Hyperbaric Oxygen Therapy Alleviates the Autoimmune Encephalomyelitis via the Reduction of IL-17a and GM-Csf Production of Autoreactive T Cells as Well as Boosting the Immunosuppressive IL-10 in the Central Nervous System Tissue Lesions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Establishment of EAE and Assessment of Clinical Manifestations
2.3. Hyperbaric Oxygen Therapy (HBOT)
2.4. Tissue Preparation
2.5. Proliferation Assay
2.6. Intracellular Cytokine Staining
2.7. ELISA Assay
2.8. Histological Analysis of Spinal Cord
2.9. Statistical Analysis
3. Results
3.1. Semi-Therapeutic Hbot Ameliorates the Disease Progression and Severity of Autoimmune Encephalomyelitis, as Well as Attenuates the Parenchymal Mononuclear Leukocytes and Demyelination
3.2. HBOT Inhibits the Proliferation Activity of T Lymphocytes of MOG-Immunized Mice
3.3. HBOT Relieves the Inflammatory Responses of Th1 and Th17 in the CNS Lesions of Autoimmune Encephalomyelitis
3.4. HBOT Diminishes the Productions of TNF-α and GM-CSF, and Boosts the Expressions of IL-4 and IL-10 of Encephalomyelitogenic CD4 T cell Subsets in the CNS Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Korn, T.; Kallies, A. T cell responses in the central nervous system. Nat. Rev. Immunol. 2017, 17, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Cohan, S.; Lucassen, E.; Smoot, K.; Brink, J.; Chen, C. Sphingosine-1-Phosphate: Its Pharmacological Regulation and the Treatment of Multiple Sclerosis: A Review Article. Biomedicines 2020, 8, 227. [Google Scholar] [CrossRef]
- Karpus, W.J. Cytokines and Chemokines in the Pathogenesis of Experimental Autoimmune Encephalomyelitis. J. Immunol. 2020, 204, 316–326. [Google Scholar] [CrossRef]
- Huang, H.; Xue, L.; Zhang, X.; Weng, Q.; Chen, H.; Gu, J.; Ye, S.; Chen, X.; Zhang, W.; Liao, H. Hyperbaric oxygen therapy provides neuroprotection following spinal cord injury in a rat model. Int. J. Clin. Exp. Pathol. 2013, 6, 1337–1342. [Google Scholar]
- Abraini, J.H.; Chazalviel, L.; Haelewyn, B.; Degoulet, M.; Blatteau, J.-E.; Vallée, N.; Risso, J.-J.; Besnard, S. Hyperbaric oxygen increases tissue-plasminogen activator-induced thrombolysis in vitro, and reduces ischemic brain damage and edema in rats subjected to thromboembolic brain ischemia. Med. Gas Res. 2016, 6, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Li, Y.; Chen, W.; Nong, Z.; Huang, J.; Chen, C. Protective Effect of Hyperbaric Oxygen on Cognitive Impairment Induced by d-Galactose in Mice. Neurochem. Res. 2016, 41, 3032–3041. [Google Scholar] [CrossRef]
- Fischer, B.H.; Marks, M.; Reich, T. Hyperbaric-Oxygen Treatment of Multiple Sclerosis. N. Engl. J. Med. 1983, 308, 181–186. [Google Scholar] [CrossRef]
- Eggleton, P.; Smerdon, G.R.; Holley, J.E.; Gutowski, N.J. Manipulation of Oxygen and Endoplasmic Reticulum Stress Factors as Possible Interventions for Treatment of Multiple Sclerosis: Evidence for and Against. Adv. Exp. Med. Biol. 2017, 958, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.; Eggleton, P.; Smerdon, G.; Newcombe, J.; Holley, J.E.; Gutowski, N.J.; Smallwood, M. Engagement of people with multiple sclerosis to enhance research into the physiological effect of hyperbaric oxygen therapy. Mult. Scler. Relat. Disord. 2020, 43, 102084. [Google Scholar] [CrossRef] [PubMed]
- Khalatbary, A.; Ahmadi, F. A review on the neuroprotective effects of hyperbaric oxygen therapy. Med. Gas Res. 2021, 11, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.-A.; Chang, C.-K.; Niu, K.-C.; Lin, M.-T.; Chiu, W.-T.; Lin, C.-M. Attenuating Experimental Spinal Cord Injury by Hyperbaric Oxygen: Stimulating Production of Vasculoendothelial and Glial Cell Line-Derived Neurotrophic Growth Factors and Interleukin-10. J. Neurotrauma 2010, 27, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, X.-H.; Qu, S.-D.; Yang, J.; Wang, Z.-W.; Gao, C.-J.; Su, Q.-J. Hyperbaric oxygen intervention on expression of hypoxia-inducible factor-1α and vascular endothelial growth factor in spinal cord injury models in rats. Chin. Med. J. 2013, 126, 3897–3903. [Google Scholar]
- Vlodavsky, E.; Palzur, E.; Feinsod, M.; Soustiel, J.F. Evaluation of the apoptosis-related proteins of the BCL-2 family In the traumatic penumbra area of the rat model of cerebral contusion, treated by hyperbaric oxygen therapy: A quantitative immunohistochemical study. Acta Neuropathol. 2005, 110, 120–126. [Google Scholar] [CrossRef]
- Harpur, G.D.; Suke, R.; Bass, B.H.; Bass, M.J.; Bull, S.B.; Reese, L.; Noseworthy, J.H.; Rice, G.P.A.; Ebers, G.C. Hyperbaric oxygen therapy in chronic stable multiple sclerosis: Double-blind study. Neurology 1986, 36, 988. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.; Mathieu, C.; Chacornac, R.; Aimard, G.; Devic, M. Ineffectiveness of hyperbaric oxygen therapy in multiple sclerosis. A randomized placebo-controlled double-blind study. Presse Med. (Paris Fr. 1983) 1986, 15, 1319–1322. [Google Scholar]
- Meneghetti, G.; Spartà, S.; Rusca, F.; Facco, E.; Martini, A.; Comacchio, F.; Schiraldi, C. Hyperbaric oxygen therapy in the treatment of multiple sclerosis. A clinical and electrophysiological study in a 2 year follow-up. Riv. di Neurol. 1990, 60, 67–71. [Google Scholar]
- Kleijnen, J.; Knipschild, P. Hyperbaric oxygen for multiple sclerosis. Review of controlled trials. Acta Neurol. Scand. 1995, 91, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.; Heard, R. Hyperbaric oxygen therapy for multiple sclerosis. Cochrane Database Syst. Rev. 1999. [Google Scholar] [CrossRef]
- Bittner, S.; Afzali, A.M.; Wiendl, H.; Meuth, S.G. Myelin Oligodendrocyte Glycoprotein (MOG35-55) Induced Experimental Autoimmune Encephalomyelitis (EAE) in C57BL/6 Mice. J. Vis. Exp. 2014, e51275. [Google Scholar] [CrossRef] [Green Version]
- Glatigny, S.; Bettelli, E. Experimental Autoimmune Encephalomyelitis (EAE) as Animal Models of Multiple Sclerosis (MS). Cold Spring Harb. Perspect. Med. 2018, 8, a028977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loos, J.; Schmaul, S.; Noll, T.M.; Paterka, M.; Schillner, M.; Löffel, J.T.; Zipp, F.; Bittner, S. Functional characteristics of Th1, Th17, and ex-Th17 cells in EAE revealed by intravital two-photon microscopy. J. Neuroinflamm. 2020, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Duarte, J.H.; Veldhoen, M.; Hornsby, E.; Li, Y.; Cua, D.J.; Ahlfors, H.; Wilhelm, C.; Tolaini, M.; Menzel, U.; et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 2011, 12, 255–263. [Google Scholar] [CrossRef]
- Jäger, A.; Dardalhon, V.; Sobel, R.A.; Bettelli, E.; Kuchroo, V.K. Th1, Th17, and Th9 Effector Cells Induce Experimental Autoimmune Encephalomyelitis with Different Pathological Phenotypes. J. Immunol. 2009, 183, 7169–7177. [Google Scholar] [CrossRef]
- Langrish, C.L.; Chen, Y.; Blumenschein, W.M.; Mattson, J.; Basham, B.; Sedgwick, J.D.; McClanahan, T.; Kastelein, R.A.; Cua, D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005, 201, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Codarri, L.; Gyülvészi, G.; Tosevski, V.; Hesske, L.; Fontana, A.; Magnenat, L.; Suter, T.; Becher, B. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 2011, 12, 560–567. [Google Scholar] [CrossRef] [PubMed]
- El-Behi, M.; Ciric, B.; Dai, H.; Yan, Y.; Cullimore, M.; Safavi, F.; Zhang, G.X.; Dittel, B.N.; Rostami, A. The encephalitogenici-ty of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 2011, 12, 568–575. [Google Scholar] [CrossRef] [Green Version]
- Chiou, H.-Y.C.; Lin, M.-W.; Hsiao, P.-J.; Chen, C.-L.; Chiao, S.; Lin, T.-Y.; Chen, Y.-C.; Wu, D.-C. Dulaglutide Modulates the Development of Tissue-Infiltrating Th1/Th17 Cells and the Pathogenicity of Encephalitogenic Th1 Cells in the Central Nervous System. Int. J. Mol. Sci. 2019, 20, 1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haase, S.; Linker, R.A. Inflammation in multiple sclerosis. Ther. Adv. Neurol. Disord. 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-L.; Hai, Y.; Zhang, X.-N.; Wang, Y.-S.; Liu, X.-H.; Ma, L.-L.; Yue, R.; Xu, G.; Li, Z. Hyperbaric oxygen improves functional recovery of rats after spinal cord injury via activating stromal cell-derived factor-1/CXC chemokine receptor 4 axis and promoting brain-derived neurothrophic factor expression. Chin. Med. J. 2019, 132, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Li, Q.; Shi, W. Hyperbaric oxygen alleviates the activation of NLRP-3-inflammasomes in traumatic brain injury. Mol. Med. Rep. 2017, 16, 3922–3928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Fang, L.; Huang, S.; Yang, Z.; Nandi, J.; Thomas, S.; Chen, C.; Camporesi, E. Hyperbaric Oxygenation Therapy Alleviates Chronic Constrictive Injury–Induced Neuropathic Pain and Reduces Tumor Necrosis Factor-Alpha Production. Anesthesia Analg. 2011, 113, 626–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, K.-C.; Niu, K.-C.; Tsai, K.-J.; Kuo, J.-R.; Wang, L.-C.; Chio, C.-C.; Chang, C.-P. Attenuating inflammation but stimulating both angiogenesis and neurogenesis using hyperbaric oxygen in rats with traumatic brain injury. J. Trauma Inj. Infect. Crit. Care 2012, 72, 650–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Duan, X.-S.; Xu, L.-J.; Zhao, J.-J.; She, Z.-F.; Chen, W.-W.; Zheng, Z.-J.; Jiang, G.-D. Interleukin-10 mediates the neuroprotection of hyperbaric oxygen therapy against traumatic brain injury in mice. Neuroscience 2014, 266, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.-K.; Cao, H.-H.; Ying, X.; Zhang, H.-T.; Yu, H.-L. The effects of hyperbaric oxygen on macrophage polarization after rat spinal cord injury. Brain Res. 2015, 1606, 68–76. [Google Scholar] [CrossRef]
- Li, H.-Z.; Chen, J.-F.; Liu, M.; Shen, J. Effect of hyperbaric oxygen on the permeability of the blood-brain barrier in rats with global cerebral ischemia/reperfusion injury. Biomed. Pharmacother. 2018, 108, 1725–1730. [Google Scholar] [CrossRef]
- Geng, F.; Zhuang, X.; Yao, L.; Ma, Y.; Xing, T.; Zhu, J. Effects of Hyperbaric Oxygen Therapy on Inflammasome Signaling after Traumatic Brain Injury. Neuroimmunomodulation 2016, 23, 122–129. [Google Scholar] [CrossRef]
- Li, Z.; Liang, F.; Li, C.; Gao, C.; Yang, J.; Liu, X.; Wang, Y. Effects of hyperbaric oxygen therapy on NACHT domain-leucine-rich-repeat- and pyrin domain-containing protein 3 inflammasome expression in rats following spinal cord injury. Mol. Med. Rep. 2015, 11, 4650–4656. [Google Scholar] [CrossRef]
- Qin, Z.; Karabiyikoglu, M.; Hua, Y.; Silbergleit, R.; He, Y.; Keep, R.F.; Xi, G. Hyperbaric Oxygen-Induced Attenuation of Hemorrhagic Transformation After Experimental Focal Transient Cerebral Ischemia. Stroke 2007, 38, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, N.H.; Marchetti, L.; Moalli, F.; Duc, D.; Basso, C.; Tardent, H.; Kaba, E.; Deutsch, U.; Pot, C.; Sallusto, F.; et al. Intercellular Adhesion Molecule-1 (ICAM-1) and ICAM-2 Differentially Contribute to Peripheral Activation and CNS Entry of Autoaggressive Th1 and Th17 Cells in Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2020, 10. [Google Scholar] [CrossRef]
- Balasa, R.; Barcutean, L.; Balasa, A.; Motataianu, A.; Roman-Filip, C.; Manu, D. The action of TH17 cells on blood brain barrier in multiple sclerosis and experimental autoimmune encephalomyelitis. Hum. Immunol. 2020, 81, 237–243. [Google Scholar] [CrossRef]
- Dusi, S.; Angiari, S.; Pietronigro, E.C.; Lopez, N.; Angelini, G.; Zenaro, E.; Della Bianca, V.; Tosadori, G.; Paris, F.; Amoruso, A.; et al. LFA-1 Controls Th1 and Th17 Motility Behavior in the Inflamed Central Nervous System. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Lutz, S.E.; Smith, J.R.; Kim, D.H.; Olson, C.V.L.; Ellefsen, K.; Bates, J.M.; Gandhi, S.P.; Agalliu, D. Caveolin1 Is Required for Th1 Cell Infiltration, but Not Tight Junction Remodeling, at the Blood-Brain Barrier in Autoimmune Neuroinflammation. Cell Rep. 2017, 21, 2104–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, D.Y.S.; Kooij, G.; Heijnen, P.D.A.M.; Breur, M.; Peferoen, L.A.N.; Van Der Valk, P.; De Vries, H.E.; Amor, S.; Dijkstra, C.D. GM-CSF promotes migration of human monocytes across the blood brain barrier. Eur. J. Immunol. 2015, 45, 1808–1819. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.W.; Cahill, J.; Yamaguchi-Okada, M.; Zhang, J.H. Oxygen treatment after experimental hypoxia-ischemia in neonatal rats alters the expression of HIF-1α and its downstream target genes. J. Appl. Physiol. 2006, 101, 853–865. [Google Scholar] [CrossRef]
- Hu, Q.; Liang, X.; Chen, D.; Chen, Y.; Doycheva, D.; Tang, J.; Tang, J.; Zhang, J.H. Delayed Hyperbaric Oxygen Therapy Promotes Neurogenesis Through Reactive Oxygen Species/Hypoxia-Inducible Factor-1α/β-Catenin Pathway in Middle Cerebral Artery Occlusion Rats. Stroke 2014, 45, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Dang, E.; Barbi, J.; Yang, H.-Y.; Jinasena, D.; Yu, H.; Zheng, Y.; Bordman, Z.; Fu, J.; Kim, Y.; Yen, H.-R.; et al. Control of TH17/Treg Balance by Hypoxia-Inducible Factor 1. Cell 2011, 146, 772–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda, K.; Takeuchi, Y.; Hirota, K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019, 41, 283–297. [Google Scholar] [CrossRef]
- Haak, S.; Croxford, A.L.; Kreymborg, K.; Heppner, F.; Pouly, S.; Becher, B.; Waisman, A. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J. Clin. Investig. 2008, 119, 61–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQualter, J.L.; Darwiche, R.; Ewing, C.; Onuki, M.; Kay, T.W.; A Hamilton, J.; Reid, H.H.; Bernard, C.C. Granulocyte Macrophage Colony-Stimulating Factor. J. Exp. Med. 2001, 194, 873–882. [Google Scholar] [CrossRef] [Green Version]
- Griffin, G.K.; Newton, G.; Tarrio, M.L.; Bu, D.-X.; Maganto-Garcia, E.; Azcutia, V.; Alcaide, P.; Grabie, N.; Luscinskas, F.; Croce, K.J.; et al. IL-17 and TNF-α Sustain Neutrophil Recruitment during Inflammation through Synergistic Effects on Endothelial Activation. J. Immunol. 2012, 188, 6287–6299. [Google Scholar] [CrossRef] [PubMed]
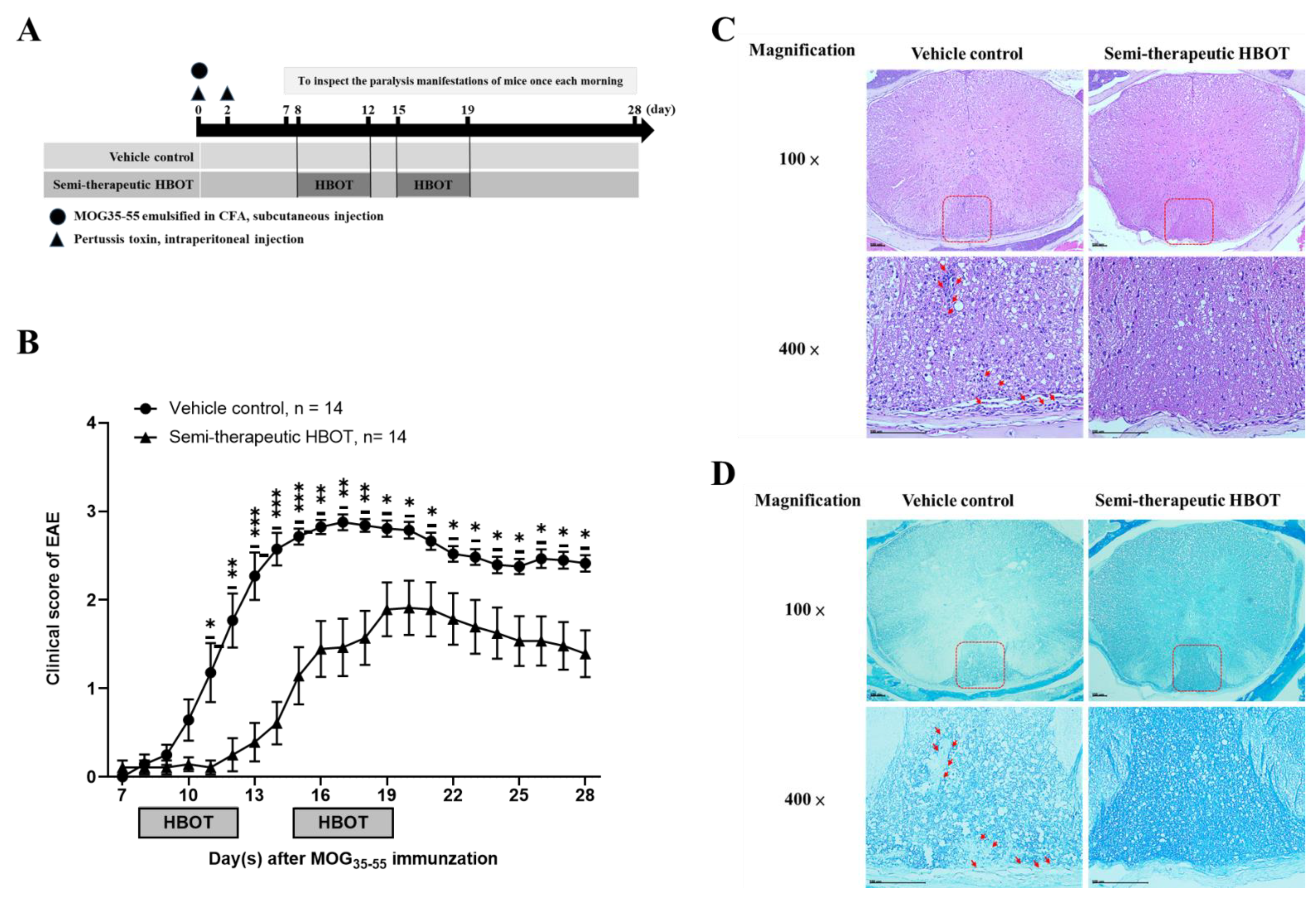
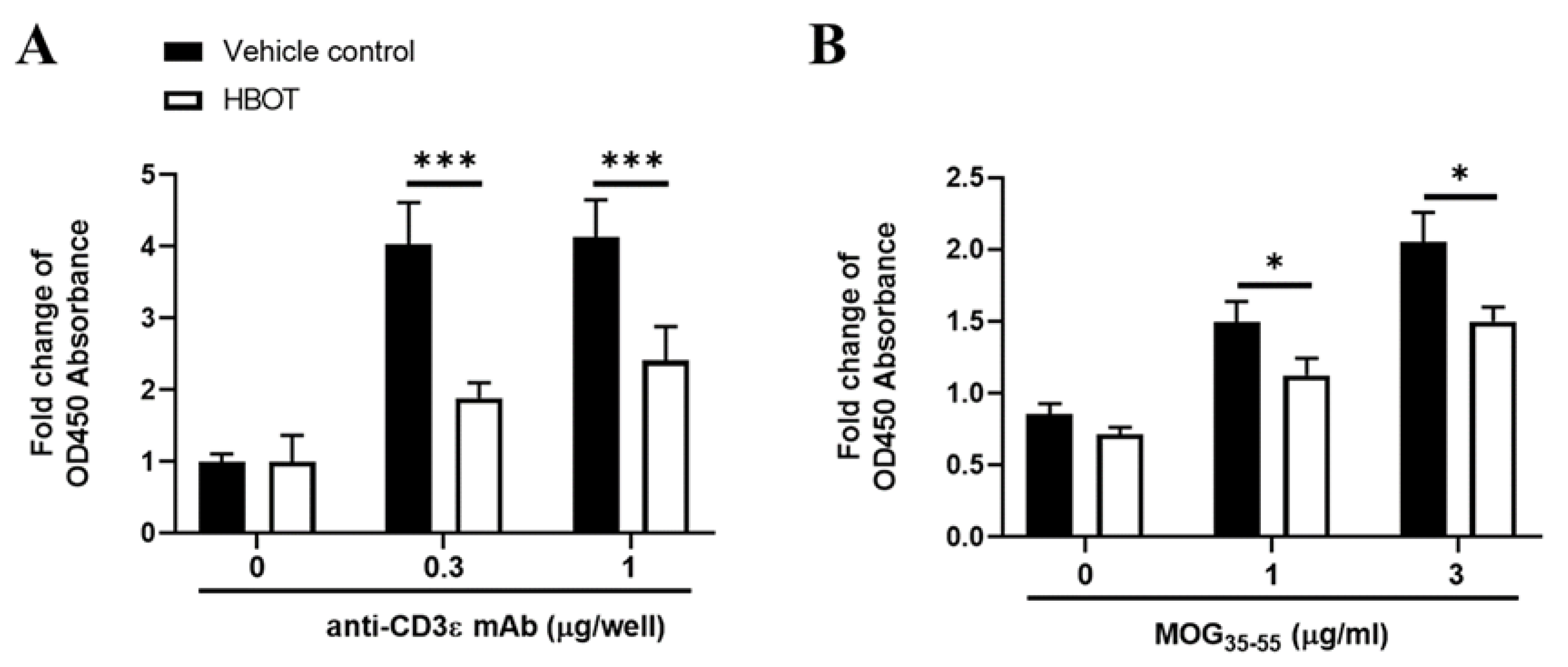
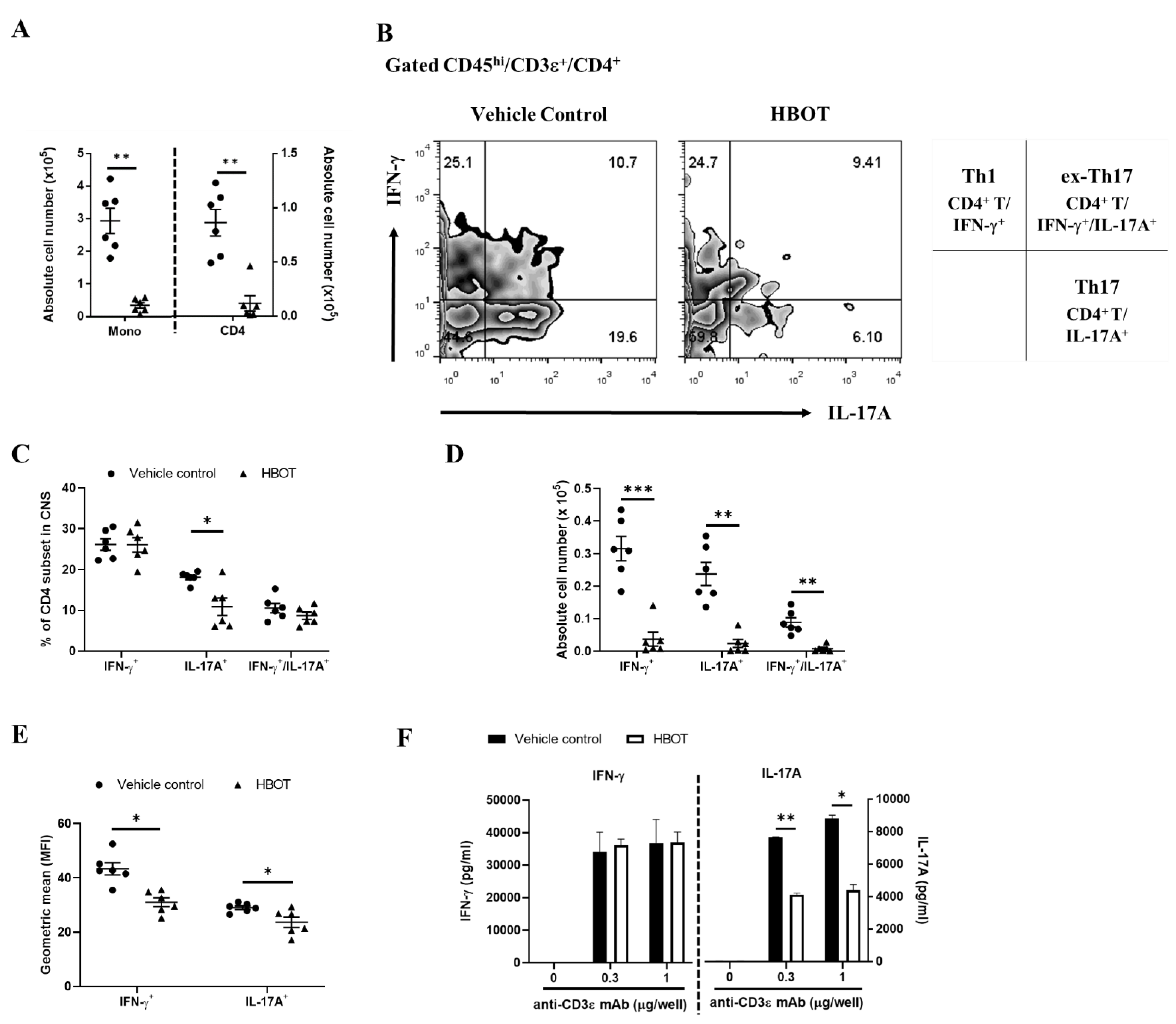
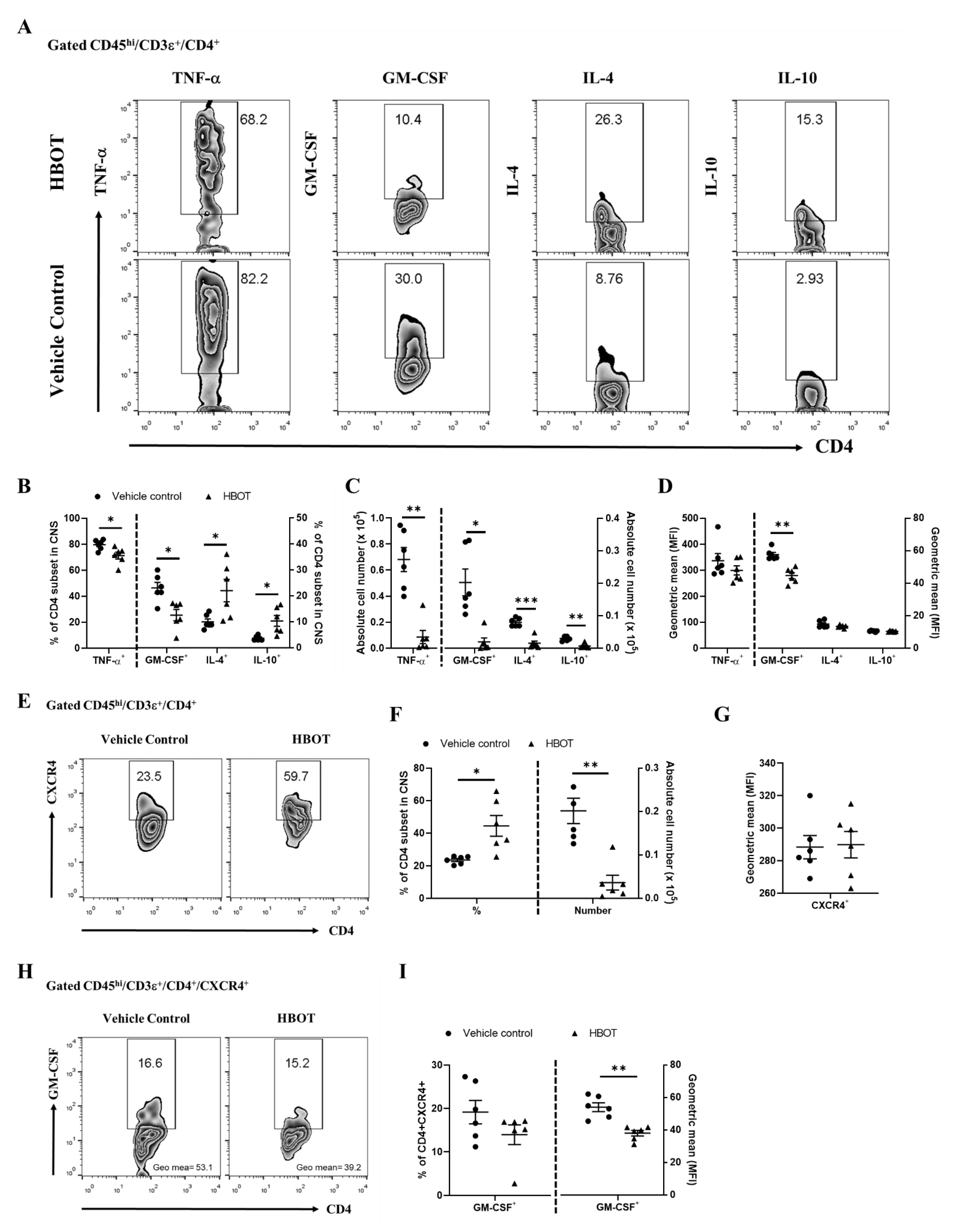
| Treatment Group | EAE Incidence | Day(s) of Onset (Mean ± SEM) | Maximal Clinical Score (Mean ± SEM) | Peak Phase with Maximal Clinical Score (Mean ± SEM) |
|---|---|---|---|---|
| Vehicle control | 14/14 (100%) | 10.6 ± 0.5 | 3.1 ± 0.1 | 3.2 ± 0.8 |
| HBOT | 12/14 (86%) | 12.3 ± 1.0 | 2.2 ± 0.3 | 2.4 ± 0.4 |
| p value | NA | 0.1439, ns | 0.0095 * | 0.7694, ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiou, H.-Y.C.; Huang, S.-H.; Hung, C.-H.; Tsai, S.-M.; Kuo, H.-R.; Huang, Y.-R.; Wang, J.-W.; Chen, S.-C.; Kuo, C.-H.; Wu, D.-C.; et al. Hyperbaric Oxygen Therapy Alleviates the Autoimmune Encephalomyelitis via the Reduction of IL-17a and GM-Csf Production of Autoreactive T Cells as Well as Boosting the Immunosuppressive IL-10 in the Central Nervous System Tissue Lesions. Biomedicines 2021, 9, 943. https://doi.org/10.3390/biomedicines9080943
Chiou H-YC, Huang S-H, Hung C-H, Tsai S-M, Kuo H-R, Huang Y-R, Wang J-W, Chen S-C, Kuo C-H, Wu D-C, et al. Hyperbaric Oxygen Therapy Alleviates the Autoimmune Encephalomyelitis via the Reduction of IL-17a and GM-Csf Production of Autoreactive T Cells as Well as Boosting the Immunosuppressive IL-10 in the Central Nervous System Tissue Lesions. Biomedicines. 2021; 9(8):943. https://doi.org/10.3390/biomedicines9080943
Chicago/Turabian StyleChiou, Hsin-Ying Clair, Shu-Hung Huang, Chih-Hsing Hung, Su-Min Tsai, Hui-Ru Kuo, Yu-Rui Huang, Jiunn-Wei Wang, Szu-Chia Chen, Chao-Hung Kuo, Deng-Chyang Wu, and et al. 2021. "Hyperbaric Oxygen Therapy Alleviates the Autoimmune Encephalomyelitis via the Reduction of IL-17a and GM-Csf Production of Autoreactive T Cells as Well as Boosting the Immunosuppressive IL-10 in the Central Nervous System Tissue Lesions" Biomedicines 9, no. 8: 943. https://doi.org/10.3390/biomedicines9080943
APA StyleChiou, H.-Y. C., Huang, S.-H., Hung, C.-H., Tsai, S.-M., Kuo, H.-R., Huang, Y.-R., Wang, J.-W., Chen, S.-C., Kuo, C.-H., Wu, D.-C., Huang, S.-K., Hsu, S.-H., & Lin, M.-H. (2021). Hyperbaric Oxygen Therapy Alleviates the Autoimmune Encephalomyelitis via the Reduction of IL-17a and GM-Csf Production of Autoreactive T Cells as Well as Boosting the Immunosuppressive IL-10 in the Central Nervous System Tissue Lesions. Biomedicines, 9(8), 943. https://doi.org/10.3390/biomedicines9080943






