A Review of the Clinical and Therapeutic Implications of Neuropathic Pain
Abstract
1. Introduction
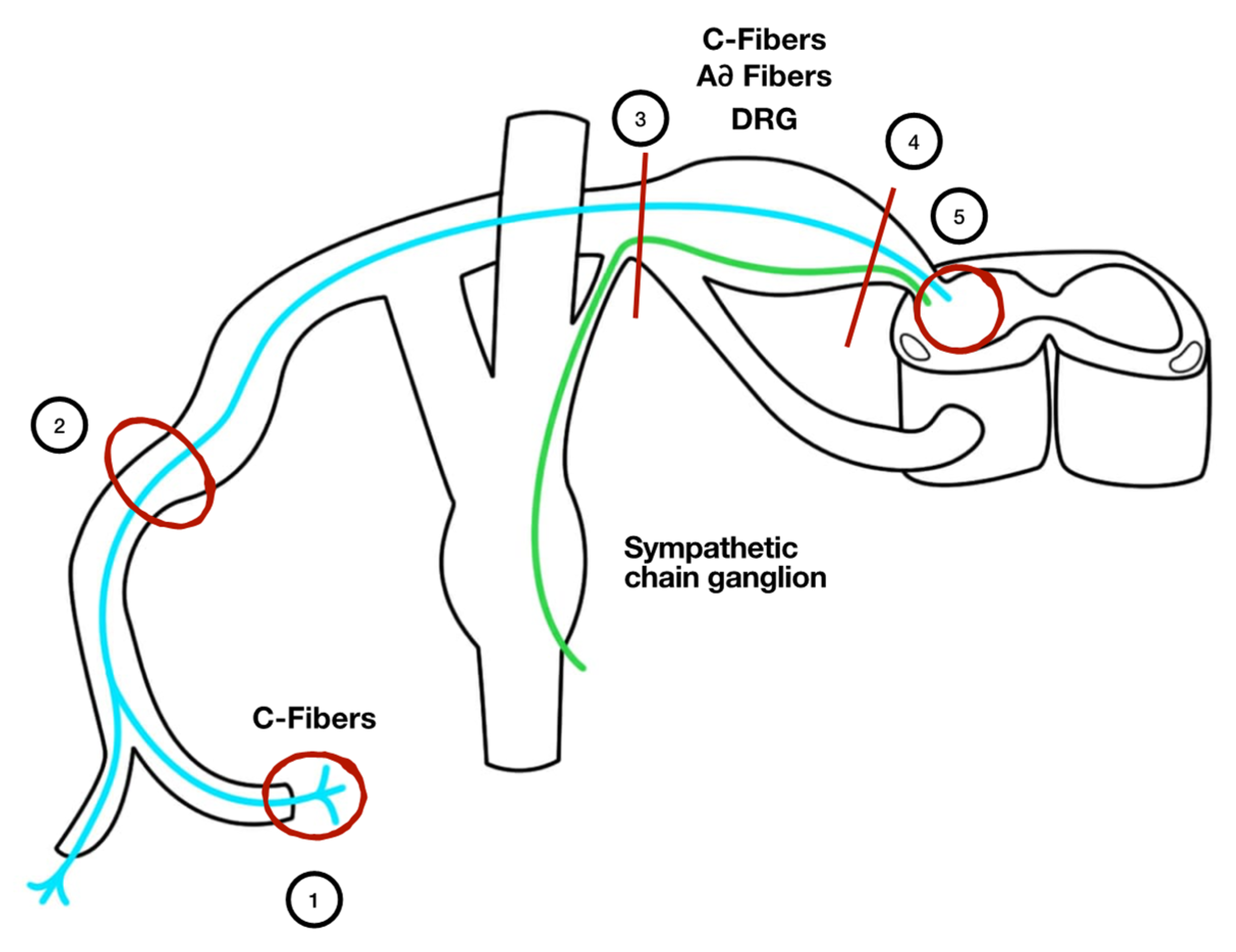
2. Pathophysiologic Mechanisms Underlying Neuropathic Pain
3. Diagnosing Neuropathic Pain
4. Treatment of Neuropathic Pain
5. Biomarkers and Neuropathic Pain
6. Future Perspectives: Molecular Alterations and Tailored Therapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neuropathic Pain. Available online: https://www.iasp-pain.org/GlobalYear/NeuropathicPain (accessed on 31 July 2021).
- Bannister, K.; Sachau, J.; Baron, R.; Dickenson, A.H. Neuropathic Pain: Mechanism-Based Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 257–274. [Google Scholar] [CrossRef]
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef]
- Bouhassira, D.; Attal, N.; Alchaar, H.; Boureau, F.; Brochet, B.; Bruxelle, J.; Cunin, G.; Fermanian, J.; Ginies, P.; Grun-Overdyking, A.; et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005, 114, 29–36. [Google Scholar] [CrossRef]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.; et al. Neuropathic pain. Nat. Rev. Dis. Prim. 2017, 3, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; Lanteri-Minet, M.; Laurent, B.; Fermanian, J.; Bouhassira, D. The specific disease burden of neuropathic pain: Results of a French nationwide survey. Pain 2011, 152, 2836–2843. [Google Scholar] [CrossRef]
- Torrance, N.; Smith, B.; Bennett, M.; Lee, A.J. The Epidemiology of Chronic Pain of Predominantly Neuropathic Origin. Results From a General Population Survey. J. Pain 2006, 7, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Orlandini, G. La Semeiotica del Dolore; Antonio Delfino Editore: Sassari, Italy, 2013. [Google Scholar]
- Devor, M. The pathophysiology and anathomy of damaged nerve. In Textbook of Pain; Wall, P.D., Melzack, R., Eds.; Churchill Livingstone: London, UK, 1984; pp. 49–64. [Google Scholar]
- Woolf, C.J. The Pathophysiology of Peripheral Neuropathic Pain—Abnormal Peripheral Input and Abnormal Central Processing. Acta Neurochir. Suppl. 1993, 58, 125–130. [Google Scholar] [CrossRef] [PubMed]
- IASP (Subcommittee and Taxonomy). Classification of Chronic Pain: Description of Chronic Pain Syndromes and Definitions of Pain Terms; IASP Press: Seattle, WA, USA, 1994. [Google Scholar]
- Fisher, A.S.; Lanigan, M.T.; Upton, N.; Lione, L.A. Preclinical Neuropathic Pain Assessment; the Importance of Translatability and Bidirectional Research. Front. Pharmacol. 2021, 11, 2308. [Google Scholar] [CrossRef]
- Sène, D. Small fiber neuropathy: Diagnosis, causes, and treatment. Jt. Bone Spine 2018, 85, 553–559. [Google Scholar] [CrossRef]
- Viswanath, O.; Urits, I.; Burns, J.; Charipova, K.; Gress, K.; McNally, A.; Urman, R.D.; Welschmeyer, A.; Berger, A.A.; Kassem, H.; et al. Central Neuropathic Mechanisms in Pain Signaling Pathways: Current Evidence and Recommendations. Adv. Ther. 2020, 37, 1946–1959. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.I.; Attal, N.; Backonja, M.M.; Baron, R.; Bouhassira, D.; Freynhagen, R.; Scholz, J.; Tölle, T.R.; Wittchen, H.-U.; Jensen, T.S. Using screening tools to identify neuropathic pain. Pain 2007, 127, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Mannion, R.J.; Hord, D.E.; Griffin, R.S.; Rawal, B.; Zheng, H.; Scoffings, D.; Phillips, A.; Guo, J.; Laing, R.J.C.; et al. A Novel Tool for the Assessment of Pain: Validation in Low Back Pain. PLoS Med. 2009, 6, e1000047. [Google Scholar] [CrossRef]
- Padua, L.; Briani, C.; Truini, A.; Aprile, I.; Bouhassira, D.; Cruccu, G.; Jann, S.; Nobile-Orazio, E.; Pazzaglia, C.; Morini, A.; et al. Consistence and discrepancy of neuropathic pain screening tools DN4 and ID-Pain. Neurol. Sci. 2012, 34, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.; Richter, H.; Rogausch, J.P.; Frettlöh, J.; Lungenhausen, M.; Maier, C. A modified score to identify and discriminate neuropathic pain: A study on the German version of the neuropathic pain symptom inventory (NPSI). BMC Neurol. 2011, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Mejdahl, M.K.; Christoffersens, K.B.; Andersen, K.G. Development and Validation of a Screening Tool for Surgery-Specific Neu-ropathic Pain: Neuropathic Pain Scale for Postsurgical Patients. Pain Phys. 2019, 22, E81–E90. [Google Scholar]
- Finnerup, N.B.; Haroutounian, S.; Kamerman, P.; Baron, R.; Bennett, D.; Bouhassira, D.; Cruccu, G.; Freeman, R.; Hansson, P.; Nurmikko, T.; et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain 2016, 157, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Schultheis, B.C.; Hanes, M.C.; Jolly, S.M.; Chakravarthy, K.V.; Deer, T.R.; Levy, R.M.; Hunter, C.W. A Comprehensive Algorithm for Management of Neuropathic Pain. Pain Med. 2019, 20, S2–S12. [Google Scholar] [CrossRef]
- Mu, A.; Weinberg, E.; Moulin, D.E.; Clarke, H. Pharmacologic management of chronic neuropathic pain: Review of the Canadian Pain Society consensus statement. Can. Fam. Phys. 2017, 63, 844–852. [Google Scholar]
- Finch, P.M.; Knudsen, L.; Drummond, P.D. Reduction of allodynia in patients with complex regional pain syndrome: A double-blind placebo-controlled trial of topical ketamine. Pain 2009, 146, 18–25. [Google Scholar] [CrossRef]
- Chapparo, L.E.; Wiffen, P.J.; Moore, R.A.; Gilron, I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst. Rev. 2012, 7, CD08943. [Google Scholar] [CrossRef]
- Tesfaye, S.; Wilhelm, S.; Lledo, A.; Schacht, A.; Tölle, T.; Bouhassira, D.; Cruccu, G.; Skljarevski, V.; Freynhagen, R. Duloxetine and pregabalin: High-dose monotherapy or their combination? The “COMBO-DN study”—A multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain 2013, 154, 2616–2625. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; O’Connor, A.; Audette, J.; Baron, R.; Gourlay, G.K.; Haanpää, M.L.; Kent, J.L.; Krane, E.J.; LeBel, A.A.; Levy, R.M.; et al. Recommendations for the Pharmacological Management of Neuropathic Pain: An Overview and Literature Update. Mayo Clin. Proc. 2010, 85, S3–S14. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Hansson, P.; Jensen, T.S.; Kamerman, P.R.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis and updated NeuPSIG recommendations. Lancet Neurol. 2015, 142, 162–173. [Google Scholar] [CrossRef]
- Chou, R.; Loeser, J.D.; Owens, D.K.; Rosenquist, R.W.; Atlas, S.J.; Baisden, J.; Carragee, E.J.; Grabois, M.; Murphy, D.R.; Resnick, D.K.; et al. Interventional Therapies, Surgery, and Interdisciplinary Rehabilitation for Low Back Pain: An evidence-based clinical practice guideline from the American Pain Society. Spine 2009, 34, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; O’Connor, A.; Kent, J.; Mackey, S.; Raja, S.N.; Stacey, B.R.; Levy, R.M.; Backonja, M.; Baron, R.; Harke, H.; et al. Interventional management of neuropathic pain: NeuPSIG recommendations. Pain 2013, 154, 2249–2261. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, N.E.; Wand, B.M.; Gibson, W.; Carr, D.B.; Birklein, F.; Stanton, T.R. Local anaesthetic sympathetic blockade for complex regional pain syndrome. Cochrane Database Syst. Rev. 2016, 2016, CD004598. [Google Scholar] [CrossRef]
- Duarte, R.V.; Nevitt, S.; McNicol, E.; Taylor, R.S.; Buchser, E.; North, R.B.; Eldabe, S. Systematic review and meta-analysis of placebo/sham controlled randomised trials of spinal cord stimulation for neuropathic pain. Pain 2020, 161, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Khoromi, S.; Cui, L.; Nackers, L.; Max, M.B. Morphine, nortriptyline and their combination vs. placebo in patients with chronic lumbar root pain. Pain 2007, 130, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. CDC Guideline for Prescribing Opioids for Chronic Pain. Available online: https://www.cdc.gov/drugoverdose/pdf/Guidelines_Factsheet-a.pdf (accessed on 31 July 2021).
- Busse, J.W.; Craigie, S.; Juurlink, D.N.; Buckley, D.N.; Wang, L.; Couban, R.; Agoritsas, T.; Akl, E.A.; Carrasco-Labra, A.; Cooper, L.; et al. Guideline for opioid therapy and chronic noncancer pain. Can. Med. Assoc. J. 2017, 189, E659–E666. [Google Scholar] [CrossRef] [PubMed]
- Raffaeli, W.; Sarti, D.; Demartini, L.; Sotgiu, A.; Bonezzi, C.; Italian Ziconotide Group. Italian registry on long-term intrathecal zi-conotide treatment. Pain Phys. 2011, 14, 15–24. [Google Scholar] [CrossRef]
- Attal, N.; Ayache, S.; de Andrade, D.C.; Mhalla, A.; Baudic, S.; Jazat, F.; Ahdab, R.; Neves, D.O.; Sorel, M.; Lefaucheur, J.-P.; et al. Repetitive transcranial magnetic stimulation and transcranial direct-current stimulation in neuropathic pain due to radiculopathy: A randomized sham-controlled comparative study. Pain 2016, 157, 1224–1231. [Google Scholar] [CrossRef]
- Akyuz, G.; Kenis, O. Physical Therapy Modalities and Rehabilitation Techniques in the Management of Neuropathic Pain. Am. J. Phys. Med. Rehabil. 2014, 93, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Shaygan, M.; Böger, A.; Kröner-Herwig, B. Predicting factors of outcome in multidisciplinary treatment of chronic neuropathic pain. J. Pain Res. 2018, 11, 2433–2443. [Google Scholar] [CrossRef]
- Cooper, T.E.; Chen, J.; Wiffen, P.J.; Derry, S.; Carr, D.B.; Aldington, D.; Cole, P.; Moore, R.A. Morphine for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 2019, CD011669. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, M.S.; Wilcox, M.; Levitan, B. Pain Qualities and Satisfaction with Therapy: A Survey of Subjects with Neuropathic Pain. Pain Med. 2013, 14, 1745–1756. [Google Scholar] [CrossRef][Green Version]
- Lamer, T.; Hooten, W.; Markus, B.; Gazelka, H.; Moeschler, S.; Murad, M. Spinal stimulation for the treatment of intractable spine and limb pain: A systematic review of RCTs and meta-analysis. Mayo Clin. Proc. 2019, 94, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Raffaeli, W.; Righetti, D.; Andruccioli, J.; Sarti, D. Periduroscopy: General Review of Clinical Features and Development of Operative Models. Acta Neurochir. Suppl. 2011, 108, 55–65. [Google Scholar] [PubMed]
- Saarto, T.; Wiffen, P.J. Antidepressants for neuropathic pain. Cochrane Database Syst. Rev. 2007, 4, CD005454. [Google Scholar] [CrossRef]
- Haanpää, M.L.; Gourlay, G.K.; Kent, J.L.; Miaskowski, C.; Raja, S.N.; Schmader, K.E.; Wells, C.D. Treatment Considerations for Patients with Neuropathic Pain and Other Medical Comorbidities. Mayo Clin. Proc. 2010, 85, S15–S25. [Google Scholar] [CrossRef]
- Virani, A.; Mailis, A.; Shapiro, L.E.; Shear, N.H. Drug interactions in human neuropathic pain pharmacotherapy. Pain 1997, 73, 3–13. [Google Scholar] [CrossRef]
- Von Korff, M.; Scher, A.I.; Helmick, C.; Carter-Pokras, O.; Dodick, D.W.; Goulet, J.; Hamill-Ruth, R.; LeResche, L.; Porter, L.; Tait, R.; et al. United States National Pain Strategy for Population Research: Concepts, Definitions, and Pilot Data. J. Pain 2016, 17, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.R. Intensive care medicine in 2050: Precision medicine. Intensiv. Care Med. 2017, 43, 1507–1509. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.D.; Aghaeepour, N.; Ahn, A.H.; Angst, M.S.; Borsook, D.; Brenton, A.; Burczynski, M.E.; Crean, C.; Edwards, R.; Gaudilliere, B.; et al. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: Challenges and opportunities. Nat. Rev. Neurol. 2020, 16, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, S.; Cascella, M.; Forte, C.A.; Esposito, G.; Cuomo, A. The Role of Anti-Nerve Growth Factor Monoclonal Antibodies in the Control of Chronic Cancer and Non-Cancer Pain. J. Pain Res. 2021, 14, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, G.; Zsombok, T.; Jakab, B.; Nemeth, J.; Szolcsanyi, J.; Bagdy, G. Sumatriptan Causes Parallel Decrease in Plasma Calcitonin Gene-Related Peptide (CGRP) Concentration and Migraine Headache During Nitroglycerin Induced Migraine Attack. Cephalalgia 2005, 25, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Quiding, H.; Jonzon, B.; Svensson, O.; Webster, L.; Reimfelt, A.; Karin, A.; Karlsten, R.; Segerdahl, M. TRPV1 antagonistic analgesic effect: A randomized study of AZD1386 in pain after third molar extraction. Pain 2013, 154, 808–812. [Google Scholar] [CrossRef]
- Miller, F.; Björnsson, M.; Svensson, O.; Karlsten, R. Experiences with an adaptive design for a dose-finding study in patients with osteoarthritis. Contemp. Clin. Trials 2014, 37, 189–199. [Google Scholar] [CrossRef]
- Douglas, S.R.; Shenoda, B.B.; Qureshi, R.A.; Sacan, A.; Alexander, G.M.; Perreault, M.; Barret, J.E.; Aradillas-Lopez, E.; Schwartzman, R.J.; Ajit, S.K. Analgesic Response to Intravenous Ketamine Is Linked to a Circulating microRNA Signature in Female Patients with Complex Regional Pain Syndrome. J. Pain 2015, 16, 814–824. [Google Scholar] [CrossRef]
- Starkey Lewis, P.J.; Dear, J.; Platt, V.; Simpson, K.J.; Craig, D.G.; Antoine, D.J.; French, N.S.; Dhaun, N.; Webb, D.J.; Costello, E.M.; et al. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology 2011, 54, 1767–1776. [Google Scholar] [CrossRef]
- Assi, L.; Whitley, G.; Howe, F.; Sofat, N. Novel biomarkers for osteoarthritis are linked to pain sensitization, bone marrow lesions and cartilage damage. Osteoarthr. Cartil. 2018, 26, S190–S191. [Google Scholar] [CrossRef]
- Balagué, F.; Nordin, M.; Schafer, D.; Sheikhzadeh, A.; Lenz, M.E.; Thonar, E.M. The potential value of blood biomarkers of interver-tebral disk metabolism in the follow-up of patients with sciatica. Eur. Spine J. 2006, 15, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Dietz, C.; Müller, M.; Reinhold, A.K.; Karch, L.; Schwab, B.; Forer, L.; Vlckova, E.; Brede, E.-M.; Jakubietz, R.; Meffert, R.; et al. What is normal trauma healing and what is complex regional pain syndrome I? An analysis of clinical and experimental biomarkers. Pain 2019, 160, 2278–2289. [Google Scholar] [CrossRef]
- Ramanathan, S.; Douglas, S.R.; Alexander, G.M.; Shenoda, B.B.; Barrett, J.E.; Aradillas, E.; Sacan, A.; Ajit, S.K. Exosome microRNA signatures in patients with complex regional pain syndrome undergoing plasma exchange. J. Transl. Med. 2019, 17, 81. [Google Scholar] [CrossRef] [PubMed]
- Ericson, H.; Abu Hamdeh, S.; Freyhult, E.; Stiger, F.; Bäckryd, E.; Svenningsson, A.; Torsten, G.; Kultima, K. Cerebrospinal fluid biomarkers of inflammation in trigeminal neuralgia patients operated with microvascular decompression. Pain 2019, 160, 2603–2611. [Google Scholar] [CrossRef]
- Hayakawa, K.; Kurano, M.; Ohya, J.; Oichi, T.; Kano, K.; Nishikawa, M.; Uranbileg, B.; Kuwajima, K.; Sumitani, M.; Tanaka, S.; et al. Lysophosphatidic acids and their substrate lyso-phospholipids in cerebrospinal fluid as objective biomarkers for evaluating the severity of lumbar spinal stenosis. Sci. Rep. 2019, 9, 9144. [Google Scholar] [CrossRef]
- Hider, S.L.; Konstantinou, K.; Hay, E.M.; Glossop, J.; Mattey, D.L. Inflammatory biomarkers do not distinguish between patients with sciatica and referred leg pain within a primary care population: Results from a nested study within the ATLAS cohort. BMC Musculoskelet. Disord. 2019, 20, 202. [Google Scholar] [CrossRef]
- Kallman, T.F.; Ghafouri, B.; Bäckryd, E. Salivary beta-endorphin and substance P are not biomarkers of neuropathic chronic pain propensity. Heliyon 2018, 4, e00718. [Google Scholar] [CrossRef] [PubMed]
- Karakulova, Y.V.; Filimonova, T.A. Biomarkers for the Development and Progression of Diabetic Polyneuropathy. Neurosci. Behav. Physiol. 2021, 51, 444–449. [Google Scholar] [CrossRef]
- Kwon, B.K.; Stammers, A.M.; Belanger, L.M.; Bernardo, A.; Chan, D.; Bishop, C.M.; Slobogean, G.P.; Zhang, H.; Umedaly, H.; Giffin, M.; et al. Cerebrospinal Fluid Inflammatory Cytokines and Biomarkers of Injury Severity in Acute Human Spinal Cord Injury. J. Neurotrauma 2010, 27, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Lind, A.-L.; Wu, D.; Freyhult, E.; Bodolea, C.; Ekegren, T.; Larsson, A.; Gustafsson, M.G.; Katila, L.; Bergquist, J.; Gordh, T.; et al. A Multiplex Protein Panel Applied to Cerebrospinal Fluid Reveals Three New Biomarker Candidates in ALS but None in Neuropathic Pain Patients. PLoS ONE 2016, 11, e0149821. [Google Scholar] [CrossRef]
- Radojčić, M.R.; Thudium, C.S.; Henriksen, K.; Tan, K.; Karlsten, R.; Dudley, A.; Chessell, I.; Karsdal, M.A.; Bay-Jensen, A.-C.; Crema, M.D.; et al. Biomarker of extracellular matrix remodelling C1M and proinflammatory cytokine interleukin 6 are related to synovitis and pain in end-stage knee osteoarthritis patients. Pain 2017, 158, 1254–1263. [Google Scholar] [CrossRef]
- Ri, M.; Iida, S.; Maruyama, D.; Saito, K.; Saito, Y.; Osaga, S.; Tohkin, M.; Miyata, N.; Tobinai, K.; Fukuhara, N.; et al. Lipidomic Profiling of Plasma Samples in Patients with Newly Diagnosed Multiple Myeloma; A Biomarker Study for Predicting the Response and Toxicities of Melphalan, Prednisolone and Bortezomib (MPB) Regimen: An Ancillary Study of the JCOG1105 (JCOG1105A1). Blood 2019, 134, 3156. [Google Scholar] [CrossRef]
- Ri, M.; Maekawa, K.; Nakajima, M.; Sekine, A.; Ueda, R.; Tohkin, M.; Maekawa, K.; Ri, M.; Nakajima, M.; Sekine, A.; et al. Serum lipid metabolomics as an useful biomarker predicting for the efficacy of bortezomib treatment and peripheral neuropathy in patients with multiple myeloma. HemaSphere 2018, 2, 584. [Google Scholar]
- Staats Pires, A.; Heng, B.; Tan, V.X.; Latini, A.; Russo, M.A.; Santarelli, D.M.; Bailey, D.; Wynne, K.; O’Brien, J.A.; Guillemin, G.J.; et al. Kynurenine, Tetrahydrobiopterin, and Cytokine Inflammatory Biomarkers in Individuals Affected by Diabetic Neuropathic Pain. Front. Neurosci. 2020, 14, 890. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, F.; Huang, C.; Xue, F.; Li, Y.; Gao, S.; Qiu, Z.; Li, S.; Chen, Q.; Zhou, H.; et al. Bioinformatic Analysis of Potential Biomarkers for Spinal Cord-injured Patients with Intractable Neuropathic Pain. Clin. J. Pain 2018, 34, 825–830. [Google Scholar] [CrossRef]
- Xu, J.; Xiaoqiang, E.; Liu, H.; Li, F.; Cao, Y.; Tian, J.; Yan, J. Tumor necrosis factor-alpha is a potential diagnostic biomarker for chronic neuropathic pain after spinal cord injury. Neurosci. Lett. 2015, 595, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Maier, C.; Attal, N.; Binder, A.; Bouhassira, D.; Cruccu, G.; Finnerup, N.B.; Haanpää, M.; Hansson, P.; Hüllemann, P.; et al. Peripheral neuropathic pain: A mechanism-related organizing principle based on sensory profiles. Pain 2017, 158, 261–272. [Google Scholar] [CrossRef]
- Basso, L.; Altier, C. Transient Receptor Potential Channels in neuropathic pain. Curr. Opin. Pharmacol. 2017, 32, 9–15. [Google Scholar] [CrossRef]
- Wan, L.; Su, Z.; Li, F.; Gao, P.; Zhang, X. MiR-122-5p suppresses neuropathic pain development by targeting PDK4. Neurochem. Res. 2021, 46, 957–963. [Google Scholar] [CrossRef]
- Campbell, J.N.; Meyer, R.A. Mechanisms of Neuropathic Pain. Neuron 2006, 52, 77–92. [Google Scholar] [CrossRef]
- Edwards, R.R.; Haythornthwaite, J.A.; Tella, P.; Max, M.B.; Raja, S. Basal Heat Pain Thresholds Predict Opioid Analgesia in Patients with Postherpetic Neuralgia. Anesthesiology 2006, 104, 1243–1248. [Google Scholar] [CrossRef]
- Gustorff, B.; Sycha, T.; Lieba-Samal, D.; Rolke, R.; Treede, R.-D.; Magerl, W. The pattern and time course of somatosensory changes in the human UVB sunburn model reveal the presence of peripheral and central sensitization. Pain 2013, 154, 586–597. [Google Scholar] [CrossRef]
- Demant, D.T.; Lund, K.; Vollert, J.; Maier, C.; Segerdahl, M.; Finnerup, N.; Jensen, T.S.; Sindrup, S.H. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: A randomised, double-blind, placebo-controlled phenotype-stratified study. Pain 2014, 155, 2263–2273. [Google Scholar] [CrossRef]
- Mainka, T.; Malewicz, N.; Baron, R.; Enax-Krumova, E.; Treede, R.-D.; Maier, C. Presence of hyperalgesia predicts analgesic efficacy of topically applied capsaicin 8% in patients with peripheral neuropathic pain. Eur. J. Pain 2015, 20, 116–129. [Google Scholar] [CrossRef]
- Baumgärtner, U.; Magerl, W.; Klein, T.; Hopf, H.C.; Treede, R.-D. Neurogenic hyperalgesia versus painful hypoalgesia: Two distinct mechanisms of neuropathic pain. Pain 2002, 96, 141–151. [Google Scholar] [CrossRef]
- Fields, H.L.; Rowbotham, M.; Baron, R. Postherpetic neuralgia: Irritable nociceptors and deafferentation. Neurobiol. Dis. 1998, 5, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.M.; Schifitto, G.; Clifford, D.B.; Murphy, T.K.; Cruz, E.D.-D.; Glue, P.; Whalen, E.; Emir, B.; Scott, G.N.; Freeman, R. Pregabalin for painful HIV neuropathy: A randomized, double-blind, placebo-controlled trial. Neurology 2010, 74, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Wasner, G.; Kleinert, A.; Binder, A.; Schattschneider, J.; Baron, R. Postherpetic neuralgia: Topical lidocaine is effective in nociceptor–deprived skin. J. Neurol. 2005, 252, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; Rouaud, J.; Brasseur, L.; Chauvin, M.; Bouhassira, D. Systemic lidocaine in pain due to peripheral nerve injury and predictors of response. Neurology 2004, 62, 218–225. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Sindrup, S.; Bach, F.; Johannesen, I.L.; Jensen, T.S. Lamotrigine in spinal cord injury pain: A randomized controlled trial. Pain 2002, 96, 375–383. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, C.; Wang, Z.J. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience 2011, 193, 440–451. [Google Scholar] [CrossRef]
- Andersson, D.A.; Gentry, C.; Moss, S.; Bevan, S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. Neurosci. 2008, 28, 2485–2494. [Google Scholar] [CrossRef]
- Materazzi, S.; Nassini, R.; Andre, E.; Campi, B.; Amadesi, S.; Trevisani, M.; Bunnett, N.W.; Patacchini, R.; Geppetti, P. Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA 2008, 105, 12045–12050. [Google Scholar] [CrossRef]
- Kwan, K.Y.; Allchorne, A.J.; Vollrath, M.A.; Christensen, A.P.; Zhang, D.-S.; Woolf, C.J.; Corey, D.P. TRPA1 Contributes to Cold, Mechanical, and Chemical Nociception but Is Not Essential for Hair-Cell Transduction. Neuron 2006, 50, 277–289. [Google Scholar] [CrossRef]
- Nassini, R.; Materazzi, S.; Benemei, S.; Geppetti, P. The TRPA1channel in inflammatory and neuropathic pain and migraine. Rev. Physiol. Biochem. Pharmacol. 2014, 167, 1–43. [Google Scholar] [PubMed]
- Wei, H.; Hamalainen, M.M.; Saarnilehto, M.; Koivisto, A.; Pertovaara, A. Attenuation of mechanical hypersensitivity by an antag-onist of the TRPA1 ion channel in diabetic animals. Anesthesiology 2009, 111, 147–154. [Google Scholar] [CrossRef]
- Proudfoot, C.J.; Garry, E.M.; Cottrell, D.F.; Rosie, R.; Anderson, H.; Robertson, D.C.; Fleetwood-Walker, S.M.; Mitchell, R. Analgesia me-diated by the TRPM8 cold receptor in chronic neuropathic pain. Curr. Biol. 2006, 16, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, W.M.; Palkar, R.; Lippoldt, E.K.; McCoy, D.D.; Baluch, F.; Chen, J.; McKemy, D.D. A sensory-labeled line for cold: TRPM8- expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J. Neurosci. 2013, 33, 2837–2848. [Google Scholar] [CrossRef]
- Descoeur, J.; Pereira, V.; Pizzoccaro, A.; Francois, A.; Ling, B.; Maffre, V.; Couette, B.; Busserolles, J.; Courteix, C.; Noel, J.; et al. Oxali-platininduced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol. Med. 2011, 3, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Yang, Z.; Guo, J.; Zheng, Y.; Su, X.; Wang, X. Interactions Among lncRNAs/circRNAs, miRNAs, and mRNAs in Neuropathic Pain. Neurotherapeutics 2020, 17, 917–931. [Google Scholar] [CrossRef]
- Tavares-Ferreira, D.; Lawless, N.; Bird, E.V.; Atkins, S.; Collier, D.; Sher, E.; Malki, K.; Lambert, D.W.; Boissonade, F.M. Correlation of miRNA expression with intensity of neuropathic pain in man. Mol. Pain 2019, 15, 1744806919860323. [Google Scholar] [CrossRef]
- Ju, C.; Liu, R.; Zhang, Y.; Zhang, F.; Sun, J.; Lv, X.-B.; Zhang, Z. Exosomes May Be the Potential New Direction of Research in Osteoarthritis Management. BioMed Res. Int. 2019, 2019, 7695768. [Google Scholar] [CrossRef]
- Tavasolian, F.; Moghaddam, A.S.; Rohani, F.; Abdollahi, E.; Janzamin, E.; Momtazi-Borojeni, A.A.; Moallem, S.A.; Jamialahmadi, T.; Sahebkar, A. Exosomes: Effectual players in rheumatoid arthritis. Autoimmun. Rev. 2020, 19, 102511. [Google Scholar] [CrossRef] [PubMed]
- Maumus, M.; Manferdini, C.; Toupet, K.; Peyrafitte, J.-A.; Ferreira, R.; Facchini, A.; Gabusi, E.; Bourin, P.; Jorgensen, C.; Lisignoli, G.; et al. Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. Stem Cell Res. 2013, 11, 834–844. [Google Scholar] [CrossRef]
- Jiang, L.; Shen, Y.; Guo, D.; Yang, D.; Liu, J.; Fei, X.; Yang, Y.; Zhang, B.; Lin, Z.; Yang, F.; et al. EpCAM-dependent extracellular vesicles from intestinal epithelial cells maintain intestinal tract immune balance. Nat Commun. 2016, 7, 13045. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Pulliam, L. Exosomes as mediators of neuroinflammation. J. Neuroinflamm. 2014, 11, 68. [Google Scholar] [CrossRef]
- Gupta, M.; Abdelmaksoud, A.; Jafferany, M.; Lotti, T.; Sadoughifar, R.; Goldust, M. COVID-19 and economy. Dermatol. Ther. 2020, 33, e13329. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Liu, L.; Ma, F.; Wong, C.W.; Guo, X.E.; Chacko, J.V.; Farhoodi, H.; Zhang, S.; Zimak, J.; Ségaliny, A.; et al. Elucidation of Exosome Migration Across the Blood–Brain Barrier Model In Vitro. Cell. Mol. Bioeng. 2016, 9, 509–529. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, Y.H.; Shin, Y.K.; Jo, Y.R.; Park, D.K.; Song, M.; Yoon, B.; Nam, S.H.; Kim, J.H.; Choi, B.; et al. p75 and neural cell adhesion molecule 1 can identify pathologic Schwann cells in peripheral neuropathies. Ann. Clin. Transl. Neurol. 2019, 6, 1292–1301. [Google Scholar] [CrossRef]
- McDonald, M.K.; Tian, Y.; Qureshi, R.A.; Gormley, M.; Ertel, A.; Gao, R.; Lopez, E.A.; Alexander, G.M.; Sacan, A.; Fortina, P.; et al. Functional significance of macrophage-derived exosomes in inflammation and pain. Pain 2014, 155, 1527–1539. [Google Scholar] [CrossRef]
- Bruehl, S. Complex regional pain syndrome. BMJ 2015, 351, h2730. [Google Scholar] [CrossRef]
- Jean-Toussaint, R.; Tian, Y.; Chaudhuri, A.D.; Haughey, N.J.; Sacan, A.; Ajit, S.K. Proteome characterization of small extracellular vesicles from spared nerve injury model of neuropathic pain. J. Proteom. 2019, 211, 103540. [Google Scholar] [CrossRef]
- D’Agnelli, S.; Gerra, M.C.; Bignami, E.; Arendt-Nielsen, L. Exosomes as a new pain biomarker opportunity. Mol. Pain 2020, 16, 1744806920957800. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Reddy, P.H. Are circulating microRNAs peripheral biomarkers for Alzheimer’s disease? Biochim. Biophys. Acta 2016, 1862, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Liu, Y.; Yang, Y.; Wang, H.; Xu, Y.; Zhang, Z. MSC-Derived Exosomes-Based Therapy for Peripheral Nerve Injury: A Novel Therapeutic Strategy. BioMed Res. Int. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Simeoli, R.; Montague, K.; Jones, H.R.; Castaldi, L.; Chambers, D.; Kelleher, J.H.; Vacca, V.; Pitcher, T.; Grist, J.; Al-Ahdal, H.; et al. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat. Commun. 2017, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
| Tool | Consistencies | How and When to Use It |
|---|---|---|
| Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) | It requires a physical examination. 85% sensitivity and 80% specificity [15]. | S-LANSS is the self-reported form. Positive scores on the LANSS or S-LANSS identify patients with pain of predominantly neuropathic origin. |
| Neuropathic Pain Questionnaire (NPQ) | 66% sensitivity and 74% specificity [15]. | 12 items that include 10 related to sensations or sensory responses, and 2 related to affect. |
| Douleur Neuropathique 4 Questions | It requires a physical examination. 83% sensitivity and 90% specificity [15]. | 7 items. A score of 4 out of 10 or more suggests neuropathic pain |
| painDETECT | Self-reported 85% sensitivity and 80% specificity [15]. | 9 items. It can be used in neuropathic, nociceptive pain, and low back pain. |
| Standardised Evaluation of Pain (StEPS) | It requires a physical examination. 92% sensitivity and 97% specificity [16]. | It can be used to discriminate between neuropathic (radicular) and non-neuropathic (axial) low back pain. |
| Neuropathic Pain Scale (NPS) | NA | The NPS quantifies already-diagnosed neuropathic pain. 10 items. A score of more than 4 suggests neuropathic pain |
| Pain Quality Assessment Scale (PQAS) | Self-reported | 20 items. It provides the pain qualities. |
| ID-Pain | 78% sensitivity and 74% specificity [17]. | 5 sensory descriptor items and 1 item relating joint nociceptive pain. |
| Neuropathic Pain Symptom Inventory (NPSI) | Self-reported. 91% sensitivity and 70% specificity [18]. | Characterize subgroups of neuropathic pain patients. |
| Neuropathic Pain scale for Postsurgical patients (NeuPPS) | 88% sensitivity and 59% specificity [19]. | 5 items. Measurement of neuropathic pain among postsurgical patients. |
| Author | Biomarker | Sample | Pathology | Evidence |
|---|---|---|---|---|
| Assi et al. [55] | Thrombospondin 4 | Serum | Advanced osteoarthritic neuropathic states | Correlation was demonstrated |
| Balagué et al. [56] | Keratan sulfate, hyaluronan, and cartilage oligomeric matrix protein | Peripheral blood | Sciatica | No correlation with clinical outcome |
| Dietz et al. [57] | hsa-miR-223-5p | Plasma | Complex regional pain syndrome | Correlation was demonstrated |
| Ramanathan et al. [58] | miRNAs | HEK293 cells | Complex regional pain syndrome | Correlation was demonstrated |
| Ericson et al. [59] | Tumor necrosis factor—related apoptosis inducing ligand, Tumor necrosis factor-beta | Cerebrospinal fluid | Trigeminal neuralgia | Correlation was demonstrated |
| Hayakawa et al. [60] | Lysophospholipids | Cerebrospinal fluid | Lumbar spinal stenosis | Correlation was demonstrated |
| Hider et al. [61] | Tumor necrosis factor-alpha, IL-6 and matrix metalloproteinases | Serum | Sciatica | No correlation with clinical outcome |
| Kallman et al. [62] | Beta-endorphin and substance P | Saliva and salivary-to-plasma quotients | Chronic neuropathic pain patients | No correlation with clinical outcome |
| Karakulova et al. [63] | Brain-derived neurotrophic factor and vascular endothelial growth factor and TrkB, VEGFR2 | Serum | Diabetic polyneuropathy | Correlation with clinical outcome |
| Kwon et al. [64] | IL-6, IL-8, and MCP-1 | Cerebrospinal fluid | Spinal cord injury | Correlation with clinical outcome |
| Lind et al. [65] | Follistatin, interleukin-1 alpha, and kallikrein-5 | Cerebrospinal fluid | Neuropathic pain patients | No correlation with clinical outcome |
| Radojcic et al. [66] | C1M and IL-6 | Serum | End-stage knee osteoarthritis | Correlation with clinical outcome |
| Ri et al. [67] | Lysophosphatidylcholine and phosphatidylcholine | Serum/plasma | Bortezomib-induced peripheral neuropathy | Correlation with clinical outcome |
| Ri et al. [68] | Lipid metabolites (1 ether-type lysophosphatidylcholine, 1 PC, 1 ceramide, 1 diacylglycerol, 1 triacylglycerol, and 9 oxFAs) | Serum | Bortezomib-induced peripheral neuropathy | Correlation with clinical outcome |
| Staats Pires et al. [69] | Major kynurenine and tetrahydrobiopterin pathway metabolites | Serum | Diabetic polyneuropathy | Correlation with clinical outcome |
| Wang et al. [70] | microRNAs (mir-204-5p, mir-519d-3p, mir-20b-5p, mir-6838-5p) | Peripheral blood sample | Spinal cord injury | Not clear correlation |
| Xu et al. [71] | Tumor necrosis factor-alpha and interleukin-6 | Peripheral blood sample | Spinal cord injury | Correlation with tumor necrosis factor-alpha and clinical outcome |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balzani, E.; Fanelli, A.; Malafoglia, V.; Tenti, M.; Ilari, S.; Corraro, A.; Muscoli, C.; Raffaeli, W. A Review of the Clinical and Therapeutic Implications of Neuropathic Pain. Biomedicines 2021, 9, 1239. https://doi.org/10.3390/biomedicines9091239
Balzani E, Fanelli A, Malafoglia V, Tenti M, Ilari S, Corraro A, Muscoli C, Raffaeli W. A Review of the Clinical and Therapeutic Implications of Neuropathic Pain. Biomedicines. 2021; 9(9):1239. https://doi.org/10.3390/biomedicines9091239
Chicago/Turabian StyleBalzani, Eleonora, Andrea Fanelli, Valentina Malafoglia, Michael Tenti, Sara Ilari, Annette Corraro, Carolina Muscoli, and William Raffaeli. 2021. "A Review of the Clinical and Therapeutic Implications of Neuropathic Pain" Biomedicines 9, no. 9: 1239. https://doi.org/10.3390/biomedicines9091239
APA StyleBalzani, E., Fanelli, A., Malafoglia, V., Tenti, M., Ilari, S., Corraro, A., Muscoli, C., & Raffaeli, W. (2021). A Review of the Clinical and Therapeutic Implications of Neuropathic Pain. Biomedicines, 9(9), 1239. https://doi.org/10.3390/biomedicines9091239







