Poulopoulos. 2022. "Comparative Study on UV-AOPs for Efficient Continuous Flow Removal of 4-tert-Butylphenol" Processes 10, no. 1: 8. https://doi.org/10.3390/pr10010008
APA StyleMergenbayeva, S., & Poulopoulos, S. G. (2022). Comparative Study on UV-AOPs for Efficient Continuous Flow Removal of 4-tert-Butylphenol. Processes, 10(1), 8. https://doi.org/10.3390/pr10010008





