Whey-Derived Porous Carbon Scaffolds for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Scaffolds
2.2. Characterization Techniques
2.3. Biocompatibility Test
2.3.1. Cell Culture
2.3.2. In Vitro Cytotoxicity Indirect Assays
2.4. Degradation and Bioactivity
3. Results
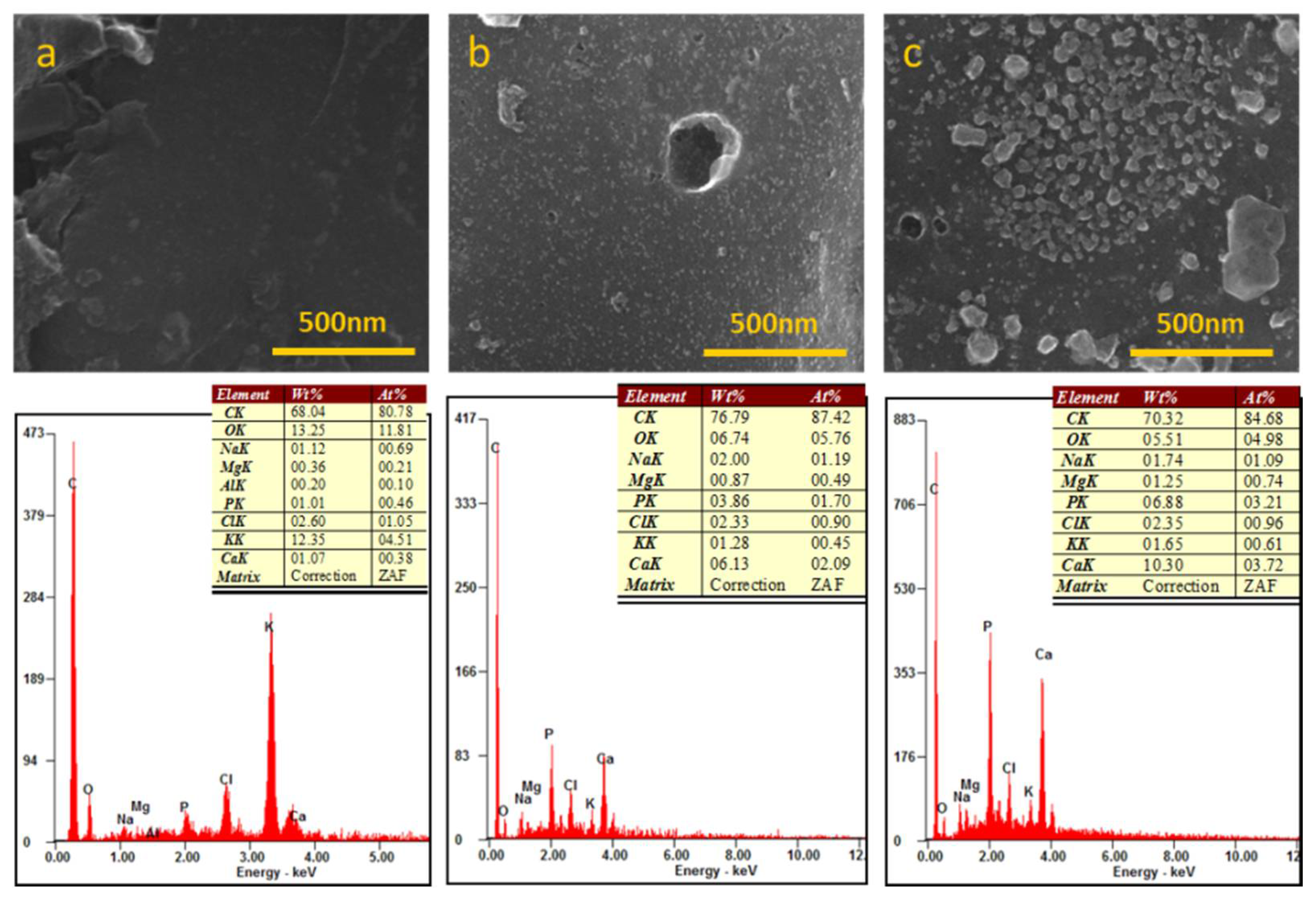
3.1. Morpho-Structural Characterization of the Whey-Derived Carbon Scaffolds
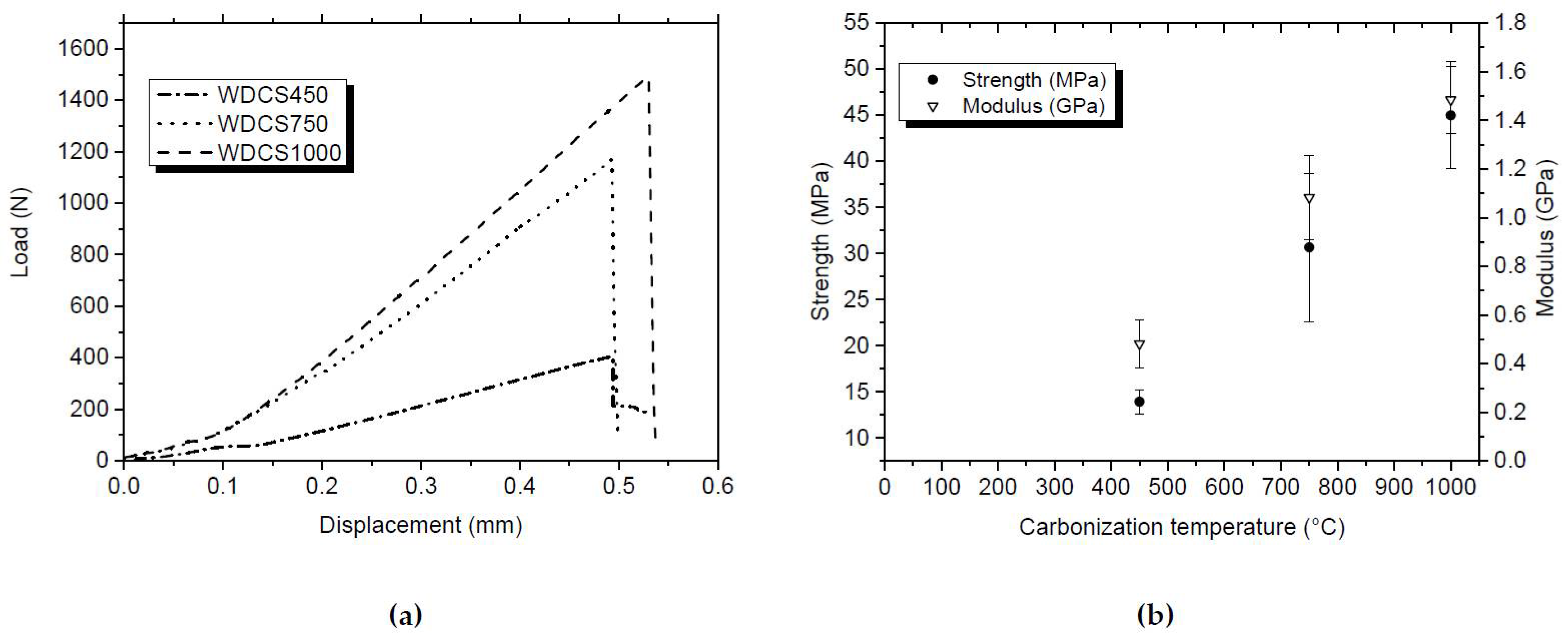
3.2. Mechanical Characterization of the Whey-Derived Carbon Scaffolds
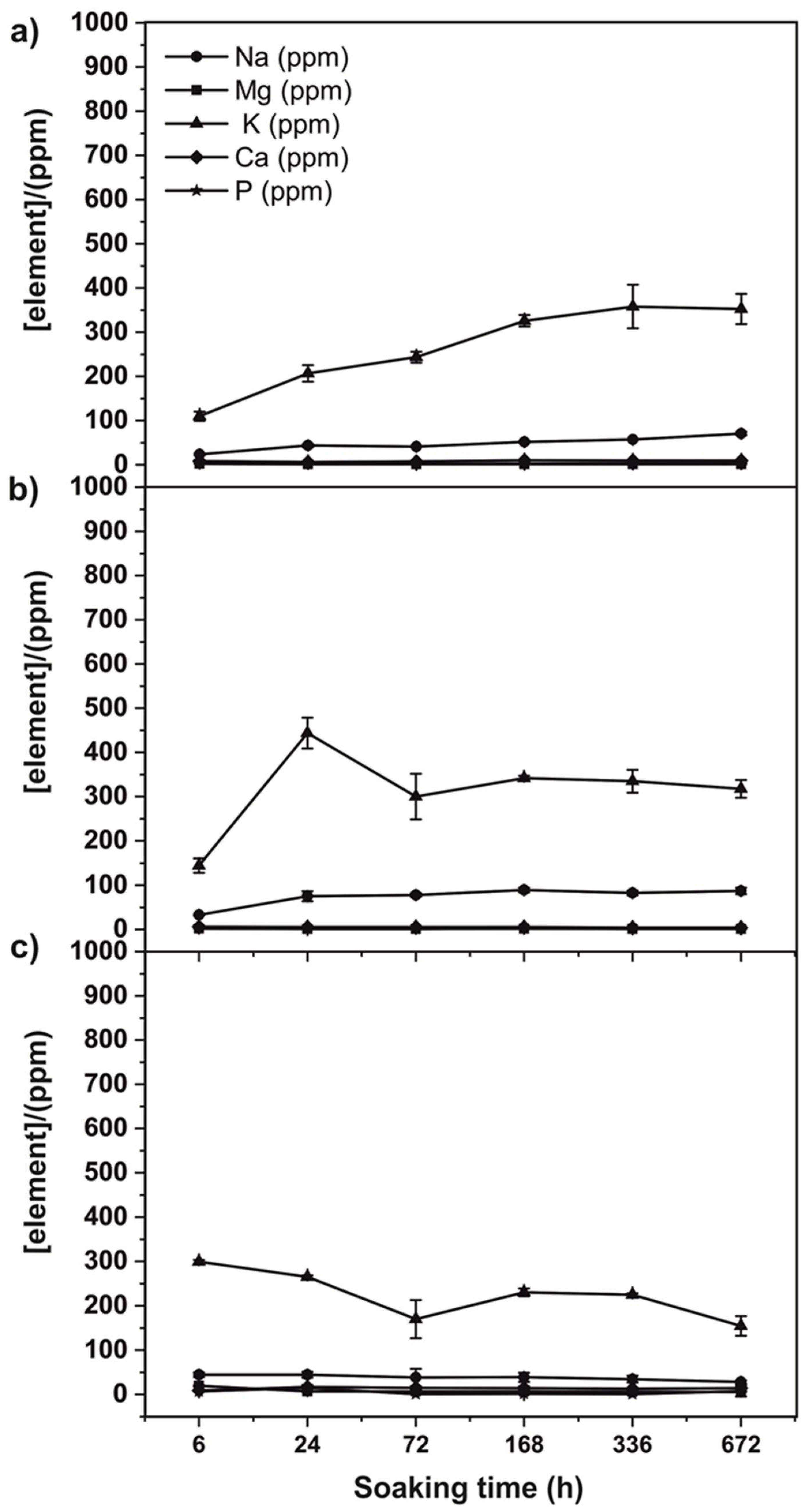
3.3. Chemical Analysis
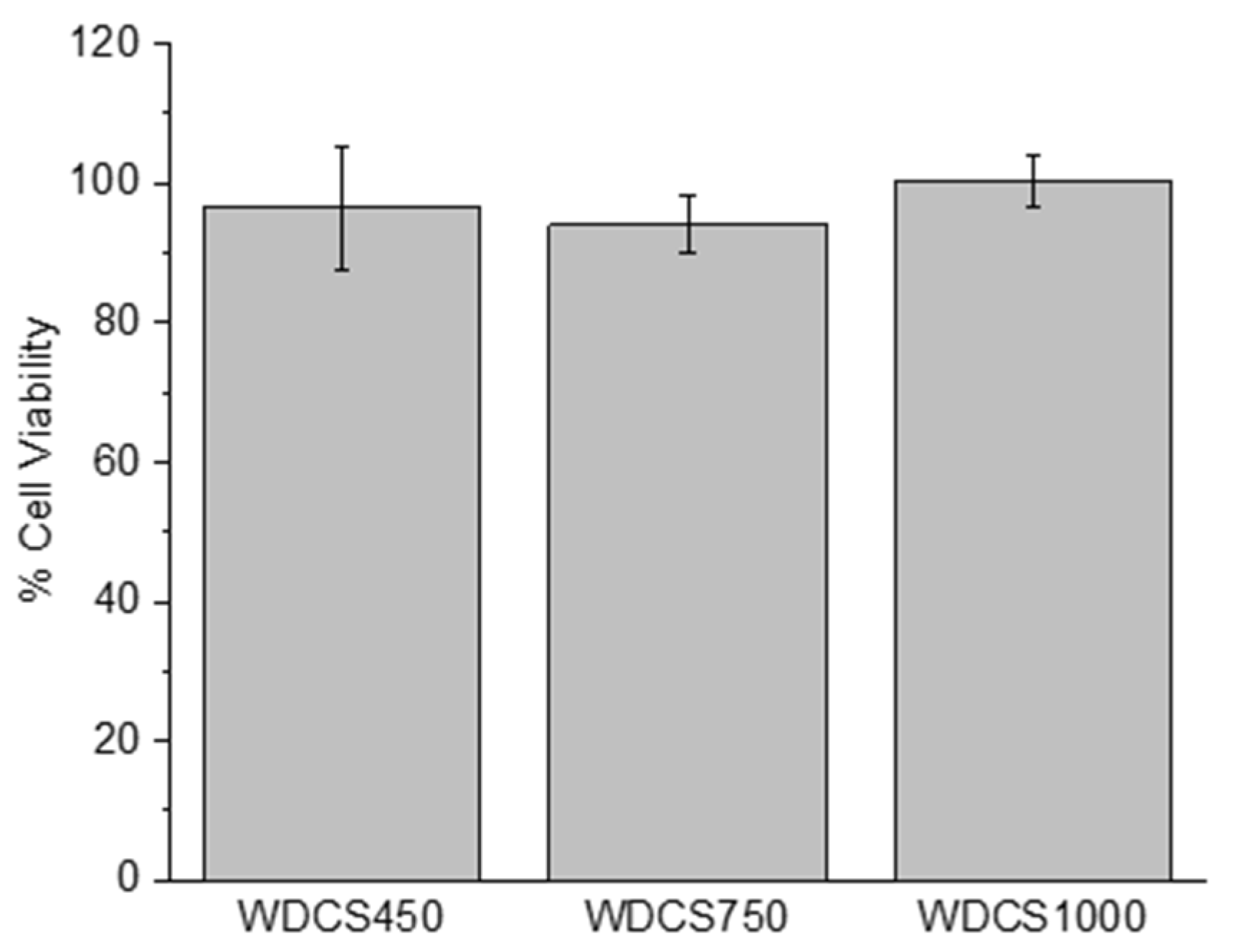
3.4. Biological Response
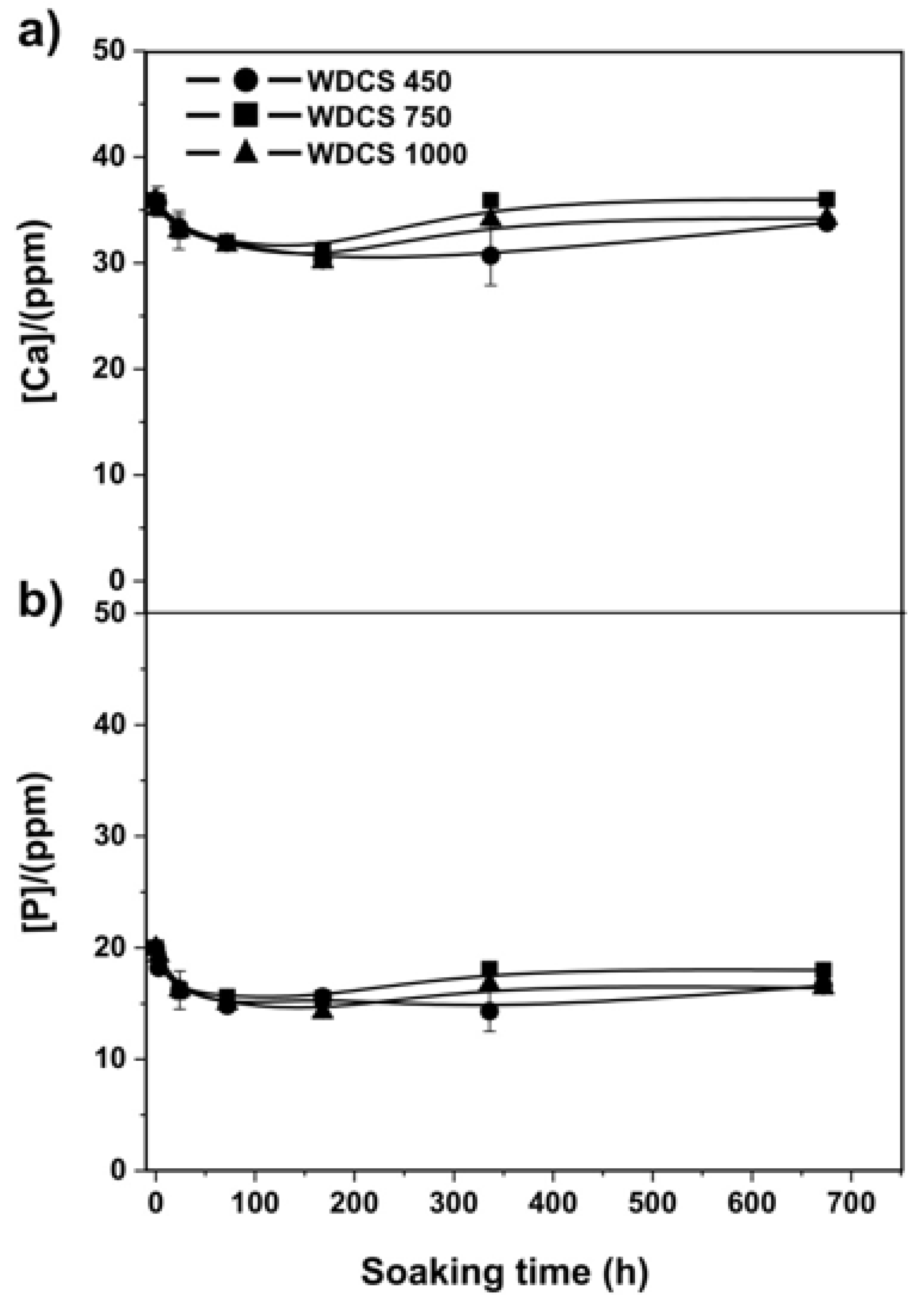
3.5. Biodegradation and Bioactivity Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tayebi, L.; Shahini, A.; Yazdimamaghani, M.; Walker, K.J.; Eastman, M.; Hatami-Marbini, H.; Smith, B.; Ricci, J.L.; Madihally, S.; Vashaee, D. 3D conductive nanocomposite scaffold for bone tissue engineering. Int. J. Nanomed. 2014, 9, 167–181. [Google Scholar] [CrossRef] [Green Version]
- Abarrategi, A.; Gutierrez, M.C.; Moreno-Vicente, C.; Hortigüela, M.J.; Ramos, V.; López-Lacomba, J.L.; Ferrer, M.L.; del Monte, F. Multiwall carbon nanotube scaffolds for tissue engineering purposes. Biomaterials 2008, 29, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, S.; Kumar, M.; Mandal, B.B.; Karak, N. A renewable resource based carbon dot decorated hydroxyapatite nanohybrid and its fabrication with waterborne hyperbranched polyurethane for bone tissue engineering. RSC Adv. 2016, 6, 26066–26076. [Google Scholar] [CrossRef]
- Bacakova, L.; Grausova, L.; Vacik, J.; Fraczek, A.; Blazewicz, S.; Kromka, A.; Vanecek, M.; Svorcik, V. Improved adhesion and growth of human osteoblast-like MG 63 cells on biomaterials modified with carbon nanoparticles. Diam. Relat. Mater. 2007, 16, 2133–2140. [Google Scholar] [CrossRef]
- Liang, C.; Luo, Y.; Yang, G.; Xia, D.; Liu, L.; Zhang, X.; Wang, H. Graphene oxide hybridized nHAC/PLGA scaffolds facilitate the proliferation of MC3T3-E1 cells. Nanoscale Res. Lett. 2018, 13, 15. [Google Scholar] [CrossRef] [Green Version]
- Dai, C.; Li, Y.; Pan, W.; Wang, G.; Huang, R.; Bu, Y.; Liao, X.; Guo, K.; Gao, F. Three-dimensional high-porosity chitosan/honeycomb porous carbon/hydroxyapatite scaffold with enhanced osteoinductivity for bone regeneration. ACS Biomater. Sci. Eng. 2019, 6, 575–586. [Google Scholar] [CrossRef]
- Prazeres, A.; Carvalho, M.d.F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef]
- Baldasso, C.; Barros, T.; Tessaro, I. Concentration and purification of whey proteins by ultrafiltration. Desalination 2011, 278, 381–386. [Google Scholar] [CrossRef]
- Zadow, J. Whey and Lactose Processing; Elsevier Science Publishers Ltd.: Essex, UK, 1992. [Google Scholar]
- Betz, M.; A García-González, C.; Subrahmanyam, R.; Smirnova, I.; Kulozik, U. Preparation of novel whey protein-based aerogels as drug carriers for life science applications. J. Supercrit. Fluids 2012, 72, 111–119. [Google Scholar] [CrossRef]
- Dziadek, M.; Douglas, T.E.L.; Dziadek, K.; Zagrajczuk, B.; Serafim, A.; Stancu, I.-C.; Cholewa-Kowalska, K. Novel whey protein isolate-based highly porous scaffolds modified with therapeutic ion-releasing bioactive glasses. Mater. Lett. 2020, 261, 127115. [Google Scholar] [CrossRef]
- Gupta, D.; Kocot, M.; Tryba, A.M.; Serafim, A.; Stancu, I.C.; Jaegermann, Z.; Pamuła, E.; Reilly, G.C.; Douglas, T. Novel naturally derived whey protein isolate and aragonite biocomposite hydrogels have potential for bone regeneration. Mater. Des. 2020, 188, 108408. [Google Scholar] [CrossRef]
- Guo, M.; Wang, G. Whey protein polymerisation and its applications in environmentally safe adhesives. Int. J. Dairy Technol. 2016, 69, 481–488. [Google Scholar] [CrossRef]
- Tan, S.; Chen, X.; Zhai, S.; Ebrahimi, A.; Langrish, T.; Chen, Y. Spray drying assisted synthesis of porous carbons from whey powders for capacitive energy storage. Energy 2018, 147, 308–316. [Google Scholar] [CrossRef]
- Llamas-Unzueta, R.; Menéndez, J.A.; Ramírez-Montoya, L.A.; Viña, J.; Argüelles, A.; Montes-Morán, M.A. 3-D structured porous carbons with virtually any shape from whey powders. Carbon 2021, 175, 403–412. [Google Scholar] [CrossRef]
- Standard, A. C1424-15” Standard Test Method for Monotonic Compressive Strength of Advanced Ceramics at Ambient Temperature; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Menéndez, J.; Illán-Gómez, M.; León, C.Y.; Radovic, L. On the difference between the isoelectric point and the point of zero charge of carbons. Carbon 1995, 33, 1655–1657. [Google Scholar] [CrossRef]
- The International Organization for Standardization. ISO-10993-5: Biological Evaluation of Medical Devices. Part 5: Tests for Cytotoxicity: In Vitro Methods; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Ellis, G.P. The maillard reaction. Adv. Carbohydr. Chem. 1959, 14, 63–134. [Google Scholar]
- Gaucheron, F. The minerals of milk. Reprod. Nutr. Dev. 2005, 45, 473–483. [Google Scholar] [CrossRef]
- Lampila, L.E. Applications and functions of food-grade phosphates. Ann. N. Y. Acad. Sci. 2013, 1301, 37–44. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef]
- Lim, T.C.; Chian, K.S.; Leong, K.F. Cryogenic prototyping of chitosan scaffolds with controlled micro and macro architecture and their effect on in vivo neo-vascularization and cellular infiltration. J. Biomed. Mater. Res. Part A 2010, 94, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.; Jamison, R.D.; Johnson, A.J.W. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef]
- Hing, K.A.; Annaz, B.; Saeed, S.; Revell, P.A.; Buckland, T. Microporosity enhances bioactivity of synthetic bone graft substitutes. J. Mater. Sci. Mater. Med. 2005, 16, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Will, J.; Detsch, R.; Boccaccini, A.R. Structural and biological characterization of scaffolds. In Characterization of Biomaterials; Elsevier: Waltham, MA, USA, 2013; pp. 299–310. [Google Scholar]
- Li, S.; De Wijn, J.R.; Li, J.; Layrolle, P.; De Groot, K. Macroporous biphasic calcium phosphate scaffold with high permeability/porosity ratio. Tissue Eng. 2003, 9, 535–548. [Google Scholar] [CrossRef] [Green Version]
- Grimm, M.J.; Williams, J.L. Measurements of permeability in human calcaneal trabecular bone. J. Biomech. 1997, 30, 743–745. [Google Scholar] [CrossRef]
- Soon, Y.-M.; Shin, K.-H.; Koh, Y.-H.; Lee, J.-H.; Choi, W.-Y.; Kim, H.-E. Fabrication and compressive strength of porous hydroxyapatite scaffolds with a functionally graded core/shell structure. J. Eur. Ceram. Soc. 2011, 31, 13–18. [Google Scholar] [CrossRef]
- Mosekilde, L.; Mosekilde, L. Normal vertebral body size and compressive strength: Relations to age and to vertebral and iliac trabecular bone compressive strength. Bone 1986, 7, 207–212. [Google Scholar] [CrossRef]
- McCalden, R.W.; McGeough, J.A. Age-related changes in the compressive strength of cancellous bone. The relative importance of changes in density and trabecular architecture. J. Bone Jt. Surg. 1997, 79, 421–427. [Google Scholar] [CrossRef]
- Macchetta, A.; Turner, I.G.; Bowen, C.R. Fabrication of HA/TCP scaffolds with a graded and porous structure using a camphene-based freeze-casting method. Acta Biomater. 2009, 5, 1319–1327. [Google Scholar] [CrossRef]
- Kopperdahl, D.L.; Keaveny, T.M. Yield strain behavior of trabecular bone. J. Biomech. 1998, 31, 601–608. [Google Scholar] [CrossRef]
- Turner, C.H.; Rho, J.; Takano, Y.; Tsui, T.Y.; Pharr, G.M. The elastic properties of trabecular and cortical bone tissues are similar: Results from two microscopic measurement techniques. J. Biomech. 1999, 32, 437–441. [Google Scholar] [CrossRef]
- Rho, J.-Y.; Tsui, T.Y.; Pharr, G.M. Elastic properties of human cortical and trabecular lamellar bone measured by nanoindentation. Biomaterials 1997, 18, 1325–1330. [Google Scholar] [CrossRef]
- Bayraktar, H.H.; Morgan, E.F.; Niebur, G.; Morris, G.E.; Wong, E.K.; Keaveny, T.M. Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J. Biomech. 2004, 37, 27–35. [Google Scholar] [CrossRef]
- Zioupos, P.; Cook, R.B.; Hutchinson, J.R. Some basic relationships between density values in cancellous and cortical bone. J. Biomech. 2008, 41, 1961–1968. [Google Scholar] [CrossRef]
- Kohles, S.S.; Roberts, J.B.; Upton, M.L.; Wilson, C.G.; Bonassar, L.J.; Schlichting, A.L. Direct perfusion measurements of cancellous bone anisotropic permeability. J. Biomech. 2001, 34, 1197–1202. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, Q.; Shi, M.; Zhang, Y.; Tao, W.; Li, M. Effect of pore size on the biodegradation rate of silk fibroin scaffolds. Adv. Mater. Sci. Eng. 2015, 2015, 315397. [Google Scholar] [CrossRef] [Green Version]
- Martín, C.; Kostarelos, K.; Prato, M.; Bianco, A. Biocompatibility and biodegradability of 2D materials: Graphene and beyond. Chem. Commun. 2019, 55, 5540–5546. [Google Scholar] [CrossRef]
- Zhang, K.; Fan, Y.; Dunne, N.; Li, X. Effect of microporosity on scaffolds for bone tissue engineering. Regen. Biomater. 2018, 5, 115–124. [Google Scholar] [CrossRef]
- Kim, H.-M.; Himeno, T.; Kokubo, T.; Nakamura, T. Process and kinetics of bonelike apatite formation on sintered hydroxyapatite in a simulated body fluid. Biomaterials 2005, 26, 4366–4373. [Google Scholar] [CrossRef] [PubMed]




| ρHg a | ρHe c | Porosity d | k f | dp a,b | Max dp a | Min dp a | Shrinkage | |
|---|---|---|---|---|---|---|---|---|
| (g/cm3) | (g/cm3) | (%) | (m2) | (µm) | (µm) | (µm) | (%) | |
| WDCS450 | 0.78 | 1.61 | 52 | 8.76 × 10−13 | 33.3 | 248.8 | 5 × 10−3 | 15.9 ± 0.7 |
| WDCS750 | 0.80 | 1.91 | 58 | 1.46 × 10−12 | 27.7 | 162.8 | 5 × 10−3 | 22.9 ± 0.3 |
| WDCS1000 | 1.06 | 2.04 | 48 | 8.90 × 10−13 | 25.3 | 162.8 | 5 × 10−3 | 21.9 ± 2.4 |
| Element 1 (wt%) | |
|---|---|
| C 2 | 40.7 |
| H 2 | 6.3 |
| N 2 | 2.3 |
| O 2 | 46.1 |
| S 2 | 0.2 |
| Na 3 | 0.3 |
| K 3 | 1.2 |
| Mg 3 | 0.1 |
| Ca 3 | 0.4 |
| P 3 | 0.6 |
| Cl 4 | 0.2 |
| (wt%) a | WDCS450 | WDCS750 | WDCS1000 |
|---|---|---|---|
| C | 70.9 | 74.3 | 76.1 |
| H | 3.2 | 1.1 | 0.6 |
| N | 3.6 | 3.0 | 2.0 |
| O | 14 | 13.6 | 11.2 |
| S | 0.2 | 0.1 | 0.1 |
| Ash | 11.3 | 13.6 | 13.7 |
| SAMPLE | % Calcium | % Phosphorus |
|---|---|---|
| WDCS450 | 15.6 | 28.5 |
| WDCS750 | 13.4 | 22.5 |
| WDCS1000 | 16.1 | 29 |
| Scaffold Composition | Porosity | Pore Size | Compressive Strength | Permeability | Ref |
|---|---|---|---|---|---|
| (%) | (µm) | (MPa) | (m2) | ||
| WDCS750 | 58 | 1–200 | 30 | 1.46 × 10−12 | This work |
| HA | 50 | 250 | - | 7.5 × 10−11 | [31] |
| HA | 60 | 50 | 12 | - | [32] |
| HA/TCP | 60 | 100–200 | 5 | - | [33,34,35] |
| WPI/aragonite | 15.7 | 18–369 | 3.16 | - | [14] |
| Cancellous bone | 30–90 | 100–600 | 0.70–15 | 0.12–8 × 10−11 | [34,36,37,38,39,40,41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llamas-Unzueta, R.; Suárez, M.; Fernández, A.; Díaz, R.; Montes-Morán, M.A.; Menéndez, J.A. Whey-Derived Porous Carbon Scaffolds for Bone Tissue Engineering. Biomedicines 2021, 9, 1091. https://doi.org/10.3390/biomedicines9091091
Llamas-Unzueta R, Suárez M, Fernández A, Díaz R, Montes-Morán MA, Menéndez JA. Whey-Derived Porous Carbon Scaffolds for Bone Tissue Engineering. Biomedicines. 2021; 9(9):1091. https://doi.org/10.3390/biomedicines9091091
Chicago/Turabian StyleLlamas-Unzueta, Raúl, Marta Suárez, Adolfo Fernández, Raquel Díaz, Miguel A. Montes-Morán, and J. Angel Menéndez. 2021. "Whey-Derived Porous Carbon Scaffolds for Bone Tissue Engineering" Biomedicines 9, no. 9: 1091. https://doi.org/10.3390/biomedicines9091091
APA StyleLlamas-Unzueta, R., Suárez, M., Fernández, A., Díaz, R., Montes-Morán, M. A., & Menéndez, J. A. (2021). Whey-Derived Porous Carbon Scaffolds for Bone Tissue Engineering. Biomedicines, 9(9), 1091. https://doi.org/10.3390/biomedicines9091091







