Complementary Use of Presepsin with the Sepsis-3 Criteria Improved Identification of High-Risk Patients with Suspected Sepsis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Measurement of Presepsin
2.3. Data Collection and Definitions
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Presepsin Levels
3.3. Multivariable Logistic Regression Analysis for 28-Day Mortality
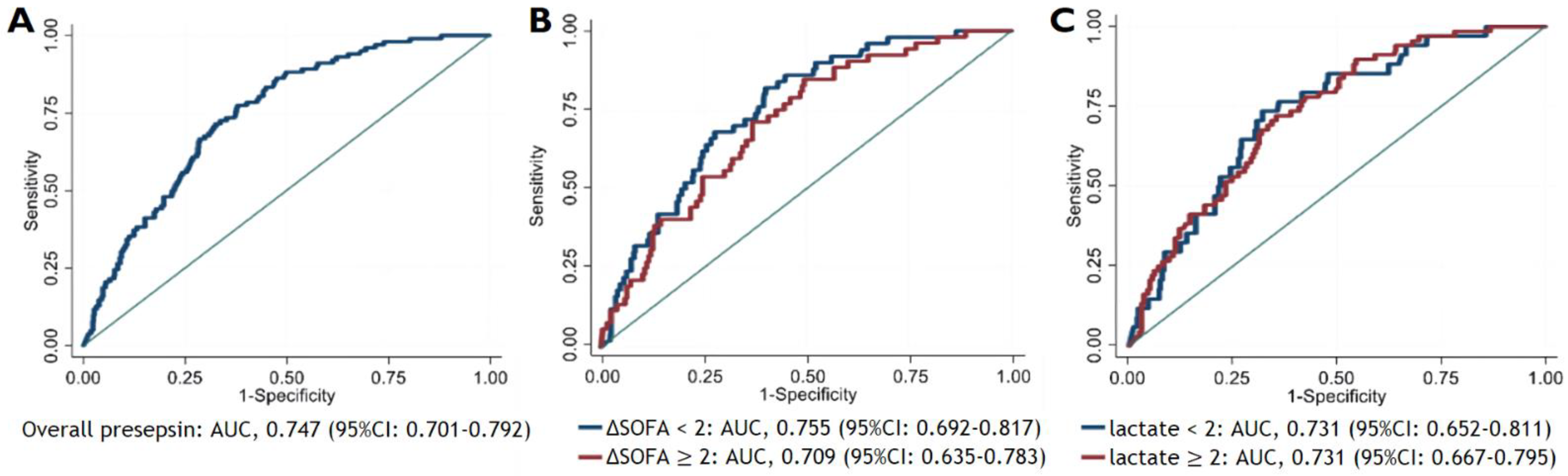
3.4. Discriminating Ability of Presepsin for 28-Day Mortality Based on Delta SOFA Score and Lactate Level
3.5. Combined Interpretation of the High Presepsin Cut-Off, Delta SOFA Score ≥ 2 Points, and Lactate ≥ 2 mmol/L
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROC | Receiver operating characteristic |
| AUC | Area under the curve |
| IQR | Interquartile range |
| SOFA | Sequential organ failure assessment |
| PCT | Procalcitonin |
| CRP | C-reactive protein |
| LPS | Lipopolysaccharide |
| ED | Emergency department |
| ICU | Intensive care unit |
| PST | Plasma separating tube |
| ALP | Alkaline phosphatase |
| QC | Quality control |
| CV | Coefficient of variation |
| CI | Confidence interval |
| MAP | Mean arterial pressure |
| PPV | Positive predictive value |
| NPV | Negative predictive value |
| COVID-19 | Coronavirus disease 2019 |
| OR | Odds ratio |
| aOR | Adjusted odds ratio |
References
- Vincent, J.L.; Jones, G.; David, S.; Olariu, E.; Cadwell, K.K. Frequency and mortality of septic shock in Europe and North America: A systematic review and meta-analysis. Crit. Care 2019, 23, 196. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, X.; Guo, P.; Chen, Y.; Li, J.; Tao, T. The accuracy assessment of presepsin (sCD14-ST) for mortality prediction in adult patients with sepsis and a head-to-head comparison to PCT: A meta-analysis. Ther. Clin. Risk Manag. 2019, 15, 741–753. [Google Scholar] [CrossRef] [Green Version]
- Heilmann, E.; Gregoriano, C.; Schuetz, P. Biomarkers of Infection: Are They Useful in the ICU? Semin. Respir. Crit. Care Med. 2019, 40, 465–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.S.; Hur, M.; Yi, A.; Kim, H.; Lee, S.; Kim, S.N. Prognostic value of presepsin in adult patients with sepsis: Systematic review and meta-analysis. PLoS ONE 2018, 13, e0191486. [Google Scholar] [CrossRef] [PubMed]
- Yaegashi, Y.; Shirakawa, K.; Sato, N.; Suzuki, Y.; Kojika, M.; Imai, S.; Takahashi, G.; Miyata, M.; Furusako, S.; Endo, S. Evaluation of a newly identified soluble CD14 subtype as a marker for sepsis. J. Infect. Chemother. 2005, 11, 234–238. [Google Scholar] [CrossRef]
- Shozushima, T.; Takahashi, G.; Matsumoto, N.; Kojika, M.; Okamura, Y.; Endo, S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J. Infect. Chemother. 2011, 17, 764–769. [Google Scholar] [CrossRef]
- Kahveci, U.; Ozkan, S.; Melekoglu, A.; Usul, E.; Ozturk, G.; Cetin, E.; Abatay, K.; Sahin, A. The role of plasma presepsin levels in determining the incidence of septic shock and mortality in patients with sepsis. J. Infect. Dev. Ctries 2021, 15, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Masson, S.; Caironi, P.; Spanuth, E.; Thomae, R.; Panigada, M.; Sangiorgi, G.; Fumagalli, R.; Mauri, T.; Isgro, S.; Fanizza, C.; et al. Presepsin (soluble CD14 subtype) and procalcitonin levels for mortality prediction in sepsis: Data from the Albumin Italian Outcome Sepsis trial. Crit. Care 2014, 18, R6. [Google Scholar] [CrossRef] [Green Version]
- Koizumi, Y.; Shimizu, K.; Shigeta, M.; Okuno, T.; Minamiguchi, H.; Kito, K.; Hodohara, K.; Yamagishi, Y.; Andoh, A.; Fujiyama, Y.; et al. Plasma presepsin level is an early diagnostic marker of severe febrile neutropenia in hematologic malignancy patients. BMC Infect. Dis. 2017, 17, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamura, Y.; Yokoi, H. Development of a point-of-care assay system for measurement of presepsin (sCD14-ST). Clin. Chim. Acta 2011, 412, 2157–2161. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Okamura, Y. Usefulness of Presepsin Measurement: A New Biomarker for Sepsis. Rinsho Byori Jpn. J. Clin. Pathol. 2015, 63, 62–71. [Google Scholar]
- Wu, J.; Hu, L.; Zhang, G.; Wu, F.; He, T. Accuracy of Presepsin in Sepsis Diagnosis: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0133057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Hu, Z.D.; Song, J.; Shao, J. Diagnostic Value of Presepsin for Sepsis: A Systematic Review and Meta-Analysis. Medicine 2015, 94, e2158. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Liu, Y.N.; Wang, R.; Xie, L.X. The accuracy of presepsin (sCD14-ST) for the diagnosis of sepsis in adults: A meta-analysis. Crit. Care 2015, 19, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.; Jiang, L.; Ye, L.; Gao, Y.; Tang, L.; Zhang, M. The accuracy of presepsin for the diagnosis of sepsis from SIRS: A systematic review and meta-analysis. Ann. Intensive Care 2015, 5, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Seymour, C.W.; Gesten, F.; Prescott, H.C.; Friedrich, M.E.; Iwashyna, T.J.; Phillips, G.S.; Lemeshow, S.; Osborn, T.; Terry, K.M.; Levy, M.M. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N. Engl. J. Med. 2017, 376, 2235–2244. [Google Scholar] [CrossRef]
- Liu, V.X.; Fielding-Singh, V.; Greene, J.D.; Baker, J.M.; Iwashyna, T.J.; Bhattacharya, J.; Escobar, G.J. The Timing of Early Antibiotics and Hospital Mortality in Sepsis. Am. J. Respir. Crit. Care Med. 2017, 196, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Mizugishi, K.; Nonomura, K.; Naitoh, K.; Takaori-Kondo, A.; Yamashita, K. Phagocytosis by human monocytes is required for the secretion of presepsin. J. Infect. Chemother. 2015, 21, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, G.; Bolke, E.; Grunert, A.; Storck, M.; Orth, K. Procalcitonin in patients with acute and chronic renal insufficiency. Wien. Klin. Wochenschr. 2004, 116, 849–853. [Google Scholar] [CrossRef]
- Jeppesen, J.B.; Mortensen, C.; Bendtsen, F.; Moller, S. Lactate metabolism in chronic liver disease. Scand. J. Clin. Lab. Investig. 2013, 73, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ishikura, H.; Nishida, T.; Kawano, Y.; Yuge, R.; Ichiki, R.; Murai, A. Usefulness of presepsin in the diagnosis of sepsis in patients with or without acute kidney injury. BMC Anesth. 2014, 14, 88. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.C.; Vincent, J.L.; Guyatt, G.; Angus, D.C.; Abraham, E.; Bernard, G.; Bombardier, C.; Calandra, T.; Jorgensen, H.S.; Sylvester, R.; et al. Outcome measures for clinical research in sepsis: A report of the 2nd Cambridge Colloquium of the International Sepsis Forum. Crit. Care Med. 2005, 33, 1708–1716. [Google Scholar] [CrossRef] [PubMed]




| Overall (n = 755) | Survivors (n = 653, 86.5%) | Non-Survivors (n = 102, 13.5%) | p-Value | |
|---|---|---|---|---|
| Age, years | 63.2 (51.9–72.1) | 62.3 (49.8–72.1) | 65.8 (59.8–71.7) | 0.004 |
| Sex, male | 425 (56.3) | 352 (54.0) | 73 (71.6) | 0.001 |
| Comorbidities | ||||
| Diabetes | 153 (20.3) | 131 (20.1) | 22 (21.6) | 0.725 |
| Cerebrovascular disease | 63 (8.4) | 57 (8.7) | 6 (5.9) | 0.334 |
| Chronic cardiac disease | 101 (13.4) | 83 (13.5) | 13 (12.8) | 0.840 |
| Chronic lung disease | 41 (5.4) | 34 (5.2) | 7 (6.9) | 0.492 |
| Chronic liver disease | 101 (13.4) | 83 (12.7) | 18 (17.7) | 0.173 |
| Chronic renal disease | 74 (9.8) | 66 (10.1) | 8 (7.8) | 0.472 |
| Malignancy | 497 (65.8) | 409 (62.7) | 88 (86.3) | <0.001 |
| Infection focus | ||||
| Respiratory | 185 (24.5) | 138 (21.1) | 47 (46.1) | <0.001 |
| Urinary tract | 117 (15.5) | 107 (16.4) | 10 (9.8) | 0.087 |
| Gastrointestinal | 107 (14.2) | 94 (14.4) | 13 (12.8) | 0.657 |
| Hepatobiliary and pancreatic | 101 (13.4) | 88 (13.5) | 13 (12.8) | 0.840 |
| Bone and soft tissues | 66 (8.7) | 62 (9.5) | 4 (3.9) | 0.063 |
| Others | 82 (10.9) | 74 (11.3) | 8 (7.8) | 0.292 |
| Positive blood culture | 110 (14.6) | 85 (13.0) | 25 (24.5) | 0.002 |
| Gram-positive bacteria | 35 (4.6) | 27 (4.1) | 8 (7.8) | 0.098 |
| Gram-negative bacteria | 72 (9.5) | 58 (8.9) | 14 (13.7) | 0.121 |
| Fungus | 5 (0.7) | 1 (0.2) | 4 (3.9) | <0.001 |
| SOFA score | 2 (1.0–5.0) | 2 (1.0–4.0) | 5 (3.0–9.0) | <0.001 |
| Patients with sepsis | 318 (42.1) | 245 (37.5) | 73 (71.6) | <0.001 |
| Patients with septic shock | 44 (5.8) | 26 (4.0) | 18 (17.7) | <0.001 |
| Laboratory tests | ||||
| WBC (103/μL) | 7.8 (3.9–12.4) | 7.7 (4.0–12.0) | 9.3 (3.1–17.4) | 0.030 |
| Hemoglobin (g/dL) | 10.4 (9.0–12.3) | 10.5 (9.1–12.5) | 9.5 (8.5–10.8) | <0.001 |
| Platelet (103/μL) | 152 (75.0–242.0) | 159 (84.0–245.0) | 101 (36.0–226.0) | 0.001 |
| Total bilirubin (mg/dL) | 0.8 (0.5–1.5) | 0.7 (0.4–1.3) | 1.3 (0.6–3.8) | <0.001 |
| Creatinine (mg/dL) | 0.8 (0.6–1.2) | 0.8 (0.6–1.1) | 1.1 (0.6–1.9) | 0.001 |
| Lactate (mmol/L) | 1.5 (1.1–2.3) | 1.4 (1.1–2.1) | 2.4 (1.7–4.4) | <0.001 |
| CRP (mg/dL) | 6.8 (2.9–14.4) | 6.4 (2.6–13.2) | 11.2 (6.1–18.9) | <0.001 |
| Procalcitonin (ng/mL) | 0.4 (0.1–1.6) | 0.3 (0.1–1.5) | 1.0 (0.4–4.3) | <0.001 |
| Monocytopenia | 348 (46.1) | 298 (45.6) | 50 (49.0) | 0.524 |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Presepsin > 755 pg/mL | 5.61 | 3.44–9.16 | <0.001 | 5.17 | 2.92–9.16 | <0.001 |
| Age, years | 1.03 | 1.01–1.04 | <0.001 | 1.02 | 1.01–1.04 | 0.016 |
| Sex, male | 2.15 | 1.36–3.39 | <0.001 | 1.14 | 0.67–1.95 | 0.626 |
| Malignancy * | 3.75 | 2.09–6.74 | <0.001 | 5.34 | 2.71–10.53 | <0.001 |
| Respiratory infection | 3.19 | 2.07–4.91 | <0.001 | 3.34 | 1.98–5.65 | <0.001 |
| Delta SOFA score ≥ 2 | 4.19 | 2.65–6.63 | <0.001 | 2.83 | 1.65–4.83 | <0.001 |
| Lactate ≥ 2 mmol/L | 4.60 | 2.95–7.17 | <0.001 | 3.37 | 2.04–5.57 | <0.001 |
| Procalcitonin > 0.5 ng/mL | 2.49 | 1.61–3.84 | <0.001 | 0.99 | 0.57–1.71 | 0.967 |
| Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | |
|---|---|---|---|---|
| Presepsin > 755 pg/mL | 77.5 (68.1–85.1) | 62 (58.2–65.8) | 24.2 (19.6–29.2) | 94.6 (92–96.6) |
| ΔSOFA ≥ 2 | 71.6 (61.8–80.1) | 62.5 (58.6–66.2) | 23 (18.4–28) | 93.4 (90.6–95.5) |
| Lactate ≥ 2 mmol/L | 66.7 (56.6–75.7) | 69.7 (66–73.2) | 25.6 (20.4–31.2) | 93.0 (90.4–95.1) |
| Presepsin > 755 pg/mL or ΔSOFA ≥ 2 | 89.2 (81.5–94.5) | 44.6 (40.7–48.5) | 20.1 (16.5–24.1) | 96.4 (93.6–98.2) |
| Presepsin > 755 pg/mL or lactate ≥ 2 mmol/L | 92.2 (85.1–96.6) | 44.6 (40.7–48.5) | 20.6 (17–24.6) | 97.3 (94.8–98.8) |
| Presepsin > 755 pg/mL or ΔSOFA ≥ 2 or lactate ≥ 2 mmol/L | 94.1 (87.6–97.8) | 34.3 (30.7–38.1) | 18.3 (15.1–21.9) | 97.4 (94.4–99) |
| Presepsin > 755 pg/mL and ΔSOFA ≥ 2 | 59.8 (49.6–69.4) | 79.9 (76.7–82.9) | 31.8 (25.3–38.9) | 92.7 (90.3–94.7) |
| Presepsin > 755 pg/mL and lactate ≥ 2 mmol/L | 52.0 (41.8–62.0) | 87.1 (84.3–89.6) | 38.7 (30.5–47.4) | 92.1 (89.7–94.1) |
| Presepsin > 755 pg/mL and ΔSOFA ≥ 2 and lactate ≥ 2 mmol/L | 43.1 (33.4–53.2) | 93.7 (91.6–95.5) | 51.8 (40.7–62.7) | 91.3 (89.0–93.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.E.; Lee, B.; Yoon, S.J.; Park, C.-M.; Jung, C.W.; Ahn, M.-J.; Park, H.-D.; Hwang, S.Y.; Shin, T.G.; Kang, E.-S. Complementary Use of Presepsin with the Sepsis-3 Criteria Improved Identification of High-Risk Patients with Suspected Sepsis. Biomedicines 2021, 9, 1076. https://doi.org/10.3390/biomedicines9091076
Park JE, Lee B, Yoon SJ, Park C-M, Jung CW, Ahn M-J, Park H-D, Hwang SY, Shin TG, Kang E-S. Complementary Use of Presepsin with the Sepsis-3 Criteria Improved Identification of High-Risk Patients with Suspected Sepsis. Biomedicines. 2021; 9(9):1076. https://doi.org/10.3390/biomedicines9091076
Chicago/Turabian StylePark, Jong Eun, Beomki Lee, Sun Joo Yoon, Chi-Min Park, Chul Won Jung, Myung-Ju Ahn, Hyung-Doo Park, Sung Yeon Hwang, Tae Gun Shin, and Eun-Suk Kang. 2021. "Complementary Use of Presepsin with the Sepsis-3 Criteria Improved Identification of High-Risk Patients with Suspected Sepsis" Biomedicines 9, no. 9: 1076. https://doi.org/10.3390/biomedicines9091076
APA StylePark, J. E., Lee, B., Yoon, S. J., Park, C.-M., Jung, C. W., Ahn, M.-J., Park, H.-D., Hwang, S. Y., Shin, T. G., & Kang, E.-S. (2021). Complementary Use of Presepsin with the Sepsis-3 Criteria Improved Identification of High-Risk Patients with Suspected Sepsis. Biomedicines, 9(9), 1076. https://doi.org/10.3390/biomedicines9091076







