Cryoconcentration by Centrifugation–Filtration: A Simultaneous, Efficient and Innovative Method to Increase Thermosensitive Bioactive Compounds of Aqueous Maqui (Aristotelia chilensis (Mol.) Stuntz) Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
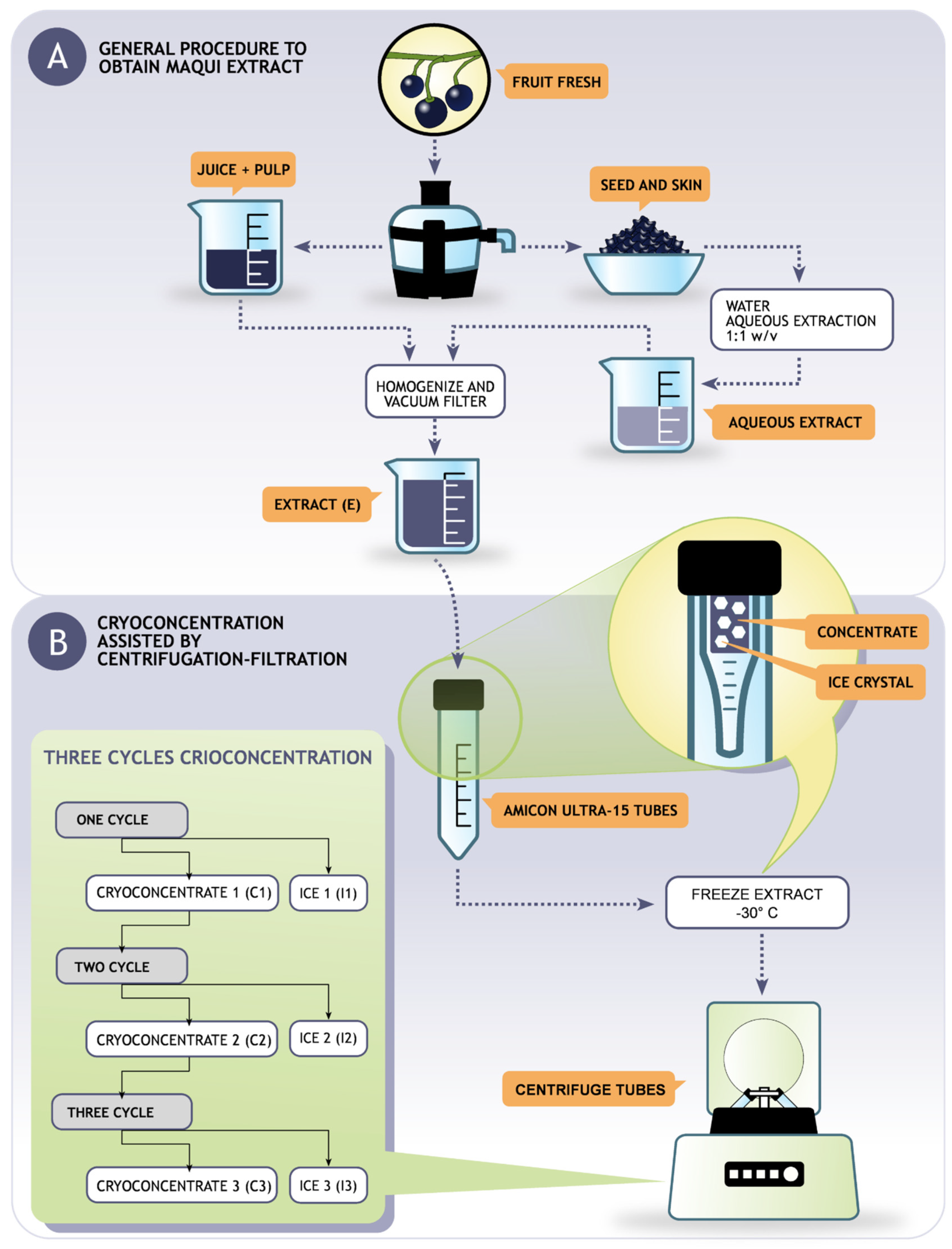
2.2. Production of the Juice and Aqueous Extract
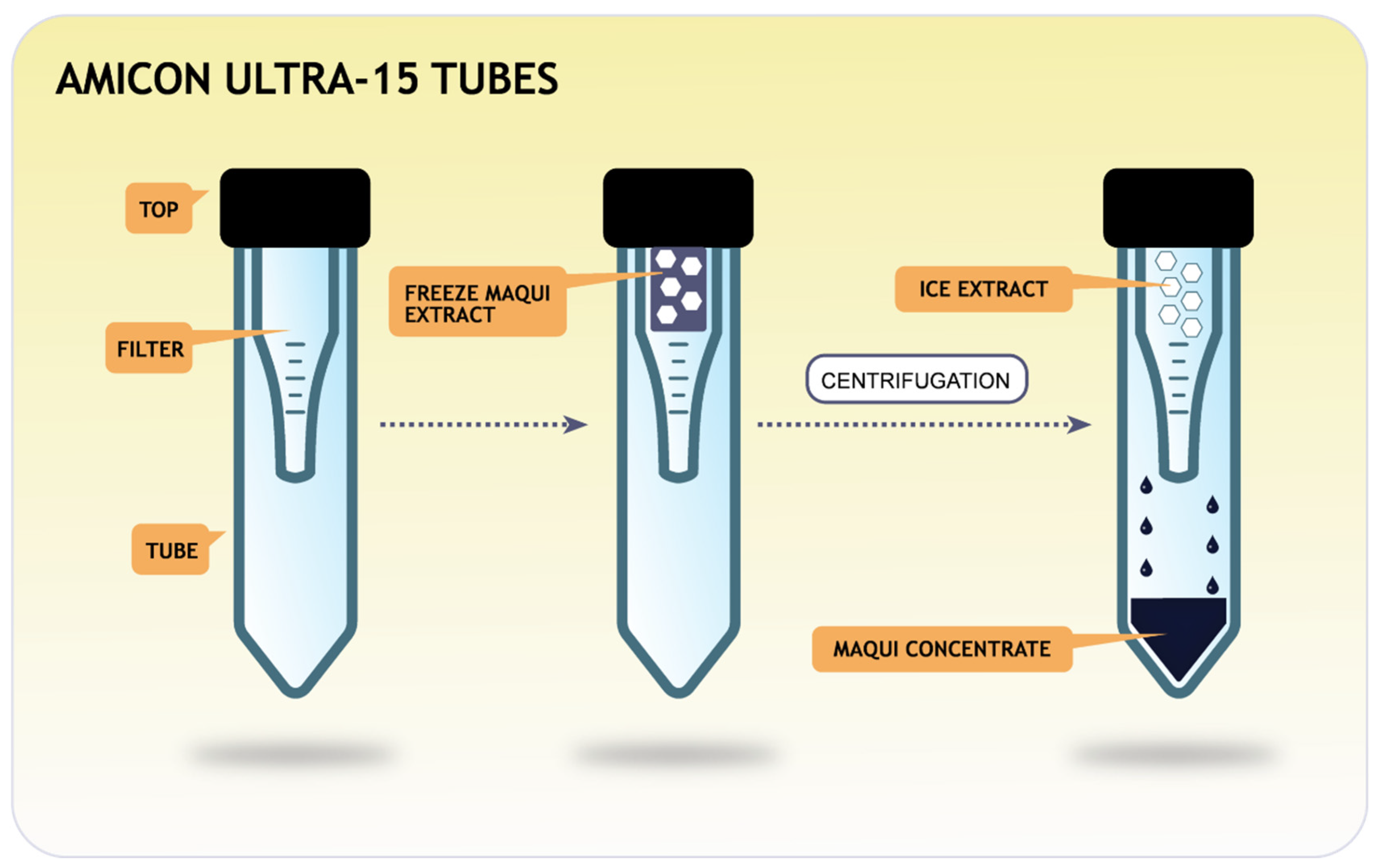
2.3. Production of Cryoconcentrates by Freezing and Centrifugation-Filtration
2.4. General Procedure for Cryoconcentration Cycles
2.5. Evaporation Concentration Process
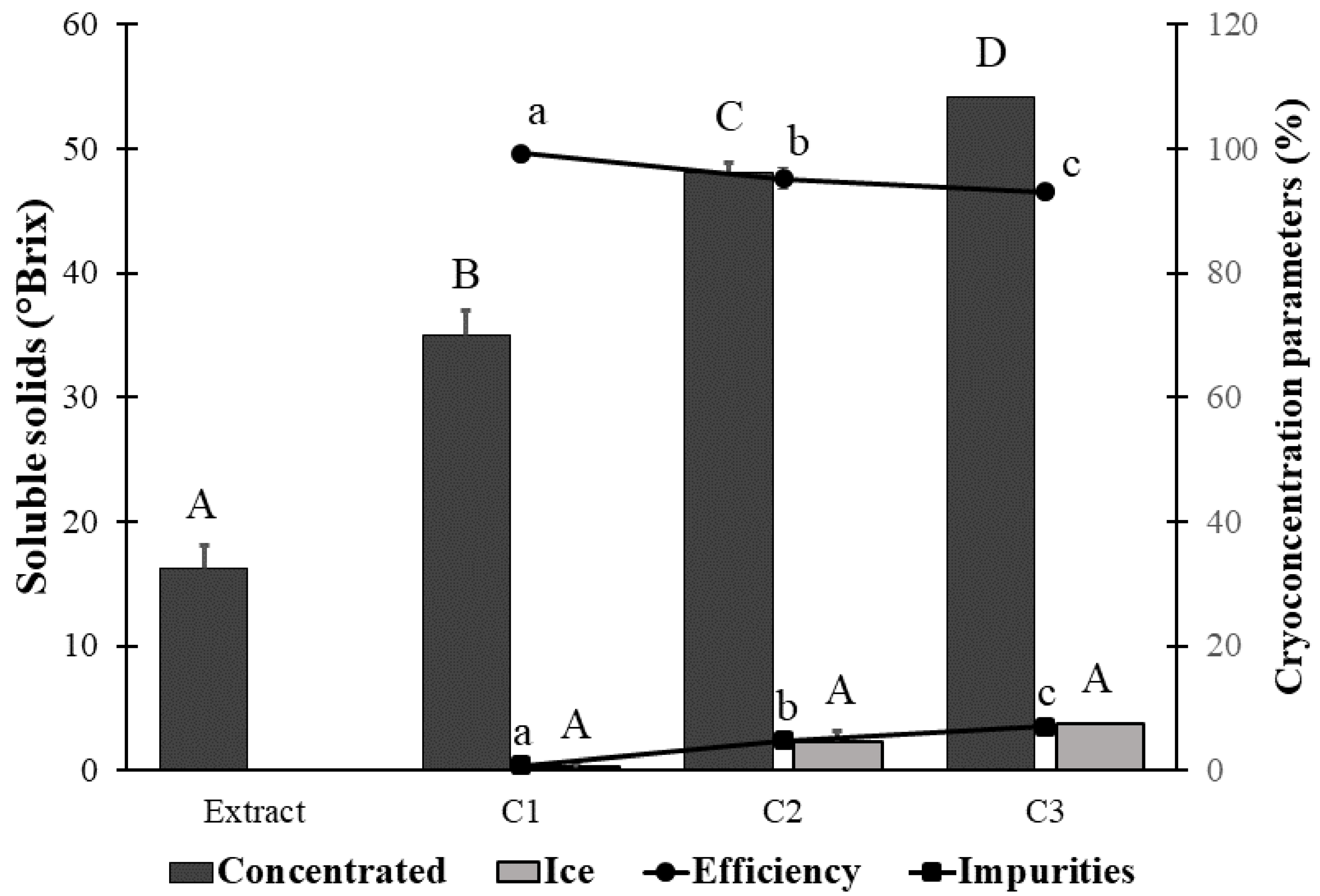
2.6. Determination of Cryoconcentration Parameters
Concentration Efficiency
2.7. Determination of Total Polyphenols, Anthocyanins, Antioxidant Capacity by the Diphenylpicrylhydrazyl (DPPH) and Oxygen Radical Absorption Capacity (ORAC) Methods
2.7.1. Total Polyphenols
2.7.2. Quantification and Identification of Anthocyanins
2.7.3. Determination of Antioxidant Capacity by the Diphenylpicrylhydrazyl (DPPH) and Oxygen Radical Absorption Capacity (ORAC) Methods
2.8. Determination of Color
2.9. Statistical Analysis
3. Results and Discussion
3.1. Condition for the Centrifugation–Filtration Process
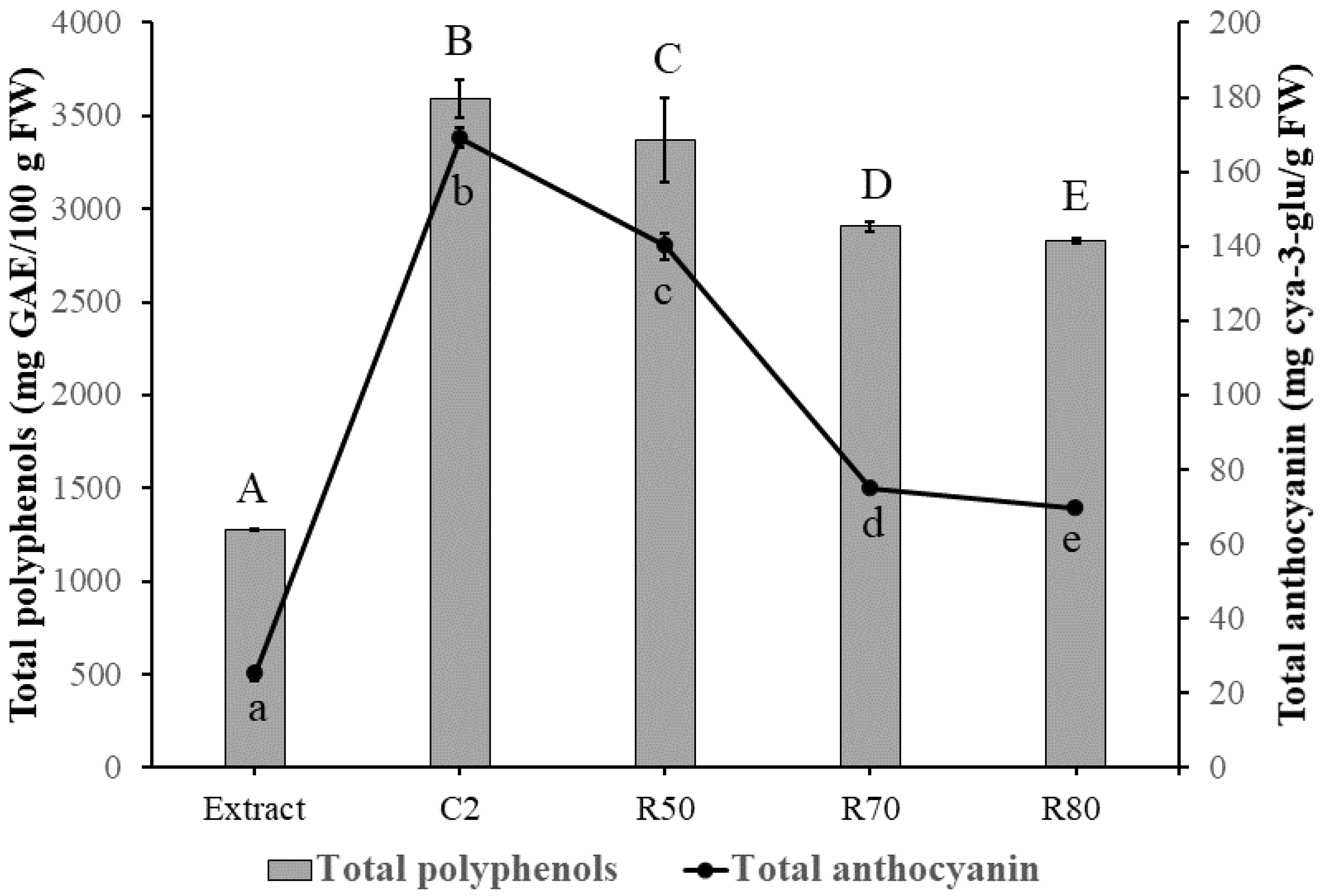
3.2. Determination of Total Polyphenols
3.3. Determination of Total Anthocyanins and Identification by High Performance Liquid Chromatography (HPLC)
3.4. Determination of Antioxidant Capacity by the Diphenylpicrylhydrazyl (DPPH) and Oxygen Radical Absorption Capacity (ORAC) Methods
3.5. Determination of Color
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Putnik, P.; Bursac Kovacevic, D.; Herceg, K.; Levaj, B. Influence of antibrowning solution, air exposure, and ultrasound on color changes in fresh-cut apples during storage. J. Food Process. Preserv. 2017, 64, 13288. [Google Scholar] [CrossRef]
- Jin, T.; Yu, Y.; Gurtler, J. Effects of pulsed electric field processing on microbial survival, quality change and nutritional characteristics of blueberries. LWT—Food Sci. Technol. 2017, 77, 517–524. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Szajdek, A.; Borowska, E. Bioactive compounds and health-promoting properties of berry fruits: A review. Plant Foods Hum. Nutr. 2008, 63, 147–156. [Google Scholar] [CrossRef]
- Juurlink, B.; Azouz, H.; Aldalati, A.; Altinawi, B.; Ganguly, P. Hydroxybenzoic acid isomers and the cardiovascular system. Nutr. J. 2014, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Rangsriwong, P.; Rangkadilok, N.; Satayavivad, J.; Goto, M.; Shotipruk, A. Subcritical water extraction of polyphenolic compounds from Terminalia chebula Retz. fruits. Sep. Purif. Technol. 2009, 66, 51–56. [Google Scholar] [CrossRef]
- Morison, K.R.; Hartel, R.W. Evaporation and freeze concentration. In Handbook of Food Engineering; Heldman, D.R., Lund, D.B., Eds.; CRC Press: New York, NY, USA, 2007; pp. 495–552. [Google Scholar]
- Sánchez, J.; Ruiz, Y.; Auleda, J.M.; Hernández, E.; Raventós, M. Review. Freeze concentration in the fruit juices industry. Food Sci. Technol. Int. 2009, 15, 303–315. [Google Scholar] [CrossRef]
- Petzold, G.; Orellana, P.; Moreno, J.; Cerda, E.; Parra, P. Vacumm-assisted block freeze concentration applied to wine. Innov. Food Sci. Emerg. Technol. 2016, 36, 330–335. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Petzold, G.; Guerra-Valle, M.; Astudillo-Lagos, M. Impact of block cryoconcentration on polyphenol retention in blueberry juice. Food Biosci. 2017, 20, 149–158. [Google Scholar] [CrossRef]
- Sánchez, J.; Ruiz, Y.; Raventós, M.; Auleda, J.M.; Hernández, E. Progressive freeze concentration of orange juice in a pilot plant falling film. Innov. Food Sci. Emerg. Technol. 2010, 11, 644–651. [Google Scholar] [CrossRef]
- Raventós, M.; Hernández, E.; Auleda, J. Freeze concentration applications in fruit processing. In Advances in Fruit Processing Technologies; Rodríguez, S., Fernandes, F.A.N., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 263–286. [Google Scholar]
- Aider, M.; De Halleux, D. Passive and microwave-assisted thawing in maple sap cryoconcentration technology. J. Food Eng. 2008, 85, 65–72. [Google Scholar] [CrossRef]
- Aider, M.; De Halleux, D. Production of concentrated cherry and apricot juices by cryoconcentration technology. LWT—Food Sci. Technol. 2008, 41, 1768–1775. [Google Scholar] [CrossRef]
- Amran, N.; Samsuri, S.; Safiei, N.; Zakaria, Z.; Jusoh, M. Review: Parametric study on the performance of progressive cryoconcentration system. Chem. Eng. Commun. 2016, 203, 957–975. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, B.; Deng, H.; Prinz, M.; Siegel, D. Body fluid identification by mass spectrometry. Int. J. Leg. Med. 2013, 127, 1065–1077. [Google Scholar] [CrossRef]
- Kranes, S.; Sterling, S.; Mason, K.; Anex, D.; Hart, B.; Parker, G.; Prinz, M. Simultaneous DNA and protein extraction using trypsin. Forensic Sci. Int. Genet. 2017, 6, e203–e204. [Google Scholar] [CrossRef]
- Wu, Y.; Sha, Q.; Wang, C.; Liu, B.F.; Wang, S.; Liu, X. Development of a filter-aided extraction method coupled with glycosylamine labeling to simplify and enhance high performance liquid chromatography-based N-glycan analysis. J. Chromatogr. A 2019, 1600, 105–111. [Google Scholar] [CrossRef]
- Bastías, J.; Vidal, C.; Muñoz, O.; Petzold, G.; Quevedo, R.; Hongxun, W.; Yi, Y.; Cespedes, C.L. Cryoconcentration procedure for aqueous extracts of maqui fruits prepared by centrifugation and filtration from fruits harvested in different years from the same localities. J. Berry Res. 2019, 9, 377–394. [Google Scholar] [CrossRef]
- Doran, A.; Foran, D. Assessment and mitigation of DNA loss utilizing centrifugal filtration devices. Forensic Sci. Int. Genet. 2014, 13, 187–190. [Google Scholar] [CrossRef]
- Hernández, E.; Raventós, M.; Auleda, J.; Ibarz, A. Freeze concentration of mustin a pilot plant falling film concentrator. Innov. Food Sci. Emerg. Technol. 2010, 11, 130–136. [Google Scholar] [CrossRef]
- Silveira, N.; Vargas, P.; Rosa, C. Polyphenol content and chemical composition of blueberry highbush group. Aliment. E Nutr. Araraquara 2007, 18, 365–370. [Google Scholar]
- Ríos, V.; Pereira, P.; Teodoro, T.; De Oliveira, L.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar]
- Zheng, Y.; Wang, S.; Wang, C.; Zheng, W. Changes in strawberry phenolics, anthocyanins, and antioxidant capacity in response to high oxygen treatments. Food Sci. Technol. 2007, 4, 49–57. [Google Scholar] [CrossRef]
- Giusti, M.; Wrolstad, R. Characterization and Measurement with UV-Visible Spectroscopy. In Handbook of Food Analytical Chemistry; Unit F1.2; Wrolstad, R.E., Schwartz, S.J., Eds.; Wiley: New York, NY, USA, 2005; pp. 19–31. [Google Scholar]
- Gaviria, C.; Cifuentes, O.; Monsalve, C.; Rojano, B. Actividad antioxidante de extractos metanólicos de Attalea butyracea. Sci. Tech. 2007, 33, 297–299. [Google Scholar]
- Takana, J.; Ogawa, K.; Hitoe, S.; Shimoda, H.; Hara, H. Maqui berry (Aristotelia chilensis) and the constituent delphinidin glycoside inhibit photoreceptor cell death induced by visible light. Food Chem. 2013, 139, 129–137. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm.-Wiss. Technol. 1995, 28, 25–30. [Google Scholar]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Prior, R. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Statgraphics. Statgraphics Centurion XVI; Stat Point Technologies, Inc.: Warrenton, VA, USA, 2009. [Google Scholar]
- Petzold, G.; Moreno, J.; Lastra, P.; Rojas, K.; Orellana, P. Block freeze concentration assisted by centrifugation applied to blueberry and pineapple juices. Innov. Food Sci. Emerg. Technol. 2015, 30, 192–197. [Google Scholar] [CrossRef]
- Nonthanum, P.; Tansakul, A. Freeze concentration of lime juice. Maejo International. J. Sci. Technol. 2008, 1, 27–37. [Google Scholar]
- Hernández, E.; Raventós, M.; Auleda, J.M.; Ibarz, A. Concentration of apple and pear juices in a multi-plate freeze concentrator. Innov. Food Sci. Emerg. Technol. 2009, 10, 348–355. [Google Scholar] [CrossRef]
- Jaeger, L.; Bento, C.; Gava, J.; Abadío, F. Commercial sterilization of fruit juices by ultrafiltration/microfiltration membranes. Alimentaria 2002, 39, 123–127. [Google Scholar]
- Cassano, A.; Drioli, E.; Galaverna, G.; Marchelli, R.; Di Silvestro, G.; Cagnasso, P. Clarification and concentration of citrus and carrot juices by integrated membrane processes. J. Food Sci. Technol. 2003, 57, 153–163. [Google Scholar] [CrossRef]
- Cisse, M.; Vaillant, F.; Pérez, A.; Dornier, M.; Reynes, M. The quality of orange juice processed by coupling crossflow microfiltration and osmotic evaporation. Int. J. Food Sci. Technol. 2005, 40, 105–116. [Google Scholar] [CrossRef]
- Tadapaneni, R.; Banaszewski, K.; Patazca, E.; Edirisinghe, I.; Cappozzo, J.; Jackson, L.; Burton-Freeman, B. Effect of high-pressure processing and Milk on the anthocyanin composition and antioxidant capacity of strawberry-based beverages. J. Agric. Food Chem. 2012, 60, 5795–5802. [Google Scholar] [CrossRef]
- Brauch, J.; Buchweitz, M.; Schweiggert, R.; Carle, R. Detailed analyses of fresh and dried maqui (Aristotelia chilensis (Mol.) Stuntz) Berries and juice. Food Chem. 2016, 190, 308–316. [Google Scholar] [CrossRef]
- Capanoglu, E.; De Vos, C.; Hall, R.; Boyacioglu, D.; Beekwilder, J. Changes in polyphenol content during production of grape juice concentrate. Food Chem. 2013, 139, 521–526. [Google Scholar] [CrossRef]
- Buckow, R.; Kastell, A.; Terefe, N.; Versteeg, C. Pressure and temperature effects on degradation kinetics and storage stability of total anthocyanins in blueberry juice. J. Agric. Food Chem. 2010, 58, 10076–10084. [Google Scholar] [CrossRef] [PubMed]
- Saeeduddin, M.; Abid, M.; Jabbar, S.; Wu, T.; Muhammad, M.; Nureldin, F.; Hu, B.; Lei, S.; Zeng, X. Quality assessment of pear juice under ultrasound and commercial pasteurization processing conditions. Food Sci. Technol. 2015, 64, 452–458. [Google Scholar] [CrossRef]
- Rawson, A.; Patras, A.; Tiwari, B.; Noci, F.; Koutchma, T.; Brunton, N. Effect of thermal and non-thermal processing technologies on the bioactive content of exotic fruits and their products: Review of recent advances. Food Res. Int. 2011, 44, 875–1887. [Google Scholar] [CrossRef]
- Rodríguez, K.; Ah-Hen, K.; Vega-Galvez, A.; Vasquez, V.; Quispe-Fuentes, I.; Rojas, P.; Lemus-Mondaca, R. Changes in bioactive components and antioxidant capacity of maqui, Aristotelia chilensis. LWT—Food Sci. Technol. 2016, 65, 537–542. [Google Scholar] [CrossRef]
- Fredes, C.; Yousef, G.; Robert, P.; Grace, M.; Lila, M.A.; Gomez, M. Anthocyanin profiling of wild maqui berries (Aristotelia chilensis [Mol.] Stuntz) from different geographical regions in Chile. J. Sci. Food Agric. 2014, 94, 2639–2648. [Google Scholar] [CrossRef]
- Bowling, B. Berry Grower’s Companion; Timber Press: Portland, OR, USA, 2000; p. 284. [Google Scholar]
- Ghafoor, K.; Park, J.; Choi, Y. Optimization of supercritical fluid extraction of bioactive compounds from grape (Vitis labrusca B.) peel by using response surface methodology. Innov. Food Sci. Emerg. Technol. 2010, 11, 485–490. [Google Scholar] [CrossRef]
- Pojer, E.M.; Mattivi, F.; Johnson, D.; Stockley, C.S. The Case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 583–1508. [Google Scholar] [CrossRef] [PubMed]
- Romero-González, J.; Ah-Hen, K.; Lemus-Mondaca, R.; Muñoz-Fariña, O. Total phenolics, anthocyanin profile and antioxidant activity of maqui, Aristotelia chilensis (Mol.) Stuntz, berries extract in freeze-dried polysaccharides microcapsules. Food Chem. 2020, 313, 126115. [Google Scholar] [CrossRef]
- Betz, M.; Kulozik, U. Microencapsulation of bioactive bilberry anthocyanins by means of whey protein gels. Procedia Food Sci. 2011, 1, 2047–2056. [Google Scholar] [CrossRef]
- Cespedes, C.; Valdez, M.; Avila, J.; El-Hafidi, M.; Alarcón, J.; Paredes, O. Phytochemical profile and the antioxidant activity of Chilean wild black-berry fruits, Aristotelia chilensis (Mol) Stuntz (Elaeocarpaceae). Food Chem. 2010, 119, 886–895. [Google Scholar] [CrossRef]
- Schreckinger, M.; Lotton, J.; Lila, M.; de Mejia, E. Berries from South America: A comprehensive review on chemistry, health potential, and commercialization. J. Med. Food 2010, 13, 233–246. [Google Scholar] [CrossRef]
- Miranda-Rottmann, S.; Aspillaga, A.; Pérez, D.; Vasquez, L.; Martinez, A.; Leighton, F. Juice and phenolic fractions of the Berry Aristotelia chilensis inhibit LDL oxidation in vitro and protect human endotelial cells against oxidative stress. J. Agric. Food Chem. 2002, 50, 7542–7547. [Google Scholar] [CrossRef]
- Céspedes, C.L.; El-Hafidi, M.; Pavon, N.; Alarcon, J. Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), maqui. Food Chem. 2008, 107, 820–829. [Google Scholar] [CrossRef]
- Polo-Insfran, D.; Brenes, C.; Talcott, S. Phytochemical composition and pigment stability of açai (Euterpe oleraceae Mart.). J. Agric. Food Chem. 2004, 52, 1539–1545. [Google Scholar] [CrossRef]
- Martínez, J.; Rojas, H.; Borda, G.; Hastamorir, A.; Medina, M. Stability of Anthocyanins in Juice and Concentrate of Agraz (Vaccinium meridionale Sw.). Rev. Fac. Nac. De Agron. Medellín 2011, 64, 6015–6022. [Google Scholar]
- Moreno, F.; Robles, C.; Sarmiento, Z.; Ruiz, Y.; Pardo, J. Effect of separation and thawing mode on block freeze-concentration of coffee brews. Food Bioprod. Process. 2013, 91, 396–402. [Google Scholar] [CrossRef]
- Pardo, M.; Sánchez, R. Block freeze concentration intensification by means of vacuum and microwave pulses. Eng. Compet. 2015, 17, 143–151. [Google Scholar]
- Garzón, G.; Wrolstad, R. Comparison of the stability of pelargonidin-based anthocyanins in strawberry juice and concentrate. J. Food Sci. 2002, 67, 1288–1299. [Google Scholar] [CrossRef]
- Escribano-Bailón, M.; Alcalde-Eon, C.; Muñoz, O.; Rivas-Gonzalo, J.; Santos-Buelga, C. Anthocyanins in berries of maqui (Aristotelia chilensis (Mol.) Stuntz). Phytochem. Anal. 2006, 1, 8–14. [Google Scholar] [CrossRef]
- Cespedes, C.L.; Pavon, N.; Dominguez, M.; Alarcon, J.; Balbontin, C.; Kubo, I.; El-Hafidi, M.; Avila, J.G. The chilean superfruit black-berry Aristotelia chilensis (Elaeocarpaceae), Maqui as mediator in inflammation-associated disorders. Food Chem. Toxicol. 2017, 108, 438–450. [Google Scholar] [CrossRef]
- Speisky, H.; López-Alarcón, C.; Gómez, M.; Fuentes, J.; Sandoval -Acuna, C. First web-based database on total phenolics and oxygen radical absorbance capacity (ORAC) of fruits produced and consumed within the South Andes Region of South America. J. Agric. Food Chem. 2012, 60, 8851–8859. [Google Scholar] [CrossRef]
- González, B.; Vogel, H.; Razmilic, I.; Wolfram, E. Polyphenol, anthocyanin and antioxidant content in different parts of maqui fruits (Aristotelia chilensis) during ripening and conservation treatments after harvest. Ind. Crops Prod. 2015, 76, 158–165. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Mena, P.; Moreno, D.; García-Viguera, C. Evaluation of sensorial, phytochemical and biological properties of new isotonic beverages enriched with lemon and berries during shelf life. J. Sci. Food Agric. 2014, 94, 1090–1100. [Google Scholar] [CrossRef]
- Gifkins, D.; Olson, S.H.; Demissie, K.; Lu, S.E.; Kong, A.N.; Bandera, E.V. Total and individual antioxidant intake and endometrial cancer risk; Results from a population-based case-control study in New Jersey. Cancer Causes Control 2012, 23, 887–895. [Google Scholar] [CrossRef][Green Version]
- Holtan, S.G.; O´Connor, H.M.; Fredericksen, Z.S.; Liebow, M.; Thompson, C.A.; Macon, W.R.; Micallef, I.N.; Wang, A.H.; Slager, S.L.; Habermann, T.M.; et al. Food-Frecuency questionnaire-based estimates of total antioxidant capacity and risk od non-hodgkin lymphoma. Int. J. Cancer 2012, 131, 1158–1168. [Google Scholar] [CrossRef]
- Farvid, M.S.; Homayouni, F.; Kashkalani, F.; Shirzadeh, L.; Valipour, G.; Farahnak, Z. The associations between oxygen radical absorbance capacity of dietary in take and hypertension in type 2 diabetic patients. J. Hum. Hypertens. 2013, 27, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Golzarand, M.; Mirmiran, P.; Shiva, N.; Azizi, F. Dietary total antioxidant capacity and the occurrence of metabolic syndrome and its components after a 3-year follow-up in adults: Tehran Lipid and Glucose Study. Nutr. Metab. 2012, 9, 70. [Google Scholar] [CrossRef]
- Ou, B.; Chang, T.; Huang, D.; Prior, R. Determination of Total Antioxidant Capacity by Oxygen Radical Absorbance Capacity (ORAC) Using Fluorescein as the Fluorescence Probe: First Action 2012.23. J. AOAC Int. 2013, 96, 1372–1376. [Google Scholar] [CrossRef]
- Kraujalyte, V.; Venskutonis, P.; Pukalskas, A.; Cesoniene, L.; Daubaras, R. Antioxidant properties, phenolic composition and potentiometric sensor array evaluation of commercial and new blueberry (Vaccinium corymbosum) and bog blueberry (Vaccinium uliginosum) genotypes. Food Chem. 2015, 188, 583–590. [Google Scholar] [CrossRef]
- Tiwari, B.; O´Donnell, C.; Muthukumarappan, K.; Cullen, P. Anthocyanin and colour degradation in ozone treated blackberry juice. Innov. Food Sci. Emerg. Technol. 2009, 10, 70–75. [Google Scholar] [CrossRef]
- Khajehei, F.; Niakousari, M.; Eskandari, M.; Sarshar, M. Production of pomegranate juice concentrate by complete block cryoconcentration process. J. Food Process. Eng. 2015, 38, 1745–4530. [Google Scholar] [CrossRef]
- Martínez, J.; Melgosa, M.; Pérez, M.; Hita, E.; Negueruela, A. Visual and instrumental color evaluation in red wines. Food Sci. Technol. Int. 2001, 7, 439–444. [Google Scholar] [CrossRef]
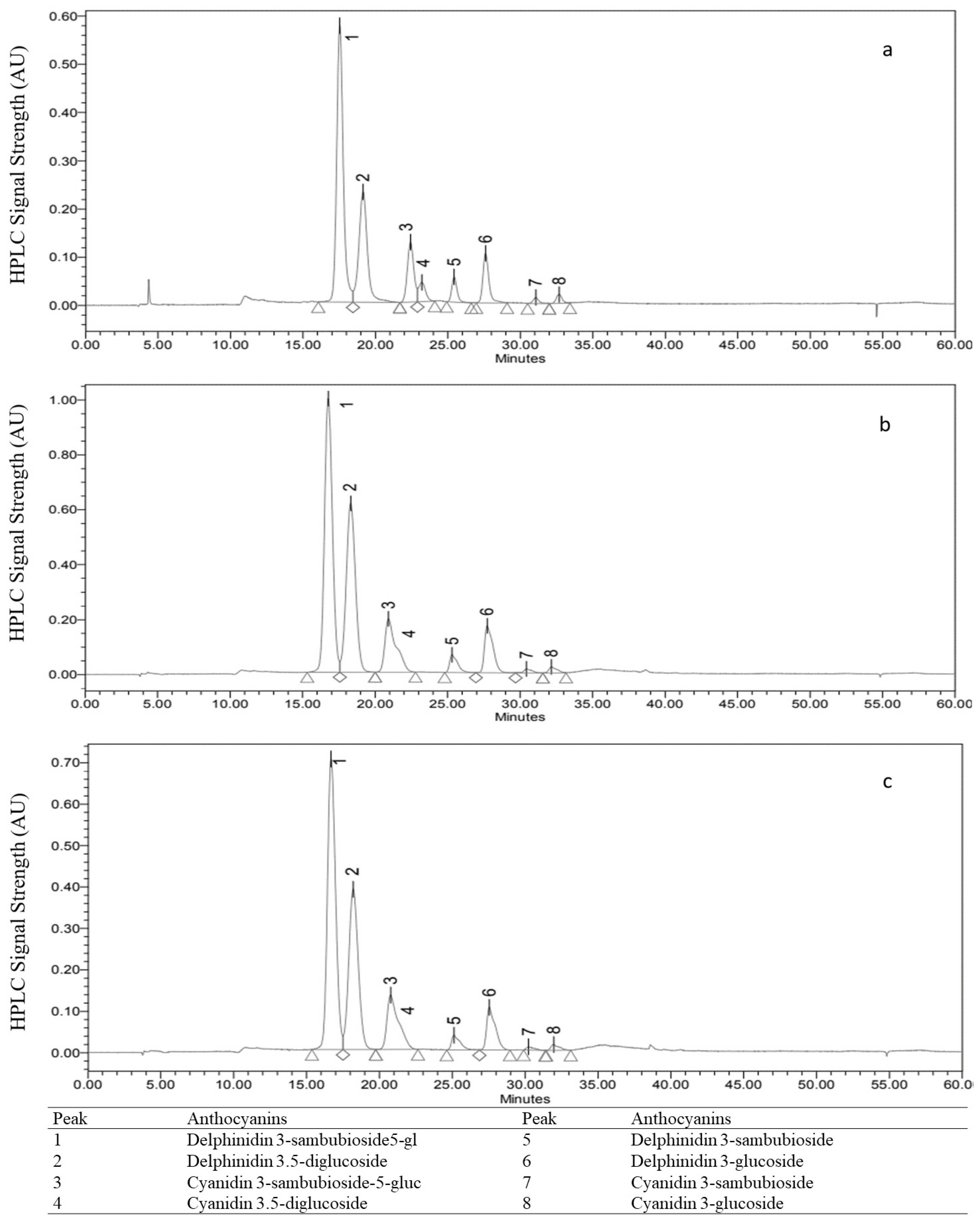

| Anthocyanin | LoD (µg/mL) | LoQ (µg/mL) | |
|---|---|---|---|
| Delphinidin 3-sambubioside 5-glucoside |  | 19.53 | 58.6 |
| Delphinidin 3,5-diglucoside | 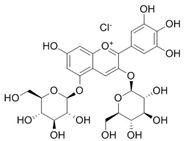 | 47.39 | 142.2 |
| Cyanidin 3-sambubioside-5-glucoside |  | 22.18 | 66.5 |
| Cyanidin 3,5-diglucoside | 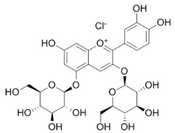 | 8.78 | 26.3 |
| Delphinidin 3-sambubioside | 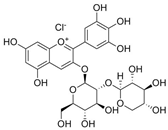 | 9.76 | 29.3 |
| Delphinidin 3-glucoside | 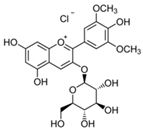 | 27.26 | 81.8 |
| Cyanidin 3-sambubioside | 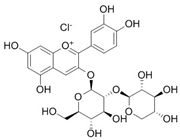 | 3.54 | 10.6 |
| Cyanidin 3-glucoside |  | 4.73 | 14.2 |
| Anthocyanin | Extract | C | R50 | R70 | R80 |
|---|---|---|---|---|---|
| Delphinidin 3-sambubioside 5-glucoside | 9.68 ± 1.68 c | 79.60 ± 0.8 a | 92.07 ± 22.76 a | 32.77 ± 0.06 b | 33.95 ± 2.38 b |
| Delphinidin 3.5-diglucoside | 7.24 ± 0.96 c | 41.90 ± 0.1 a | 16.31 ± 6.31 cb | 23.71 ± 0.09 b | 21.93 ± 1.52 b |
| Cyanidin 3-sambubioside-5-glucoside | 2.79 ± 0.63 c | 18.98 ± 2.1 a | 17.79 ± 0.65 a | 9.07 ± 0.02 b | 6.27 ± 3.25 b |
| Cyanidin 3.5-diglucoside | 1.73 ± 0.61 b | 5.43 ± 1.91 a | 1.14 ± 0.14 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| Delphinidin 3-sambubioside | 0.76 ± 0.05 b | 6.17 ± 0.27 a | 2.32 ± 0.32 b | 2.15 ± 0.01 b | 1.33 ± 0.58 b |
| Delphinidin 3-glucoside | 2.33 ± 0.20 d | 13.16 ± 0.31 a | 9.08 ± 1.09 b | 6.03 ± 0.02 c | 5.25 ± 0.33 c |
| Cyanidin 3-sambubioside | 0.25 ± 0.20 b | 1.48 ± 0.08 a | 0.50 ± 0.05 b | 0.47 ± 0.03 b | 0.27 ± 0.11 b |
| Cyanidin 3-glucoside | 0.33 ± 0.24 b | 2.29 ± 0.04 a | 0.76 ± 0.06 b | 0.72 ± 0.02 b | 0.45 ± 0.32 b |
| Total Anthocyanin content | 25.11 ± 4.57 c | 169.01 ± 5.61 a | 139.97 ± 31.38 a | 74.92 ± 0.25 b | 69.45 ± 8.49 b |
| Coordinates/Products | Extract | C | R50 | R70 | R80 |
|---|---|---|---|---|---|
| L* | 0.14 ± 0.04 a | 0.023 ± 0.01 b | 0.023 ± 0.02 b | 0.017 ± 0.01 b | 0.013 ± 0.01 b |
| a* | 0.11 ± 0.03 a | −0.08 ± 0.02 b | −0.023 ± 0.01 c | −0.08 ± 0.02 b | 0.01 ± 0.00 d |
| b* | −0.10 ± 0.01 a | 0.03 ± 0.01 b | −0.017 ± 0.01 c | −0.03 ± 0.01 c | 0.02 ± 0.01 b |
| Hab* | 318.60 ± 4.58 a | 160.90 ± 1.22 b | 233.90 ± 15.34 c | 248.60 ± 5.32 c | 59.99 ± 13.61 d |
| Cab* | 0.15 ± 0.00 a | 0.081 ± 0.02 b | 0.029 ± 0.01 c | 0.083 ± 0.02 b | 0.023 ± 0.02 c |
| ΔE | - | 0.252 ± 0.04 a | 0.107 ± 0.06 b | 0.241 ± 0.02 a | 0.204 ± 0.02 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastías-Montes, J.M.; Vidal-San-Martín, C.; Tamarit-Pino, Y.; Muñoz-Fariña, O.; García-Figueroa, O.; Quevedo-León, R.; Wei, Z.-J.; Lv, X.; Cespedes-Acuña, C.L. Cryoconcentration by Centrifugation–Filtration: A Simultaneous, Efficient and Innovative Method to Increase Thermosensitive Bioactive Compounds of Aqueous Maqui (Aristotelia chilensis (Mol.) Stuntz) Extract. Processes 2022, 10, 25. https://doi.org/10.3390/pr10010025
Bastías-Montes JM, Vidal-San-Martín C, Tamarit-Pino Y, Muñoz-Fariña O, García-Figueroa O, Quevedo-León R, Wei Z-J, Lv X, Cespedes-Acuña CL. Cryoconcentration by Centrifugation–Filtration: A Simultaneous, Efficient and Innovative Method to Increase Thermosensitive Bioactive Compounds of Aqueous Maqui (Aristotelia chilensis (Mol.) Stuntz) Extract. Processes. 2022; 10(1):25. https://doi.org/10.3390/pr10010025
Chicago/Turabian StyleBastías-Montes, José Miguel, Carla Vidal-San-Martín, Yanara Tamarit-Pino, Ociel Muñoz-Fariña, Olga García-Figueroa, Roberto Quevedo-León, Zhao-Jun Wei, Xingang Lv, and Carlos L. Cespedes-Acuña. 2022. "Cryoconcentration by Centrifugation–Filtration: A Simultaneous, Efficient and Innovative Method to Increase Thermosensitive Bioactive Compounds of Aqueous Maqui (Aristotelia chilensis (Mol.) Stuntz) Extract" Processes 10, no. 1: 25. https://doi.org/10.3390/pr10010025
APA StyleBastías-Montes, J. M., Vidal-San-Martín, C., Tamarit-Pino, Y., Muñoz-Fariña, O., García-Figueroa, O., Quevedo-León, R., Wei, Z.-J., Lv, X., & Cespedes-Acuña, C. L. (2022). Cryoconcentration by Centrifugation–Filtration: A Simultaneous, Efficient and Innovative Method to Increase Thermosensitive Bioactive Compounds of Aqueous Maqui (Aristotelia chilensis (Mol.) Stuntz) Extract. Processes, 10(1), 25. https://doi.org/10.3390/pr10010025







