High-Fat Diets Modify the Proteolytic Activities of Dipeptidyl-Peptidase IV and the Regulatory Enzymes of the Renin–Angiotensin System in Cardiovascular Tissues of Adult Wistar Rats
Abstract
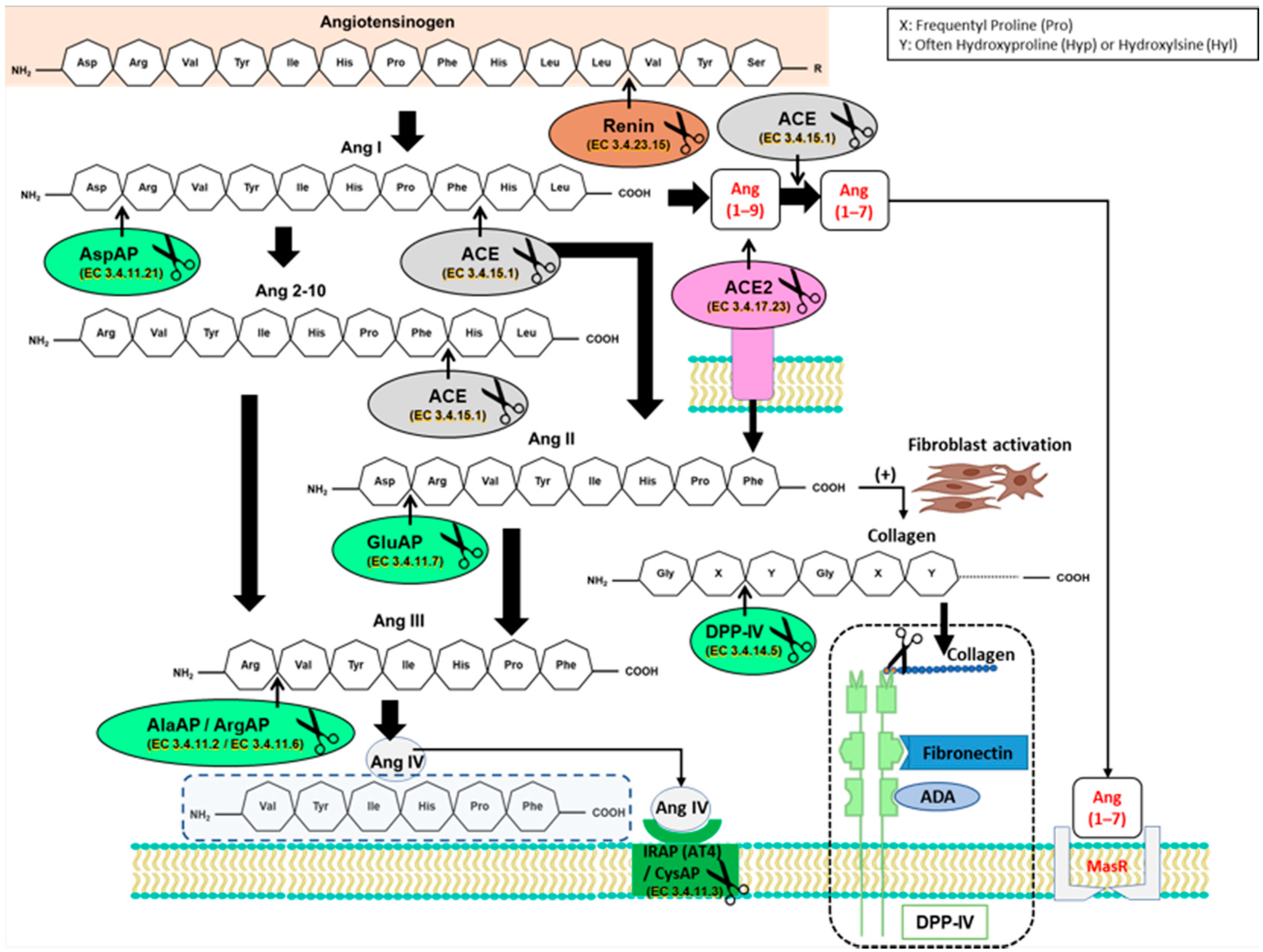
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Assay of Aminopeptidase Activities
2.3. Protein Measurement
2.4. Statistical Analysis
3. Results
3.1. Angiotensinase Activities
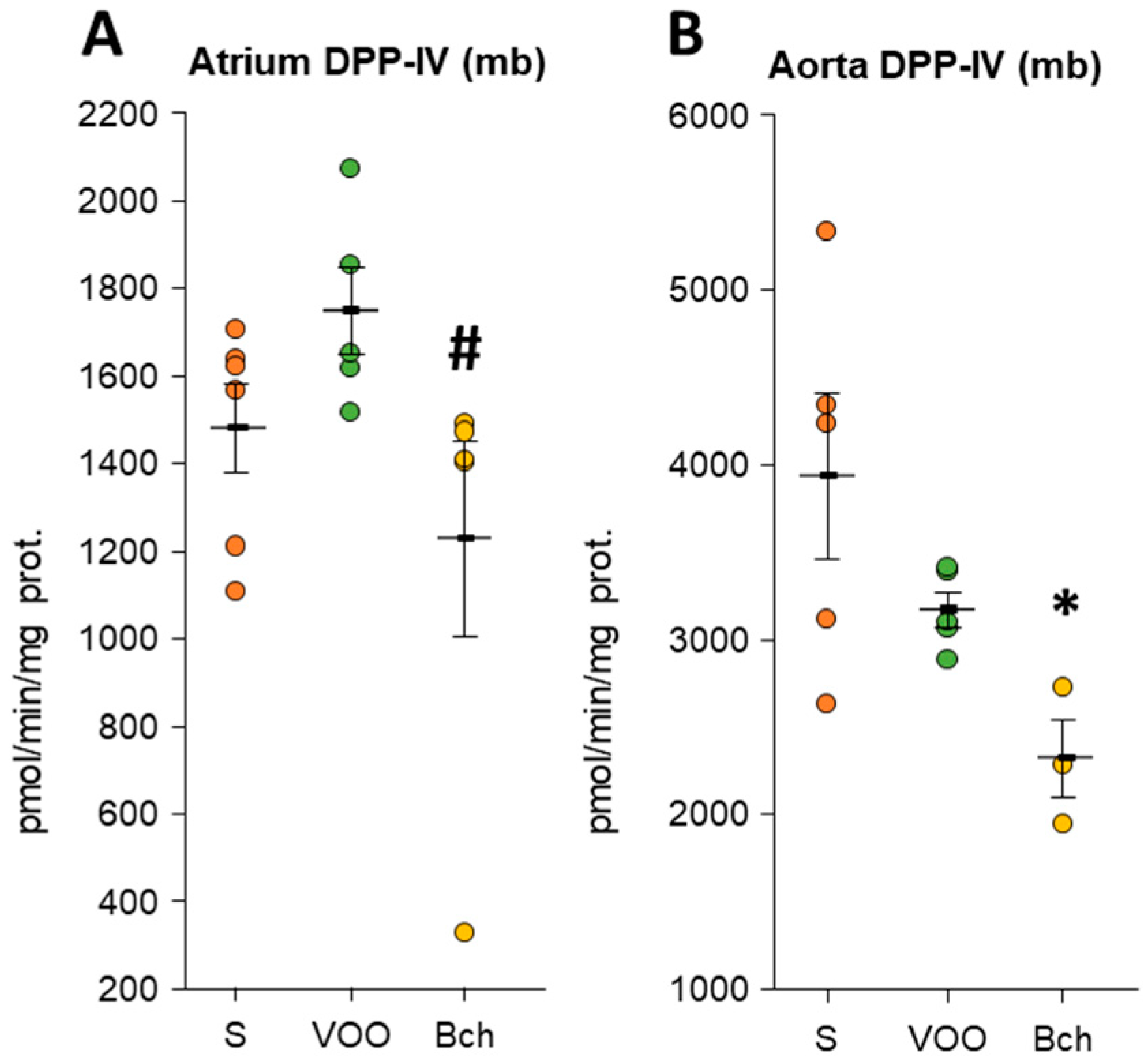
3.2. Dipeptidyl Peptidase IV Activity
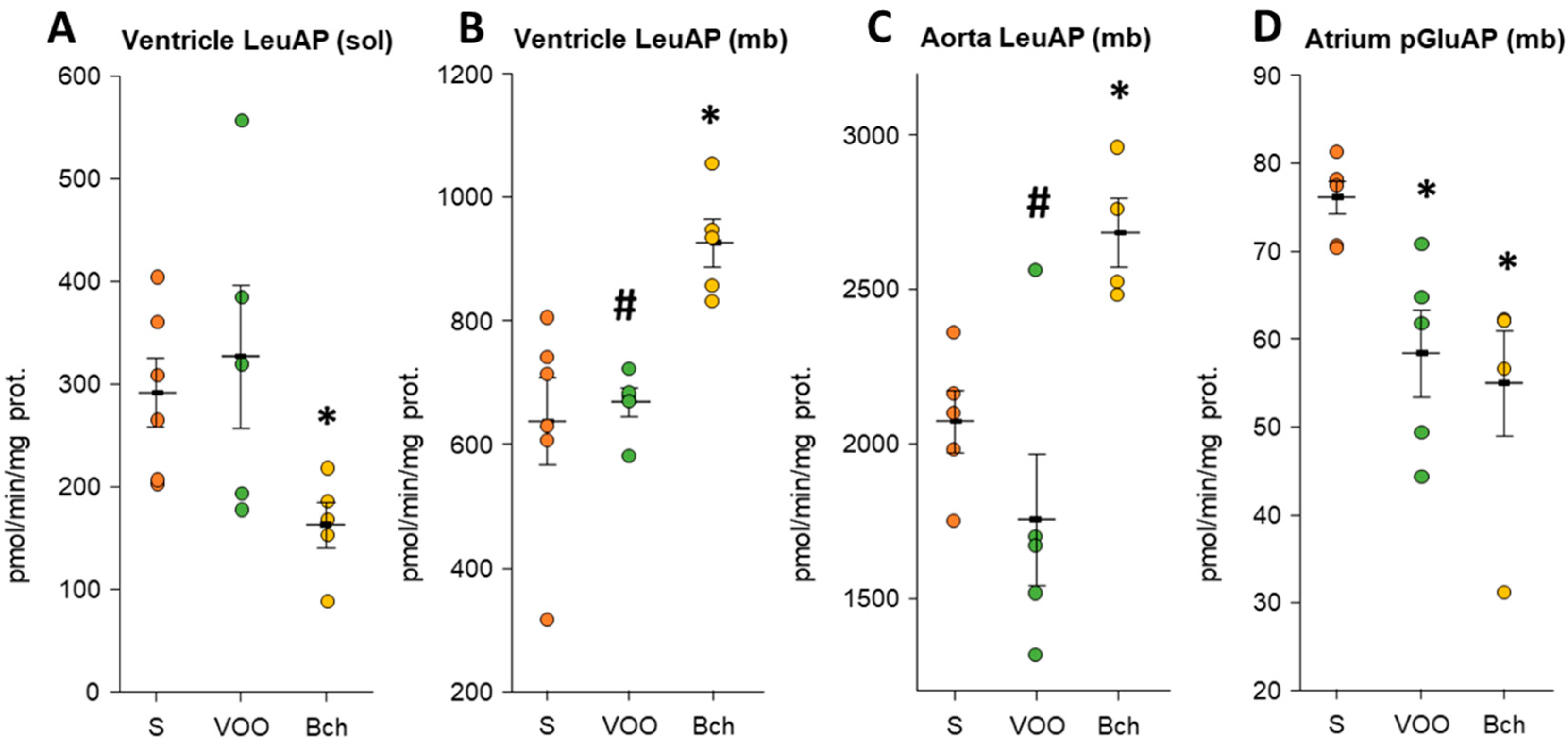
3.3. Leucyl Aminopeptidase, Gamma-Glutamyl Transferase, and Pyroglutamyl Aminopeptidase Activities
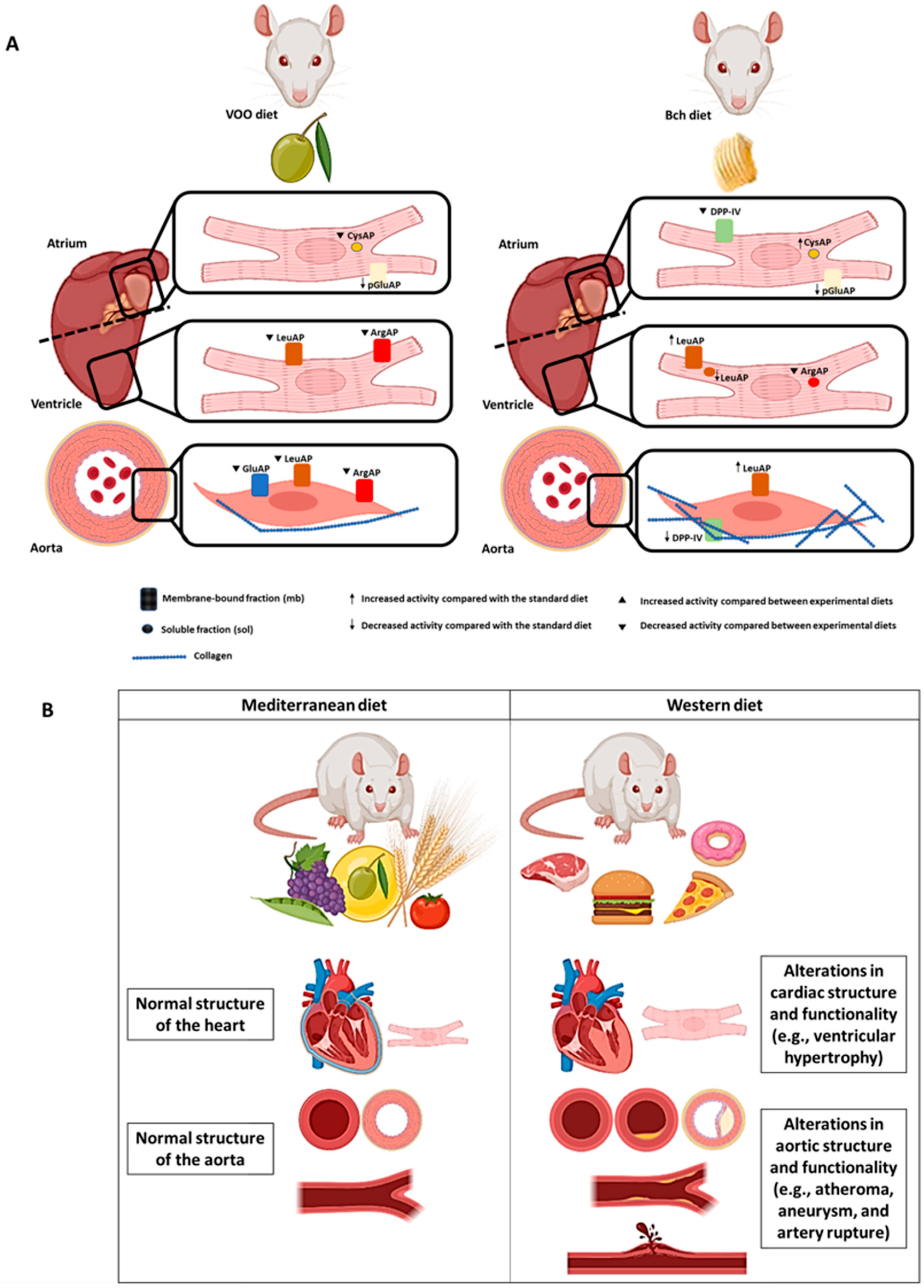
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AlaAP | alanyl-aminopeptidase |
| Ang | angiotensin |
| AP | aminopeptidase |
| ArgAP | arginyl-aminopeptidase |
| AspAP | aspartyl-aminopeptidase |
| Bch | butter plus cholesterol |
| BP | blood pressure |
| CysAP | cystine aminopeptidase |
| CVD | cardiovascular diseases |
| DPP-IV | dipeptidyl-peptidase IV |
| GGT | gamma-glutamyl transferase |
| GluAP | glutamyl-aminopeptidase |
| HFD | high-fat diet |
| IRAP | insulin-regulated aminopeptidase |
| LeuAP | leucyl-aminopeptidase |
| MUFA | monounsaturated fatty acids |
| pGluAP | pyroglutamyl-aminopeptidase |
| RAS | renin–angiotensin system |
| S | standard |
| SAFA | saturated fatty acids |
| VOO | virgin olive oil |
References
- Vasilopoulou, D.; Markey, O.; Kliem, K.E.; Fagan, C.C.; Grandison, A.S.; Humphries, D.J.; Todd, S.; Jackson, K.G.; Givens, D.I.; Lovegrove, J.A. Reformulation initiative for partial replacement of saturated with unsaturated fats in dairy foods attenuates the increase in LDL cholesterol and improves flow-mediated dilatation compared with conventional dairy: The randomized, controlled REplacement of SaturatEd fat in dairy on Total cholesterol (RESET) study. Am. J. Clin. Nutr. 2020, 111, 739–748. [Google Scholar] [CrossRef]
- Cerf, M.E. High Fat Programming and Cardiovascular Disease. Medicina 2018, 54, 86. [Google Scholar] [CrossRef] [Green Version]
- Wali, J.A.; Jarzebska, N.; Raubenheimer, D.; Simpson, S.J.; Rodionov, R.N.; O’Sullivan, J.F. Cardio-Metabolic Effects of High-Fat Diets and Their Underlying Mechanisms—A Narrative Review. Nutrients 2020, 12, 1505. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Vías, G.; Segarra, A.B.; Ramírez-Sánchez, M.; Prieto, I. Effects of Virgin Olive Oil on Blood Pressure and Renal Aminopeptidase Activities in Male Wistar Rats. Int. J. Mol. Sci. 2021, 22, 5388. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.B.; Ramirez, M.; Banegas, I.; Alba, F.; Vives, F.; Gasparo, M.d.; Ortega, E.; Ruiz, E.; Prieto, I. Dietary fat influences testosterone, cholesterol, aminopeptidase A, and blood pressure in male rats. Horm. Metab. Res. 2008, 40, 289–291. [Google Scholar] [CrossRef]
- Villarejo, A.B.; Ramírez-Sánchez, M.; Segarra, A.B.; Martínez-Cañamero, M.; Prieto, I. Influence of extra virgin olive oil on blood pressure and kidney angiotensinase activities in spontaneously hypertensive rats. Planta Med. 2015, 81, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Vías, G.; Segarra, A.B.; Martínez-Cañamero, M.; Ramírez-Sánchez, M.; Prieto, I. Influence of a Virgin Olive Oil versus Butter Plus Cholesterol-Enriched Diet on Testicular Enzymatic Activities in Adult Male Rats. Int. J. Mol. Sci. 2017, 18, 1701. [Google Scholar] [CrossRef] [Green Version]
- Martínez, N.; Prieto, I.; Hidalgo, M.; Segarra, A.B.; Martínez-Rodríguez, A.M.; Cobo, A.; Ramírez, M.; Gálvez, A.; Martínez-Cañamero, M. Refined versus Extra Virgin Olive Oil High-Fat Diet Impact on Intestinal Microbiota of Mice and Its Relation to Different Physiological Variables. Microorganisms 2019, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Vías, G.; Segarra, A.B.; Ramírez-Sánchez, M.; Prieto, I. The Role of High Fat Diets and Liver Peptidase Activity in the Development of Obesity and Insulin Resistance in Wistar Rats. Nutrients 2020, 12, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segarra, A.B.; Domínguez-Vías, G.; Redondo, J.; Martínez-Cañamero, M.; Ramírez-Sánchez, M.; Prieto, I. Hypothalamic Renin–Angiotensin System and Lipid Metabolism: Effects of Virgin Olive Oil versus Butter in the Diet. Nutrients 2021, 13, 480. [Google Scholar] [CrossRef]
- Sánchez-Aguilar, M.; Ibarra-Lara, L.; Del Valle-Mondragón, L.; Soria-Castro, E.; Torres-Narváez, J.C.; Carreón-Torres, E.; Sánchez-Mendoza, A.; Rubio-Ruíz, M.E. Nonclassical Axis of the Renin-Angiotensin System and Neprilysin: Key Mediators That Underlie the Cardioprotective Effect of PPAR-Alpha Activation during Myocardial Ischemia in a Metabolic Syndrome Model. PPAR Res. 2020, 2020, 8894525. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.; Prieto, I.; Alba, F.; Vives, F.; Banegas, I.; de Gasparo, M. Role of central and peripheral aminopeptidase activities in the control of blood pressure: A working hypothesis. Heart Fail Rev. 2008, 13, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.; Prieto, I.; Martinez, J.M.; Vargas, F.; Alba, F. Renal aminopeptidase activities in animal models of hypertension. Regul. Pept. 1997, 72, 155–159. [Google Scholar] [CrossRef]
- Prieto, I.; Martinez, A.; Martinez, J.M.; Ramírez, M.J.; Vargas, F.; Alba, F.; Ramírez, M. Activities of aminopeptidases in a rat saline model of volume hypertension. Horm. Metab. Res. 1998, 30, 246–248. [Google Scholar] [CrossRef]
- Prieto, I.; Martínez, J.M.; Hermoso, F.; Ramírez, M.J.; de Gasparo, M.; Vargas, F.; Alba, F.; Ramírez, M. Effect of valsartan on angiotensin II- and vasopressin-degrading activities in the kidney of normotensive and hypertensive rats. Horm. Metab. Res. 2001, 33, 559–563. [Google Scholar] [CrossRef]
- Prieto, I.; Arechaga, G.; Segarra, A.B.; Alba, F.; de Gasparo, M.; Ramirez, M. Effects of dehydration on renal aminopeptidase activities in adult male and female rats. Regul. Pept. 2002, 106, 27–32. [Google Scholar] [CrossRef]
- Prieto, I.; Hermoso, F.; de Gasparo, M.; Vargas, F.; Alba, F.; Segarra, A.B.; Banegas, I.; Ramírez, M. Aminopeptidase activity in renovascular hypertension. Med. Sci. Monit. 2003, 9, 31–36. [Google Scholar]
- Prieto, I.; Hermoso, F.; Gasparo, M.d.; Vargas, F.; Alba, F.; Segarra, A.B.; Banegas, I.; Ramírez, M. Angiotensinase activities in the kidney of renovascular hypertensive rats. Peptides 2003, 24, 755–760. [Google Scholar] [CrossRef] [Green Version]
- Segarra, A.B.; Prieto, I.; Villarejo, A.B.; Banegas, I.; Wangensteen, R.; de Gasparo, M.; Vives, F.; Ramírez-Sánchez, M. Effects of antihypertensive drugs on angiotensinase activities in the testis of spontaneously hypertensive rats. Horm. Metab. Res. 2013, 45, 344–348. [Google Scholar] [CrossRef]
- Prieto, I.; Villarejo, A.B.; Segarra, A.B.; Banegas, I.; Wangensteen, R.; Martinez-Cañamero, M.; de Gasparo, M.; Vives, F.; Ramírez-Sánchez, M. Brain, heart and kidney correlate for the control of blood pressure and water balance: Role of angiotensinases. Neuroendocrinology 2014, 100, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Villarejo, A.B.; Prieto, I.; Segarra, A.B.; Banegas, I.; Wangensteen, R.; Vives, F.; de Gasparo, M.; Ramírez-Sánchez, M. Relationship of angiotensinase and vasopressinase activities between hypothalamus, heart, and plasma in L-NAME-treated WKY and SHR. Horm. Metab. Res. 2014, 46, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Prieto, I.; Villarejo, A.B.; Segarra, A.B.; Wangensteen, R.; Banegas, I.; de Gasparo, M.; Vanderheyden, P.; Zorad, S.; Vives, F.; Ramírez-Sánchez, M. Tissue distribution of CysAP activity and its relationship to blood pressure and water balance. Life Sci. 2015, 134, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Prieto, I.; Segarra, A.B.; de Gasparo, M.; Martínez-Cañamero, M.; Ramírez-Sánchez, M. Divergent profile between hypothalamic and plasmatic aminopeptidase activities in WKY and SHR. Influence of beta-adrenergic blockade. Life Sci. 2018, 192, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Keck, M.; Hmazzou, R.; Llorens-Cortes, C. Orally Active Aminopeptidase A Inhibitor Prodrugs: Current State and Future Directions. Curr. Hypertens. Rep. 2019, 21, 50. [Google Scholar] [CrossRef] [PubMed]
- Llorens-Cortes, C.; Touyz, R.M. Evolution of a New Class of Antihypertensive Drugs: Targeting the Brain Renin-Angiotensin System. Hypertension 2020, 75, 6–15. [Google Scholar] [CrossRef]
- Segarra, A.B.; Ruiz-Sanz, J.I.; Ruiz-Larrea, M.B.; Ramírez-Sánchez, M.; de Gasparo, M.; Banegas, I.; Martínez-Cañamero, M.; Vives, F.; Prieto, I. The profile of fatty acids in frontal cortex of rats depends on the type of fat used in the diet and correlates with neuropeptidase activities. Horm. Metab. Res. 2011, 43, 86–91. [Google Scholar] [CrossRef]
- Boitard, S.E.; Marc, Y.; Keck, M.; Mougenot, N.; Agbulut, O.; Balavoine, F.; Llorens-Cortes, C. Brain renin-angiotensin system blockade with orally active aminopeptidase A inhibitor prevents cardiac dysfunction after myocardial infarction in mice. J. Mol. Cell Cardiol. 2019, 127, 215–222. [Google Scholar] [CrossRef]
- Turgut, O.; Yilmaz, A.; Yalta, K.; Karadas, F.; Birhan-Yilmaz, M. Gamma-Glutamyltransferase is a promising biomarker for cardiovascular risk. Med. Hypotheses. 2006, 67, 1060–1064. [Google Scholar] [CrossRef]
- Danziger, R.S. Aminopeptidase N in arterial hypertension. Heart Fail. Rev. 2008, 13, 293–298. [Google Scholar] [CrossRef]
- Tsujimoto, M.; Goto, Y.; Maruyama, M.; Hattori, A. Biochemical and enzymatic properties of the M1 family of aminopeptidases involved in the regulation of blood pressure. Heart Fail Rev. 2008, 13, 285–291. [Google Scholar] [CrossRef]
- Mason, J.E.; Starke, R.D.; Van Kirk, J.E. Gamma-glutamyl transferase: A novel cardiovascular risk biomarker. Prev. Cardiol. 2010, 13, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.; Vargas, F.; Montoro-Molina, S.; O’Valle, F.; Rodríguez-Martínez, M.D.; Osuna, A.; Prieto, I.; Ramírez, M.; Wangensteen, R. Urinary aminopeptidase activities as early and predictive biomarkers of renal dysfunction in cisplatin-treated rats. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Gao, Y.; Xiong, H.; Lv, W.; Yang, G.; Lu, C.; Nie, J.; Ma, C.; Chen, Z.; Ren, J.; et al. A near-infrared fluorescent probe for monitoring leucine aminopeptidase in living cells. Analyst 2019, 144, 463–467. [Google Scholar] [CrossRef]
- Vargas, F.; Wangesteen, R.; Rodríguez-Gómez, I.; García-Estañ, J. Aminopeptidases in Cardiovascular and Renal Function. Role as Predictive Renal Injury Biomarkers. Int. J. Mol. Sci. 2020, 21, 5615. [Google Scholar] [CrossRef]
- Du, K.; Sheng, L.; Luo, X.; Fan, G.; Shen, D.; Wu, C.; Shen, R. A ratiometric fluorescent probe based on quinoline for monitoring and imaging of Leucine aminopeptidase in liver tumor cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 249, 119328. [Google Scholar] [CrossRef] [PubMed]
- Vauquelin, G.; Michotte, Y.; Smolders, I.; Sarre, S.; Ebinger, G.; Dupont, A.; Vanderheyden, P. Cellular targets for angiotensin II fragments: Pharmacological and molecular evidence. J. Renin Angiotensin Aldosterone Syst. 2002, 3, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Wallis, M.G.; Lankford, M.F.; Keller, S.R. Vasopressin is a physiological substrate for the insulin-regulated aminopeptidase IRAP. Am. J. Physiol. Endocrinol. Metab. 2007, 293, 1092–1102. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Sánchez, M.; Prieto, I.; Wangensteen, R.; Banegas, I.; Segarra, A.B.; Villarejo, A.B.; Vives, F.; Cobo, J.; de Gasparo, M. The renin-angiotensin system: New insight into old therapies. Curr. Med. Chem. 2013, 20, 1313–1322. [Google Scholar] [CrossRef]
- Segarra, A.B.; Prieto, I.; Martinez-Canamero, M.; Vargas, F.; De Gasparo, M.; Vanderheyden, P.; Zorad, S.; Ramirez-Sanchez, M. Cystinyl and pyroglutamyl-beta-naphthylamide hydrolyzing activities are modified coordinately between hypothalamus, liver and plasma depending on the thyroid status of adult male rats. J. Physiol. Pharmacol. 2018, 69, 197–204. [Google Scholar] [CrossRef]
- Weber, K.T. Fibrosis and hypertensive heart disease. Curr. Opin. Cardiol. 2000, 15, 264–272. [Google Scholar] [CrossRef]
- Weber, K.T. Fibrosis in hypertensive heart disease: Focus on cardiac fibroblasts. J. Hypertens. 2004, 22, 47–50. [Google Scholar] [CrossRef]
- Aroor, A.R.; Habibi, J.; Kandikattu, H.K.; Garro-Kacher, M.; Barron, B.; Chen, D.; Hayden, M.R.; Whaley-Connell, A.; Bender, S.B.; Klein, T.; et al. Dipeptidyl peptidase-4 (DPP-4) inhibition with linagliptin reduces western diet-induced myocardial TRAF3IP2 expression, inflammation and fibrosis in female mice. Cardiovasc. Diabetol. 2017, 16, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Zhang, J.; Lu, L.; Chen, S.S.; Quinn, M.T.; Weber, K.T. Aldosterone-induced inflammation in the rat heart: Role of oxidative stress. Am. J. Pathol. 2002, 161, 1773–1781. [Google Scholar] [CrossRef]
- Li, C.; Han, R.; Kang, L.; Wang, J.; Gao, Y.; Li, Y.; He, J.; Tian, J. Pirfenidone controls the feedback loop of the AT1R/p38 MAPK/renin-angiotensin system axis by regulating liver X receptor-α in myocardial infarction-induced cardiac fibrosis. Sci. Rep. 2017, 7, 40523. [Google Scholar] [CrossRef] [Green Version]
- Laurent, G.J. Dynamic state of collagen: Pathways of collagen degradation in vivo and their possible role in regulation of collagen mass. Am. J. Physiol. 1987, 252, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kou, J.; Zhang, H.; Wang, C.; Li, H.; Ren, Y.; Zhang, Y. The renin-angiotensin system in the synovium promotes periarticular osteopenia in a rat model of collagen-induced arthritis. Int. Immunopharmacol. 2018, 65, 550–558. [Google Scholar] [CrossRef]
- Lijnen, P.J.; Petrov, V.V.; Fagard, R.H. Collagen production in cardiac fibroblasts during inhibition of angiotensin-converting enzyme and aminopeptidases. J. Hypertens. 2004, 22, 209–216. [Google Scholar] [CrossRef]
- Mizutani, T.; Mizutani, H.; Kaneda, T.; Hagihara, M.; Nagatsu, T. Activity of dipeptidyl peptidase II and dipeptidyl peptidase IV in human gingiva with chronic marginal periodontitis. Arch. Oral Biol. 1990, 35, 891–894. [Google Scholar] [CrossRef]
- Kamori, M.; Hagihara, M.; Nagatsu, T.; Iwata, H.; Miura, T. Activities of dipeptidyl peptidase II, dipeptidyl peptidase IV, prolyl endopeptidase, and collagenase-like peptidase in synovial membrane from patients with rheumatoid arthritis and osteoarthritis. Biochem. Med. Metab. Biol. 1991, 45, 154–160. [Google Scholar] [CrossRef]
- Hatanaka, T.; Kawakami, K.; Uraji, M. Inhibitory effect of collagen-derived tripeptides on dipeptidylpeptidase-IV activity. J. Enzyme Inhib. Med. Chem. 2014, 29, 823–828. [Google Scholar] [CrossRef] [Green Version]
- Kenny, A.J.; Booth, A.G.; George, S.G.; Ingram, J.; Kershaw, D.; Wood, E.J.; Young, A.R. Dipeptidyl peptidase IV, a kidney brush-border serine peptidase. Biochem. J. 1976, 157, 169–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nistala, R.; Savin, V. Diabetes, hypertension, and chronic kidney disease progression: Role of DPP4. Am. J. Physiol. Renal Physiol. 2017, 312, 661–670. [Google Scholar] [CrossRef]
- Zhong, J.; Rao, X.; Rajagopalan, S. An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: Potential implications in cardiovascular disease. Atherosclerosis 2013, 226, 305–314. [Google Scholar] [CrossRef]
- Wolke, C.; Teumer, A.; Endlich, K.; Endlich, N.; Rettig, R.; Stracke, S.; Fiene, B.; Aymanns, S.; Felix, S.B.; Hannemann, A.; et al. Serum protease activity in chronic kidney disease patients: The GANI_MED renal cohort. Exp. Biol. Med. 2017, 242, 554–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, T.; Watanabe, A.; Tanaka, M.; Shiota, M.; Osada-Oka, M.; Sano, S.; Yoshiyama, M.; Miura, K.; Kitajima, S.; Matsunaga, S.; et al. A dipeptidyl peptidase-4 (DPP-4) inhibitor, linagliptin, attenuates cardiac dysfunction after myocardial infarction independently of DPP-4. J. Pharmacol. Sci. 2019, 139, 112–119. [Google Scholar] [CrossRef]
- Shi, Z.X.; Xu, W.; Mergner, W.J.; Li, Q.L.; Cole, K.H.; Wilber, J.F. Localization of thyrotropin-releasing hormone mRNA expression in the rat heart by in situ hybridization histochemistry. Pathobiology 1996, 64, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Arechaga, G.; Prieto, I.; Segarra, A.B.; Alba, F.; Ruiz-Larrea, M.B.; Ruiz-Sanz, J.I.; de Gasparo, M.; Ramirez, M. Dietary fatty acid composition affects aminopeptidase activities in the testes of mice. Int. J. Androl. 2002, 25, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, S.; Chen, K.; Hua, W.; Zhang, S. Predictive value of gamma-glutamyltransferase for ventricular arrhythmias and cardiovascular mortality in implantable cardioverter-defibrillator patients. BMC Cardiovasc. Disord. 2019, 19, 129. [Google Scholar] [CrossRef]
- Neuman, M.G.; Malnick, S.; Chertin, L. Gamma glutamyl transferase—An underestimated marker for cardiovascular disease and the metabolic syndrome. J. Pharm. Pharm. Sci. 2020, 23, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Ruttmann, E.; Brant, L.J.; Concin, H.; Diem, G.; Rapp, K.; Ulmer, H.; Vorarlberg Health Monitoring and Promotion Program Study Group. Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: An epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation 2005, 112, 2130–2137. [Google Scholar] [CrossRef] [Green Version]
- Koenig, G.; Seneff, S. Gamma-Glutamyltransferase: A Predictive Biomarker of Cellular Antioxidant Inadequacy and Disease Risk. Dis. Markers 2015, 2015, 818570. [Google Scholar] [CrossRef] [Green Version]
- Kreutzer, F.; Krause, D.; Klaassen-Mielke, R.; Trampisch, H.J.; Diehm, C.; Rudolf, H. Gamma-glutamyl transferase as a risk factor for mortality and cardiovascular events in older adults—Results from a prospective cohort study in a primary care setting (getABI). Vasa 2019, 48, 313–319. [Google Scholar] [CrossRef]
- Lee, D.S.; Evans, J.C.; Robins, S.J.; Wilson, P.W.; Albano, I.; Fox, C.S.; Wang, T.J.; Benjamin, E.J.; D’Agostino, R.B.; Vasan, R.S. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: The Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Ndrepepa, G.; Colleran, R.; Kastrati, A. Gamma-glutamyl transferase and the risk of atherosclerosis and coronary heart disease. Clin. Chim. Acta 2018, 476, 130–138. [Google Scholar] [CrossRef]
- Greenberg, L.J. Fluorometric measurement of alkaline phosphatase and aminopeptidase activities in the order of 10-14 mole. Biochem. Biophys. Res. Commun. 1962, 9, 430–435. [Google Scholar] [CrossRef]
- Cheung, H.S.; Cushman, D.W. A soluble aspartate aminopeptidase from dog kidney. Biochim. Biophys. Acta 1971, 242, 190–193. [Google Scholar] [CrossRef]
- Tobe, H.; Kojima, F.; Aoyagi, T.; Umezawa, H. Purification by affinity chromatography using amastatin and properties of aminopeptidase A from pig kidney. Biochim. Biophys. Acta 1980, 613, 459–468. [Google Scholar] [CrossRef]
- Ramírez, M.; Prieto, I.; Banegas, I.; Segarra, A.B.; Alba, F. Neuropeptidases. Methods Mol. Biol. 2011, 789, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sá, J.M.; Barbosa, R.M.; Menani, J.V.; De Luca, L.A., Jr.; Colombari, E.; Almeida-Colombari, D.S. Cardiovascular and hidroelectrolytic changes in rats fed with high-fat diet. Behav. Brain Res. 2019, 373, 112075. [Google Scholar] [CrossRef]
- Clifton, P.M.; Keogh, J.B. A systematic review of the effect of dietary saturated and polyunsaturated fat on heart disease. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1060–1080. [Google Scholar] [CrossRef] [PubMed]
- Arechaga, G.; Martínez, J.M.; Prieto, I.; Ramírez, M.J.; Sánchez, M.J.; Alba, F.; De Gasparo, M.; Ramírez, M. Serum aminopeptidase A activity of mice is related to dietary fat saturation. J. Nutr. 2001, 131, 1177–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summerhill, V.; Karagodin, V.; Grechko, A.; Myasoedova, V.; Orekhov, A. Vasculoprotective Role of Olive Oil Compounds via Modulation of Oxidative Stress in Atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Agostino, R.; Barberio, L.; Gatto, M.; Muzzalupo, I.; Mandalà, M. Extra Virgin Olive Oil Phenols Dilate the Rat Mesenteric Artery by Activation of BKCa2+ Channels in Smooth Muscle Cells. Molecules 2020, 25, 2601. [Google Scholar] [CrossRef]
- Paknahad, Z.; Moosavian, S.P.; Mahdavi, R.; Rajabi, P. The effects of olive oil and cholesterol enriched diets on aortic fatty streak development and lipid peroxidation in rabbits. Nutr. Health 2021, 18, 2601060211022260. [Google Scholar] [CrossRef]
- Khan, S.A.; Sattar, M.Z.; Abdullah, N.A.; Rathore, H.A.; Abdulla, M.H.; Ahmad, A.; Johns, E.J. Obesity depresses baroreflex control of renal sympathetic nerve activity and heart rate in Sprague Dawley rats: Role of the renal innervation. Acta Physiol. 2015, 214, 390–401. [Google Scholar] [CrossRef]
- Segarra, A.B.; Prieto, I.; Martinez-Canamero, M.; Ruiz-Sanz, J.I.; Ruiz-Larrea, M.B.; De Gasparo, M.; Banegas, I.; Zorad, S.; Ramirez-Sanchez, M. Enkephalinase activity is modified and correlates with fatty acids in frontal cortex depending on fish, olive or coconut oil used in the diet. Endocr. Regul. 2019, 53, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Brilla, C.G.; Maisch, B.; Weber, K.T. Renin-angiotensin system and myocardial collagen matrix remodeling in hypertensive heart disease: In vivo and in vitro studies on collagen matrix regulation. Clin. Investig. 1993, 71, 35–41. [Google Scholar] [CrossRef]
- Liu, T.; Wen, H.; Li, H.; Xu, H.; Xiao, N.; Liu, R.; Chen, L.; Sun, Y.; Song, L.; Bai, C.; et al. Oleic Acid Attenuates Ang II (Angiotensin II)-Induced Cardiac Remodeling by Inhibiting FGF23 (Fibroblast Growth Factor 23) Expression in Mice. Hypertension 2020, 75, 680–692. [Google Scholar] [CrossRef]
- Mascolo, A.; Sessa, M.; Scavone, C.; De Angelis, A.; Vitale, C.; Berrino, L.; Rossi, F.; Rosano, G.; Capuano, A. New and old roles of the peripheral and brain renin-angiotensin-aldosterone system (RAAS): Focus on cardiovascular and neurological diseases. Int. J. Cardiol. 2017, 227, 734–742. [Google Scholar] [CrossRef]
- Marc, Y.; Boitard, S.E.; Balavoine, F.; Azizi, M.; Llorens-Cortes, C. Targeting Brain Aminopeptidase A: A New Strategy for the Treatment of Hypertension and Heart Failure. Can. J. Cardiol. 2020, 36, 721–731. [Google Scholar] [CrossRef]
- Weber, K.T.; Sun, Y.; Tyagi, S.C.; Cleutjens, J.P. Collagen network of the myocardium: Function, structural remodeling and regulatory mechanisms. J. Mol. Cell. Cardiol. 1994, 26, 279–292. [Google Scholar] [CrossRef]
- Harikrishnan, V.; Titus, A.S.; Cowling, R.T. Collagen receptor cross-talk determines α-smooth muscle actin-dependent collagen gene expression in angiotensin II-stimulated cardiac fibroblasts. J. Biol. Chem. 2019, 294, 19723–19739. [Google Scholar] [CrossRef]
- Manrique, C.; Habibi, J.; Aroor, A.R.; Sowers, J.R.; Jia, G.; Hayden, M.R.; Garro, M.; Martinez-Lemus, L.A.; Ramirez-Perez, F.I.; Klein, T.; et al. Dipeptidyl peptidase-4 inhibition with linagliptin prevents western diet-induced vascular abnormalities in female mice. Cardiovasc. Diabetol. 2016, 15, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Cuevas, J.; Sandoval-Rodriguez, A.; Meza-Rios, A.; Monroy-Ramírez, H.C.; Galicia-Moreno, M.; García-Bañuelos, J.; Santos, A.; Armendariz-Borunda, J. Molecular Mechanisms of Obesity-Linked Cardiac Dysfunction: An Up-Date on Current Knowledge. Cells 2021, 10, 629. [Google Scholar] [CrossRef]
- Santana, A.B.; de Souza Oliveira, T.C.; Bianconi, B.L.; Barauna, V.G.; Santos, E.W.; Alves, T.P.; Silva, J.C.; Fiorino, P.; Borelli, P.; Irigoyen, M.C.; et al. Effect of high-fat diet upon inflammatory markers and aortic stiffening in mice. BioMed Res. Int. 2014, 2014, 914102. [Google Scholar] [CrossRef] [Green Version]
- Arendse, L.B.; Danser, A.; Poglitsch, M.; Touyz, R.M.; Burnett, J.C., Jr.; Llorens-Cortes, C.; Ehlers, M.R.; Sturrock, E.D. Novel Therapeutic Approaches Targeting the Renin-Angiotensin System and Associated Peptides in Hypertension and Heart Failure. Pharmacol. Rev. 2019, 71, 539–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.C.; Abdel-Ghany, M.; Elble, R.C.; Pauli, B.U. Lung endothelial dipeptidyl peptidase IV promotes adhesion and metastasis of rat breast cancer cells via tumor cell surface-associated fibronectin. J. Biol. Chem. 1998, 273, 24207–24215. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.M.; Holz, L.E.; Chowdhury, S.; Cordoba, S.P.; Evans, K.A.; Gall, M.G.; Vieira de Ribeiro, A.J.; Zheng, Y.Z.; Levy, M.T.; Yu, D.M.; et al. The pro-fibrotic role of dipeptidyl peptidase 4 in carbon tetrachloride-induced experimental liver injury. Immunol. Cell. Biol. 2017, 95, 443–453. [Google Scholar] [CrossRef]
- Oparil, S.; Schmieder, R.E. New approaches in the treatment of hypertension. Circ. Res. 2015, 116, 1074–1095. [Google Scholar] [CrossRef] [Green Version]
- Stewart, M.H.; Lavie, C.J.; Ventura, H.O. Future pharmacological therapy in hypertension. Curr. Opin. Cardiol. 2018, 33, 408–415. [Google Scholar] [CrossRef] [PubMed]

| Sample | DPP-IV (Fraction) | Angiotensinase (Fraction) | p-Value | R | Intra-Group Correlations | p-Value | R |
|---|---|---|---|---|---|---|---|
| Atrium | DPP-IV (sol) vs. | AlaAP (sol) | 0.004 | 0.692 | VOO–VOO | 0.007 | 0.969 |
| ArgAP (sol) | 0.002 | 0.734 | |||||
| CysAP (sol) | <0.001 | 0.777 | S–S VOO–VOO | 0.008 0.023 | 0.927 0.928 | ||
| Atrium | DPP-IV (mb) vs. | AlaAP (mb) | 0.012 | 0.609 | S–S Bch-Bch | 0.014 0.043 | 0.902 0.890 |
| ArgAP (mb) | 0.012 | 0.609 | S–S | 0.011 | 0.912 | ||
| CysAP (mb) | 0.040 | 0.517 | |||||
| GluAP (mb) | 0.037 | 0.526 | S-S | 0.006 | 0.936 | ||
| Ventricle | DPP-IV (sol) vs. | AlaAP (sol) | <0.001 | 0.942 | VOO–VOO Bch–Bch | 0.002 0.018 | 0.984 0.940 |
| ArgAP (sol) | <0.001 | 0.923 | VOO–VOO | 0.004 | 0.979 | ||
| AspAP (sol) | <0.001 | 0.813 | S–S VOO–VOO | 0.040 0.046 | 0.833 0.954 | ||
| CysAP (sol) | <0.001 | 0.756 | VOO–VOO | 0.023 | 0.929 | ||
| GluAP (sol) | <0.001 | 0.904 | VOO–VOO | 0.002 | 0.986 | ||
| Aorta | DPP-IV (sol) vs. | ArgAP (sol) | 0.048 | 0.542 | |||
| AlaAP (sol) | 0.044 | 0.589 | |||||
| Aorta | DPP-IV (mb) vs. | CysAP (mb) | 0.019 | 0.638 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Vías, G.; Segarra, A.B.; Ramírez-Sánchez, M.; Prieto, I. High-Fat Diets Modify the Proteolytic Activities of Dipeptidyl-Peptidase IV and the Regulatory Enzymes of the Renin–Angiotensin System in Cardiovascular Tissues of Adult Wistar Rats. Biomedicines 2021, 9, 1149. https://doi.org/10.3390/biomedicines9091149
Domínguez-Vías G, Segarra AB, Ramírez-Sánchez M, Prieto I. High-Fat Diets Modify the Proteolytic Activities of Dipeptidyl-Peptidase IV and the Regulatory Enzymes of the Renin–Angiotensin System in Cardiovascular Tissues of Adult Wistar Rats. Biomedicines. 2021; 9(9):1149. https://doi.org/10.3390/biomedicines9091149
Chicago/Turabian StyleDomínguez-Vías, Germán, Ana Belén Segarra, Manuel Ramírez-Sánchez, and Isabel Prieto. 2021. "High-Fat Diets Modify the Proteolytic Activities of Dipeptidyl-Peptidase IV and the Regulatory Enzymes of the Renin–Angiotensin System in Cardiovascular Tissues of Adult Wistar Rats" Biomedicines 9, no. 9: 1149. https://doi.org/10.3390/biomedicines9091149
APA StyleDomínguez-Vías, G., Segarra, A. B., Ramírez-Sánchez, M., & Prieto, I. (2021). High-Fat Diets Modify the Proteolytic Activities of Dipeptidyl-Peptidase IV and the Regulatory Enzymes of the Renin–Angiotensin System in Cardiovascular Tissues of Adult Wistar Rats. Biomedicines, 9(9), 1149. https://doi.org/10.3390/biomedicines9091149






