Exploring Hyperoxia Effects in Cancer—From Perioperative Clinical Data to Potential Molecular Mechanisms
Abstract
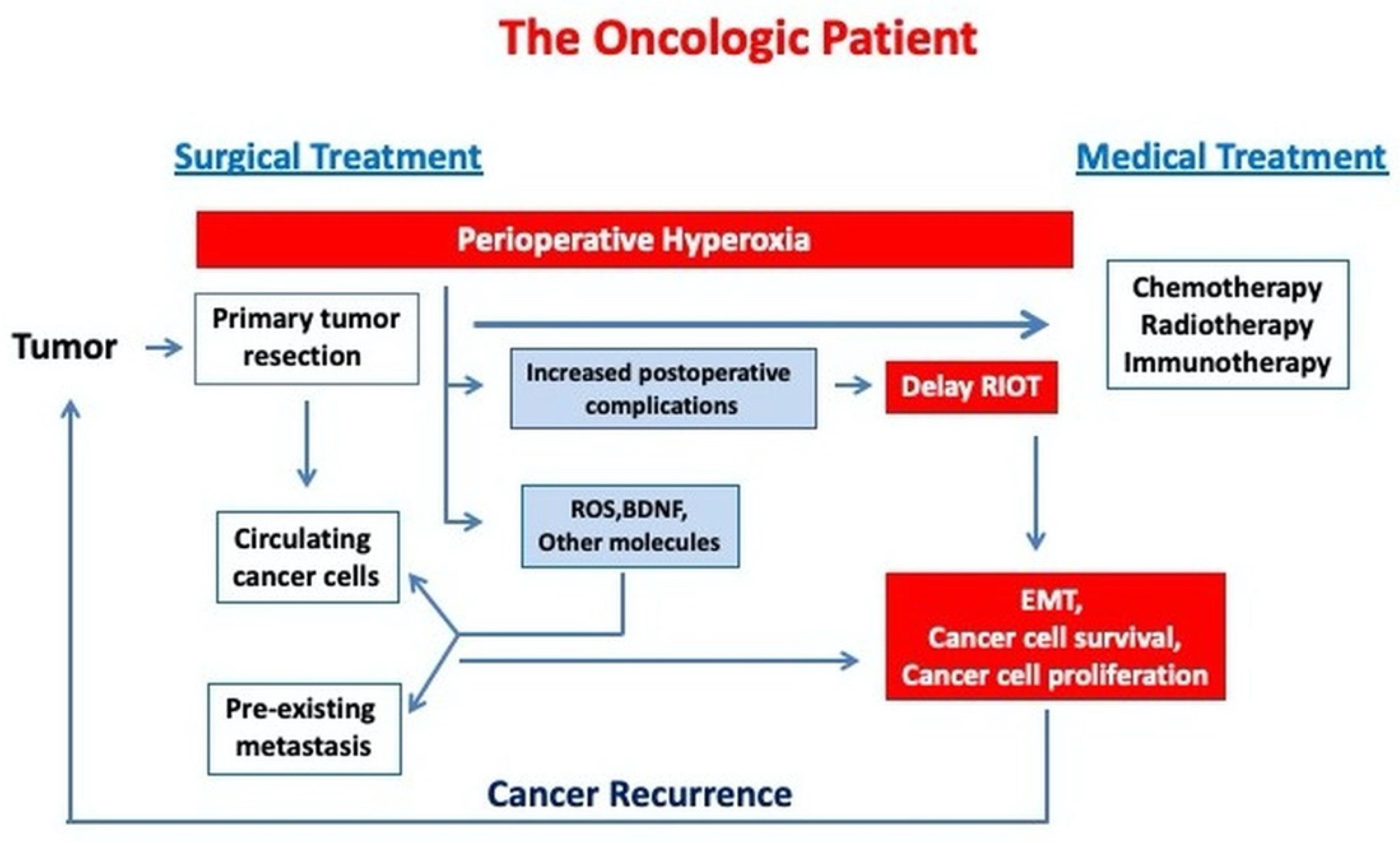
1. Background
2. Hyperoxia Effects on Surgical Cancer Patients—Clinical and Experimental Data
2.1. Short-Term Effects of Hyperoxia in Surgical Patients
2.1.1. Respiratory Effects
2.1.2. Cardiovascular Effects
2.1.3. Cerebral Effects
2.2. Long-Term Effects of Hyperoxia on Surgical Cancer Patients
3. Potential Molecular Mechanisms Exploring Hyperoxia Effects on Cancer Progression
3.1. ROS Production and Oxidative Stress
3.2. Hyperoxia and the Immune System
3.3. Angiogenesis and Epithelial Mesenchymal Transition (EMT)
3.4. Brain-Derived Neurotrophic Factor (BDNF)
3.5. Hyperoxic-Hypoxic Paradox
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sullivan, R.; Alatise, O.I.; Anderson, B.O.; Audisio, R.; Autier, P.; Aggarwal, A.; Balch, C.; Brennan, M.; Dare, A.; D’Cruz, A.; et al. Global cancer surgery: Delivering safe, affordable, and timely cancer surgery. Lancet Oncol. 2015, 16, 1193–1224. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Anderson, R.N. The Leading Causes of Death in the US for 2020. JAMA 2021, 325, 1829. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Treatment & Survivorship, Facts & Figures 2019–2021; American Cancer Society: Atlanta, GA, USA, 2019. [Google Scholar]
- Horowitz, M.; Neeman, E.; Sharon, E.; Ben-Eliyahu, S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat. Rev. Clin. Oncol. 2015, 12, 213–226. [Google Scholar] [CrossRef]
- Hiller, J.G.; Perry, N.J.; Poulogiannis, G.; Riedel, B.; Sloan, E. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 2017, 15, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Ristescu, I.; Grigoraș, I.; Dumitras, E.; Dimofte, G. Perioperative immune response alteration. can it influence cancer recurrence? Rev. Med. Chir. Soc. Med. Nat. Iasi 2016, 120, 861–865. [Google Scholar]
- Wigmore, T.; Gottumukkala, V.; Riedel, B. Making the Case for the Subspecialty of Onco-Anesthesia. Int. Anesthesiol. Clin. 2016, 54, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Wigmore, T.J.; Mohammed, K.; Jhanji, S. Long-term Survival for Patients Undergoing Volatile versus IV Anesthesia for Cancer Surgery. Anesthesiology 2016, 124, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Yap, A.; Lopez-Olivo, M.A.; Dubowitz, J.; Hiller, J.; Riedel, B.; Wigmore, T.; Ferguson, M.; Shan, D.; Yee, K.; Meyer, I.; et al. Anesthetic technique and cancer outcomes: A meta-analysis of total intravenous versus volatile anesthesia. Can. J. Anesth. 2019, 66, 546–561. [Google Scholar] [CrossRef]
- I Sessler, D.; Pei, L.; Huang, Y.; Fleischmann, E.; Marhofer, P.; Kurz, A.; Mayers, D.B.; A Meyer-Treschan, T.; Grady, M.; Tan, E.Y.; et al. Recurrence of breast cancer after regional or general anaesthesia: A randomised controlled trial. Lancet 2019, 394, 1807–1815. [Google Scholar] [CrossRef]
- Wall, T.P.; Crowley, P.D.; Sherwin, A.; Foley, A.G.; Buggy, D.J. Effects of Lidocaine and Src Inhibition on Metastasis in a Murine Model of Breast Cancer Surgery. Cancers 2019, 11, 1414. [Google Scholar] [CrossRef]
- Forget, P.; Aguirre, J.A.; Bencic, I.; Borgeat, A.; Cama, A.; Condron, C.; Eintrei, C.; Eroles, P.; Gupta, A.; Hales, T.G.; et al. How Anesthetic, Analgesic and Other Non-Surgical Techniques during Cancer Surgery Might Affect Postoperative Oncologic Outcomes: A Summary of Current State of Evidence. Cancers 2019, 11, 592. [Google Scholar] [CrossRef] [PubMed]
- Tiron, C.; Patrașcanu, E.; Postu, P.; Trandafir, I.V.; Tiron, A.; Grigoras, I. Sevoflurane Modulates AKT Isoforms in Triple Negative Breast Cancer Cells. An Experimental Study. Curr. Issues Mol. Biol. 2021, 43, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Damiani, E.; Adrario, E.; Girardis, M.; Romano, R.; Pelaia, P.; Singer, M.; Donati, A. Arterial hyperoxia and mortality in critically ill patients: A systematic review and meta-analysis. Crit. Care 2014, 18, 711. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2017, 39, 119–177. [Google Scholar] [CrossRef]
- Writing Committee Members; O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; De Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. Circulation 2013, 127, e362–e425. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e99. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Czlonkowska, A.; Ford, G.A.; Fonseca, A.C.; Luijckx, G.J.; Korv, J.; de la Ossa, N.P.; Price, C.; Russell, D.; Tsiskaridze, A.; et al. European Academy of Neurology and European Stroke Organization consensus statement and practical guidance for pre-hospital management of stroke. Eur. J. Neurol. 2017, 25, 425–433. [Google Scholar] [CrossRef]
- Elmer, J.; Scutella, M.; Pullalarevu, R.; Wang, B.; Vaghasia, N.; Trzeciak, S.; Rosario-Rivera, B.L.; Guyette, F.; Rittenberger, J.C.; Dezfulian, C.; et al. The association between hyperoxia and patient outcomes after cardiac arrest: Analysis of a high-resolution database. Intensive Care Med. 2014, 41, 49–57. [Google Scholar] [CrossRef]
- Helmerhorst, H.J.; Roos-Blom, M.J.; van Westerloo, D.J.; de Jonge, E. Association between arterial hyperoxia and outcome in subsets of critical illness: A systematic review, meta- analysis, and meta-regression of cohort studies. Crit. Care Med. 2015, 43, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, H.J.F.; Arts, D.; Schultz, M.J.; Van Der Voort, P.H.J.; Abu-Hanna, A.; De Jonge, E.; Van Westerloo, D.J. Metrics of Arterial Hyperoxia and Associated Outcomes in Critical Care. Crit. Care Med. 2017, 45, 187–195. [Google Scholar] [CrossRef]
- Chu, D.; Kim, L.H.-Y.; Young, P.; Zamiri, N.; A Almenawer, S.; Jaeschke, R.; Szczeklik, W.; Schünemann, H.J.; Neary, J.D.; Alhazzani, W. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): A systematic review and meta-analysis. Lancet 2018, 391, 1693–1705. [Google Scholar] [CrossRef]
- Siemieniuk, R.A.C.; Chu, D.; Kim, L.H.-Y.; Güell-Rous, M.-R.; Alhazzani, W.; Soccal, P.M.; Karanicolas, P.J.; Farhoumand, P.D.; Siemieniuk, J.L.K.; Satia, I.; et al. Oxygen therapy for acutely ill medical patients: A clinical practice guideline. BMJ 2018, 363, k4169. [Google Scholar] [CrossRef]
- de Jonge, S.; Egger, M.; Latif, A.; Loke, Y.K.; Berenholtz, S.; Boermeester, M.; Allegranzi, B.; Solomkin, J. Effectiveness of 80% vs. 30–35% fraction of inspired oxygen in patients undergoing surgery: An updated systematic review and meta-analysis. Br. J. Anaesth. 2019, 122, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Weenink, R.P.; de Jonge, S.W.; van Hulst, R.A.; Wingelaar, T.T.; van Ooij, P.J.A.; Immink, R.V.; Hollmann, M.W. Perioperative hyperoxyphobia: Justified or not? Benefits and harms of hyperoxia during surgery. J. Clin. Med. 2020, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Kallet, R.H.; Matthay, M.A. Hyperoxic Acute Lung Injury. Respir. Care 2012, 58, 123–141. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, W.; Yang, J.; Sun, F.; Zhou, H. Interleukin-3 plays a vital role in hyperoxic acute lung injury in mice via mediating inflammation. BMC Pulm. Med. 2018, 18, 164. [Google Scholar] [CrossRef]
- Hedenstierna, G.; Edmark, L. Effects of anesthesia on the respiratory system. Best Pract. Res. Clin. Anaesthesiol. 2015, 29, 273–284. [Google Scholar] [CrossRef]
- Cohen, B.; Ruetzler, K.; Kurz, A.; Leung, S.; Rivas, E.; Ezell, J.; Mao, G.; Sessler, D.I.; Turan, A. Intra-operative high inspired oxygen fraction does not increase the risk of postoperative respiratory complications: Alternating intervention clinical trial. Eur. J. Anaesthesiol. 2019, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kurz, A.; Kopyeva, T.; Suliman, I.; Podolyak, A.; You, J.; Lewis, B.; Vlah, C.; Khatib, R.; Keebler, A.; Reigert, R.; et al. Supplemental oxygen and surgical-site infections: An alternating intervention controlled trial. Br. J. Anaesth. 2018, 120, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Mattishent, K.; Thavarajah, M.; Sinha, A.; Peel, A.; Egger, M.; Solomkin, J.; de Jonge, S.; Latif, A.; Berenholtz, S.; Allegranzi, B.; et al. Safety of 80% vs. 30–35% fraction of inspired oxygen in patients undergoing surgery: A systematic review and meta-analysis. Br. J. Anaesth. 2019, 122, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Staehr-Rye, A.K.; Meyhoff, C.; Scheffenbichler, F.T.; Melo, M.F.V.; Gätke, M.R.; Walsh, J.L.; Ladha, K.S.; Grabitz, S.D.; Nikolov, M.I.; Kurth, T.; et al. High intraoperative inspiratory oxygen fraction and risk of major respiratory complications. Br. J. Anaesth. 2017, 119, 140–149. [Google Scholar] [CrossRef]
- Smit, B.; Smulders, Y.M.; Van Der Wouden, J.C.; Straaten, H.M.O.-V.; Man, A.M.E.S.-D. Hemodynamic effects of acute hyperoxia: Systematic review and meta-analysis. Crit. Care 2018, 22, 1–10. [Google Scholar] [CrossRef]
- Smit, B.; Smulders, Y.M.; De Waard, M.C.; Straaten, H.M.O.; Girbes, A.R.J.; Eringa, E.; De Man, A.M.E.S. Hyperoxia does not directly affect vascular tone in isolated arteries from mice. PLoS ONE 2017, 12, e0182637. [Google Scholar] [CrossRef] [PubMed]
- Damiani, E.; Casarotta, E.; Orlando, F.; Carsetti, A.; Scorcella, C.; Domizi, R.; Adrario, E.; Ciucani, S.; Provinciali, M.; Donati, A. Effects of Normoxia, Hyperoxia, and Mild Hypoxia on Macro-Hemodynamics and the Skeletal Muscle Microcirculation in Anesthetised Rats. Front. Med. 2021, 8, 672257. [Google Scholar] [CrossRef] [PubMed]
- Fonnes, S.; Gögenur, I.; Søndergaard, E.S.; Siersma, V.D.; Jorgensen, L.N.; Wetterslev, J.; Meyhoff, C.S. Perioperative hyperoxia—Long-term impact on cardiovascular complications after abdominal surgery, a post hoc analysis of the PROXI trial. Int. J. Cardiol. 2016, 215, 238–243. [Google Scholar] [CrossRef]
- Peng, Y.-W.; Mohammed, A.; Deatrick, K.B.; Major, T.; Cheng, D.; Charpie, I.; Charpie, J.R. Differential Effects of Normoxic and Hyperoxic Reperfusion on Global Myocardial Ischemia-Reperfusion Injury. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 188–198. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, D.H.; Kim, S.C.; Kang, C.; Lee, S.H.; Jeong, J.H.; Lee, S.B.; Park, Y.J.; Lim, D. Impact of early hyperoxia on 28-day in-hospital mortality in patients with myocardial injury. PLoS ONE 2018, 13, e0201286. [Google Scholar] [CrossRef]
- López, H.V.; Vivas, M.F.; Ruiz, R.N.; Martínez, J.R.; Navaridas, B.G.-V.; Villa, M.G.; Lázaro, C.L.; Rubio, R.J.; Ortiz, A.M.; Lacal, L.A.; et al. Association between post-procedural hyperoxia and poor functional outcome after mechanical thrombectomy for ischemic stroke: An observational study. Ann. Intensive Care 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Kongebro, E.K.; Jorgensen, L.N.; Siersma, V.D.; Meyhoff, C.S. Association between perioperative hyperoxia and cerebrovascular complications after laparotomy-A post-hoc follow-up study. Acta Anaesthesiol. Scand. 2018, 63, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, A.C.; Lee, R.M.; Turgeon, M.K.; Bs, C.V.; Regenbogen, S.E.; Hrebinko, K.A.; Holder-Murray, J.; Wiseman, J.T.; Ejaz, A.; Ba, M.P.F.; et al. Impact of Postoperative Complications on Oncologic Outcomes After Rectal Cancer Surgery: An Analysis of the US Rectal Cancer Consortium. Ann. Surg. Oncol. 2020, 28, 1712–1721. [Google Scholar] [CrossRef]
- Aoyama, T.; Murakawa, M.; Katayama, Y.; Yamaoku, K.; Kanazawa, A.; Higuchi, A.; Shiozawa, M.; Morimoto, M.; Yoshikawa, T.; Morinaga, S.; et al. Impact of post-operative complications on survival and recurrence after resection of colorectal liver metastases: Systematic review and meta-analysis. Ann. Surg. 2019, 270, 1018–1027. [Google Scholar]
- Khuri, S.F.; Henderson, W.G.; DePalma, R.G.; Mosca, C.; Healey, N.A.; Kumbhani, D.J. Determinants of Long-Term Survival after Major Surgery and the Adverse Effect of Postoperative Complications. Ann. Surg. 2005, 242, 326–343. [Google Scholar] [CrossRef]
- Wu, W.; He, J.; Cameron, J.L.; Makary, M.; Soares, K.; Ahuja, N.; Rezaee, N.; Herman, J.; Zheng, L.; Laheru, D.; et al. The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann. Surg. Oncol. 2014, 21, 2873–2881. [Google Scholar] [CrossRef]
- Aloia, T.A.; Zimmitti, G.; Conrad, C.; Gottumukalla, V.; Kopetz, S.; Vauthey, J.-N. Return to intended oncologic treatment (RIOT): A novel metric for evaluating the quality of oncosurgical therapy for malignancy. J. Surg. Oncol. 2014, 110, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Finnerty, D.T.; Buggy, D.J. Return to intended oncologic therapy: A potentially valuable endpoint for perioperative research in cancer patients? Br. J. Anaesth. 2020, 124, 508–510. [Google Scholar] [CrossRef]
- Lillemoe, H.A.; Marcus, R.K.; Kim, B.; Narula, N.; Davis, C.; Aloia, T.A. Detours on the Road to Recovery: What Factors Delay Readiness to Return to Intended Oncologic Therapy (RIOT) after Liver Resection for Malignancy? J. Gastrointest. Surg. 2019, 23, 2362–2371. [Google Scholar] [CrossRef]
- Ramos, M.F.K.P.; De Castria, T.B.; Pereira, M.A.; Dias, A.R.; Antonacio, F.F.; Zilberstein, B.; Hoff, P.M.G.; Ribeiro, U.; Cecconello, I. Return to Intended Oncologic Treatment (RIOT) in Resected Gastric Cancer Patients. J. Gastrointest. Surg. 2019, 24, 19–27. [Google Scholar] [CrossRef]
- Meyhoff, C.S.; Wetterslev, J.; Jorgensen, L.N.; Henneberg, S.W.; Hogdall, C.; Lundvall, L.; Svendsen, P.E.; Mollerup, H.; Lunn, T.H.; Simonsen, I.; et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: The PROXI randomized clinical trial. JAMA 2009, 302, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Meyhoff, C.S.; Jorgensen, L.N.; Wetterslev, J.; Christensen, K.B.; Rasmussen, L.S.; PROXI Trial Group. Increased long-term mortality after a high perioperative inspiratory oxygen fraction during abdominal surgery: Follow-up of a randomized clinical trial. Anesth. Analg. 2012, 115, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Meyhoff, C.S.; Jorgensen, L.N.; Wetterslev, J.; Siersma, V.D.; Rasmussen, L.S. Risk of new or recurrent cancer after a high perioperative inspiratory oxygen fraction during abdominal surgery. Br. J. Anaesth. 2014, 113 (Suppl. 1), i74–i81. [Google Scholar] [CrossRef]
- Podolyak, A.; Sessler, D.I.; Reiterer, C.; Fleischmann, E.; Akça, O.; Mascha, E.J.; Greif, R.; Kurz, A. Perioperative Supplemental Oxygen Does Not Worsen Long-Term Mortality of Colorectal Surgery Patients. Anesth. Analg. 2016, 122, 1907–1911. [Google Scholar] [CrossRef]
- Greif, R.; Akça, O.; Horn, E.-P.; Kurz, A.; Sessler, D.I. Supplemental Perioperative Oxygen to Reduce the Incidence of Surgical-Wound Infection. N. Engl. J. Med. 2000, 342, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Kurz, A.; Fleischmann, E.; Sessler, D.; Buggy, D.; Apfel, C.; Akça, O.; Erdik, E.; Eredics, K.; Kabon, B.; Herbst, F.; et al. Effects of supplemental oxygen and dexamethasone on surgical site infection: A factorial randomized trial. Br. J. Anaesth. 2015, 115, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Kurz, A.; Zhang, X.; Liu, L.; Yang, D.; Sessler, D.I. Supplemental Intraoperative Oxygen and Long-term Mortality: Subanalysis of a Multiple Crossover Cluster Trial. Anesthesiology 2021, 134, 709–721. [Google Scholar] [CrossRef]
- Li, L.-F.; Liao, S.-K.; Ko, Y.-S.; Lee, C.-H.; Quinn, D.A. Hyperoxia increases ventilator-induced lung injury via mitogen-activated protein kinases: A prospective, controlled animal experiment. Crit. Care 2007, 11, R25. [Google Scholar] [CrossRef]
- Thiel, M.; Chouker, A.; Ohta, A.; Jackson, E.; Caldwell, C.; Smith, P.; Lukashev, D.; Bittmann, I.; Sitkovsky, M.V. Oxygenation Inhibits the Physiological Tissue-Protecting Mechanism and Thereby Exacerbates Acute Inflammatory Lung Injury. PLoS Biol. 2005, 3, e174. [Google Scholar] [CrossRef] [PubMed]
- Nagato, A.; Bezerra, F.; Lanzetti, M.; Lopes, A.D.A.; Silva, M.A.D.S.; Porto, L.C.; Valença, S.S. Time course of inflammation, oxidative stress and tissue damage induced by hyperoxia in mouse lungs. Int. J. Exp. Pathol. 2012, 93, 269–278. [Google Scholar] [CrossRef]
- Potteti, H.R.; Rajasekaran, S.; Rajamohan, S.B.; Tamatam, C.R.; Reddy, N.M.; Reddy, S.P. Sirtuin 1 Promotes Hyperoxia-Induced Lung Epithelial Cell Death Independent of NF-E2–Related Factor 2 Activation. Am. J. Respir. Cell Mol. Biol. 2016, 54, 697–706. [Google Scholar] [CrossRef]
- Terraneo, L.; Paroni, R.; Bianciardi, P.; Giallongo, T.; Carelli, S.; Gorio, A.; Samaja, M. Brain adaptation to hypoxia and hyperoxia in mice. Redox Biol. 2016, 11, 12–20. [Google Scholar] [CrossRef]
- Kupiec, A.; Adamik, B.; Forkasiewicz-Gardynik, K.; Goździk, W. Intra-operative hyperoxia and the risk of delirium in elderly patients after cardiac surgery. Aging 2020, 12, 7006–7014. [Google Scholar] [CrossRef]
- Li, K.C.; Tam, C.W.Y.; Shum, H.-P.; Yan, W.W. Impact of Hyperoxia and Hypocapnia on Neurological Outcomes in Patients with Aneurysmal Subarachnoid Hemorrhage: A Retrospective Study. Crit. Care Res. Pract. 2019, 2019, 7584573. [Google Scholar] [CrossRef]
- Li, L.-F.; Yang, C.-T.; Huang, C.-C.; Liu, Y.-Y.; Kao, K.-C.; Lin, H.-C. Low-molecular-weight heparin reduces hyperoxia-augmented ventilator-induced lung injury via serine/threonine kinase-protein kinase B. Respir. Res. 2011, 12, 90. [Google Scholar] [CrossRef]
- Chen, C.; Weng, H.; Zhang, X.; Wang, S.; Lu, C.; Jin, H.; Chen, S.; Liu, Y.; Sheng, A.; Sun, Y. Low-Dose Vitamin D Protects Hyperoxia-Induced Bronchopulmonary Dysplasia by Inhibiting Neutrophil Extracellular Traps. Front. Pediatr. 2020, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Tung, Y.T.; Wei, C.H.; Lee, P.Y.; Chen, W. Anti-inflammatory and reactive oxygen species suppression through aspirin pretreatment to treat hyperoxia-induced acute lung injury in NF-κB–luciferase inducible transgenic mice. Antioxidants 2020, 9, 429. [Google Scholar] [CrossRef] [PubMed]
- Shenberger, J.S.; Myers, J.L.; Zimmer, S.G.; Powell, R.J.; Barchowsky, A. Hyperoxia alters the expression and phosphorylation of multiple factors regulating translation initiation. Am. J. Physiol. Cell. Mol. Physiol. 2005, 288, L442–L449. [Google Scholar] [CrossRef][Green Version]
- Climent, M.; Viggiani, G.; Chen, Y.-W.; Coulis, G.; Castaldi, A. MicroRNA and ROS Crosstalk in Cardiac and Pulmonary Diseases. Int. J. Mol. Sci. 2020, 21, 4370. [Google Scholar] [CrossRef]
- Zhang, X.; Chu, X.; Gong, X.; Zhou, H.; Cai, C. The expression of miR-125b in Nrf2-silenced A549 cells exposed to hyperoxia and its relationship with apoptosis. J. Cell. Mol. Med. 2019, 24, 965–972. [Google Scholar] [CrossRef]
- Cifci, S.P.; Tapan, Y.U.; Erkul, B.T.; Savran, Y.; Comert, B. The Impact of Hyperoxia on Outcome of Patients Treated with Noninvasive Respiratory Support. Can. Respir. J. 2020, 2020, 3953280. [Google Scholar] [CrossRef]
- Helmerhorst, H.J.F.; Schultz, M.J.; van der Voort, P.H.J.; de Jonge, E.; van Westerloo, D.J. Bench-to-bedside review: The effects of hyperoxia during critical illness. Crit. Care 2015, 19, 284. [Google Scholar] [CrossRef]
- Ottolenghi, S.; Rubino, F.M.; Sabbatini, G.; Coppola, S.; Veronese, A.; Chiumello, D.; Paroni, R. Oxidative Stress Markers to Investigate the Effects of Hyperoxia in Anesthesia. Int. J. Mol. Sci. 2019, 20, 5492. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 2009, 29, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Tavare, A.N.; Perry, N.J.; Benzonana, L.L.; Takata, M.; Ma, D. Cancer recurrence after surgery: Direct and indirect effects of anesthetic agents. Int. J. Cancer 2011, 130, 1237–1250. [Google Scholar] [CrossRef]
- Sun, S.; Lee, D.; Lee, N.P.; Pu, J.K.S.; Wong, T.S.; Lui, W.M.; Fung, C.F.; Leung, G.K.K. Hyperoxia resensitizes chemoresistant human glioblastoma cells to temozolomide. J. Neuro-Oncol. 2012, 109, 467–475. [Google Scholar] [CrossRef]
- Wang, P.; Wan, W.; Xiong, S.; Wang, J.; Zou, D.; Lan, C.; Yu, S.; Liao, B.; Feng, H.; Wu, N. HIF1α regulates glioma chemosensitivity through the transformation between differentiation and dedifferentiation in various oxygen levels. Sci. Rep. 2017, 7, 7965. [Google Scholar] [CrossRef] [PubMed]
- De Bels, D.; Tillmans, F.; Corazza, F.; Bizzarri, M.; Germonpre, P.; Radermacher, P.; Orman, K.G.; Balestra, C. Hyperoxia Alters Ultrastructure and Induces Apoptosis in Leukemia Cell Lines. Biomolecules 2020, 10, 282. [Google Scholar] [CrossRef]
- Raa, A.; Stansberg, C.; Steen, V.M.; Bjerkvig, R.; Reed, R.K.; Stuhr, L.E.B. Hyperoxia retards growth and induces apoptosis and loss of glands and blood vessels in DMBA-induced rat mammary tumors. BMC Cancer 2007, 7, 23. [Google Scholar] [CrossRef][Green Version]
- Moen, I.; Øyan, A.M.; Kalland, K.-H.; Tronstad, K.J.; Akslen, L.A.; Chekenya, M.; Sakariassen, P.; Reed, R.K.; Stuhr, L.E.B. Hyperoxic Treatment Induces Mesenchymal-to-Epithelial Transition in a Rat Adenocarcinoma Model. PLoS ONE 2009, 4, e6381. [Google Scholar] [CrossRef]
- Qian, X.; Zhang, Q.; Shao, N.; Shan, Z.; Cheang, T.; Zhang, Z.; Su, Q.; Wang, S.; Lin, Y. Respiratory hyperoxia reverses immunosuppression by regulating myeloid-derived suppressor cells and PD-L1 expression in a triple-negative breast cancer mouse model. Am. J. Cancer Res. 2019, 9, 529–545. [Google Scholar] [PubMed]
- Yamamoto, N.; Oyaizu, T.; Enomoto, M.; Horie, M.; Yuasa, M.; Okawa, A.; Yagishita, K. VEGF and bFGF induction by nitric oxide is associated with hyperbaric oxygen-induced angiogenesis and muscle regeneration. Sci. Rep. 2020, 10, 2744. [Google Scholar] [CrossRef]
- Huang, X.; Liang, P.; Jiang, B.; Zhang, P.; Yu, W.; Duan, M.; Guo, L.; Cui, X.; Huang, M.; Huang, X. Hyperbaric oxygen potentiates diabetic wound healing by promoting fibroblast cell proliferation and endothelial cell angiogenesis. Life Sci. 2020, 259, 118246. [Google Scholar] [CrossRef]
- Chang, H.-C.; Yang, Y.-R.; Wang, R.-Y. Effects of repetitive hyperbaric oxygen therapy on neuroprotection in middle cerebral artery occlusion rats. Brain Res. 2020, 1748, 147097. [Google Scholar] [CrossRef]
- Hatfield, S.M.; Kjaergaard, J.; Lukashev, D.; Schreiber, T.; Belikoff, B.; Abbott, R.; Sethumadhavan, S.; Philbrook, P.; Ko, K.; Cannici, R.; et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci. Transl. Med. 2015, 7, 277ra30. [Google Scholar] [CrossRef]
- Kumar, V.H.S.; Wang, H.; Nielsen, L. Adaptive immune responses are altered in adult mice following neonatal hyperoxia. Physiol. Rep. 2018, 6, e13577. [Google Scholar] [CrossRef]
- Kiers, D.; Gerretsen, J.; Janssen, E.; John, A.; Groeneveld, R.; Van Der Hoeven, J.G.; Scheffer, G.-J.; Pickkers, P.; Kox, M. Short-term hyperoxia does not exert immunologic effects during experimental murine and human endotoxemia. Sci. Rep. 2015, 5, 17441. [Google Scholar] [CrossRef]
- Ash, S.A.; Valchev, G.I.; Looney, M.; Ni Mhathuna, A.; Crowley, P.D.; Gallagher, H.C.; Buggy, D.J. Xenon decreases cell migration and secretion of a pro-angiogenesis factor in breast adenocarcinoma cells: Comparison with sevoflurane. Br. J. Anaesth. 2014, 113, i14–i21. [Google Scholar] [CrossRef]
- Crowley, P.D.; Stuttgen, V.; O’Carroll, E.; A Ash, S.; Buggy, D.J.; Gallagher, H.C. Exposure to 60% oxygen promotes migration and upregulates angiogenesis factor secretion in breast cancer cells. Med. Gas Res. 2017, 7, 226–235. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2018, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Aiello, N.; Kang, Y. Context-dependent EMT programs in cancer metastasis. J. Exp. Med. 2019, 216, 1016–1026. [Google Scholar] [CrossRef]
- Tadokoro, A.; Kanaji, N.; Liu, D.; Yokomise, H.; Haba, R.; Ishii, T.; Takagi, T.; Watanabe, N.; Kita, N.; Kadowaki, N.; et al. Vimentin Regulates Invasiveness and Is a Poor Prognostic Marker in Non-small Cell Lung Cancer. Anticancer Res. 2016, 36, 1545–1551. [Google Scholar]
- Du, L.; Li, J.; Lei, L.; He, H.; Chen, E.; Dong, J.; Yang, J. High Vimentin Expression Predicts a Poor Prognosis and Progression in Colorectal Cancer: A Study with Meta-Analysis and TCGA Database. BioMed Res. Int. 2018, 2018, 6387810. [Google Scholar] [CrossRef]
- Chaw, S.Y.; Majeed, A.A.; Dalley, A.J.; Chan, A.; Stein, S.; Farah, C.S. Epithelial to mesenchymal transition EMT) biomarkers–E-cadherin, beta-catenin, APC and Vimentin–in oral squamous cell carcinogenesis and transformation. Oral Oncol. 2012, 48, 997–1006. [Google Scholar] [CrossRef]
- Tiron, A.; Ristescu, I.; Postu, P.A.; Tiron, C.E.; Zugun-Eloae, F.; Grigoras, I. Long-Term Deleterious Effects of Short-term Hyperoxia on Cancer Progression—Is Brain-Derived Neurotrophic Factor an Important Mediator? An Experimental Study. Cancers 2020, 12, 688. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, I.K.; Ha, J.H.; Yeo, C.D.; Kang, H.H.; Kim, J.W.; Lee, S.H. Normobaric hyperoxia inhibits the progression of lung cancer by inducing apoptosis. Exp. Biol. Med. 2018, 243, 739–748. [Google Scholar] [CrossRef]
- Yao, Q.; Haxhiu, M.A.; Zaidi, S.I.; Liu, S.; Jafri, A.; Martin, R.J. Hyperoxia enhances brain-derived neurotrophic factor and tyrosine kinase B receptor expression in peribronchial smooth muscle of neonatal rats. Am. J. Physiol. Cell. Mol. Physiol. 2005, 289, L307–L314. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kwon, H.-J.; Lee, J.-E.; Lee, Y.; Seoh, J.-Y.; Han, P.-L. Hyperoxygenation revitalizes Alzheimer’s disease pathology through the upregulation of neurotrophic factors. Aging Cell 2019, 18, e12888. [Google Scholar] [CrossRef] [PubMed]
- Kujawski, S.; Słomko, J.; Morten, K.J.; Murovska, M.; Buszko, K.; Newton, J.L.; Zalewski, P. Autonomic and Cognitive Function Response to Normobaric Hyperoxia Exposure in Healthy Subjects. Preliminary Study. Medicina 2020, 56, 172. [Google Scholar] [CrossRef] [PubMed]
- Paris, A.J.; Hayer, K.E.; Oved, J.H.; Avgousti, D.C.; Toulmin, S.A.; Zepp, J.A.; Zacharias, W.J.; Katzen, J.B.; Basil, M.C.; Kremp, M.M.; et al. STAT3–BDNF–TrkB signalling promotes alveolar epithelial regeneration after lung injury. Nat. Cell Biol. 2020, 22, 1197–1210. [Google Scholar] [CrossRef]
- Lee, B.; Cao, R.; Choi, Y.-S.; Cho, H.-Y.; Rhee, A.D.; Hah, C.K.; Hoyt, K.; Obrietan, K. The CREB/CRE transcriptional pathway: Protection against oxidative stress-mediated neuronal cell death. J. Neurochem. 2009, 108, 1251–1265. [Google Scholar] [CrossRef]
- Yoo, J.-M.; Lee, B.D.; Sok, D.-E.; Ma, J.Y.; Kim, M.R. Neuroprotective action of N-acetyl serotonin in oxidative stress-induced apoptosis through the activation of both TrkB/CREB/BDNF pathway and Akt/Nrf2/Antioxidant enzyme in neuronal cells. Redox Biol. 2017, 11, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Hacioglu, G.; Senturk, A.; Ince, I.; Alver, A. Assessment of oxidative stress parameters of brain-derived neurotrophic factor heterozygous mice in acute stress model. Iran. J. Basic Med. Sci. 2016, 19, 388–393. [Google Scholar]
- Jiang, J.M.; Zhou, C.F.; Gao, S.L.; Tian, Y.; Wang, C.Y.; Wang, L.; Gu, H.F.; Tang, X.Q. BDNF-TrkB pathway mediates neuroprotection of hydrogen sulfide against formaldehyde-induced toxicity to PC12 cells. PLoS ONE 2015, 10, e0119478. [Google Scholar]
- Wu, C.L.; Chen, S.D.; Yin, J.H.; Hwang, C.S.; Yang, D.I. Nuclear factor-kappaB-dependent sestrin2 induction mediates the antioxidant effects of BDNF against mitochondrial inhibition in rat cortical neurons. Mol. Neurobiol. 2016, 53, 4126–4142. [Google Scholar] [CrossRef]
- Boyadjieva, N.I.; Sarkar, D.K. Cyclic Adenosine Monophosphate and Brain-Derived Neurotrophic Factor Decreased Oxidative Stress and Apoptosis in Developing Hypothalamic Neuronal Cells: Role of Microglia. Alcohol. Clin. Exp. Res. 2013, 37, 1370–1379. [Google Scholar] [CrossRef]
- Huang, Y.Z.; McNamara, J.O. Neuroprotective Effects of Reactive Oxygen Species Mediated by BDNF-Independent Activation of TrkB. J. Neurosci. 2012, 32, 15521–15532. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Naruo, A.; Okada, M.; Hayabe, Y.; Yamawaki, H. Brain-derived neurotrophic factor promotes angiogenic tube formation through generation of oxidative stress in human vascular endothelial cells. Acta Physiol. 2014, 211, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhou, B.; Zhang, P.; Lei, D.; Wang, Y.; Yao, G.; Hayashi, T.; Xia, M.; Tashiro, S.-I.; Onodera, S.; et al. Protective Effect of Silibinin on Learning and Memory Impairment in LPS-Treated Rats via ROS–BDNF–TrkB Pathway. Neurochem. Res. 2016, 41, 1662–1672. [Google Scholar] [CrossRef]
- Jia, S.; Wang, W.; Hu, Z.; Shan, C.; Wang, L.; Wu, B.; Yang, Z.; Yang, X.; Lei, D. BDNF mediated TrkB activation contributes to the EMT progression and the poor prognosis in human salivary adenoid cystic carcinoma. Oral Oncol. 2015, 51, 64–70. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, W.S.; Jeong, J.; Kim, S.J.; Jin, W. Induction of metastatic potential by TrkB via activation of IL6/JAK2/STAT3 and PI3K/AKT signaling in breast cancer. Oncotarget 2015, 6, 40158. [Google Scholar] [CrossRef]
- Kawamura, K.; Kawamura, N.; Okamoto, N.; Manabe, M. Suppression of choriocarcinoma invasion and metastasis following blockade of BDNF /TrkB signaling. Cancer Med. 2013, 2, 849–861. [Google Scholar] [CrossRef]
- Götz, R.; Sendtner, M. Cooperation of Tyrosine Kinase Receptor TrkB and Epidermal Growth Factor Receptor Signaling Enhances Migration and Dispersal of Lung Tumor Cells. PLoS ONE 2014, 9, e100944. [Google Scholar] [CrossRef]
- Tanaka, K.; Okugawa, Y.; Toiyama, Y.; Inoue, Y.; Saigusa, S.; Kawamura, M.; Araki, T.; Uchida, K.; Mohri, Y.; Kusunoki, M. Brain-Derived Neurotrophic Factor (BDNF)-Induced Tropomyosin-Related Kinase B (Trk B) Signaling Is a Potential Therapeutic Target for Peritoneal Carcinomatosis Arising from Colorectal Cancer. PLoS ONE 2014, 9, e96410. [Google Scholar] [CrossRef]
- Polakowski, N.; Terol, M.J.; Hoang, K.; Nash, I.; Laverdure, S.; Gazon, H.; Belrose, G.; Mesnard, J.-M.; Césaire, R.; Peloponese, J.-M.; et al. HBZ Stimulates Brain-Derived Neurotrophic Factor/TrkB Autocrine/Paracrine Signaling To Promote Survival of Human T-Cell Leukemia Virus Type 1-Infected T Cells. J. Virol. 2014, 88, 13482–13494. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Hung, S.-Y.; Chen, H.-T.; Tsou, H.-K.; Fong, Y.-C.; Wang, S.-W.; Tang, C.-H. Brain-derived neurotrophic factor increases vascular endothelial growth factor expression and enhances angiogenesis in human chondrosarcoma cells. Biochem. Pharmacol. 2014, 91, 522–533. [Google Scholar] [CrossRef]
- Huang, S.-M.; Lin, C.; Lin, H.-Y.; Chiu, C.-M.; Fang, C.-W.; Liao, K.-F.; Chen, D.-R.; Yeh, W.-L. Brain-derived neurotrophic factor regulates cell motility in human colon cancer. Endocr.-Relat. Cancer 2015, 22, 455–464. [Google Scholar] [CrossRef]
- Nakamura, K.; Martin, K.C.; Jackson, J.K.; Beppu, K.; Woo, C.-W.; Thiele, C.J. Brain-Derived Neurotrophic Factor Activation of TrkB Induces Vascular Endothelial Growth Factor Expression via Hypoxia-Inducible Factor-1α in Neuroblastoma Cells. Cancer Res. 2006, 66, 4249–4255. [Google Scholar] [CrossRef]
- Terraneo, L.; Virgili, E.; Caretti, A.; Bianciardi, P.; Samaja, M. In vivo hyperoxia induces hypoxia-inducible factor-1α overexpression in LNCaP tumors without affecting the tumor growth rate. Int. J. Biochem. Cell Biol. 2014, 51, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.A.; Geiger, T.R.; Song, J.Y.; Gitelman, I.; Peeper, D.S. A Twist-Snail axis critical for TrkB-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis. Mol. Cell Biol. 2009, 29, 3722–3737. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Qiu, H.; Yang, T.; Luo, X.; Zhang, H.; Wan, X. Upregulation of TrkB Promotes Epithelial-Mesenchymal Transition and Anoikis Resistance in Endometrial Carcinoma. PLoS ONE 2013, 8, e70616. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Ye, H.; Ren, Q. Proliferative role of BDNF/TrkB signaling is associated with anoikis resistance in cervical cancer. Oncol. Rep. 2018, 40, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yu, Y.; Song, Y.; Li, X.; Lan, D.; Zhang, P.; Xiao, Y.; Xing, Y. Activation of BDNF/TrkB pathway promotes prostate cancer progression via induction of epithelial-mesenchymal transition and anoikis resistance. FASEB J. 2020, 34, 9087–9101. [Google Scholar] [CrossRef] [PubMed]
- Parekh, A.; Das, S.; Parida, S.; Das, C.K.; Dutta, D.; Mallick, S.K.; Wu, P.-H.; Kumar, B.N.P.; Bharti, R.; Dey, G.; et al. Multi-nucleated cells use ROS to induce breast cancer chemo-resistance in vitro and in vivo. Oncogene 2018, 37, 4546–4561. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Paget, J.T.E.; Khan, A.; Harrington, K. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Jarosz-Biej, M.; Smolarczyk, R.; Cichoń, T.; Kułach, N. Tumor microenvironment as a “game changer” in cancer radiotherapy. Int. J. Mol. Sci. 2019, 20, 3212. [Google Scholar] [CrossRef] [PubMed]
- Agliano, A.; Balarajah, G.; Ciobota, D.M.; Sidhu, J.; Clarke, P.; Jones, C.; Workman, P.; Leach, M.O.; Al-Saffar, N.M. Pediatric and adult glioblastoma radiosensitization induced by PI3K/mTOR inhibition causes early metabolic alterations detected by nuclear magnetic resonance spectroscopy. Oncotarget 2017, 8, 47969–47983. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; He, Z.; Xing, D. Low-level laser therapy rescues dendrite atrophy via upregulating BDNF expression: Implications for Alzheimer’s disease. J. Neurosci. 2013, 33, 13505–13517. [Google Scholar] [CrossRef]
- Cui, M.; Xiao, H.; Li, Y.; Dong, J.; Luo, D.; Li, H.; Feng, G.; Wang, H.; Fan, S. Total abdominal irradiation exposure impairs cognitive function involving miR-34a-5p/BDNF axis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 2333–2341. [Google Scholar] [CrossRef]
- Amir, H.; Shai, E. The Hyperoxic-Hypoxic Paradox. Biomolecules 2020, 10, 958. [Google Scholar] [CrossRef]
| Author, Year, Reference | Study Type | Patients Number | Type of Patients | Hyperoxia Exposure | Primary Outcome | Results |
|---|---|---|---|---|---|---|
| Cohen, B. et al., 2019 [29] | Post-hoc analysis of [30] | 5056 | Colorectal surgery | Intraoperative, 39 vs. 80% O2 | Postoperative pulmonary complications | No difference |
| Mattishent, K. et al., 2019 [31] | Meta-analysis | 3839, 3458 | Surgical patients | Perioperative, 30 vs. 80% O2 | Postoperative atelectasis, pneumonia | No difference |
| Staehr-Rye, A.K. et al., 2017 [32] | Retrospective registry study | 73,922 | Non-cardiotoracic surgery | Intraoperative, 30%, 40%, 51%, 58%, 79% O2 | Major respiratory complication (re-intubation, respiratory failure, pulmonary oedema, pneumonia) | Hyperoxia increased risk, in a dose dependent manner |
| Smit, B. et al., 2018 [33] | Meta-analysis | 408 392 | Healthy volunteers, medical and surgical patients | PaO2 = 234–617 mmHg | Hemodynamic effects | Hyperoxia decreased cardiac output, increased systemic vascular resistance |
| Fonnes, S. et al., 2016 [37] | Post-hoc analysis of [49] | 1386 | Abdominal surgery | Intraoperative, 2 h postoperative 30 vs. 80% O2 | Long-term major cardiovascular complication | Hyperoxia (80% O2) increased acute coronary syndrome |
| Meyhoff, C. et al., 2009 [49] | RCT | 1386 | Abdominal surgery | Intraoperative, 2 h postoperative 30 vs. 80% O2 | Surgical site infection within 14 days | No difference |
| Meyhoff, C. et al., 2012 [50] | 2.3 years follow-up | 1386 352 | Abdominal surgery Cancer patients | Intraoperative, 2 h postoperative 30 vs. 80% O2 | Long-term mortality | Hyperoxia increased long term mortality in cancer patients |
| Meyhoff, C. et al., 2014 [51] | 3.9 year follow-up | 1377 | Abdominal surgery Cancer patients | Intraoperative, 2 h postoperative 30% vs. 80% O2 | Risk of new or recurrent cancer at 3.9 years follow-up | Shorter cancer-free survival time |
| Podolyak, A et al., 2016 [52] | Follow-up [53,54] | 927 (432 + 495) | Colorectal surgery | Intraoperative, 2 h postoperative 30% vs. 80% O2 | Long-term mortality analysis | No difference |
| Greif, R. et al., 2000 [53] | RCT | 500 | Colorectal surgery | Intraoperative, 2 h postoperative 30% vs. 80% O2 | Surgical site infection within 30 days | Decreased in hyperoxia group |
| Kurz, A. et al., 2015 [54] | RCT | 585 | Colorectal surgery | Intraoperative, 2 h postoperative 30% vs. 80% O2 | Surgical site infection within 30 days | No difference |
| Kurz, A. et al., 2018 [30] | Alternating intervention controlled trial | 5749 | Major intestinal surgery | Intraoperative 30% and 80% O2, alternating at 2-week intervals for 39 months | 30-day composite of deep tissue or organ-space SSI, healing-related wound complications, mortality | No difference |
| Jiang, Q. et al., 2021 [55] | Post-hoc analysis of [30], 3 years follow-up | 2800 995 | Colorectal surgery Cancer patients | Intraoperative 30% and 80% O2, alternating at 2-week intervals for 39 months | Long term mortality | No difference |
| Author, Year, References | Study Type | Experimental Design | Hyperoxia Exposure | Primary Outcome | Results |
|---|---|---|---|---|---|
| Li, L. et al., 2007 [56] | In vivo | C57BL/6 mice exposed to high-VT mechanical ventilation | 21 vs. 95% O2 for 1–5 h | Ventilator-induced lung injury | Hyperoxia increases lung and inflammation ventilator-induced lung injury |
| Tiron, A. et al., 2020 [93] | In vitro, In vivo | MCF10A, MDA-MB-231, 4T1 breast cancer cells 4T1 TNBC Murine model | 21%, 40%, 60%, 80% O2 for 6 h 21%, 40%, 60%, 80% O2 for 6 h perioperative | Effects on breast cancer growth | Hyperoxia (80%) increases ROS, BDNF, EMT and angiogenesis molecules Increases size and number of lung metastasis |
| Crowley et al., 2018 [87] | In vitro | MDA-MB-231 MCF-7 breast cancer cells | 21%, 30%, 60%, 80% O2 for 3 h | Effects on breast cancer cell migration and angiogenesis | Hyperoxia (60%) promotes migration and upregulates angiogenesis factor secretion |
| Ash et al., 2014 [86] | In vitro | MDA-MB-231 and MCF-7 breast cancer cells | Xenon 70% + O2 25%, sevoflurane 2.5% + 65% O2 for 5 h | Effects on breast cancer cells migration and angiogenesis | Hyperoxia (65%) promotes breast cancer cell migration |
| Kim et al., 2018 [94] | In vitro In vivo | A549 lung cancer cells Murine model of lung cancer | 85% O2 for 24 h | Morphological changes in lung cancer | Hyperoxia increased ROS, apoptosis Decreases size and number of lung tumors |
| Qian et al., 2018 [79] | In vivo | 4T1 TNBC murine model | 21%, vs. 65% O2 for 21 days | Effects on tumor microenvironment | Hyperoxia reverses immunosuppression by regulating myeloid-derived suppressor cells and PD-L1 expression |
| Hatfield et al., 2015 [83] | In vitro In vivo | MCA205 tumor cell line 4T1 TNBC Murine model | 40%, 60% O2 for 2 days | Effects on tumor microenvironment | Hyperoxia increases tumoral infiltration with CD8+ T cells, proinflammatory cytokines, decreased TGF-β and immunosuppression by T-reg cells. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ristescu, A.I.; Tiron, C.E.; Tiron, A.; Grigoras, I. Exploring Hyperoxia Effects in Cancer—From Perioperative Clinical Data to Potential Molecular Mechanisms. Biomedicines 2021, 9, 1213. https://doi.org/10.3390/biomedicines9091213
Ristescu AI, Tiron CE, Tiron A, Grigoras I. Exploring Hyperoxia Effects in Cancer—From Perioperative Clinical Data to Potential Molecular Mechanisms. Biomedicines. 2021; 9(9):1213. https://doi.org/10.3390/biomedicines9091213
Chicago/Turabian StyleRistescu, Anca Irina, Crina Elena Tiron, Adrian Tiron, and Ioana Grigoras. 2021. "Exploring Hyperoxia Effects in Cancer—From Perioperative Clinical Data to Potential Molecular Mechanisms" Biomedicines 9, no. 9: 1213. https://doi.org/10.3390/biomedicines9091213
APA StyleRistescu, A. I., Tiron, C. E., Tiron, A., & Grigoras, I. (2021). Exploring Hyperoxia Effects in Cancer—From Perioperative Clinical Data to Potential Molecular Mechanisms. Biomedicines, 9(9), 1213. https://doi.org/10.3390/biomedicines9091213







