Derivation and Characterization of EGFP-Labeled Rabbit Limbal Mesenchymal Stem Cells and Their Potential for Research in Regenerative Ophthalmology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Obtaining of Rabbit L-MSCs with Overexpression of Green Fluorescence Protein (rbL-MSC-EGFP)
2.3. Cell Proliferation Assay
2.4. Cell Surface Marker Analysis
2.5. Immunofluorescence
2.6. In Vitro Multilineage Differentiation
2.7. Epithelial Transdifferentiation
2.8. Quantitative RT-PCR Analysis
2.9. Lifetime Evaluation of Scaffolds Biocompatibility
2.10. Statistical Analysis
3. Results
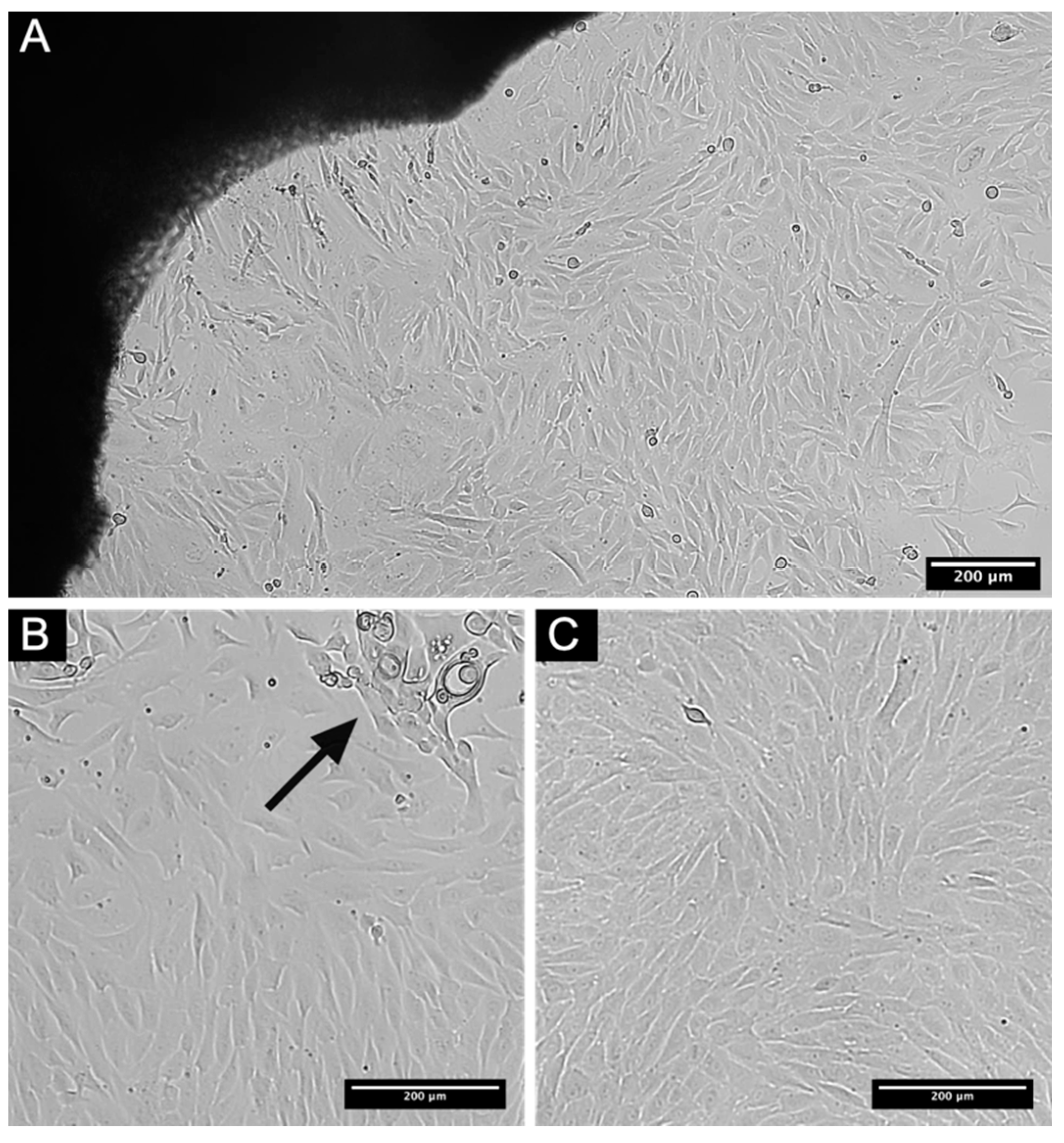
3.1. Establishment of L-MSCs Culture of Rabbit
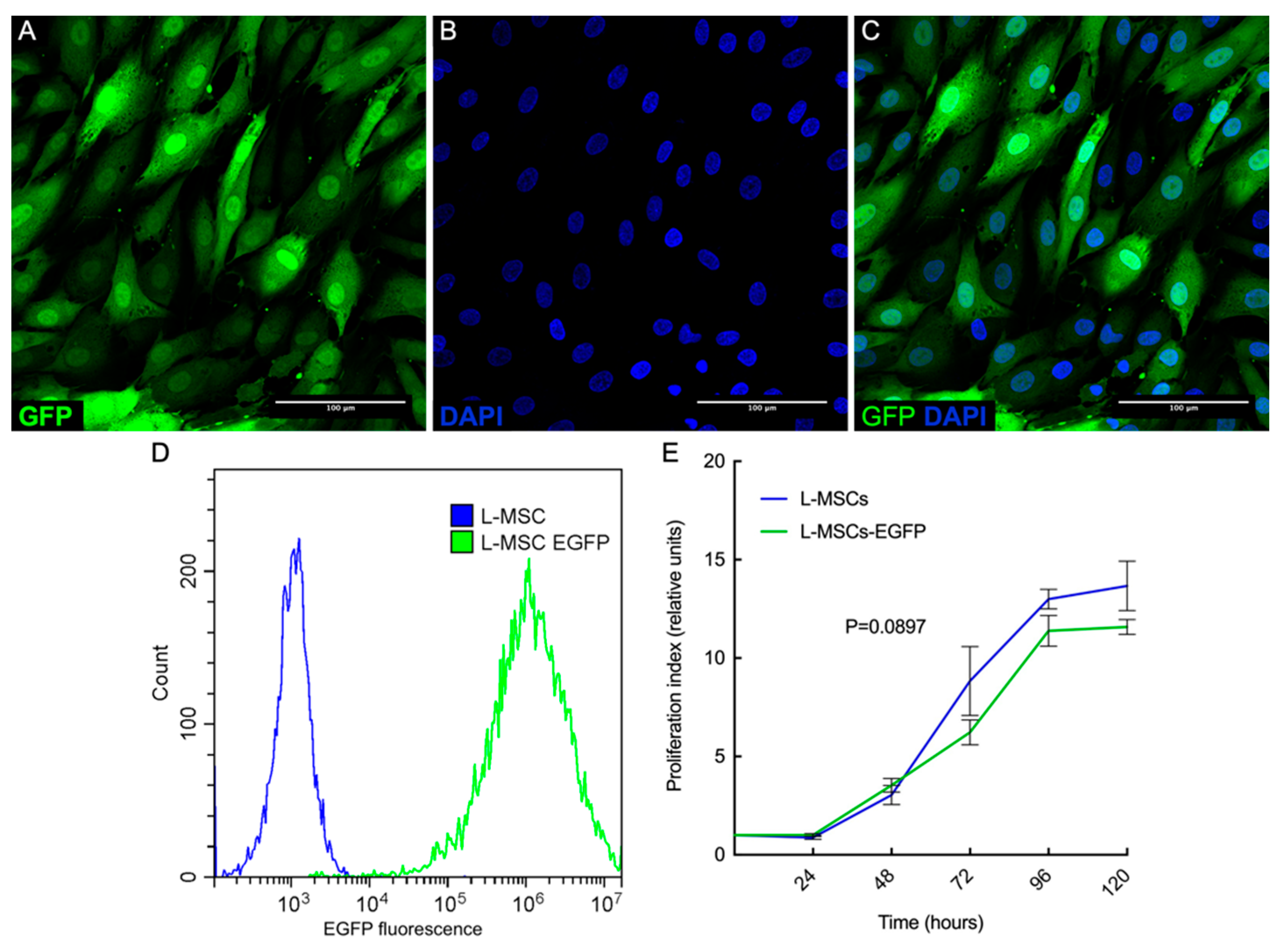
3.2. Obtaining of Rabbit L-MSCs-EGFP Culture
3.3. Characterization and Comparison of Rabbit L-MSCs and L-MSCs-EGFP
3.3.1. Comparison of Cell Proliferation Rates
3.3.2. Cell Surface Marker Analysis
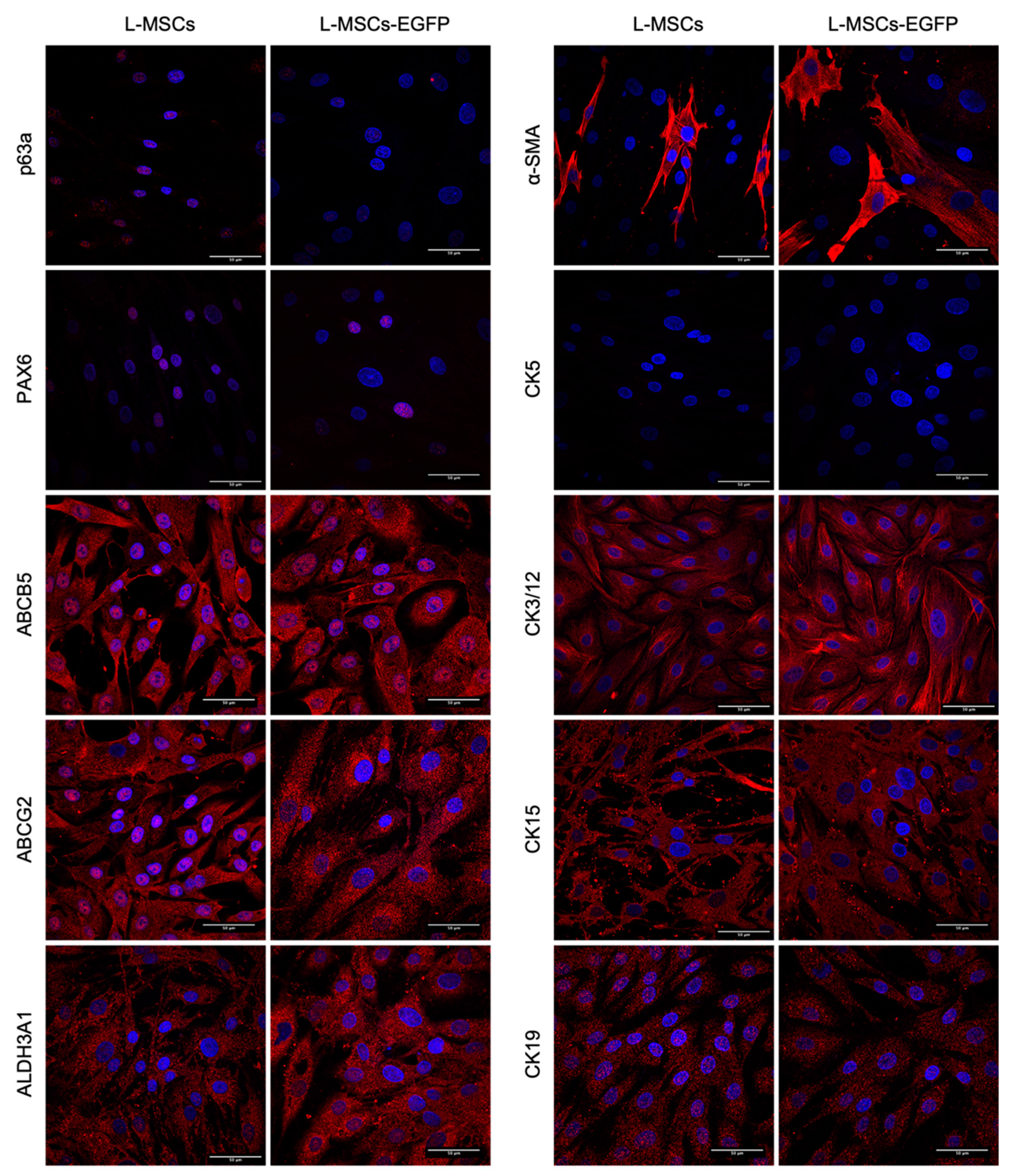
3.3.3. L-MSCs and L-MSCs-EGFP Characterization and Comparison by Immunocytochemistry
3.4. Ability of L-MSCs-EGFP to Multilineage Differentiation
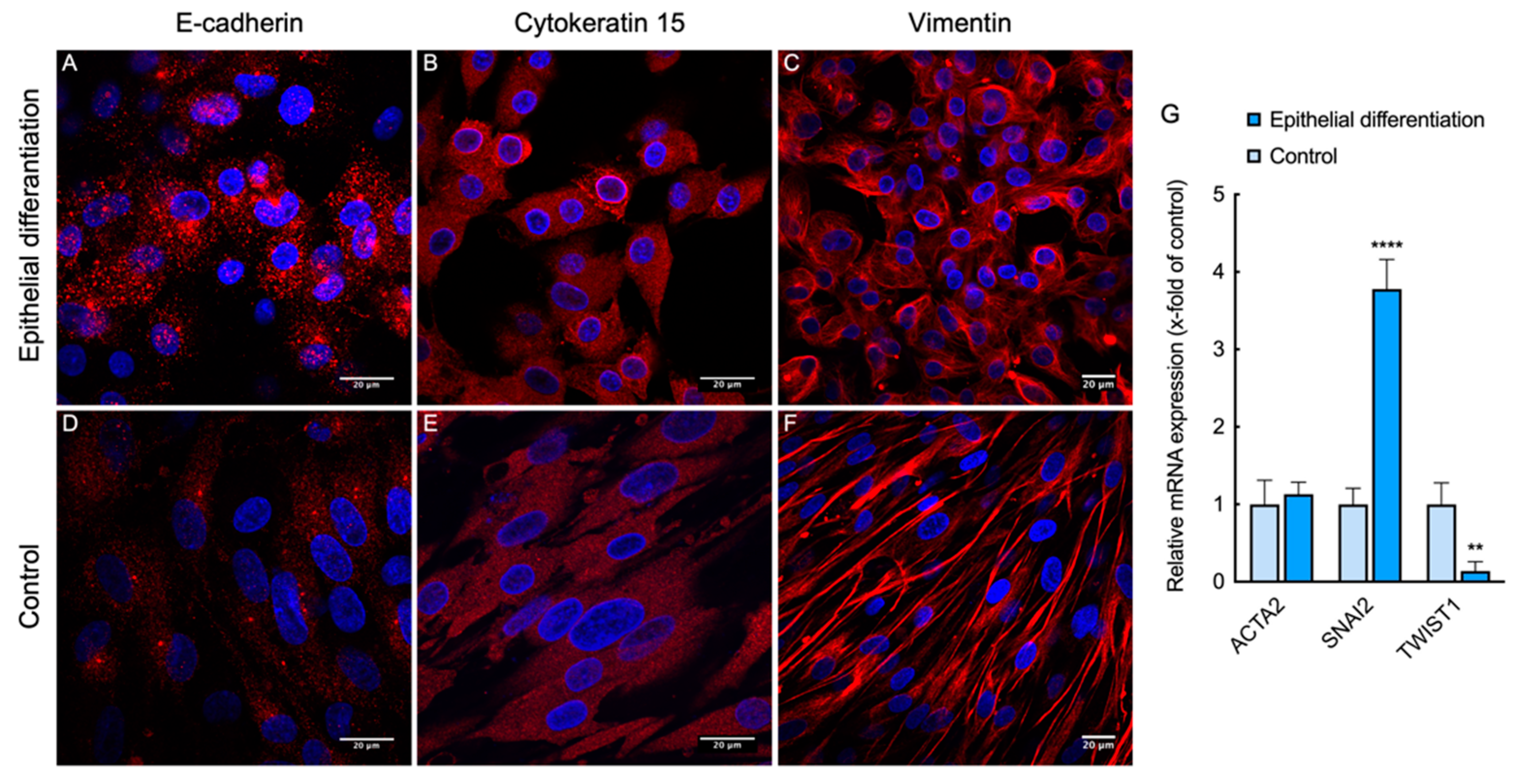
3.5. Direct Epithelial Transdifferentiation of L-MSCs-EGFP
3.6. Lifetime Evaluation of Biomedical Scaffolds Biocompatibility with L-MSCs-EGFP
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Barut Selver, Ö.; Yağcı, A.; Eğrilmez, S.; Gürdal, M.; Palamar, M.; Çavuşoğlu, T.; Ateş, U.; Veral, A.; Güven, Ç.; Wolosin, J.M. Limbal Stem Cell Deficiency and Treatment with Stem Cell Transplantation. Turk. J. Ophthalmol. 2017, 47, 285–291. [Google Scholar] [CrossRef]
- Daniels, J.T.; Harris, A.R.; Mason, C. Corneal epithelial stem cells in health and disease. Stem Cell Rev. 2006, 2, 247–254. [Google Scholar] [CrossRef]
- Di Girolamo, N. Stem cells of the human cornea. Br. Med. Bull. 2011, 100, 191–207. [Google Scholar] [CrossRef]
- Boulton, M.; Albon, J.; Grant, M.B. Stem Cells in the Eye, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780123983589. [Google Scholar]
- Deng, S.X.; Borderie, V.; Chan, C.C.; Dana, R.; Figueiredo, F.C.; Gomes, J.A.P.; Pellegrini, G.; Shimmura, S.; Kruse, F.E.; The International Limbal Stem Cell Deficiency Working Group. Global Consensus on Definition, Classification, Diagnosis, and Staging of Limbal Stem Cell Deficiency. Cornea 2019, 38, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; De Luca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Nakamura, T.; Sotozono, C.; Bentley, A.J.; Mano, S.; Inatomi, T.; Koizumi, N.; Fullwood, N.J.; Kinoshita, S. Long-term phenotypic study after allogeneic cultivated corneal limbal epithelial transplantation for severe ocular surface diseases. Ophthalmology 2010, 117, 2247–2254.e1. [Google Scholar] [CrossRef] [PubMed]
- Calonge, M.; Pérez, I.; Galindo, S.; Nieto-Miguel, T.; López-Paniagua, M.; Fernández, I.; Alberca, M.; García-Sancho, J.; Sánchez, A.; Herreras, J.M. A proof-of-concept clinical trial using mesenchymal stem cells for the treatment of corneal epithelial stem cell deficiency. Transl. Res. 2019, 206, 18–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Funderburgh, M.L.; Mann, M.M.; SundarRaj, N.; Funderburgh, J.L. Multipotent stem cells in human corneal stroma. Stem Cells 2005, 23, 1266–1275. [Google Scholar] [CrossRef] [Green Version]
- Funderburgh, M.L.; Du, Y.; Mann, M.M.; SundarRaj, N.; Funderburgh, J.L. PAX6 expression identifies progenitor cells for corneal keratocytes. FASEB J. 2005, 19, 1371–1373. [Google Scholar] [CrossRef] [Green Version]
- Pinnamaneni, N.; Funderburgh, J.L. Concise review: Stem cells in the corneal stroma. Stem Cells 2012, 30, 1059–1063. [Google Scholar] [CrossRef] [Green Version]
- Polisetty, N.; Fatima, A.; Madhira, S.L.; Sangwan, V.S.; Vemuganti, G.K. Mesenchymal cells from limbal stroma of human eye. Mol. Vis. 2008, 14, 431. [Google Scholar]
- Shukla, S.; Shanbhag, S.S.; Tavakkoli, F.; Varma, S.; Singh, V.; Basu, S. Limbal Epithelial and Mesenchymal Stem Cell Therapy for Corneal Regeneration. Curr. Eye Res. 2020, 45, 265–277. [Google Scholar] [CrossRef] [Green Version]
- Agorogiannis, G.I.; Alexaki, V.-I.; Castana, O.; Kymionis, G.D. Topical application of autologous adipose-derived mesenchymal stem cells (MSCs) for persistent sterile corneal epithelial defect. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Reinshagen, H.; Auw-Haedrich, C.; Sorg, R.V.; Boehringer, D.; Eberwein, P.; Schwartzkopff, J.; Sundmacher, R.; Reinhard, T. Corneal surface reconstruction using adult mesenchymal stem cells in experimental limbal stem cell deficiency in rabbits. Acta Ophthalmol. 2011, 89, 741–748. [Google Scholar] [CrossRef]
- Holan, V.; Trosan, P.; Cejka, C.; Javorkova, E.; Zajicova, A.; Hermankova, B.; Chudickova, M.; Cejkova, J. A Comparative Study of the Therapeutic Potential of Mesenchymal Stem Cells and Limbal Epithelial Stem Cells for Ocular Surface Reconstruction. Stem Cells Transl. Med. 2015, 4, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Hashmani, K.; Branch, M.J.; Sidney, L.E.; Dhillon, P.S.; Verma, M.; McIntosh, O.D.; Hopkinson, A.; Dua, H.S. Characterization of corneal stromal stem cells with the potential for epithelial transdifferentiation. Stem Cell Res. Ther. 2013, 4, 75. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.-S.; Cai, L.; Ji, W.-Y.; Hui, Y.-N.; Wang, Y.-S.; Hu, D.; Zhu, J. Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol. Vis. 2010, 16, 1304–1316. [Google Scholar]
- Nieto-Miguel, T.; Galindo, S.; Reinoso, R.; Corell, A.; Martino, M.; Pérez-Simón, J.A.; Calonge, M. In vitro simulation of corneal epithelium microenvironment induces a corneal epithelial-like cell phenotype from human adipose tissue mesenchymal stem cells. Curr. Eye Res. 2013, 38, 933–944. [Google Scholar] [CrossRef]
- Katikireddy, K.R.; Dana, R.; Jurkunas, U.V. Differentiation Potential of Limbal Fibroblasts and Bone Marrow Mesenchymal Stem Cells to Corneal Epithelial Cells. Stem Cells 2014, 32, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Nicolau, N.; Martín-Antonio, B.; Müller-Sánchez, C.; Casaroli-Marano, R.P. In vitro potential of human mesenchymal stem cells for corneal epithelial regeneration. Regen. Med. 2020, 15, 1409–1426. [Google Scholar] [CrossRef] [PubMed]
- Bray, L.J.; Heazlewood, C.F.; Munster, D.J.; Hutmacher, D.W.; Atkinson, K.; Harkin, D.G. Immunosuppressive properties of mesenchymal stromal cell cultures derived from the limbus of human and rabbit corneas. Cytotherapy 2014, 16, 64–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kachkin, D.V.; Khorolskaya, J.I.; Ivanova, J.S.; Rubel, A.A. An Efficient Method for Isolation of Plasmid DNA for Transfection of Mammalian Cell Cultures. Methods Protoc. 2020, 3, 69. [Google Scholar] [CrossRef] [PubMed]
- Campeau, E.; Ruhl, V.E.; Rodier, F.; Smith, C.L.; Rahmberg, B.L.; Fuss, J.O.; Campisi, J.; Yaswen, P.; Cooper, P.K.; Kaufman, P.D. A versatile viral system for expression and depletion of proteins in mammalian cells. PLoS ONE 2009, 4, e6529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debyser, Z. Biosafety of Lentiviral Vectors. Curr. Gene Ther. 2003, 3, 517–525. [Google Scholar] [CrossRef]
- Roth, V. Doubling Time Computing. Available online: https://www.doubling-time.com/compute.php (accessed on 16 July 2021).
- Alexandrova, O.I.; Gavriluk, I.O.; Mashel, T.V.; Chernysh, V.F.; Churashov, S.V.; Kulikov, A.N.; Blinova, M.I. The preparation of the amniotic membrane as a scaffold cell cultures for the bioengineered corneal structures. Saratov J. Med. Sci. Res. 2019, 15, 409–413. (In Russian) [Google Scholar]
- Nashchekina, Y.; Samusenko, I.; Zorin, I.; Kukhareva, L.; Bilibin, A.; Blinova, M. Poly(D,L-lactide)/PEG blend films for keratinocyte cultivation and skin reconstruction. Biomed. Mater. 2019, 14, 65005. [Google Scholar] [CrossRef]
- Khorolskaya, J.I.; Aleksandrova, O.I.; Samusenko, I.A.; Mikhailova, N.A.; Lobov, I.B.; Yudintceva, N.M.; Blinova, M.I. The Effect of Soluble Recombinant Protein Dll4-Fc on the Functional Activity of Endothelial Cells In Vitro and Vascularization In Vivo. Cell Tissue Biol. 2019, 13, 276–282. [Google Scholar] [CrossRef]
- Chandrakasan, G.; Torchia, D.A.; Piez, K.A. Preparation of intact monomeric collagen from rat tail tendon and skin and the structure of the nonhelical ends in solution. J. Biol. Chem. 1976, 251, 6062–6067. [Google Scholar] [CrossRef]
- Mimura, T.; Amano, S.; Yokoo, S.; Uchida, S.; Usui, T.; Yamagami, S. Isolation and distribution of rabbit keratocyte precursors. Mol. Vis. 2008, 14, 197–203. [Google Scholar]
- Gu, S.; Xing, C.; Han, J.; Tso, M.O.M.; Hong, J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol. Vis. 2009, 15, 99–107. [Google Scholar]
- Guo, P.; Sun, H.; Zhang, Y.; Tighe, S.; Chen, S.; Su, C.W.; Liu, Y.; Zhao, H.; Hu, M.; Zhu, Y. Limbal niche cells are a potent resource of adult mesenchymal progenitors. J. Cell. Mol. Med. 2018, 22, 3315–3322. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Hertsenberg, A.J.; Funderburgh, M.L.; Burrow, M.K.; Mann, M.M.; Du, Y.; Lathrop, K.L.; Syed-Picard, F.N.; Adams, S.M.; Birk, D.E.; et al. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci. Transl. Med. 2014, 6, 266ra172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branch, M.J.; Hashmani, K.; Dhillon, P.; Jones, D.R.E.; Dua, H.S.; Hopkinson, A. Mesenchymal Stem Cells in the Human Corneal Limbal Stroma. Investig. Opthalmol. Vis. Sci. 2012, 53, 5109. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Li, G.-G.; Zhu, Y.-T.; Xie, H.-T.; Chen, S.-Y.; Tseng, S.C.G. Mesenchymal Stem Cells Derived from Human Limbal Niche Cells. Investig. Opthalmol. Vis. Sci. 2012, 53, 5686. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.-C.; Lee, T.-H.; Huang, Y.-H.; Chang, N.-K.; Lin, Y.-J.; Chien, P.-W.C.; Yang, W.-H.; Lin, M.H.-C. Comparison of Surface Markers between Human and Rabbit Mesenchymal Stem Cells. PLoS ONE 2014, 9, e111390. [Google Scholar] [CrossRef] [Green Version]
- Dravida, S.; Pal, R.; Khanna, A.; Tipnis, S.P.; Ravindran, G.; Khan, F. The transdifferentiation potential of limbal fibroblast-like cells. Dev. Brain Res. 2005, 160, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Ainscough, S.L.; Linn, M.L.; Barnard, Z.; Schwab, I.R.; Harkin, D.G. Effects of fibroblast origin and phenotype on the proliferative potential of limbal epithelial progenitor cells. Exp. Eye Res. 2011, 92, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.-T.; Chen, S.-Y.; Li, G.-G.; Tseng, S.C.G. Isolation and Expansion of Human Limbal Stromal Niche Cells. Investig. Opthalmol. Vis. Sci. 2012, 53, 279. [Google Scholar] [CrossRef]
- Shaharuddin, B.; Osei-Bempong, C.; Ahmad, S.; Rooney, P.; Ali, S.; Oldershaw, R.; Meeson, A. Human limbal mesenchymal stem cells express ABCB5 and can grow on amniotic membrane. Regen. Med. 2016, 11, 273–286. [Google Scholar] [CrossRef] [Green Version]
- Pappa, A.; Estey, T.; Manzer, R.; Brown, D.; Vasiliou, V. Human aldehyde dehydrogenase 3A1 (ALDH3A1): Biochemical characterization and immunohistochemical localization in the cornea. Biochem. J. 2003, 376, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-Q.; Wang, Z.; Yoon, K.-C.; Bian, F. Characterization, isolation, expansion and clinical therapy of human corneal epithelial stem/progenitor cells. J. Stem Cells 2014, 9, 79–91. [Google Scholar]
- Sareen, D.; Saghizadeh, M.; Ornelas, L.; Winkler, M.A.; Narwani, K.; Sahabian, A.; Funari, V.A.; Tang, J.; Spurka, L.; Punj, V.; et al. Differentiation of human limbal-derived induced pluripotent stem cells into limbal-like epithelium. Stem Cells Transl. Med. 2014, 3, 1002–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, A.W.; Weinberg, R.A. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat. Rev. Cancer 2021, 21, 325–338. [Google Scholar] [CrossRef]
- Wawruszak, A.; Kalafut, J.; Okon, E.; Czapinski, J.; Halasa, M.; Przybyszewska, A.; Miziak, P.; Okla, K.; Rivero-Muller, A.; Stepulak, A. Histone Deacetylase Inhibitors and Phenotypical Transformation of Cancer Cells. Cancers 2019, 11, 148. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.Q.; Ma, C.; Wang, Q.; Song, Y.; Lv, T. The role of TWIST1 in epithelial-mesenchymal transition and cancers. Tumor Biol. 2016, 37, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.K.; Jia, D.; Boareto, M.; Mani, S.A.; Pienta, K.J.; Ben-Jacob, E.; Levine, H. Coupling the modules of EMT and stemness: A tunable ‘stemness window’ model. Oncotarget 2015, 6, 25161–25174. [Google Scholar] [CrossRef] [Green Version]
- Fernandes-Cunha, G.M.; Na, K.S.; Putra, I.; Lee, H.J.; Hull, S.; Cheng, Y.C.; Blanco, I.J.; Eslani, M.; Djalilian, A.R.; Myung, D. Corneal Wound Healing Effects of Mesenchymal Stem Cell Secretome Delivered within a Viscoelastic Gel Carrier. Stem Cells Transl. Med. 2019, 8, 478–489. [Google Scholar] [CrossRef] [Green Version]
- Arnhold, S.; Absenger, Y.; Klein, H.; Addicks, K.; Schraermeyer, U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 414–422. [Google Scholar] [CrossRef] [PubMed]



| Antigen | Dilution | Manufacturer, Country | Reactivity |
|---|---|---|---|
| CD34 (GTX75414) | 1:100 | Genetex, Irvine, CA, USA | Human, Mouse, Rabbit |
| CD44 (12-0441-82) | 1:150 | eBioscience, San Diego, CA, USA | Human, Mouse |
| CD45 (IM1834U) | 1:10 | Beckman Coulter, Brea, CA, USA | Human |
| CD73 (550257) | 1:30 | BD Pharmingen, San Diego, CA, USA | Human |
| CD90 (555596) | 1:100 | BD Pharmingen, San Diego, CA, USA | Human |
| CD105 (560839) | 1:200 | BD Pharmingen, San Diego, CA, USA | Human |
| Iso PE (554680) | 1:400 | BD Pharmingen, San Diego, CA, USA | - |
| Antigen | ICC Dilution | Manufacturer, Country |
|---|---|---|
| CK3/12 (ab68260) | 1/200 | Abcam, Cambridge, UK |
| CK5 (VP-C400) | 1/50 | Vector, Burlingame, CA, USA |
| CK15 (MA5-15567) | 1/500 | Thermo Fisher Scientific, Waltham, MA, USA |
| CK19 (ab7754) | 1/200 | Abcam, Cambridge, UK |
| P63α (4892S) | 1/100 | Cell Signalling Technology, Danvers, MA, USA |
| PAX6 (60433) | 1/100 | Santa Cruz Biotechnology, Dallas, TX, USA |
| ALDH3A1 (ab76976) | 1/200 | Abcam, Cambridge, UK |
| ABCG2 (ab3380) | 1/200 | Abcam, Cambridge, UK |
| αSMA (ab7817) | 1/200 | Abcam, Cambridge, UK |
| E-cadherin (ab76066) | 1/200 | Abcam, Cambridge, UK |
| Vimentin (ab8978) | 1/200 | Abcam, Cambridge, UK |
| Anti-Mouse IgG H&L (ab150114) | 1/500 | Abcam, Cambridge, UK |
| Anti-Rabbit IgG H&L (ab150083) | 1/500 | Abcam, Cambridge, UK |
| Primer Name | Sequence 5′-3′ | T °C |
|---|---|---|
| ACTA2-For | GTTACTACTGCTGAGCGTGAG | 60 °C |
| ACTA2-Rev | CAGGCAACTCGTAACTCTTC | 60 °C |
| HPRT1-For | CTGGCGTCGTGATTAGTGATGA | 60 °C |
| HPRT1-Rev | ACGTTCAGTCCTGTCCATAATT | 60 °C |
| SNAI1-For | CTCTTTCCTCGTCAGGAAGC | 60 °C |
| SNAI1-Rev | GGCTGCTGGAAGGTAAACTC | 60 °C |
| TWIST1-For | AGCAGGGCCGGAGACCTAGAT | 60 °C |
| TWIST1-Rev | GCCCCACGCCCTGTTTCTTTGA | 60 °C |
| Antigen | BM-MSCs | L-MSCs | L-MSCs-EGFP |
|---|---|---|---|
| CD44 | 97.71% | 91.34% | 73.84% |
| CD73 | 2.11% | 2.41% | 3.73% |
| CD90 | 67.64% | 92.86% | 74.07% |
| CD105 | 88.97% | 52.01% | 36.59% |
| CD34 | 6.39% | 4.97% | 6.33% |
| CD45 | 2.27% | 3.30% | 3.07% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khorolskaya, J.I.; Perepletchikova, D.A.; Kachkin, D.V.; Zhurenkov, K.E.; Alexander-Sinkler, E.I.; Ivanova, J.S.; Mikhailova, N.A.; Blinova, M.I. Derivation and Characterization of EGFP-Labeled Rabbit Limbal Mesenchymal Stem Cells and Their Potential for Research in Regenerative Ophthalmology. Biomedicines 2021, 9, 1134. https://doi.org/10.3390/biomedicines9091134
Khorolskaya JI, Perepletchikova DA, Kachkin DV, Zhurenkov KE, Alexander-Sinkler EI, Ivanova JS, Mikhailova NA, Blinova MI. Derivation and Characterization of EGFP-Labeled Rabbit Limbal Mesenchymal Stem Cells and Their Potential for Research in Regenerative Ophthalmology. Biomedicines. 2021; 9(9):1134. https://doi.org/10.3390/biomedicines9091134
Chicago/Turabian StyleKhorolskaya, Julia I., Daria A. Perepletchikova, Daniel V. Kachkin, Kirill E. Zhurenkov, Elga I. Alexander-Sinkler, Julia S. Ivanova, Natalia A. Mikhailova, and Miralda I. Blinova. 2021. "Derivation and Characterization of EGFP-Labeled Rabbit Limbal Mesenchymal Stem Cells and Their Potential for Research in Regenerative Ophthalmology" Biomedicines 9, no. 9: 1134. https://doi.org/10.3390/biomedicines9091134
APA StyleKhorolskaya, J. I., Perepletchikova, D. A., Kachkin, D. V., Zhurenkov, K. E., Alexander-Sinkler, E. I., Ivanova, J. S., Mikhailova, N. A., & Blinova, M. I. (2021). Derivation and Characterization of EGFP-Labeled Rabbit Limbal Mesenchymal Stem Cells and Their Potential for Research in Regenerative Ophthalmology. Biomedicines, 9(9), 1134. https://doi.org/10.3390/biomedicines9091134








