Abstract
Coronavirus disease 2019 (COVID-19) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) constitute one of the deadliest pandemics in modern history demonstrating cardiovascular, gastrointestinal, hematologic, mucocutaneous, respiratory, neurological, renal and testicular manifestations and further complications. COVID-19-induced excessive immune response accompanied with uncontrolled release of cytokines culminating in cytokine storm seem to be the common pathogenetic mechanism of these complications. The aim of this narrative review is to elucidate the relation between anaphylaxis associated with profound hypotension or hypoxemia with pro-inflammatory cytokine release. COVID-19 relation with Kounis syndrome and post-COVID-19 vaccination correlation with heparin-induced thrombocytopenia with thrombosis (HITT), especially serious cerebral venous sinus thrombosis, were also reviewed. Methods: A current literature search in PubMed, Embase and Google databases was performed to reveal the pathophysiology, prevalence, clinical manifestation, correlation and treatment of COVID-19, anaphylaxis with profuse hypotension, Kounis acute coronary syndrome and thrombotic events post vaccination. Results: The same key immunological pathophysiology mechanisms and cells seem to underlie COVID-19 cardiovascular complications and the anaphylaxis-associated Kounis syndrome. The myocardial injury in patients with COVID-19 has been attributed to coronary spasm, plaque rupture and microthrombi formation, hypoxic injury or cytokine storm disposing the same pathophysiology with the three clinical variants of Kounis syndrome. COVID-19-interrelated vaccine excipients as polysorbate, polyethelene glycol (PEG) and trometamol constitute potential allergenic substances. Conclusion: Better acknowledgement of the pathophysiological mechanisms, clinical similarities, multiorgan complications of COVID-19 or other viral infections as dengue and human immunodeficiency viruses along with the action of inflammatory cells inducing the Kounis syndrome could identify better immunological approaches for prevention, treatment of the COVID-19 pandemic as well as post-COVID-19 vaccine adverse reactions.
Keywords:
anaphylaxis; COVID-19; cytokine storm; heparin; Kounis syndrome; thrombocytopenia; thrombosis 1. Introduction
The latest threat to global health is the ongoing outbreak of the respiratory disease caused by SARS-CoV-2, named COVID-19, firstly recognized in December 2019 in the city of Wuhan, in Hubei province, China [1]. COVID-19, caused by SARS-CoV-2, constitutes one of the deadliest pandemics inmodern history. In a modern overpopulated world of almost 8 billion people, characterized by dramatic changes in environmental conditions, in conjunction with rapid development of intercontinental transportation and inadequate global public health mechanisms, viral diseases with significant infectivity may turn into global health threats. Whereas, the cardiovascular, gastrointestinal, hematologic, mucocutaneous, respiratory, neurological, renal and testicular manifestations, and further complications that concern the whole human pathology, can provide also the substrate for elucidation of the disease pathophysiology. The COVID-19 pandemic has been spreading worldwide, including to all of Europe and the United States. Careful identification of any similarities regarding clinical manifestation and subsequent multiorgan complications could provide a better acknowledgement of the underlying pathophysiology and trigger mechanisms, elucidating potential prevention and therapeutic strategies.
2. Methods
A literature search was conducted on the PubMed, MedLine, Embase databases and Google and updated on 28 February 2021 with the keywords “COVID-19”, “Kounis syndrome”, “cytokine storm”, “SARS-CoV-2”, “SARS-CoV”, “MERS”, “allergy”, “anaphylaxis”, “coronaviruses”, “mast cells”.
Bibliographic search was also undertaken. Articles in this review needed to be published up to end of June 2021, available as full text in English, categorized as original research, reviews, meta-analyses or letters to the editor. Database screening was closed on 28 June 2021.
Titles and abstracts were reviewed to verify these criteria. The articles were read in full if all inclusion requirements were present or if this remained unclear.
Searching references included in the manuscripts was an additional literature. The abstracts were scanned to assess their appropriateness to be included in this narrative review.
3. Results
3.1. Virology and Origin
Coronaviruses are enveloped, positive single-stranded RNA viruses (+)RNA) with a genome of 27–32 kb. COVID-19 belongs to the beta-coronavirus genera, while evolutionary analyses have demonstrated bats and rodents as gene sources [2]. Regarding its origin, theories for laboratory construction have been widely spread through social media, but genetic data are not suggestive of this scenario. The receptor-binding domain in the spike protein is the most variable part of the coronavirus genome. Genetic manipulations in laboratories have been performed on available viral reverse-genetic systems, allowing researchers to introduce scheduled mutations. However, genetic data clearly reveal that COVID-19 is not derived from any previously used virus backbone, supporting the evidence that COVID-19 is a novel coronavirus, originated from natural selection, either in an animal host pre or post zoonotic transfer [3].
Infection is initiated with virus attachment to its cellular receptor on the host cell surface. COVID-19 spike protein (S-protein) binds with the angiotensin-converting enzyme 2 (ACE2) receptor on the epithelial cells’ membrane. COVID-19 transmission via the respiratory system could be facilitated by the abundant ACE2 expression by human respiratory epithelium [4]. Given that ACE2 is also expressed in vascular endothelium, cardiovascular, gastrointestinal, brain, and renal tissue as well as testicular epithelium, these organs are rendered potential targets for infection [5]. ACE-2 metabolizes angiotensin II to the vasodilatory and anti-inflammatory peptide angiotensin. SARS-CoV-2 interrupts the metabolism of angiotensin II, which further results in increased angiotensin II that can cause vasoconstriction, endothelial and platelet activation, and proinflammatory cytokine release.
Although patients infected with COVID-19 can be asymptomatic or present mild symptoms, some patients may develop moderate or severe complications, such as acute respiratory distress syndrome (ARDS), acute cardiac injury, acute coronary syndromes, coagulopathy with thromboses, renal and hepatic dysfunction, accompanied by increased risk of mortality [6]. A common pathogenetic mechanism for these complications seems to be COVID-19-induced pro-inflammatory state, the so-called cytokine storm syndrome (CSS) [6].
3.2. SARS-CoV-2 and Other Human Coronaviruses
Up to now, seven coronaviruses have been identified that can cause human disease. Coronaviruses hCoV-HKU1, hCoV-OC43, hCoV-NL63 andhCoV-229E principally cause asymptomatic or mild respiratory and gastrointestinal infections, accounting for approximately 5–30% of common colds [7]. However, in the last two decades, three human coronaviruses resulted in outbreaks that raised considerable global health consternation. First, the severe acute respiratory syndrome coronavirus (SARS-CoV) was recognized in 2002 as the cause of the severe acute respiratory syndrome (SARS) infecting over 8000 people the next year (2003) and causing 774 deaths with a case fatality ratio of 9.5%. Nine years later, a new coronavirus appeared in the Middle East, causing a highly lethal respiratory disease (34% case fatality rate), and this virus was named MERS-CoV [7]. Finally, since December 2019, the new human coronavirus SARS-CoV-2 has rapidly spread from Wuhan, China, to over 223 countries and regions in the world, causing a global pandemic affecting 114 million people, with over 2.5 million deaths by 1 March 2021 and a case fatality ratio ranging from 1 to 3.5% in most countries [8]. The higher reproduction number (R0) estimated as 3.1 (0.58 for SARS-CoV and 0.69 for MERS) has contributed to this global distribution [9] (Table 1). It is anticipated that these rates will change over time.

Table 1.
Characteristics, similarities and differences among three coronaviruses [7,9].
Regarding SARS-CoV-2 biological features, its genome consists of 6 common major ORFs for coronaviruses, with SARS-CoV-2 sequence disposing a 70% similarity to SARS-CoV and nearly 40% to that of MERS-CoV.ORF1a and the sequence of gene spike coding protein-S, the pivotal protein that interacts with the target cells, constitute the main differences among these three viruses [10]. SARS-CoV and SARS-CoV-2 S proteins are both attractive to ACE2 by electrostatic forces; however, mutations between SARS-CoV S protein and SARS-CoV-2 S protein lead to electric field line different densities, which results in a stronger interaction between SARS-CoV-2 and ACE2 [11].
These highly pathogenic human coronaviruses have evolutionarily acquired the ability to encode numerous proteins that allow them to escape the immune response, but also to overactivate inflammatory and immune cells, inducing a cytokine storm [7]. Severe systemic complications, such as coagulopathy with thromboses, acute cardiac injury, brain, liver injury and multiple-organ dysfunction have been associated with the cytokine storm syndrome, leading to increased risk of mortality. The characteristics and clinical manifestations of this cytokine storm syndrome have been described with SARS-CoV-2 owing to the abundance of human cases observed.
3.3. The Cytokine Storm
Cytokine storm is a complex and excessive immune response accompanied by excessive or uncontrolled release of pro-inflammatory cytokines that can be triggered by a variety of external stimuli such as severe viral infection as influenza [12], certain drugs [13] and cancer immunotherapeutic agents [14].
Cytokine storm has also been described as an unfortunate consequence and adverse effect of some monoclonal antibody medications as well as adoptiveT-cell therapies [14,15,16]. In addition, severe anaphylactic reactions with profuse hypotension or hypoxemia can be also associated with the release of pro-inflammatory cytokines [17].
The term “cytokine storm” was first used in 1993, in a case of a graft-versus-host disease [18]. Its association with infectious diseases started in early 2000 in several reports related to hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS) [19], group A streptococcus [20], influenza virus [21], variola virus infections [22], and SARS-CoV-2 [23]. In 2005, it was applied in the context of the avian H5N1 influenza virus infection [24]. During the H1N1 influenza pandemic, the cytokine storm was also considered as the cause of death among several victims [25].
In cancer immunotherapy, with chimeric antigen receptor T-cells (CAR-T) treatment, autoimmune toxicity manifesting as CSS results from an antigen-specific attack on host tissues when the targeted tumor-associated antigen is expressed as well on non malignant tissues [26].
Another condition, the so-called cytokine release syndrome (CRS), is increasingly recognized, and it is caused by high-level immune activation, constituting anon-antigen-specific toxicity. Such high immune activation is necessary to mediate clinical beneficial effects via modern immunotherapies [14].
In severe anaphylaxis with either hypotension or hypoxemia, a number of cytokines are released and elevated in blood serum during the acute phase, correlating with the presence of hypotension [27]. During the allergic respiratory reactions, mediators may be largely confined to the airways’ tissue, without concomitant increase in blood serum levels. Alternatively, other mediators such as anaphylatoxins may play a significant role during respiratory reactions [28].
The cytokine storm is considered to be one of the major causes of ARDS, usually associated with multiple-organ failure [29], playing an important role inthe process of disease aggravation [30,31].
In critically ill patients, suffering from COVID-19 infection, the cytokine storm can deteriorate even more their clinical condition; therefore, its effective detection and suppression is of paramount importance for a positive outcome.
3.4. The Cytokine “Network”
The cytokine storm and the consecutive dangerous complications arise from the combined effects of several immune-active molecules (Table 2). The different types of cytokines associated with the cytokine storm include [13,32,33]:

Table 2.
Cytokines constituting the cytokine storm.
- Interleukins (ILs), so named because their fundamental function appears to be the communication between (inter-) various populations of white blood cells (leucocytes-leukin) and further regulation of the immune cell differentiation and activation. They are produced by almost all stromal cells, mast cells, B lymphocytes, T lymphocytes, macrophages, monocytes, dendritic cells, and other non-lymphocytic cells, such as fibroblasts, endothelial cells, keratinocytes, glomerular mesangial cells and tumor cells [34]. They induce an increase of the acute phase signaling, activate epithelial cells, and mediate the transportation of immune cells to the infection site, triggering also the production of secondary cytokines [35]. Interleukins comprise the largest group of cytokines.
- Chemokines are small secreted proteins that can bind to one or more of 21 G-protein-coupled receptors. Chemokines serve as chemoattractants to recruit inflammatory cells from endothelium and epithelium into the inflammation site [36], especially those of the immune system, contributing to innate and adaptive immune function and development. The role of one specific chemokine, CXCL10 (previously referred to as interferon-γ inducible protein of 10 kDa, or IP-10), has been highlighted in ARDS and coronary syndromes [37]. Chemokines are emerging as the second largest family of cytokines.
- Interferons (IFNs), so named because they interfere with virus replication and play the central role in establishing innate immunity to viruses and other microbial pathogens [38,39].
- Tumor necrosis factors (TNFs), called by their ability to cause a hemorrhagic tumor necrosis post injection into experimental animals, are involved in the pathogenesis of septic shock. They are regarded, today, as the central cytokines in acute viral diseases, including influenza virus, dengue virus, and Ebola virus diseases. Immune cells can release TNFs in the acute inflammation and infection phase, while they have been correlated with several chronic inflammatory and autoimmune diseases [40,41].
- Colony-stimulating factors (CSFs) play an important role supporting the growth and differentiation of various bone marrow elements. They are proteinic components of a cascade that induces cytokine production by macrophages at sites of inflammation, further perpetuating the inflammatory reaction [42]. They divide into different subtypes based on their action as: granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF), and granulocyte–macrophage colony-stimulating factor (GM-CSF). Ongoing trials will help to inform whether blunting the inflammatory signaling provided by the G-CSF axis in COVID-19 is beneficial [43].
3.5. Cytokine Storm Clinical Manifestations
The cytokine storm clinically manifests during activation and further release of inflammatory cytokines by large numbers of lymphocytes (B lymphocytes, T lymphocytes, and/or natural killer cells) and/or myeloid cells (macrophages, mast cells, monocytes, eosinophils, dendritic cells, or even endothelial cells lining blood vessels) [44]. Pleiotropism characterizes each individual cytokine that can exert multiple functions depending upon the cell that produces it and the target cell(s) upon which it acts. The severity of cytokine storm and the symptom onset may vary according to the inducing cause and the magnitude of immune cell activation. The clinical symptomatology of the cytokine storm includes a variety of symptoms and signs (Table 3) that range from mild, flu-like symptoms to severe life-threatening manifestations, depending on the severity of inflammatory response. Arthralgia, fatigue, fever, headache, muscular pains and rash constitute mild symptoms, but in severe cases, hypotension, hypoxemia and fever can further lead to severe and uncontrolled systemic inflammatory response accompanied by vasodepressor-circulatory shock, and vascular leakage, as in anaphylactic shock [45]. Furthermore, thrombotic vascular events, disseminated intravascular coagulation, and multiorgan system failure may be induced.

Table 3.
Clinical signs, symptoms and laboratory findings associated with cytokine storm.
Respiratory complications can often occur in patients with cytokine storm. Cough and tachypnea may be prevalent in mild cases that can also progress to ARDS characterized by dyspnea, hypoxemia, and evidence of bilateral opacities on chest X-ray [44]. Mechanical ventilation might also be required in ARDS due to airway protection inability secondary to neurotoxicity rather than actual respiratory distress [46].
Neurologic symptoms and signs are also encountered during cytokine storm [47] and range from mild confusion, hallucinations, headaches, word-finding difficulty, to aphasia, cranial nerve palsies, hemiparesis, seizures, and somnolence.
Cardiovascular complications of cytokine storm in general, and in viral infections in particular, may include arrhythmias, myocarditis, myocardial ischemia, pericarditis, and type 1 (atherosclerotic plaque rupture) and type 2 (secondary to an ischemic imbalance) myocardial infarction and heart failure [48].
3.6. COVID-19, Kawasaki Disease and Chimeric Antigen Receptor T-Cell (CAR-T) Therapy-Associated Cytokine Storm
Recent reports have demonstrated cases of children suffering from COVID-19 with unusual febrile illnesses, acute abdominal conditions, toxic shock syndrome, encephalopathy, elevated inflammatory markers, and multisystem involvement that resemble the features of Kawasaki’s disease (KD). Kawasaki disease is an acute inflammatory disease characterized by medium-sized vasculitis with predilection for coronary arteries, predominantly affecting children. Coronary artery aneurysms from KD may manifest in adult life, accounting for 5% of acute coronary syndromes in respective individuals. Kawasaki syndrome is associated with a variety of viruses [49] such as adenovirus, Coxsackie B3 virus, dengue virus, Epstein–Barr virus, herpes virus, human bocavirus, human coronavirus, human immunodeficiency virus, influenza virus, measles, para-influenza type 3, parvovirus B19, rotavirus, Varicella-Zoster Virus and even 2009 H1N1 virus.
Bacteria and viruses have been sporadically isolated from KD patients and their proteins acting as superantigens proposed as possible triggers of a dysregulated immune response.The presence of tissue intracytoplasmic inclusion bodies and RNA virus-like inclusion bodies in KD patients supports the hypothesis that RNA viruses are closely involved in the disease etiopathogenesis [50].
In many pediatric COVID-19 patients, a new nosological entity of a multisystem cytokine storm temporally associated with SARS-CoV-2 has been documented and recognized as a multisystem inflammatory syndrome in children (MIS-C). This typically occurs 2–6 weeks after acute SARS-CoV-2 infection. Symptoms include persistent fever, gastrointestinal issues, multiorgan inflammation, low blood pressure, high inflammatory markers, and a variety of other symptoms, including respiratory. Hospital admission is mandatory and approximately 60% of patients are admitted to intensive care unit and approximately 2% die.
The pathogenic mechanisms are not well understood, but the delay between infection/exposure and onset suggests inappropriate innate immune responses elicited by infection, sometimes leading to a “cytokine storm syndrome” (CSS) [51]. A condition resembling MIS-C, called MIS-A, has also been reported, but more rarely, in adults [52]. It is not clear, however, that this condition pathogenesis is identical to MIS-C. MIS-C shares several features with Kawasaki disease, but differs from the cytokine storm of severe acute COVID-19 [53]. It also differs from this condition with respect to T-cell subsets, interleukin (IL)-17A, and biomarkers associated with arterial damage. Additionally, MIS-C occurs in older children (median age 9–10 years in largest series of African origin, while KD predominantly occurs in children ≤5 years of age of Asian descent. MIS-C is associated with more severe lymphopenia, higher levels of the inflammatory markers C-reactive protein and ferritin, and lower platelet counts compared to Kawasaki disease. Significantly elevated inflammatory cytokines are present in MIS-C including IL-6, IL-17A, and IL-18, and elevated chemokines as CCL3, 4, 20, and 28, and CXCL10. There are also reductions of subsets of natural killer and T lymphocytes. Multiple autoantibodies against self-antigens, such as La, a lupus autoantigen, and Jo-1, seen in idiopathic inflammatory myopathies, might be present. Thus, MIS-C appears to be both autoinflammatory and autoimmune in nature. Interestingly, treatment with IL-6R antibody or IV immunoglobulin has led to disease resolution [54]. Other treatments commonly used include IL-1RA or a high dose of steroids.
Another condition associated with cytokine storm that is related to the COVD-19 pandemic is the Chimeric antigen receptor T-cell (CAR-T) therapy used to patients with relapsed/refractory hematologic malignancies. Whereas such a therapy is a successful therapeutic strategy for improving clinical outcomes, unexpected complications and challenges amid the COVID-19 pandemic may be encountered. Indeed, patients with hematologic malignancies could be severely affected by SARS-CoV-2 infection mainly because of immunosuppression. The most common adverse effect of CAR-T therapy is CSS, which is triggered by the binding of chimeric antigen receptors with their target antigens [55]. Generally, CSS is reversible and only requires supportive therapy, but in some extreme cases, it can be life-threatening. CSS is characterized by the activation of epithelial cells, which may result in pulmonary edema, eventually leading to acute respiratory failure in severe cases. Previous studies have explored the correlation between cytokine profiles and the severity of CSS after CAR T-cell infusion. Early elevated serum levels of multiple cytokines, including interleukins IL-6, IL-8, and IL-10, monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein-1β (MIP-1β), and interferon-g (IFN-g), can be associated with the development of severe CRS [56]. Patients who have completed CAR-T treatment and suffered as well from confirmed COVID-19 infection, should be treated based on well-acknowledged guidelines [57] and local medical guidelines for the diagnosis and treatment of COVID-19. On the other hand, high-dose steroids should be avoided, as this could disrupt CAR-T cell persistence and may affect the outcomes of CAR-T therapy. In addition, tocilizumab or artificial liver treatment is recommended for severe COVID-19 cases with elevated cytokine levels. Indeed, the artificial liver is recommended to inhibit pro-inflammatory cytokine cascade. All medical staff should use preventive measures and rules during ward rounds to avoid cross-infection, whereas the use of disposable caps, surgical masks, and other garments is imperative. Proper hand washing and disinfection with alcohol or hydrogen peroxide should also be performed by both staff members and patients [57]. A comparison of characteristics of SARS-CoV-2 CSS, CAR-T cell therapy-associated CRS and HLH/MAS is shown in Table 4.

Table 4.
Characteristics of SARS-CoV-2 cytokine storm syndrome (CSS), CAR-T cell therapy-associated cytokine release syndrome (CRS) and hemophagocytic lymphohistiocytosis /macrophage activation syndrome (HLH/MAS) [58,59,60,61].
3.7. Cytokine Storm in COVID-19, Cytokine Surge in Anaphylaxis
The immediate-type of generalized hypersensitivity reaction that affects multiple organ systems and manifests, at its most severe form, by bronchospasm, upper airway obstruction, hypoxemia, hypotension, collapseand cardiovascular complications including Kounis syndrome [62] is anaphylaxis (an-aphylaxis means ‘no-prophylaxis’, ‘opposite protection’ or ‘against protection’, whereas prophylaxis in Greek means ‘protection’).
Since the era of histamine thought to be the principal mediator of anaphylaxis, a range of other mediators, similar to those of cytokine storm in COVID-19 patients, have been implicated in human anaphylaxis, in vitro cell stimulation studies, and in animal models [63]. Mast cells and other inflammatory cells can release anaphylaxis-implicated mediators resembling cytokine storm molecules. These mediators include beyond histamine, mast cell tryptase, chymase, and cathepsin-D, newly synthesized cytokines/chemokines such as tumor necrosis factor-a (TNF-a), IL-1, IL-4, IL-5, IL-6, IL-8, IL-9, IL-13, complement breakdown products such as the anaphylatoxins C3a, C4a, and C5a, contact system activation products including bradykinin, lipid-derived mediators (platelet activating factor, prostaglandin D2, leukotriene LT B4, cysteinyl leukotrienes LTC4, LTD4, and LTE4), and a variety of pro- or anti-inflammatory products stemmed from eosinophil activation [64].
Indeed, mast cells and other inflammatory cells can activate each other via multidirectional signals like a “ball of thread”, participating in an inflammatory vicious cycle (Figure 1). For instance, mast cells can induce macrophage activation [65] and enhance T-cell activation [66], whereas mast cells might be activated by inducible macrophage protein 1a [67]. In addition, CD169-macrophages can induce CD8 T-cell activation [68], which, in turn, may mediate mast-cell activation and proliferation [69] and regulate macrophage activity [70].
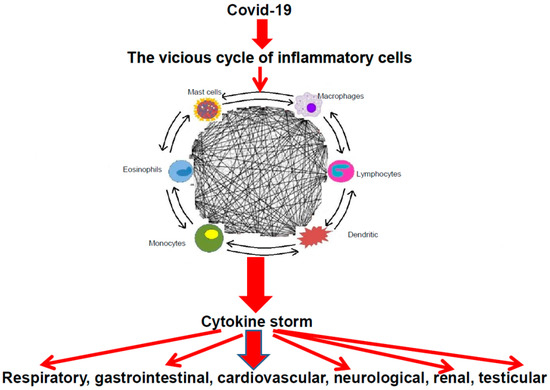
Figure 1.
The cascade of COVID-19 and the vicious cycle of inflammatory cells leading to cytokine storm with their clinical consequences.
Monocyte chemotactic protein 3 is a most effective basophil-and eosinophil-activating chemokine [71]. Dendritic cells may initiate autoimmune responses and stimulate T cells with resultant macrophage activation [72].
Interactions between lymphocytes, monocytes, macrophages, and mast cells increase vascular permeability, via release of TNF, IL-1β, IL-6, CXCL8 (IL-8), macrophage migration inhibitory factor, CCL2 (also known as monocyte chemoattractant protein-1, MCP-1), high mobility group box-1 and matrix metalloproteinases [73]. The latter can promote plaque disruption or rupture, leading to myocardial infarction via activation of their zymogen forms interstitial collagenase, gelatinase, and stromelysin [74].
3.8. Viral Infections and Mast Cells
Viral infections seem to interplay mast cell activation cascade [75], as mast cells participate in certain viruses host responses, but their precise role remains to be elucidated [76]. Moreover, mast cells can be insulted by some viruses as dengue virus and human immunodeficiency virus (HIV) [77,78]. There is evidence that human mast cells can constitute a persistent viral infection reservoir as mast cell exposure to TLR2 (toll-like receptors), −4, or −9 ligands can trigger virus replication in latently infected cells. Moreover, virus co-receptor expression by mast cells might be influenced by IgE-FcεRI interactions, thus establishing their susceptibility to infection with CXCR4-tropic and R5X4-tropic variants [79].
Indeed, human and rodent mast cells express TLR3s that constitute a class of proteins expressed on sentinel cells such as macrophages and dendritic cells, which can recognize structurally conserved molecules derived from microbes [80]. These receptors can be activated by viral double-stranded RNA to release various chemokines and cytokines, including IFN-α and IFN-β [81].
Viral activation of mast cell-associated-cytokine production might also insult the airway tissue and the coronary arteries, culminating in ARDS and in immunology-associated Kounis syndrome [82].
It has been proposed that co-stimulation of human and rodent mast cell populations in vitro with FcεRI and certain TLRs can induce release of various pro-inflammatory mediators, suggesting another mechanism through which bacterial or viral infections might exacerbate atopic asthma and other IgE- and mast cell-associated disorders such as Kounis syndrome in vivo [76,81].
3.9. The Dengue and Human Immunodeficiency Viruses (HIV) Paradigm
Surprisingly, the same clinical manifestations as in COVID-19, namely fever, headache, bone pain, skin rash, shock syndrome with major features of high levels of pro-inflammatory cytokines, vascular leakage, thrombocytopenia, hypotensive shock, myocarditis, myocardial infarction and to a lesser extent hemorrhage [83], are encountered in other viral diseases including dengue fever-a mosquito-transmitted viral infection [84].
Dengue virus has been associated with immunological mechanisms such as antibody-dependent enhancement [85]. Indeed, opsonization of dengue particles can bound in immune complexes with non-neutralizing antibodies, leading to an increase in infection rate in FcR-bearing cells, such as mast cells, dendritic cells and monocytes and higher levels of viral replication [86].
In subjects infected with HIV, the IgEs, HIV-1 gp120 and Tat proteins were demonstrated to be increased [86]. These molecules can induce human mast cells migration, functional activation in vitro, and further release of IL-4 and IL-13 from human mast cells and basophils. Moreover, Tat protein also can upregulate the β-chemokine receptor CCR3, expressed on lung mast cells, that it also constitutes a co-receptor of HIV-1 infection [87].
Therefore, this cascade of viruses, coronaviruses, mast cells, other interacting inflammatory cells and platelets lead to releasing mediators acting on specific receptors. The final result is myocardial injury, coagulopathy, thrombosis, coronary complications and, in particular, the immunity-associated, induced by mast cell activation Kounis syndrome.
3.10. The Kounis Syndrome
Anaphylactic, allergic, hypersensitivity, or anaphylactoid reactions correlated with cardiovascular disorders were initially characterized as acute carditis, morphologic cardiac reactions, or rheumatic carditis of unknown pathophysiology and were attributed to serum pathology. The allergic angina syndrome, a coronary spasm leading to allergic acute myocardial infarction constituting an endothelial dysfunction or microvascular manifestation, was first introduced 30 years ago [88] and in 2005 was named Kounis syndrome [89].
The pathophysiology of this syndrome [90] includes inflammatory mediators released during an anaphylactic event from mast cell activation and degranulation in the context of other interacting cells, including lymphocytes, macrophages, eosinophils, monocytes, eosinophils and dendritic cells (Table 5). Although mast cells consist numerically as a minority in this inflammatory cascade, they influence decisively the inflammatory process. Platelets also participate in this cascade via activation of their corresponding receptors by thromboxane, histamine, and platelet-activating factor. Moreover, platelets express also high affinity IgE (FcεRI) and low affinity IgE (FcεRII/CD32) receptors that constitute potential targets for many antigens [91]. Potential trigger factors of the Kounis syndrome include drugs, metals, foods, environmental exposures, and clinical conditions.

Table 5.
Mast cell: the “pleiotropic cell”and its inflammatory mediators participating in “Cytokine storm” able to induce the Kounis syndrome.
3.11. COVID-19 and Myocardial Infarction
According to recent reports, ST segment-elevation myocardial infarction (STEMI), may represent the first clinical manifestation of COVID-19. Indeed, STEMI represented the first clinical manifestation of COVID-19 in 85.7% of patients who did not have a COVID-19 test result at the time of coronary angiography [89,90]. Therefore, further elucidation of the myocardial injury pathophysiology in patients with COVID-19 seems of paramount importance.
The Kounis syndrome induced by the release of inflammatory mediators through mast cell degranulation may manifest as coronary spasm due to endothelial dysfunction or microvascular angina in patients with normal or nearly normal coronary arteries constituting subtype MINOCA (myocardial infarction with non-obstructive coronary arteries), coronary thrombosis of type1 myocardial infarction and stent thrombosis [92]. Similarly, the myocardial injury in patients with COVID-19 has been attributed to coronary spasm, direct endothelial or vascular injury, plaque rupture and microthrombi, hypoxic injury or cytokine storm [93].
Recently, a series of patients suffering from COVID-19 revealed a higher-than-expected incidence of stent thrombosis [94], a fact that was attributed to the underlying hypercoagulable state [95]. Although thrombus aspiration was performed only in one patient, further histopathological study that may be valuable for identification of stent thrombosis cause has not taken place.
Moreover, none of the other patients were evaluated for IgEs, specific IgEs and other inflammatory mediators that could have confirmed the presence of any type of cytokine surge and Kounis syndrome [96]. Indeed, in a recent longitudinal analysis, severe COVID-19 was accompanied by an increase in effector cells including IgEs and eosinophils [97]. Furthermore, in current clinical practice, Kounis syndrome has appeared not only in COVID-19 cases [98,99], but also in cases following vaccine administration for prevention of COVID-19 disease [100,101].
Although stent thrombosis is a multifactorial complication, the implanted “antigenic complex” of nickel alloys, polymers, eluted drugs, in conjunction with concomitant oral antiplatelet drugs and environmental exposures can induce Kounis syndrome. Thus far, all clinical reports and pathologic findings in all patients and animal experimental studies point toward inflammation with thrombus infiltration of various interrelated and interacting inflammatory cells, including eosinophils, macrophages, T cells, and mast cells [102].
The histamine-2 receptor antagonist famotidine that blocks the histamine 2 receptor has significantly reduced the risk for death or intubation (adjusted hazard ratio (aHR) 0.42, 95% CI 0.21–0.85) and also the risk for death alone (aHR 0.30, 95% CI 0.11–0.80) in hospitalized patients with COVID-19 [103].
Indeed, famotidine pretreatment can prevent endothelial cell permeability attributed to histamine H2 activation [103]. It must be emphasized that famotidine and other antihistamines are drugs of choice for prevention and treatment of Kounis syndrome [104], as well as for prevention of myocardial ischemia/reperfusion injury induced by histamine H2 receptor activation [105] and further for peripheral vessel leakage prevention that is incriminated for the shock in anaphylaxis.
The value of corticosteroids is widely debated, but they have been widely used in syndromes closely related to COVID-19, including SARS-CoV, MERS, severe influenza, and community-acquired pneumonia. Corticosteroids are also the drugs of choice for the treatment of Kounis syndrome. According to RECOVERY trial, dexamethasone reduced the 28-day mortality in hospitalized patients with COVID-19 receiving invasive mechanical ventilation or oxygen at randomization to 29.3%, as compared with 41.4% in the usual care group [106].
Therefore, these similarities in pathophysiology, etiology, clinical cardiac manifestations and therapeutic approaches of the severe COVID-19 cardiac complications and anaphylaxis-associated Kounis syndrome might be proven as beneficial for future treatments based on the antigenic substrate of the latter.
The use, for example, of humanized monoclonal antibodies to clear free IgEs and downregulate FcεRI expression might represent a new era for the treatment or prevention of such events. This has been already demonstrated for Kounis syndrome via anti-Immunoglobulin E administration [96,107].
3.12. COVID-19 Vaccine-Induced Thrombotic Events
The COVID-19 pandemic necessitated the rapid development of various technologies’ vaccines. Some rare cases of anaphylaxis, immune thrombocytopenia and bleeding without thrombosis have been associated with the messenger RNA (mRNA)-based vaccines, namely the mRNA-1273 (Moderna) and BNT162b2 (Pfizer–BioNTech) [108]. However, rare delayed manifestations, mainly in women of less than sixty years of age resembling autoimmune heparin-induced thrombocytopenia with thrombosis (HITT) and especially serious cerebral venous sinus thrombosis(CVST), in the absence of heparin administration, have appeared 5–24 days after initial vaccination with both the adenoviral vector ChAdOx1 nCov-19 (AstraZeneca) and Ad26.COV2.S (Janssen/Johnson & Johnson) vaccines [109]. In the latter rare cases, almost every patient has been detected with high levels of antibodies to platelet factor 4 (PF4)-polyanion complexes.
The arising questions, therefore, include: why these HIT-like thromboses are delayed and seem to occur in the absence of heparin administration with these 2 specific COVID-19 vaccines affecting only young middle-aged women? Is there any common pathway that connects all these 4 conditions? We believe that the following may provide an answer to these questions:
- A.
- The prevalence of delayed adverse reactions and especially hypersensitivity reactions appearing days after vaccination is not new. Such reactions are antibody-independent, cell-mediated, stemming from over stimulation of T cells and monocytes/macrophages and cytokine’s release that further cause inflammation, cell death, and tissue damage [110]. They have been associated with vaccines containing anti-microbial agents and ingredients, such as thimerosal and aluminum [111] and withJapanese encephalitis and rabies vaccines [112]. A common type of delayed hypersensitivity reaction is the heparin hypersensitivity [113].
- B.
- Heparin and related polymer heparin sulfate constitute natural glycosaminoglycans that have particular relevance to allergy and inflammation. The PF4 is a highly positive protein present in the a-granules of platelets that can quickly bind to either exogenous or endogenous heparin. Indeed, the platelet released PF4 binds to surface glycosaminoglycans on hematopoietic and vascular cells [114]. The pair PF4/heparin acts as an autoantigen and induces anti-PF4/heparin antibodies of IgG class. PF4-heparin-IgG antibody complexes have been detected in 3.1–4.4% of healthy subjects and can cause thrombosis via activation of the specific low-affinity IgG (FcγRIIa/CD32) receptors on the platelet surface. However, platelet surface disposes also high affinity IgE (FcεRI) and low affinity IgE (FcεRII/CD23) receptors, a fact that is not so well known to clinical practitioners [90]. Furthermore, receptors for histamine, platelet-activating factor, thromboxan, thrombin, adenosine diphosphate (ADP) can also exist in the platelet surface (Figure 2). Upon their activation, platelets secrete pro-inflammatory pro-thrombotic, adhesive, and chemotactic mediators that propagate, amplify and sustain the thrombotic process [115]. The platelet consumption, due to extensive thrombosis, leads to thrombocytopenia [116]. The 3-component PF4-heparin-IgG antibody complex can bind to FcγRII receptors on monocytes, inducing endothelial injury that leads to tissue factor release, thrombin production and diffusion. Thrombin production plays an important role in the pathogenesis of CVST and thrombosis in general [117]. The classical HIT is not complicated with CVST, despite constituting a highly pro-thrombotic condition. In COVID-19-vaccination-associated CVST, any form of heparin (i.e., unfractionated heparin, even for line flushes, or LMWH e.g., enoxaparin) and platelet transfusion should be avoided. Treatment with Argatroban, which is a direct thrombin inhibitor together with corticosteroids (that are used for Kounis syndrome) such as dexamethasone and immunoglobulin, are recommended [118]. All this cascade of actions, reactions, interactions, together with the released mediators and inflammatory cells, constitute the pathophysiologic basis of Kounis anaphylaxis-associated thrombotic syndrome [119]. It is reasonable, therefore, to anticipate that heparin is not always required to be given externally in order to cause HIT-like thrombosis and that HIT itself behaves as a new manifestation of Kounis syndrome [120,121].
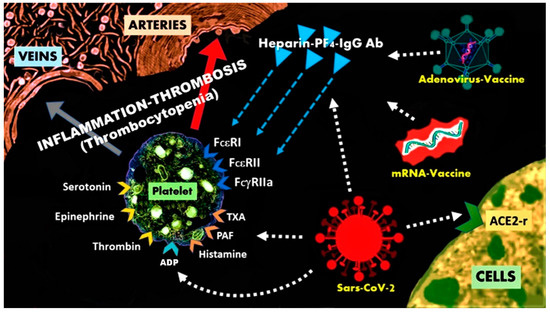 Figure 2. The heparin-PF4-IgG antibody complexes can activate platelets, via the FcεRI, FcεRII, FcγRII receptors and cause heparin-induced thrombocytopenia with thrombosis.
Figure 2. The heparin-PF4-IgG antibody complexes can activate platelets, via the FcεRI, FcεRII, FcγRII receptors and cause heparin-induced thrombocytopenia with thrombosis.
- C.
- Both adenoviral vector ChAdOx1 nCov-19(AstraZeneca) and Ad26.COV2.S (Janssen/Johnson & Johnson) vaccines contain excipients that could be potential antigens such as polysorbate, a synthetic nonionic surfactant, also known as Tween 80, which is a mixture of esters and etherates synthesized by oleic acid, ethylene oxide, sorbitan and isosorbide. Polysorbate 80 can induce systemic reactions including IgE immediate reactions as well as non-immunologic anaphylactoid reactions but also local reactions such as thrombophlebitis, pain, erythema [122]. Polysorbate can enhance membrane permeability, penetrate the blood–brain barrier and facilitate the passage of drugs from the blood compartment to the brain for therapeutic purposes, especially in oncology [123]. The release of cytokines from overstimulation of T cells, monocytes and macrophages can induce inflammatory response and delayed adverse reactions post polysorbate administration via the vaccine. Such polysorbate actions make one wonder whether or not the adenoviral vector vaccine-induced CVST is a simple coincidence. Indeed, as mentioned above, the classical HIT, which is not related to polysorbate, is not complicated with CVST.
- D.
- Younger-aged women, who routinely use creams, ointments, lotions, and other cosmetics that contain polysorbate, could have been earlier sensitized to polysorbate and will likely develop adverse reactions when exposed to the same agent. Furthermore, polysorbate is also an ingredient in various dental materials and contact sensitization in dental patients has been proven [124]. Indeed, it is estimated that 1–5.4% of the population is already sensitized to cosmetics or cosmetic ingredients [124]. We suggest, therefore, that atopic subjects and patients with a previous allergic history should carefully look at SPC (Special Product Characteristics) of their used cosmetics for any containing polysorbate excipient, and also for any other ingredient cross-reacting with polysorbate such as polyethylene glycol product. The latter is contained in the messenger RNA (mRNA)-based vaccines mRNA-1273 (Moderna) and BNT162b2 (Pfizer–BioNTech), respectively. Additionally, the Moderna vaccine contains tromethamine, also known as trometamol, that is also used in cosmetic products as an emulsifier. Contact sensitization, allergy and anaphylaxis to this compound have been already reported [125]. Individuals who present late adverse reactions following the first dose of the above vaccines could proceed to skin testing in order to elucidate the cause of the reactions and in case of being positive to polysorbate 80 to avoid the second dose as subsequent exposure to the substance could even lead to an immediate type of reactions. Pharmaceutical companies should try to produce free allergenic vaccines. Free polysorbate oncology medications have been already in the market [122]. Alternatives to polysorbate and PEG excipients such as alkylsaccharides constitute promising agents because they reduced immunogenicity, improve stability, and suppress oxidative damage problems of polysorbates or other polyoxyethylene-based excipients [126].
4. Discussion
The Food and Drug Administration (FDA) has created a special emergency program forpossible therapies, the Coronavirus Treatment Acceleration Program, in an attempt to evaluate whether new treatments might be helpful and their further application to patient management as quickly as possible.
The main representatives of agents (Table 6) together with specific and non specific intravenous immune globulins, convalescent plasma and humanized monoclonal antibodies are available in the medical armamentarium. These medications seem to exert a positive impact, especially in severe cases with laboratory evidence of host hyper-inflammatory response and or development of the macrophage-activation syndrome.

Table 6.
The variety of current COVID-19 treatments.
In previous years, multiple viral and bacterial pathogens have been associated with atherosclerosis and experimental studies demonstrating an acceleration of atherogenesis following infection in animal models [127].
Recent studies have reported that COVID-19 infection enhances activation of the immune system similar to the macrophage-activation syndrome that may further unmask any potential subclinical cardiovascular disease promoting intra-plaque inflammation and subsequent pro-thrombotic activation [128].
Based on these patho-physiological mechanisms, it appears possible that asymptomatic subjects infected with COVID-19 are predisposed to an increased risk of conversion from asymptomatic, subclinical, atherosclerotic disease into an unstable state with vulnerable plaques prone to thrombosis [129].
Viral activated mast cells release early inflammatory chemical compounds, including histamine and proteases, while late activation may provoke theactivation of pro-inflammatory IL-1 family members including IL-1, IL-6, and IL-33. It has been proposed that inflammation by coronavirus may be inhibited by anti-inflammatory cytokines belonging to the IL-1 family members [130].
The role of Vitamin D has been shown to be protective and safe against acute respiratory infections, and dedicated studies about vitamin D levels inCOVID-19 have been proposed [131].
COVID-19 has been associated with ST segment-elevation myocardial infarction [115,132], but the course of the disease after their hospital discharge has not been clarified. Indeed, respiratory infections caused by influenza, para-influenza, rhinovirus, respiratory syncytial virus, adenovirus, or humanmeta-pneumovirus have been found as independent predictors of hospital readmission for myocardial infarction [133,134], while influenza epidemics are associated with a higher risk of out-of-hospital cardiac arrest [135]. Since pathophysiology and virulence mechanisms of coronavirus, and especially of COVID-19, are correlated with the function of their nonstructural and structural proteins [136], a similar effect could be expected and in patients with COVID-19infection. Implementation, therefore, of public health interventions may reduce the risk of out-of-hospital cardiac arrest during the current COVID-19 pandemic.
Whereas the current literature on COVID-19 is replete with anecdotal reports of therapeutic successes, small clinical trials, observational cohort studies, hypotheses, ideas and suggestions that claim efficacy and suspected success, it pays little attention to the effect of unrecognized confounders [137].
Indeed, several diverse medications with antiviral or immunomodulatory activity have been used in clinical practice, several others have been proposed, but large clinical trials that are necessary to prove their efficacy are lacking.
Therefore, physicians should be always bring in mind that “empty vessels make the most noise” expressed by the English author William Baldwin’s and Aristotle’s (384–322 B.C.) dictum: “Not many is the good, but in the good, the many” (Ouk en tō Pollo to af, but en to af to poly=OΥΚ ΕΝ ΤΩ ΠOΛΛΩ ΤO ΕΥ AΛΛA ΕΝ ΤΩ ΕΥ ΤO ΠOΛΥ)
5. Conclusions
Similarities between allergy/anaphylaxis-associated Kounis syndrome and COVID-19 immunology strongly suggest that the same key immune cells might be involved in COVID-19-induced anaphylaxis and the anaphylaxis-associated Kounis syndrome. It has been already emphasized that early immunological interventions targeting inflammatory markers predictive of adverse outcomes could be more beneficial than those targeting cytokine inhibition [119].
It seems that an individualized treatment approach, tailored to each patient, is required in COVID-19 cases; as severity of the disease, comorbidities, age, clinico-laboratory characteristics, indications for hyper-inflammatory response, cardiovascular and myocardial complications and host factors are important determinants for the disease progression. General and personal protective equipment for all people, and especially for the healthcare workers, is the final line of protection [138]. The incidence of severe adverse reactions and anaphylaxis is very low [139,140] but is highly encouraged to support vaccination against COVΙD-19 because vaccines are the only means to fight this pandemic. Further investigations of relevant risk identification and management approaches for COVID-19 consequences are required. There is much to learn from this pandemic.
Author Contributions
Conceptualization, N.G.K., S.F.A., D.V., C.d.G., M.G., E.N., V.M., V.A., L.S., D.O.R., P.S., R.W.M.; methodology, N.G.K., C.G., A.B., S.N.K., G.D.S.; writing—original draft preparation, N.G.K., I.K., S.F.A., M.-Y.H., C.d.G.; writing—review and editing, N.G.K., I.K., V.A., P.S., K.N., M.-Y.H.; visualization, N.G.K., C.d.G., G.D.S.,V.M., S.F.A.; supervision, N.G.K., I.K., S.F.A., S.N.K., D.V.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The study did not report new data.
Conflicts of Interest
The authors have no financial or other interest in the product or distributor of the product. Furthermore, they have no other kinds of associations, such as consultancies, stock ownership, or other equity interests or patent-licensing arrangements.
Abbreviations
| aHR | Adjusted hazard ratio |
| ACE2 | Angiotensin-converting enzyme 2 |
| ADP | Adenosine diphosphate |
| ARDS | Acute respiratory distress syndrome |
| CAR-T | Chimeric antigen receptor T cells |
| CD8 | Cluster of differentiation 8 |
| CCL2 | Chemokine (C-C motif) ligand 2 |
| CCR3 C-C | chemokine receptor type 3 |
| COVID-19 | Coronavirus disease 2019 |
| CRS | Cytokine release syndrome |
| CSS | Cytokine storm syndrome |
| CVST | Cerebral venous sinus thrombosis |
| CSFs | Colony-stimulating factors |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| G-CSF | Granulocyte colony-stimulating factor |
| FDA | Food and Drug Administration |
| FcγRIIa | Fragment crystallizable gamma region II alpha |
| FcεRI | Fragment crystallizable epsilon region I |
| FcεRII | Fragment crystallizable epsilon region II |
| HLH/MAS | Hemophagocytic lymphohistiocytosis/ macrophage activation syndrome |
| HIV | Human immunodeficiency virus |
| HIT | Heparin-induced thrombocytopenia |
| HITT | Heparin-induced thrombocytopenia with thrombosis |
| IFNs | Interferons |
| ILs | Interleukins |
| KD | Kawasaki disease |
| MAS | Macrophage activation syndrome |
| MERS | Middle East Respiratory Syndrome |
| MCP-1 | Monocyte chemoattractant protein-1 |
| M-CSF | Macrophage colony-stimulating factor |
| MINOCA | Myocardial infarction with non-obstructive coronary arteries |
| PAF | Platelet activating factor |
| PEG | Polyethylene glycol |
| RECOVERY | Randomized Evaluation of COVID-19 therapy |
| mRNA | Messenger ribonucleic acid |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SARS-COV | Severe acute respiratory syndrome coronavirus |
| SPC | Special product characteristics |
| STEMI ST | Segment-elevation myocardial infarction |
| TNFs | Tumor necrosis factors |
| TLR TXA | Toll-like receptor Thromboxane |
References
- Li, Q.; Guan, X.; Wu, P.; Chu, H.; To, K.K.; Yuan, S.; Yuen, K.Y. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Chan, J.F.; Kok, K.H.; Zhu, Z.; Chu, H.; To, K.K.; Yuan, S.; Yuen, K.Y. Genomic characterization of the 2019 novelhuman-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef] [Green Version]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.P.; Look, D.C.; Shi, L.; Hickey, M.; Pewe, L.; Netland, J.; Farzan, M.; Wohlford-Lenane, C.; Perlman, S.; McCray, P.B., Jr. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J. Virol. 2005, 79, 14614–14621. [Google Scholar] [CrossRef] [Green Version]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Zhu, Z.; Lian, X.; Su, X.; Wu, W.; Marraro, G.A.; Zeng, Y. From SARS and MERS to COVID-19: A brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir. Res. 2020, 21, 224. [Google Scholar] [CrossRef]
- John Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/data/mortality (accessed on 25 June 2020).
- Abdelrahman, Z.; Li, M.; Wang, X. Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza a Respiratory Viruses. Front. Immunol. 2020, 11, 552909. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Karki, C.B.; Du, D.; Teng, S.; Tang, Q.; Li, L. Spike Proteins of SARS-CoV and SARS-CoV-2 Utilize Different Mechanisms to Bind with Human ACE2. Front. Mol. Biosci. 2020, 7, 591873. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.S.; Hui, K.P.; Yen, H.L. Host response to influenza virus: Protectionversus immunopathology. Curr. Opin. Immunol. 2010, 22, 475–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suntharalingam, G.; Perry, M.R.; Ward, S.; Brett, S.J.; Castello-Cortes, A.; Brunner, M.D.; Panoskaltsis, N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006, 355, 1018–1028. [Google Scholar] [CrossRef]
- Kroschinsky, F.; Stolzel, F.; von Bonin, S.; Beutel, G.; Kochanek, M.; Kiehl, M.; Schellongowski, P. New drugs, new toxicities: Severe side effects of modern targeted and immunotherapy of cancer and their management. Crit. Care 2017, 21, 89. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Bi, Y.; Sun, K.; Wang, C. IL-10-producing type 1 regulatory T cells and allergy. Cell Mol. Immunol. 2007, 4, 269–275. [Google Scholar]
- Ferrara, J.L.; Abhyankar, S.; Gilliland, D.G. Cytokine storm of graft versus host disease: A critical effector role for interleukin-1. Transplant. Proc. 1993, 25, 1216–1217. [Google Scholar]
- Imashuku, S. Clinical features and treatment strategies of Epstein-Barrvirus-associated hemophagocytic lymphohistiocytosis. Crit. Rev. Oncol. Hematol. 2002, 44, 259–272. [Google Scholar] [CrossRef]
- Bisno, A.L.; Brito, M.O.; Collins, C.M. Molecular basis of group Astreptococcal virulence. Lancet Infect. Dis. 2003, 3, 191–200. [Google Scholar] [CrossRef]
- Yokota, S. Influenza-associated encephalopathy-pathophysiology and disease mechanisms. Nippon Rinsho 2003, 61, 1953–1958. [Google Scholar] [PubMed]
- Jahrling, P.B.; Hensley, L.E.; Martinez, M.J.; Leduc, J.W.; Rubins, K.H.; Relman, D.A.; Huggins, J.W. Exploring the potential of variola virus infection of cynomolgus macaques as a model for human smallpox. Proc. Natl. Acad. Sci. USA 2004, 101, 15196–15200. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.J.; Su, I.J.; Theron, M.; Wu, Y.C.; Lai, S.K.; Liu, C.C.; Lei, H.Y. An interferon-γ-related cytokine storm in SARS patients. J. Med. Virol. 2005, 75, 185–194. [Google Scholar] [CrossRef]
- Yuen, K.Y.; Wong, S.S. Human infection by avian influenza A H5N1. Hong Kong Med. J. 2005, 11, 189–199. [Google Scholar]
- Velthuis, A.J.W.; Long, J.C.; Bauer, D.L.V.; Fan, R.L.Y.; Yen, H.L.; Sharps, J.; Siegers, J.Y.; Killip, M.J.; French, H.; Oliva-Martín, M.J. Mini viral RNAs act as innate immune agonists during influenza virus infection. Nat. Microbiol. 2018, 3, 1234–1242. [Google Scholar] [CrossRef]
- Page, D.B.; Postow, M.A.; Callahan, M.K.; Allison, J.P.; Wolchok, J.D. Immune modulation in cancer with antibodies. Annu. Rev. Med. 2014, 65, 185–202. [Google Scholar] [CrossRef]
- Van der Linden, P.W.; Hack, C.E.; Struyvenberg, A.; Roem, D.; Brouwer, M.C.; deBoer, J.P.; van der Zwan, J.K. Controlled insect-sting challenge in 55 patients: Correlation between activation of plasminogen and the development of anaphylactic shock. Blood 1993, 82, 1740–1748. [Google Scholar] [CrossRef] [Green Version]
- Castells, M. Mast cell mediators in allergic inflammation and mastocytosis. Immunol. Allergy Clin. N. Am. 2006, 26, 465–485. [Google Scholar] [CrossRef]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517528. [Google Scholar] [CrossRef]
- Wang, F.; Nie, J.; Wang, H.; Zhao, Q.; Xiong, Y.; Deng, L.; Song, S.; Ma, Z.; Mo, P.; Zhang, Y. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J. Infect. Dis. 2020, 221, 1762–1769. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Comerford, I.; McColl, S.R. Focus on chemokines. Immunol. Cell Biol. 2011, 89, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Scheller, J.; Rose-John, S. Interleukin-6 and its receptor: From bench to bedside. Med. Microbiol. Immunol. 2006, 195, 173–183. [Google Scholar] [CrossRef]
- Brocker, C.; Thompson, D.; Matsumoto, A.; Nebert, D.W.; Vasiliou, V. Evolutionary divergence and functions of the human interleukin (IL) genefamily. Hum. Genom. 2010, 5, 30–55. [Google Scholar] [CrossRef]
- Zemans, R.L.; Colgan, S.P.; Downey, G.P. Transepithelial migration of neutrophils: Mechanisms and implications for acute lung injury. Am. J. Respir. Cell Mol. Biol. 2009, 40, 519–535. [Google Scholar] [CrossRef] [Green Version]
- Neville, L.F.; Abdullah, F.; McDonnell, P.M.; Young, P.R.; Feuerstein, G.Z.; Rabinovici, R. Mob-1 expression in IL-2-induced ARDS: Regulation by TNFalpha. Am. J. Physiol. 1995, 269 Pt 1, L884–L890. [Google Scholar] [CrossRef]
- Borden, E.C.; Sen, G.C.; Uze, G.; Silverman, R.H.; Ransohoff Foster, G.R.; Stark, G.R. Interferons at age 50: Past, current and future impact on biomedicine. Nat. Rev. Drug Discov. 2007, 6, 975–990. [Google Scholar] [CrossRef]
- Pandey, S.; Bonilla, H.F.; Jacobson, K.; Parsonnet, J.; Andrews, J.R.; Weiskopf, D.; Sette, A.; Pinsky, B.A.; Singh, U.; Banaei, N. Interferon-gamma release assay for accurate detection of SARS-CoV-2 T cell response. Clin. Infect. Dis. 2020, ciaa1537. [Google Scholar] [CrossRef]
- Carswell, E.A.; Old, L.J.; Kassel, R.L.; Green, S.; Fiore, N.; Williamson, B. An endotoxin induced serum factor that causes necrosis of tumors. Proc. Natl. Acad. Sci. USA 1975, 72, 3666–3670. [Google Scholar] [CrossRef] [Green Version]
- Feldmann, M.; Maini, R.N.; Woody, J.N.; Holgate, S.T.; Winter, G.; Rowland, M.; Richards, D.; Hussell, T. Trials of anti-tumour necrosis factor therapy forCOVID-19 are urgently needed. Lancet 2020, 39, 1407–1409. [Google Scholar] [CrossRef]
- Hamilton, J.A. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 2008, 8, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Cremer, P.C.; Carey, B.; Rajendram, P.; Hudock, K.M.; Korbee, L.; Van Tassell, B.W.; Dagna, L.; Abbate, A. Targeting GM-CSF in COVID-19 Pneumonia:Rationale and Strategies. Front. Immunol. 2020, 11, 1625. [Google Scholar]
- Shimabukuro-Vornhagen, A.; Gödel, P.; Subklewe, M.; Stemmler, H.J.; Schlößer, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kounis, N.G.; Soufras, G.D.; Hahalis, G. Anaphylactic cardiac collapse, sudden death and the Kounis syndrome. J. Postgrad. Med. 2014, 60, 227–229. [Google Scholar] [CrossRef]
- Hay, K.A.; Hanafi, L.-A.; Li, D.; Gust, J.; Liles, W.C.; Wurfel, M.M.; López, J.A.; Chen, J.; Chung, D.; Harju-Baker, S. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 2017, 130, 2295–2306. [Google Scholar] [CrossRef] [Green Version]
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N. Chimeric antigen receptor T-cell therapy-assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018, 15, 47–62. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Medaglia, A.A.; Siracusa, L.; Gioè, C.; Giordano, S.; Cascio, A.; Colomba, C. Kawasaki disease recurrence in the COVID-19 era: A systematic review of the literature. Ital. J. Pediatr. 2021, 47, 95. [Google Scholar] [CrossRef]
- Rowley, A.H.; Baker, S.C.; Arrollo, D.; Gruen, L.J.; Bodnar, T.; Innocentini, N.; Hackbart, M.; Cruz-Pulido, Y.E.; Wylie, K.M.; Kim, K.A.; et al. A Protein Epitope Targeted by the Antibody Response to Kawasaki Disease. J. Infect. Dis. 2020, 222, 158–168. [Google Scholar] [CrossRef]
- Rowley, A.H. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat. Rev. Immunol. 2020, 20, 453–454. [Google Scholar] [CrossRef]
- Morris, S.B.; Schwartz, N.G.; Patel, P.; Abbo, L.; Beauchamps, L.; Balan, S.; Lee, E.H.; Paneth-Pollak, R.; Geevarughese, A.; Lash, M.K.; et al. Case Series of Multisystem Inflammatory Syndrome in Adults Associated with SARS-CoV-2 Infection—United Kingdom and United States, March-August 2020. MMWR Morb. Mortal. Wkl. Rep. 2020, 69, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, C.R.; Cotugno, N.; Sardh, F.; Pou, C.; Amodio, D.; Rodriguez, L.; Tan, Z.; Zicari, S.; Ruggiero, A.; Pascucci, G.R.; et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell 2020, 183, 968–981.e7. [Google Scholar] [CrossRef] [PubMed]
- Gruber, C.N.; Patel, R.S.; Trachtman, R.; Lepow, L.; Amanat, F.; Krammer, F.; Wilson, K.M.; Onel, K.; Geanon, D.; Tuballes, K.; et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell 2020, 183, 982–995.e14. [Google Scholar] [CrossRef]
- Yakoub-Agha, I.; Chabannon, C.; Bader, P.; Basak, G.W.; Bonig, H.; Ciceri, F.; Corbacioglu, S.; Duarte, R.F.; Einsele, H.; Hudecek, M.; et al. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: Best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Haematologica 2020, 105, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, Z.; Li, J.W.; Zhao, H.; Wang, G.Q. Cytokine release syndrome in severe COVID-19: Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents 2020, 55, 105954. [Google Scholar] [CrossRef]
- Hu, Y.; Tan Su Yin, E.; Yang, Y.; Wu, H.; Wei, G.; Su, J.; Cui, Q.; Jin, A.; Yang, L.; Fu, S.; et al. CAR T-cell treatment during the COVID-19 pandemic: Management strategies and challenges. Curr. Res. Transl. Med. 2020, 68, 111–118. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Y.; Wang, C.; Zhang, Y.; Tong, C.; Dai, G.; Wang, W.; Rasko, J.E.J.; Melenhorst, J.J.; Qian, W.; et al. The model of cytokine release syndrome in CAR T-cell treatment for B-cell non-Hodgkin lymphoma. Signal Transduct, Target. Ther. 2020, 5, 134. [Google Scholar] [CrossRef]
- Oliynyk, O.; Barg, W.; Slifirczyk, A.; Oliynyk, Y.; Gurianov, V.; Rorat, M. Efficacy of Tocilizumab Therapy in Different Subtypes of COVID-19 Cytokine Storm Syndrome. Viruses 2021, 13, 1067. [Google Scholar] [CrossRef] [PubMed]
- Yongzhi, X. COVID-19-associated cytokine storm syndrome and diagnostic principles: An old and new Issue. Emerg. Microbes Infect. 2021, 10, 266–276. [Google Scholar] [CrossRef]
- Kounis, N.G.; Mazarakis, A.; Tsigkas, G.; Giannopoulos, S.; Goudevenos, J. Kounis syndrome: A new twist on an old disease. Future Cardiol. 2011, 7, 805–824. [Google Scholar] [CrossRef]
- Stone, S.F.; Cotterell, C.; Isbister, G.K.; Holdgate, A.; Brown, S.G.; Emergency Department Anaphylaxis Investigators. Elevated serum cytokines during human anaphylaxis: Identification of potential mediators of acute allergic reactions. J. Allergy Clin. Immunol. 2009, 124, 786–792.e4. [Google Scholar] [CrossRef]
- Ogawa, Y.; Grant, J.A. Mediators of anaphylaxis. Immunol. Allergy Clin. N. Am. 2007, 27, 249–260. [Google Scholar] [CrossRef]
- Salari, H.; Chan-Yeung, M. Mast cell mediators stimulate synthesis of arachidonic acid metabolites in macrophages. J. Immunol. 1989, 142, 2821–2827. [Google Scholar]
- Kambayashi, T.; Allenspach, E.J.; Chang, J.T.; Zou, T.; Shoag, J.E.; Reiner, S.L.; Caton, A.J.; Koretzky, G.A. Inducible MHC class II expression by mast cells supports effector and regulatory T cell activation. J. Immunol. 2009, 182, 4686–4695. [Google Scholar] [CrossRef]
- Miyazaki, D.; Nakamura, T.; Toda, M.; Cheung-Chau, K.W.; Richardson, R.M.; Ono, S.J. Macrophage inflammatory protein-1a as a costimulatory signal for mast cell mediated immediate hypersensitivity reactions. J. Clin. Investig. 2005, 115, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Pomares, L.; Gordon, S. CD169_macrophages at the crossroads of antigen presentation. Trends Immunol. 2012, 33, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Mekori, Y.A.; Metcalfe, D.D. Mast cell-T cell interactions. J. Allergy Clin. Immunol. 1999, 104, 517–523. [Google Scholar] [CrossRef]
- Doherty, T.M. T cell regulation of macrophage function. Curr. Opin. Immunol. 1995, 7, 400–404. [Google Scholar] [CrossRef]
- Dahinden, C.A.; Geiser, T.; Brunner, T.; von Tscharner, V.; Caput, D.; Ferrara, P.; Minty, A.; Baggiolini, M. Monocyte chemotactic protein 3 is a most effective basophil-and eosinophil-activating chemokine. J. Exp. Med. 1994, 179, 751–756. [Google Scholar] [CrossRef] [Green Version]
- Ferenbach, D.; Hughes, J. Macrophages and dendritic cells: What is the difference? Kidney Int. 2008, 74, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Dollery, C.M.; Libby, P. Atherosclerosis and proteinase activation. Cardiovasc. Res. 2006, 69, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Newby, A.C. Metalloproteinases promote plaque rupture and myocardial infarction: A persuasive concept waiting for clinical translation. Matrix Biol. 2015, 44–46, 157–166. [Google Scholar] [CrossRef]
- Graham, A.C.; Temple, R.M.; Obar, J.J. Mast cells and influenza a virus: Association with allergic responses and beyond. Front. Immunol. 2015, 6, 238. [Google Scholar] [CrossRef] [Green Version]
- Galli, S.J.; Tsai, M. Mast cells in allergy and infection: Versatile effector and regulatory cells in innate and adaptive immunity. Eur. J. Immunol. 2010, 40, 1843–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann, A.M.; Abraham, S.N. New roles for mast cells in modulating allergic reactions and immunity against pathogens. Curr. Opin. Immunol. 2009, 21, 679–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawicki, W.; Marshall, J.S. New and emerging roles for mast cells in host defence. Curr. Opin. Immunol. 2007, 19, 31–38. [Google Scholar] [CrossRef]
- Sundstrom, J.B.; Hair, G.A.; Ansari, A.A.; Secor, W.E.; Gilfillan, A.M.; Metcalfe, D.D.; Kirshenbaum, A.S. I gE-Fcepsilon RI interaction determine HIV co-receptor usage and susceptibility to infection during ontogeny of mast cells. J. Immunol. 2009, 182, 6401–6409. [Google Scholar] [CrossRef] [PubMed]
- Orinska, Z.; Bulanova, E.; Budagian, V.; Metz, M.; Maurer, M.; Bulfone-Paus, S. TLR3-induced activation of mast cells modulates CD8+ T-cell recruitment. Blood 2005, 106, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Kulka, M.; Alexopoulou, L.; Flavell, R.A.; Metcalfe, D.D. Activation of mast cellsby double-stranded RNA: Evidence for activation through Toll-like receptor 3. J. Allergy Clin. Immunol. 2004, 114, 174–182. [Google Scholar] [CrossRef] [Green Version]
- Kounis, N.G. Coronary hypersensitivity disorder: The Kounis syndrome. Clin. Ther. 2013, 35, 563–571. [Google Scholar] [CrossRef]
- Joob, B.; Wiwanitkit, V. Hemorrhagic Problem among the Patients with COVID-19: Clinical Summary of 41 Thai Infected Patients. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620918308. [Google Scholar] [CrossRef] [Green Version]
- Malavige, G.N.; Ogg, G.S. Pathogenesis of vascular leak in dengue virus infection. Immunology 2017, 151, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Guzman-Rodriguez, M.; Acosta-Nanez, H.F.; Mantilla, J.C.; Bonelo, A. Dengue Virus and Influenza A Virus Co-Infection in Pregnancy: A Case Report. Trop. Med. Infect. Dis. 2019, 4, 84. [Google Scholar] [CrossRef] [Green Version]
- Marone, G.; Florio, G.; Petraroli, A.; Triggiani, M.; de Paulis, A. Role of human FcepsilonRI+ cells in HIV-1 infection. Immunol. Rev. 2001, 179, 128–138. [Google Scholar] [CrossRef]
- Westendorp, M.O.; Frank, R.; Ochsenbauer, C.; Stricker, K.; Dhein, J.; Walczak, H.; Debatin, K.M.; Krammer, P.H. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 1995, 37, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G.; Zavras, G.M. Histamine-induced coronary artery spasm: The concept of allergic angina. Br. J. Clin. Pract. 1991, 45, 121–128. [Google Scholar]
- Rich, M.W. Is vasospastic angina an inflammatory disease? Am. J. Cardiol 2005, 96, 1612. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G.; Kourelis, T.; Hahalis, G.; Manola, A.; Theoharides, T.C. Kounis syndrome (allergic angina and allergic myocardial infarction). In Angina Pectoris; Gallo, A.P., Ed.; Nova Biomedical Books: New York, NY, USA, 2008; pp. 6–150. [Google Scholar]
- Hasegawa, S.; Tashiro, N.; Matsubara, T.; Furukawa, S.; Ra, C. A comparison of FcepsilonRI-mediated RANTES release from human platelets between allergic patients and healthy individuals. Int. Arch. Allergy Immunol. 2001, 125 (Suppl. S1), 42–47. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; Velissaris, D.; Tzanis, G.; Hahalis, G. Kounis Syndrome—Not a Single-organ Arterial Disorder but a Multisystem and Multidisciplinary Disease. Balkan Med. J. 2019, 36, 212–221. [Google Scholar] [CrossRef]
- Tavazzi, G.; Pellegrini, C.; Maurelli, M.; Belliato, M.; Sciutti, F.; Bottazzi, A.; Sepe, P.A.; Resasco, T.; Camporotondo, R.; Bruno, R.; et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020, 22, 911–915. [Google Scholar] [CrossRef] [Green Version]
- Prieto-Lobato, A.; Ramos-Martínez, R.; Vallejo-Calcerrada, N.; Corbí-Pascual, M.; Córdoba-Soriano, J.G. A Case Series of Stent Thrombosis during the COVID-19 Pandemic. JACC Case Rep. 2020, 2, 1291–1296. [Google Scholar] [CrossRef]
- Dominguez-Erquicia, P.; Dobarro, D.; Raposeiras-Roubin, S.; Bastos-Fernandez, G.; Iniguez-Romo, A. Multivessel coronary thrombosis in a patient with COVID-19 pneumonia. Eur. Heart J. 2020, 41, 2132. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; Tsigkas, G.; Davlouros, P. Humanized Monoclonal Antibodies Against IgE Antibodies as Therapy for IgE-Mediated Coronary Syndromes: Are We There Yet? Can. J. Cardiol. 2020, 36, 816–819. [Google Scholar] [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Ertekin, A. Triggered by Covid-19? Large Vascular Occlusion Resulting in Cytokine Storm Syndrome and Kounis Syndrome: A Case Report. J. Biomed. Res. Environ. Sci. 2021, 2, 030–033. [Google Scholar]
- Wang, M.; Talon, A.; Saririan, M. COVID-19 cardiac arrest due to Prinzmetal’s angina in a previously normal heart. Clin. Case Rep. 2021, 9, e04205. [Google Scholar] [CrossRef] [PubMed]
- Tajstra, M.; Jaroszewicz, J.; Gąsior, M. Acute Coronary Tree Thrombosis after Vaccination for COVID-19. JACC Cardiovasc. Interv. 2021, 14, e103–e104. [Google Scholar] [CrossRef]
- Özdemir, İ.H.; Özlek, B.; Özen, M.B.; Gündüz, R.; Bayturan, Ö. Type 1 Kounis Syndrome Induced by Inactivated SARS-COV-2 Vaccine. J. Emerg. Med. 2021. [Google Scholar] [CrossRef]
- Riegger, J.; Byrne, R.A.; Joner, M.; Chandraratne, S.; Gershlick, A.H.; Ten Berg, J.M.; Adriaenssens, T.; Guagliumi, G.; Godschalk, T.C.; Neumann, F.J. Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter European study: A report of the prevention of late stent thrombosis by an interdisciplinary global European effort consortium. Eur. Heart J. 2016, 37, 1538–1549. [Google Scholar] [CrossRef] [Green Version]
- Freedberg, D.E.; Conigliaro, J.; Wang, T.C.; Tracey, K.J.; Callahan, M.V.; Abrams, J.A. Famotidine Use Is Associated with Improved Clinical Outcomes in Hospitalized COVID-19 Patients: A Propensity Score Matched Retrospective Cohort Study. Gastroenterology 2020, 159, 1129–1131.e3. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Hou, D.; Pendyala, L.; Goudevenos, J.A.; Kounis, N.G. Drug eluting stent thrombosis: The Kounis hypersensitivity-associated acute coronary syndrome revisited. J. Am. Coll. Cardiol. Intv. 2009, 2, 583–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, T.; Chen, B.; Zhao, Z.; He, N.; Zeng, Z.; Wu, B.; Fukushima, Y.; Dai, M.; Huang, Q.; Xu, D. Histamine H2 receptor activation exacerbates myocardial ischemia/reperfusion injury by disturbing mitochondrial and endothelial function. Basic Res. Cardiol. 2013, 108, 342. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Liu, Y.; Lu, C.; Guo, Q. Recurrent Type III Kounis Syndrome: Will Anti-Immunoglobulin E Drug Be Another Option? Can. J. Cardiol. 2020, 36, e5–e966. [Google Scholar] [CrossRef]
- Lee, E.J.; Cines, D.B.; Gernsheimer, T.; Kessler, C.; Michel, M.; Tarantino, M.D.; Semple, J.W.; Arnold, D.M.; Godeau, B.; Lambert, M.P.; et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am. J. Hematol. 2021, 96, 534–537. [Google Scholar] [CrossRef]
- Cines, D.B.; Bussel, J.B. SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021, 384, 2254–2256. [Google Scholar] [CrossRef]
- Karron, R.A.; Key, N.S.; Sharfstein, J.M. Assessing a Rare and Serious Adverse Event Following Administration of the Ad26.COV2.S Vaccine. JAMA 2021, 325, 2445–2447. [Google Scholar] [CrossRef]
- Kuder, M.M.; Lang, D.M.; Patadia, D.D. Anaphylaxis to vaccinations: A review of the literature and evaluation of the COVID-19 mRNA vaccinations. Cleve Clin. J. Med. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- McNeil, M.M.; DeStefano, F. Vaccine-associated hypersensitivity. J. Allergy Clin. Immunol. 2018, 141, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Pföhler, C.; Müller, C.S.; Pindur, G.; Eichler, H.; Schäfers, H.J.; Grundmann, U.; Tilgen, W. Delayed-type heparin allergy: Diagnostic procedures and treatment alternatives-a case series including 15 patients. World Allergy Organ. J. 2008, 1, 194–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, J.; Madeeva, D.; Hayes, V.; Ahn, H.S.; Tutwiler, V.; Arepally, G.M.; Cines, D.B.; Poncz, M.; Rauova, L. Dynamic intercellular redistribution of HIT antigen modulates heparin-induced thrombocytopenia. Blood 2018, 132, 727–773. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Sharma, A.; Slotwiner, A.; Yatskar, L.; Harari, R.; Shah, B.; Ibrahim, H.; Friedman, G.H.; Thompson, C.; Alviar, C.L.; et al. ST-Segment Elevation in Patients with Covid-19—A Case Series. N. Engl. J. Med. 2020, 382, 2478–2480. [Google Scholar] [CrossRef] [PubMed]
- Chong, B.H. Heparin-induced thrombocytopenia. Br. J. Haematol. 1995, 89, 431–439. [Google Scholar] [CrossRef]
- Kounis, N.G.; Soufras, G.D.; Almpanis, G.; Tsigkas, G.; Mazarakis, A. Acute stent thrombosis and heparin induced thrombocytopenia: Another manifestation of kounis syndrome? Korean Circ. J. 2013, 43, 221–222. [Google Scholar] [CrossRef]
- Mehta, P.R.; ApapMangion, S.; Benger, M.; Stanton, B.R.; Czuprynska, J.; Arya, R.; Sztriha, L.K. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination—A report of two UK cases. Brain Behav. Immun. 2021, 95, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G.; Koniari, I.; de Gregorio, C. COVID-19 and Kounis Syndrome: Deciphering Their Relationship. Balkan Med. J. 2021, 38, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G.; Soufras, G.D.; Lianas, D.; Patsouras, N. Heparin-induced thrombocytopenia, allergy to heparins, heart failure, thrombi, and the Kounis syndrome. Int. J. Cardiol. 2016, 214, 508–509. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; Soufras, G.D.; Tsigkas, G.; Plotas, P.; Davlouros, P.; Hahalis, G. The paradox of heparin induced thrombocytopenia-thrombosis, the role of fondaparinux and the need for new therapeutic strategies. Int. Angiol. 2020, 39, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, L.S.; Navari, R.M. Safety of polysorbate 80 in the oncology setting. Adv. Ther. 2018, 35, 754–767. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.X.; Liu, A.C.; Yu, S.W.; Wang, Z.X.; Lin, X.Q.; Zhai, G.X.; Zhang, Q.Z. The permeability of puerarin loaded poly(butylcyanoacrylate) nanoparticles coated with polysorbate 80 on the blood-brain barrier and its protective effect against cerebral ischemia/reperfusion injury. Biol. Pharm. Bull. 2013, 36, 1263–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyapina, M.G.; Stoyanova Dencheva, M. Contact sensitization to ingredients of dental materials and cosmetics in dental students: A pilot study. Cent. Eur. J. Public Health 2019, 27, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Lukawska, J.; Mandaliya, D.; Chan, A.W.E.; Foggitt, A.; Bidder, T.; Harvey, J.; Stycharczuk, L.; Bisdas, S. Anaphylaxis to trometamol excipient in gadolinium-based contrast agents for clinical imaging. J. Allergy Clin. Immunol. Pract. 2019, 7, 1086–1087. [Google Scholar] [CrossRef] [PubMed]
- Maggio, E.T. Alkylsaccharides: Circumventing oxidative damage to biotherapeutics caused by polyoxyethylene-based surfactants. Ther. Deliv. 2013, 4, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.A.; Rosenfeld, M.E. Infection and Atherosclerosis Development. Arch Med. Res. 2015, 46, 339–350. [Google Scholar] [CrossRef] [Green Version]
- McGonagle, D.; O’Donnell, J.S.; Sharif, K.; Emery, P.; Bridgewood, C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020, 2, e437–e445. [Google Scholar] [CrossRef]
- Saba, L.; Gerosa, C.; Wintermark, M.; Hedin, U.; Fanni, D.; Suri, J.S.; Faa, G. Can COVID 19 trigger the plaque vulnerability—A Kounis syndrome warning for “asymptomatic subjects”. Cardiovasc. Diagn. Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kritas, S.K.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Conti, P. Mast cells contribute to coronavirus-induced inflammation: New anti-inflammatory strategy. J. Biol. Regul. Homeost. Agents 2020, 34, 9–14. [Google Scholar] [CrossRef]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef]
- Stefanini, G.G.; Montorfano, M.; Trabattoni, D.; Andreini, D.; Ferrante, G.; Ancona, M.; Metra, M.; Curello, S.; Maffeo, D.; Pero, G. ST-Elevation Myocardial Infarction in Patients with COVID-19: Clinical and Angiographic Outcomes. Circulation 2020, 141, 2113–2116. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G.; Koniari, I.; Soufras, G.D.; Chourdakis, E.; Despotopoulos, S.; Davlouros, P.; Hahalis, G. Respiratory Infections as Predictors of Hospital Admission for Myocardial Infarction and Stroke: Pathophysiologic and Therapeutic Considerations. Clin. Infect. Dis. 2019, 68, 533. [Google Scholar] [CrossRef]
- Blackburn, R.M.; Zhao, H.; Pebody, R.; Hayward, A.C.; Warren-Gash, C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: Time-series analysis of English data for 2004–2015. Clin. Infect. Dis. 2018, 67, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Onozuka, D.; Hagihara, A. Extreme influenza epidemics and out-of-hospital cardiac arrest. Int. J. Cardiol. 2018, 263, 158–162. [Google Scholar] [CrossRef]
- Lei, J.; Kusov, Y.; Hilgenfeld, R. Nsp3 of coronaviruses: Structures and functions of a large multi-domain protein. Antiviral. Res. 2018, 149, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Lane, H.C.; Fauci, A.S. Research in the Context of a Pandemic. N. Engl. J. Med. 2021, 384, 755–757. [Google Scholar] [CrossRef]
- Ha, J.F. The COVID-19 pandemic, personal protective equipment and respirator: A narrative review. Int. J. Clin. Pract. 2020, 74, e13578. [Google Scholar] [CrossRef]
- Kim, M.A.; Lee, Y.W.; Kim, S.R.; Kim, J.H.; Min, T.K.; Park, H.S.; Shin, M.; Ye, Y.M.; Lee, S.; Lee, J.; et al. COVID-19 Vaccine-associated Anaphylaxis and Allergic Reactions: Consensus Statements of the KAAACI Urticaria/Angioedema/Anaphylaxis Working Group. Allergy Asthma Immunol. Res. 2021, 13, 526–544. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G.; Koniari, I.; de Gregorio, C.; Velissaris, D.; Petalas, K.; Brinia, A.; Assimakopoulos, S.F.; Gogos, C.; Kouni, S.N.; Kounis, G.N.; et al. Allergic Reactions to Current Available COVID-19 Vaccinations: Pathophysiology, Causality, and Therapeutic Considerations. Vaccines 2021, 9, 221. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).